Impairments in neuropsychological function have been demonstrated in people with schizophrenia at first presentation and in their unaffected relatives (Reference Cannon, Mednick and ParnasCannon et al, 1994; Reference David, Malmberg and BrandtDavid et al, 1997; Reference Heinrichs and ZakzanisHeinrichs & Zakzanis, 1998; Reference Johnson-Selfridge and ZalewskiJohnson-Selfridge & Zalewski, 2001). Some impairments are also found in people with bipolar disorder (Reference Quraishi and FrangouQuraishi & Frangou, 2002), although deficits in general intellectual function are not generally shown (Reference Robertson and TaylorRobertson & Taylor, 1985). We sought to clarify these issues by examining neuropsychological functioning in families affected by schizophrenia, bipolar disorder or both. Relatives were compared with controls to examine abnormalities contingent upon genetic liability, whereas phenotypic abnormalities were inferred from patient—relative differences. Specifically, we predicted that intellectual and executive abnormalities would be related to a genetic liability to schizophrenia, whereas memory impairments would be found in all people affected by functional psychotic illness, regardless of diagnosis.
METHOD
Sample
Patients diagnosed with schizophrenia or bipolar disorder were identified at the Royal Edinburgh Hospital and associated hospitals and their informed consent was sought. Those with a family history of one or both disorders were selected, and DSM—IV operational criteria (American Psychiatric Association, 1994) were applied to the patients and to their affected relatives wherever possible using the Operational Criteria Checklist for Psychotic Illness (OPCRIT; Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991). Healthy relatives from the families were also invited to participate. The intention was to recruit 24 people in each of the following groups.
-
(a) Patients with schizophrenia from ‘schizophrenia’ families: this group consisted of people with DSM—IV schizophrenia with at least one first- or second-degree relative with schizophrenia.
-
(b) Unaffected participants from ‘schizophrenia’ families: this group consisted of healthy people with at least two first- or second-degree relatives with schizophrenia.
-
(c) Patients with bipolar disorder from ‘bipolar’ families: this group consisted of people with DSM—IV bipolar I disorder with at least one first- or second-degree relative with bipolar disorder.
-
(d) Unaffected participants from ‘bipolar’ families: this group consisted of unaffected people with at least two first- or second-degree relatives with bipolar disorder.
-
(e) Patients with bipolar disorder from ‘mixed’ families: this group consisted of people with DSM—IV bipolar I disorder with at least one first- or second-degree relative with schizophrenia.
-
(f) Unaffected participants from ‘mixed’ families: this group consisted of unaffected people with at least one first- or second-degree relative with schizophrenia and one with bipolar disorder.
All people fulfilling study inclusion criteria were interviewed using version 9 of the Present State Examination (PSE; Reference Wing, Cooper and SartoriusWing et al, 1974). The PSE was used to supplement the information obtained from case notes, confirm the diagnosis of affected participants and confirm that apparently healthy people were indeed unaffected.
A control group consisting of 50 people with no personal or family history of schizophrenia or affective disorder was also recruited from the social network of the participants. Control status was confirmed using the Schedule for Affective Disorders and Schizophrenia, Lifetime version (SADS—L; Reference Endicott and SpitzerEndicott & Spitzer, 1978) and using data about previous medical treatment obtained at interview. Unaffected relatives were similarly interviewed to confirm the lifetime absence of major depressive disorder, bipolar disorder or schizophrenia.
Additional demographic and historical information was collected at interview on all participants using a semi-structured questionnaire. All eligible subsample members and controls were interviewed using the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987), the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) and the Young Mania Rating Scale (YMRS; Reference Young, Biggs and ZieglerYoung et al, 1978).
All relatives and controls gave informed consent to their participation. The study protocol and consent procedures were approved by the relevant ethics committees. Sample sizes were chosen on the basis of a power calculation for two independent groups at a significance level of 0.05.
Neuropsychological assessments
All neuropsychological assessments were administered by two investigators (L.H. and K.F.), masked to diagnosis. The test battery chosen included tests that had previously been shown to distinguish individuals with schizophrenia or bipolar disorder from controls and was organised according to domain of neuropsychological function (see Appendix). Genetic liability to psychosis was estimated using a continuous measure described elsewhere (Reference Lawrie, Whalley and AbukmeilLawrie et al, 2001), developed originally by Professor Sham of the Institute of Psychiatry, which assumes a liability threshold model (Reference Pearson and LeePearson & Lee, 1901) of genetic disease. Using the estimated prevalence of the disorder and published heritability estimates, the average genetic liability of someone selected at random from a population can be calculated. Using this information on average genetic liabilities combined with family history data, a revised liability estimate for individuals is given.
Statistical analysis
The distribution of each neuropsychological variable was examined for normality using a normal probability plot for each group. Where data were not normally distributed a ladder of transformations was applied, and that resulting in the greatest approximation to normal distribution was chosen. The assumption of multivariate normality was checked further using a Mahalanobis plot. Standardised residuals from the mixed-effects analyses of variance (ANOVAs) conducted were also examined for normality.
Domains of neuropsychological function (IQ, executive function and psychomotor performance) were compared between groups using a multivariate analysis of covariance (MANCOVA). All tests conducted included psychiatric symptoms and, where appropriate, age as covariates. Tests of executive function and psychomotor performance were conducted, adjusting additionally for Wechsler Abbreviated Scale of Intelligence (WASI) Full-Scale IQ (Reference WechslerWechsler, 1999). All multivariate analyses were conducted using the proc glm procedure within the statistical package SAS, version 8.2 (SAS Institute, Cary, North Carolina, USA). Memory, having only one measure, the Extended Rivermead Behavioural Memory Test (E—RBMT; Reference de Wall, Wilson and Baddeleyde Wall et al, 1994), was compared between groups using mixed-effects ANOVA.
Where the MANOVA showed an overall difference within a domain of neuropsychological function, further tests were conducted to examine first, which specific neuropsychological variable means differed between the groups, and second, specific pairwise effect sizes. Both analyses were conducted using a mixed-model ANOVA, with ‘family’ modelled as a random factor to take account of the correlation within pedigrees. Where differences were found in the overall ANOVA, controlling for WASI Full-Scale IQ (for memory and executive function) and psychiatric symptoms (HRSD, PANSS positive sub-scale and YMRS scores), the pairwise between-group contrasts were estimated, controlling for the comparison-wise error rate. Age was also included as a potential confound where this was not adjusted for in the calculation of individual test scores. All analyses were conducted using the proc mixed procedure within SAS.
The influence of medication and alcohol consumption were checked by plotting the unstandardised residuals (unexplained variation) from each analysis against current conventional antipsychotic dosage (in chlorpromazine equivalents), lithium dosage and estimated weekly alcohol consumption.
RESULTS
The flow of participants through the study is shown in Fig. 1. Over 300 patients with a diagnosis of either schizophrenia or bipolar disorder were identified. Of the 110 patients with a family history of either schizophrenia or affective disorder who were invited to participate, 102 gave their consent. On the basis of combined information from the PSE and case notes, 80 patients met study inclusion criteria and 74 provided complete clinical data and near-complete neuropsychological data. From the families of eligible patients meeting study inclusion criteria, a further 160 apparently unaffected close family members were identified; 85 of them then underwent a semi-structured interview about previous psychiatric problems using the PSE. On the basis of this information 80 persons met study inclusion criteria, of whom 76 provided complete clinical data and near-complete neuropsychological data. Fifty-four potential control participants were identified. All completed a semi-structured interview using the SADS—L. Three were excluded because of a history of previous psychiatric disorder (one with anorexia nervosa and two with a major depressive episode). Fifty individuals provided near-complete neuropsychological data. Demographic information about the participants is given in Table 1.

Fig. 1 Flow of participants through the study.
Table 1 Demographic details of participants

| Group | Number of participants/families n/n | Age; years Mean (s.d.) | Male n (%) | Education1 n (%) | Parental SES2 n (%) | Married3 n (%) |
|---|---|---|---|---|---|---|
| Control | 50/50 | 35.5 (11.2) | 23 (46) | 39 (78) | 32 (64) | 22 (44) |
| SCZ from SCZ family | 27/24 | 37.6 (14.0) | 13 (48) | 10 (37) | 16 (59) | 3 (11) |
| UA from SCZ family | 25/18 | 38.8 (12.6) | 11 (44) | 11 (44) | 13 (52) | 13 (52) |
| BPD from BPD family | 27/21 | 40.3 (11.9) | 14 (52) | 20 (74) | 15 (56) | 11 (41) |
| UA from BPD family | 24/10 | 33.5 (12.8) | 9 (38) | 17 (71) | 13 (54) | 10 (42) |
| BPD from ‘mixed’ family | 20/18 | 40.5 (9.6) | 7 (35) | 12 (60) | 10 (50) | 5 (25) |
| UA from ‘mixed’ family | 27/14 | 34.4 (12.8) | 14 (52) | 13 (48) | 9 (33) | 14 (52) |
The patient groups were closely balanced in terms of duration of illness (estimated from current age minus age at first presentation), but differed in terms of psychiatric symptom measurements and prescribed medication (Table 2). Patients with schizophrenia were prescribed more antipsychotic medication and had higher levels of (positive) psychotic and depressive symptoms than patients with bipolar disorder from either ‘bipolar’ or ‘mixed’ families. In contrast, patients with bipolar disorder from ‘bipolar’ families had the highest doses of lithium prescribed and patients with bipolar disorder from ‘mixed’ families had the highest numbers of manic symptoms compared with the other groups.
Table 2 Illness duration, prescribed medication and current symptoms
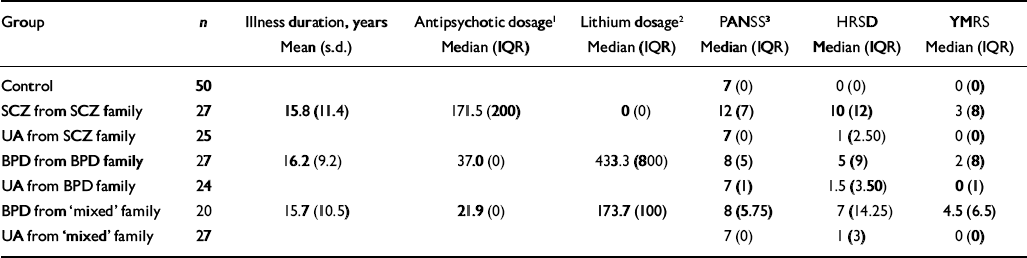
| Group | n | Illness duration, years Mean (s.d.) | Antipsychotic dosage1 Median (IQR) | Lithium dosage2 Median (IQR) | PANSS3 Median (IQR) | HRSD Median (IQR) | YMRS Median (IQR) |
|---|---|---|---|---|---|---|---|
| Control | 50 | 7 (0) | 0 (0) | 0 (0) | |||
| SCZ from SCZ family | 27 | 15.8 (11.4) | 171.5 (200) | 0 (0) | 12 (7) | 10 (12) | 3 (8) |
| UA from SCZ family | 25 | 7 (0) | 1 (2.50) | 0 (0) | |||
| BPD from BPD family | 27 | 16.2 (9.2) | 37.0 (0) | 433.3 (800) | 8 (5) | 5 (9) | 2 (8) |
| UA from BPD family | 24 | 7 (1) | 1.5 (3.50) | 0 (1) | |||
| BPD from ‘mixed’ family | 20 | 15.7 (10.5) | 21.9 (0) | 173.7 (100) | 8 (5.75) | 7 (14.25) | 4.5 (6.5) |
| UA from ‘mixed’ family | 27 | 7 (0) | 1 (3) | 0 (0) |
Neuropsychology
The vectors of intellectual function (F (20,203)=2.02, P<0.01) and psychomotor function (F (15,166)=2.0, P=0.02) differed significantly between the groups using MANCOVA. Executive function showed no evidence of variation between the groups overall (F (20,196.6)=1.29, P=0.19). Since the numbers in each group were not sufficiently large to allow the conclusion that the groups were equal in terms of executive function, further mixed-effects ANOVAs were conducted to explore whether any single measure of executive function differed between the groups, despite the absence of a statistically significant difference overall. The means and standard deviations of test performance scores are shown in Table 3; these are raw scores unadjusted for confounders and for the effects of intrafamilial clustering.
Table 3 Neuropsychological measures analysed by group membership
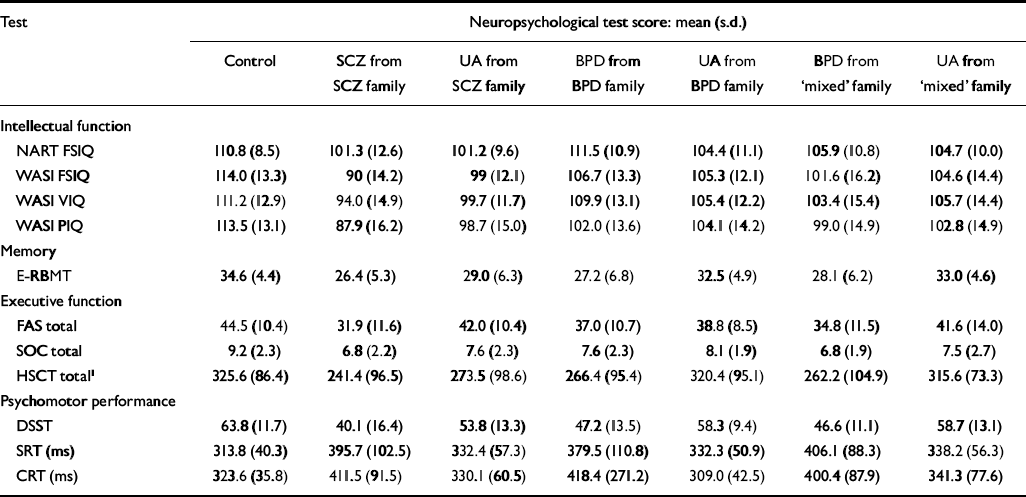
| Test | Neuropsychological test score: mean (s.d.) | ||||||
|---|---|---|---|---|---|---|---|
| Control | SCZ from SCZ family | UA from SCZ family | BPD from BPD family | UA from BPD family | BPD from ‘mixed’ family | UA from ‘mixed’ family | |
| Intellectual function | |||||||
| NART FSIQ | 110.8 (8.5) | 101.3 (12.6) | 101.2 (9.6) | 111.5 (10.9) | 104.4 (11.1) | 105.9 (10.8) | 104.7 (10.0) |
| WASI FSIQ | 114.0 (13.3) | 90 (14.2) | 99 (12.1) | 106.7 (13.3) | 105.3 (12.1) | 101.6 (16.2) | 104.6 (14.4) |
| WASI VIQ | 111.2 (12.9) | 94.0 (14.9) | 99.7 (11.7) | 109.9 (13.1) | 105.4 (12.2) | 103.4 (15.4) | 105.7 (14.4) |
| WASI PIQ | 113.5 (13.1) | 87.9 (16.2) | 98.7 (15.0) | 102.0 (13.6) | 104.1 (14.2) | 99.0 (14.9) | 102.8 (14.9) |
| Memory | |||||||
| E-RBMT | 34.6 (4.4) | 26.4 (5.3) | 29.0 (6.3) | 27.2 (6.8) | 32.5 (4.9) | 28.1 (6.2) | 33.0 (4.6) |
| Executive function | |||||||
| FAS total | 44.5 (10.4) | 31.9 (11.6) | 42.0 (10.4) | 37.0 (10.7) | 38.8 (8.5) | 34.8 (11.5) | 41.6 (14.0) |
| SOC total | 9.2 (2.3) | 6.8 (2.2) | 7.6 (2.3) | 7.6 (2.3) | 8.1 (1.9) | 6.8 (1.9) | 7.5 (2.7) |
| HSCT total1 | 325.6 (86.4) | 241.4 (96.5) | 273.5 (98.6) | 266.4 (95.4) | 320.4 (95.1) | 262.2 (104.9) | 315.6 (73.3) |
| Psychomotor performance | |||||||
| DSST | 63.8 (11.7) | 40.1 (16.4) | 53.8 (13.3) | 47.2 (13.5) | 58.3 (9.4) | 46.6 (11.1) | 58.7 (13.1) |
| SRT (ms) | 313.8 (40.3) | 395.7 (102.5) | 332.4 (57.3) | 379.5 (110.8) | 332.3 (50.9) | 406.1 (88.3) | 338.2 (56.3) |
| CRT (ms) | 323.6 (35.8) | 411.5 (91.5) | 330.1 (60.5) | 418.4 (271.2) | 309.0 (42.5) | 400.4 (87.9) | 341.3 (77.6) |
General intellectual function
Premorbid intellectual function, measured using the National Adult Reading Test (NART; Reference NelsonNelson, 1982), and current intellectual function measured by WASI Full-Scale IQ, Performance IQ and Verbal IQ scores (Reference WechslerWechsler, 1999) all differed significantly between the groups. Patients with schizophrenia and unaffected participants from families with schizophrenia had significantly lower NART IQ scores than the control group, and patients with schizophrenia also had significantly lower scores on this measure compared with people with bipolar disorder from either group. Patients with bipolar disorder from families with bipolar disorder had significantly higher NART scores than their unaffected relatives (Table 4).
Table 4 Mixed-effects analysis of variance by domain of neuropsychological function
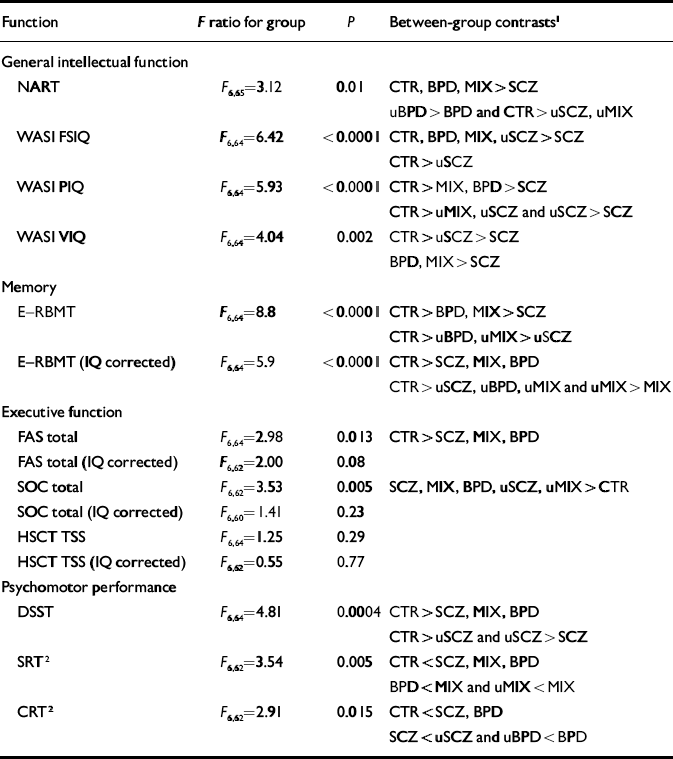
| Function | F ratio for group | P | Between-group contrasts1 |
|---|---|---|---|
| General intellectual function | |||
| NART | F 6,65=3.12 | 0.01 | CTR, BPD, MIX>SCZ |
| uBPD>BPD and CTR>uSCZ, uMIX | |||
| WASI FSIQ | F 6,64=6.42 | <0.0001 | CTR, BPD, MIX, uSCZ>SCZ |
| CTR>uSCZ | |||
| WASI PIQ | F 6,64=5.93 | <0.0001 | CTR>MIX, BPD>SCZ |
| CTR>uMIX, uSCZ and uSCZ>SCZ | |||
| WASI VIQ | F 6,64=4.04 | 0.002 | CTR>uSCZ>SCZ |
| BPD, MIX>SCZ | |||
| Memory | |||
| E—RBMT | F 6,64=8.8 | <0.0001 | CTR>BPD, MIX>SCZ |
| CTR>uBPD, uMIX>uSCZ | |||
| E—RBMT (IQ corrected) | F 6,64=5.9 | <0.0001 | CTR>SCZ, MIX, BPD |
| CTR>uSCZ, uBPD, uMIX and uMIX>MIX | |||
| Executive function | |||
| FAS total | F 6,64=2.98 | 0.013 | CTR>SCZ, MIX, BPD |
| FAS total (IQ corrected) | F 6,62=2.00 | 0.08 | |
| SOC total | F 6,62=3.53 | 0.005 | SCZ, MIX, BPD, uSCZ, uMIX>CTR |
| SOC total (IQ corrected) | F 6,60=1.41 | 0.23 | |
| HSCT TSS | F 6,64=1.25 | 0.29 | |
| HSCT TSS (IQ corrected) | F 6,62=0.55 | 0.77 | |
| Psychomotor performance | |||
| DSST | F 6,64=4.81 | 0.0004 | CTR>SCZ, MIX, BPD |
| CTR>uSCZ and uSCZ>SCZ | |||
| SRT2 | F 6,62=3.54 | 0.005 | CTR<SCZ, MIX, BPD |
| BPD<MIX and uMIX<MIX | |||
| CRT2 | F 6,62=2.91 | 0.015 | CTR<SCZ, BPD |
| SCZ<uSCZ and uBPD<BPD |
Patients with schizophrenia, patients with bipolar disorder from ‘mixed’ families and unaffected relatives from ‘schizophrenia’ families had significantly lower WASI Full-Scale IQ scores than the control group. Although unaffected relatives from ‘mixed’ families did not differ significantly from controls in terms of WASI Full-Scale IQ, the difference was similar to that found between unaffected relatives from ‘schizophrenia’ families and controls (7 IQ points ν. 10). Patients with schizophrenia also had significantly lower WASI Full-Scale IQ scores than patients with bipolar disorder from either group and indeed than their own healthy relatives.
In terms of Verbal IQ, patients with schizophrenia and their healthy relatives had significantly lower WASI scores than controls, but both bipolar disorder groups and their unaffected relatives did not differ significantly from controls. A greater difference was observed between unaffected relatives from ‘mixed’ families and controls than between unaffected relatives from ‘bipolar’ families and controls. Patients with schizophrenia also had significantly lower WASI Verbal IQ scores than either their own relatives (difference –9.8, 95% CI –17.45 to 1.83) or either group of patients with bipolar disorder. The latter two groups did not differ significantly from their unaffected relatives on this measure.
The pattern of impairment in terms of WASI Performance IQ differed from that of other intellectual measures. Patients with schizophrenia or bipolar disorder (whether from ‘bipolar’ or ‘mixed’ families) had significantly lower Performance IQ scores than controls. The effect size was greatest in those with schizophrenia (difference 25.3, 95% CI 16.68 to 33.92), intermediate in patients with bipolar disorder from ‘mixed’ families (difference 14.50, 95% CI 5.79 to 23.20) and least in patients with bipolar disorder from ‘bipolar’ families (difference 10.43, 95% CI 2.50 to 18.37). Unaffected relatives from ‘schizophrenia’ or ‘mixed’ families were also significantly more impaired in terms of Performance IQ than controls. No trend to significance was found for the unaffected relatives from ‘bipolar’ families. Patients with schizophrenia were also significantly more impaired than their own relatives. Although no significant differences between the unaffected relative groups were found, impairments were greatest in the unaffected relatives from ‘schizophrenia’ families, intermediate in the unaffected relatives from ‘mixed’ families and least in the unaffected relatives from ‘bipolar’ families.
Memory
Scores on the E—RBMT were lower in all patient and relative groups compared with controls. There was no evidence of disease specificity among affected individuals although unaffected relatives from ‘schizophrenia’ families were more impaired than unaffected relatives from either ‘bipolar’ families (difference –3.96, 95% CI –7.25 to –0.67) or ‘mixed’ families (difference – 4.11, 95% CI –7.25 to –0.97). Once WASI IQ was taken into account, the nature of the results changed little. Controls performed better than all other groups and, among affected participants, there was little evidence of disease specificity. However, patients with bipolar disorder from ‘mixed’ families performed worse than their unaffected relatives (difference 3.8, 95% CI 0.5 to 7.1).
Executive function
No difference was found between the groups in terms of performance on the Hayling Sentence Completion Test (HSCT; Reference Burgess and ShalliceBurgess & Shallice, 1996), whether controlled for current IQ or not. Total verbal fluency and performance on the Stockings of Cambridge test (Reference Sahakian and OwenSahakian & Owen, 1992) differed among the groups in the non-IQ-controlled analysis. Patients from all groups performed worse in terms of verbal fluency and the Stockings of Cambridge test than controls. Unaffected relatives from either ‘schizophrenia’ or ‘mixed’ families also performed significantly worse than controls on the Stockings of Cambridge test. Once these analyses were adjusted for current intellectual function, no significant difference remained.
Psychomotor performance
The number of correct substitutions on the Digit—Symbol Substitution Test (DSST; Reference ErberErber, 1976) differed significantly between groups (F (6,64)=4.81, P=0.0004). All patient groups were impaired relative to controls. Similarly, unaffected relatives from all families made fewer correct substitutions than controls, although only unaffected relatives from ‘schizophrenia’ families performed significantly worse than controls (difference 6.91, 95% CI 0.94 to 12.87). Patients with schizophrenia did significantly worse than their unaffected relatives (difference 10.6, 95% CI 3.7 to 17.5) and showed a tendency to do worse than the other patient groups. There was no evidence of a difference between patients from ‘mixed’ or ‘bipolar’ families.
Simple reaction time differed significantly between groups. All patient groups were significantly impaired compared with controls, although no difference was found between controls and any unaffected relative group. Patients with bipolar disorder from ‘mixed’ families did significantly worse than those from ‘bipolar’ families (difference – 56.41, 95% CI –104.94 to –7.89). Patients with schizophrenia and patients with bipolar disorder from ‘mixed’ families did significantly worse than their unaffected relatives.
Choice reaction time also differed between groups. All patient groups were significantly impaired compared with controls, although no difference was found between the control group and any unaffected relative group. Patients with schizophrenia performed less well than their unaffected relatives (difference 77.93, 95% CI 30.11 to 125.76) and patients with bipolar disorder also did less well than their unaffected relatives (difference –104.70, 95% CI –178.79 to –30.61). However, no significant difference was found between patients with bipolar disorder from ‘mixed’ families and their unaffected relatives. Differences between affected or unaffected groups showed no diagnostic or familial specificity.
Effects of medication and weekly alcohol consumption
There was no statistically significant association between weekly alcohol consumption, prescribed daily lithium or conventional antipsychotic dosages and any measure of psychomotor performance.
Relationship of neuropsychology to genetic liability
The relationship of measures of intellectual and mnemonic function (NART IQ and WASI Full-Scale, Verbal and Performance IQ scores) to genetic liability was computed for all six groups where there was a family history of psychiatric disorder. Within the group of patients with schizophrenia, genetic liability to schizophrenia was inversely related to NART IQ (r= – 70.49, P=0.01), WASI Full-Scale IQ (r= –0.33, P=0.1) and WASI Verbal IQ (r=–0.55, P=0.004) (Fig. 2) but not WASI Performance IQ (r=–0.01, P=0.94). However, within the unaffected relatives from ‘schizophrenia’ families, genetic liability to schizophrenia was positively related to Performance IQ (r=0.45, P 0.03) (Fig. 3) and Full-Scale IQ (r=0.36, P=0.08, trend only). No relationship was found between NART IQ (r=0.31, P=0.13) or WASI Verbal IQ and genetic liability (r=0.06, P=0.78).
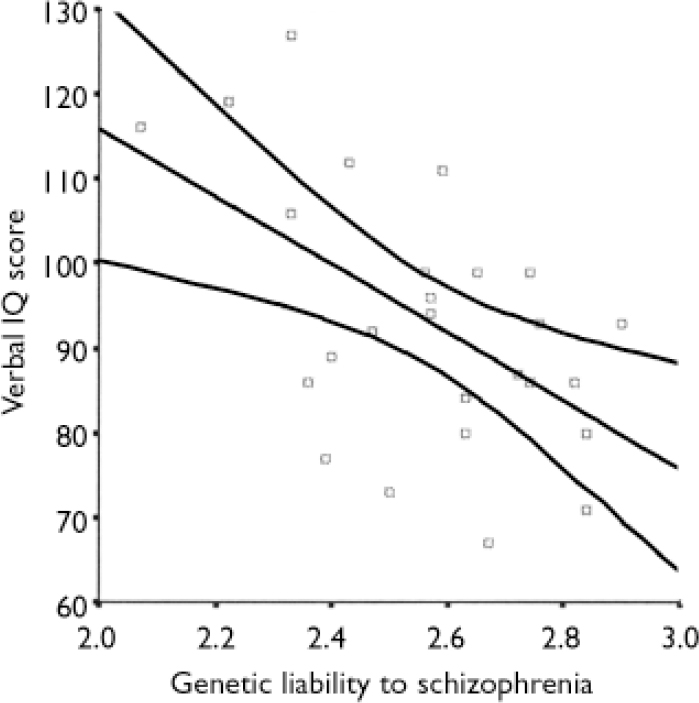
Fig. 2 Relationship between genetic liability to schizophrenia and Verbal IQ in patients with schizophrenia (r 2=0.3).

Fig. 3 Relationship between genetic liability to schizophrenia and Performance IQ in the relatives of patients with schizophrenia (r 2= 0.2).
Within the group of patients with bipolar disorder from ‘mixed’ families there was no significant relationship between genetic liability to schizophrenia and measures of IQ, and no suggestion of any trend. Within unaffected relatives from ‘mixed’ families, genetic liability was not related to NART IQ or WASI Full-Scale or Performance IQ; however, WASI Verbal IQ showed a trend to a negative association with genetic liability to schizophrenia (r=–0.39, P=0.05). There was no evidence of a relationship between genetic liability to schizophrenia and scores on the E—RBMT within any group. There was no evidence of a relationship between genetic liability to bipolar disorder and any measure of IQ within the groups of patients with bipolar disorder from ‘bipolar’ families, their unaffected relatives or patients with bipolar disorder from ‘mixed’ families. However, unaffected relatives from ‘mixed’ families showed negative associations between genetic liability to bipolar disorder and WASI Full-Scale (r=–0.50, P=0.009), Verbal IQ (r=–0.47, P=0.01) and Performance IQ (r=–0.40, P=0.04), but not NART IQ. There was no evidence of a relationship between genetic liability to bipolar disorder and scores on the E—RBMT within any group.
DISCUSSION
In a study of 200 people — patients with functional psychosis, their healthy relatives and controls – several measures of neuropsychological function were estimated with correction for current psychiatric symptoms, the non-independence of observations within families and, where appropriate, age and current IQ. Current, verbal and premorbid IQ were impaired in people with schizophrenia and in their relatives. People with bipolar disorder and their relatives were not impaired on these measures, unless at least one other family member had schizophrenia. Performance IQ, in contrast, was impaired in all the patient groups, but not in unaffected individuals who had two or more relatives with bipolar disorder only. Memory was impaired across all patient and relative groups compared with controls, although patients with schizophrenia were affected more severely than those with bipolar disorder. This difference was not accounted for by differences in IQ. Psychomotor performance was impaired in patients compared with controls, regardless of diagnosis and regardless of whether the test involved a strong motor (reaction time) or cognitive (DSST) component. However, unaffected individuals with two or more relatives with schizophrenia showed impairments on the DSST test that were not present in the other unaffected relative groups.
This study did not find an overall difference in executive function across the groups. Post hoc testing revealed possible impairments in verbal fluency within all patient groups and reductions in planning ability (Stockings of Cambridge test) in all patients and in unaffected individuals with at least one relative with schizophrenia. However, neither of these findings survived correction for current intellectual function.
These findings suggest that intellectual function, planning ability and psychomotor tests with a high cognitive component are preferentially impaired in the relatives of people with schizophrenia. The fact that, among relatives of people with schizophrenia, Verbal IQ is positively related to a genetic liability to schizophrenia is somewhat counterintuitive. However, it finds precedent in an earlier study showing that ‘obligate carriers’ (unaffected people who appear to transmit a parental diagnosis of schizophrenia to their offspring) of schizophrenia had a higher IQ than controls (Reference Steel, Whalley and MillerSteel et al, 2002). Since our sample included people who will develop schizophrenia or other psychiatric illnesses in later years, it is possible that their inclusion explains the reduced mean IQ in this group overall. Furthermore, the positive association between IQ and genetic liability provides some evidence that genes for schizophrenia may convey an advantage in unaffected individuals.
Strengths and weaknesses of the study
All groups were well balanced in terms of age, gender, paternal social class and weekly alcohol consumption. A history of compulsory education only or less was more common both in patients with schizophrenia and in their unaffected relatives than in the other five groups. Although all groups had relatively low scores on the HRSD, YMRS and PANSS, none of the groups was symptom-free and most patients were taking medication. However, allowance for current symptoms was made at the analysis stage and none of the remaining variation in test scores could be accounted for by medication.
Relationship to other research
This study confirms others that suggest that intellectual deficits are related to a genetic liability to schizophrenia, but is one of the few to study contemporaneously patients with schizophrenia and bipolar disorder. The positive association between genetic liability to schizophrenia and IQ in unaffected relatives is novel, as far as we know.
Studies of patients with schizophrenia (Reference Aleman, Hijman and de HaanAleman et al, 1999) and bipolar disorder (Reference Quraishi and FrangouQuraishi & Frangou, 2002) have shown reductions in memory compared with controls which are also evident in direct comparisons (Reference Seidman, Kremen and KorenSeidman et al, 2002; Reference McClellan, Prezbindowski and BriegerMcClellan et al, 2004) and are of similar magnitude regardless of diagnosis. However, the propensity of psychotropic medication and symptoms to confound these results has rarely been investigated.
For the HSCT, no difference was observed between any groups and controls for overall scaled score or error score. This finding is in contrast to several others, including one from the Edinburgh High-Risk Study (Reference Byrne, Clafferty and CoswayByrne et al, 2003). However, patients in our study were on average 10–20 years older than those in the Edinburgh study. The unaffected sample therefore includes fewer people likely to develop psychosis in future.
Deficits in cognitive tasks with a high attentional component have previously been found in the relatives of patients with schizophrenia (Reference Pogue-Geile, Garrett and BrunkePogue-Geile et al, 1991; Reference Franke, Maier and HardtFranke et al, 1993). This has suggested to some that attentional deficits may be a mechanism by which schizophrenia might develop. The finding of attentional deficits in unaffected individuals with relatives with schizophrenia supports this view. However, their presence in people with bipolar disorder suggests that once the disease is apparent, attentional deficits show no diagnostic specificity and may possibly act to maintain psychiatric symptoms regardless of the factors leading to their development.
Future work
Although our study examined groups of people with ‘functional psychosis’, it is unclear whether the differences observed relate only to diagnosis or whether they relate to psychotic symptoms. Most people included in this study had such symptoms, although the numbers involved do not allow enough statistical power to perform sensitivity analyses. It has also been suggested that dimensions of psychotic symptoms found in affected individuals represent the extremes of a range of variation within the population as a whole. Brain anatomy and physiology may be more closely related to these dimensions than to diagnosis. A future study could usefully re-examine symptom dimensions in a range of people with psychotic illness and relate these findings to neuro-psychological test performance, brain structure and perhaps function.
APPENDIX
Neuropsychological test battery
Current intellectual function
Wechsler Abbreviated Scale of Intelligence (Reference WechslerWechsler, 1999).
Premorbid intellectual function
National Adult Reading Test (Reference NelsonNelson, 1982).
Memory
Extended Rivermead Behavioural Memory Test, version A (Reference de Wall, Wilson and Baddeleyde Wall et al, 1994).
Executive function
-
(a) Response inhibition: Hayling Sentence Completion Test.
-
(b) Spontaneous production: verbal fluency and semantic category (‘FAS'test) (Reference Spreen and StraussSpreen & Strauss, 1991).
-
(c) Planning: CANTAB Stockings of Cambridge Test (Reference Sahakian and OwenSahakian & Owen, 1992).
Psychomotor performance
-
(a) Digit—Symbol Substitution Test (Reference ErberErber, 1976).
-
(b) CANTAB Simple and Choice Reaction Times (Reference Sahakian and OwenSahakian & Owen, 1992).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Intellectual impairments are related to increased genetic liability to schizophrenia.
-
▪ Within relatives of people with schizophrenia, genetic liability is positively associated with performance IQ.
-
▪ No single neuropsychological measure was related specifically to the expression of or liability to bipolar disorder.
LIMITATIONS
-
▪ The measure of genetic liability used may be confounded by differences in memory and IQ between groups.
-
▪ Unaffected relative groups might have included people who would develop schizophrenia or other disorders in later years.
-
▪ The small number in each group might have led to false negative results.
Acknowledgements
We thank all the participants for agreeing to take part in the study and. the UK Medical Research Council for funding the principal investigator (A.M.) through a Clinical Training Fellowship.










eLetters
No eLetters have been published for this article.