Introduction
A child neurologist can effectively treat most children with epilepsy; however, about a third will have seizures that are treatment resistant after two tolerated, appropriate, anti-epileptic medications.Reference Kwan and Brodie1, Reference Kwan, Arzimanoglou and Berg2 These children, as well as those with focal onset epilepsy associated with an epileptogenic lesion on magnetic resonance imaging (MRI), are eligible for referral to a comprehensive pediatric epilepsy program (CPEP) for assessment of surgical candidacy.Reference Cross, Jayakar and Nordli3 Epilepsy surgery has better outcomes than continued medical management alone. Limited awareness of surgical treatment options, however, leads to inappropriate patient referral and underutilization of epilepsy surgery in potential candidates.
Patients with lesions on MRI such as focal cortical dysplasia (FCD) are likely to be referred to epilepsy centers by neurologists; however, many FCD lesions go undetected on MRI when reported outside of a major epilepsy center.Reference Salamon, Kung and Shaw4 Bowen et al. also reported that out of an estimated 5170 children with drug refractory epilepsy in Ontario between 2011 and 2016, 2800 (54%) received video electroencephalograph (EEG), while 2370 (45%) were discussed at seizure conference.Reference Bowen, Snead and Chandra5
FCD is a common cause of medically refractory, focal onset epilepsy in children. It may comprise as much as 25–40% of refractory childhood epilepsies, with increasing incidence as imaging technologies improve.Reference Lee and Kim6 FCD is the most common cause of epilepsy surgery in children less than 4 years of age.Reference Harvey, Cross and Shinnar7 The International League Against Epilepsy Sub-Commission for Pediatric Epilepsy Surgery identified FCD as one of the several etiologies and syndromes in children for epilepsy surgery evaluation.Reference Cross, Jayakar and Nordli3 It is a developmental, cortical migration disorder, classified by clinical–pathological criteria.Reference Blumcke, Thom and Aronica8 These malformations are epileptogenic and can occur anywhere in the cortex, gray–white matter junction, or adjacent subcortical white matter.
FCD Type 1 has been associated with cerebral hypoplasia or mild blurring of the cortical–white matter junction on MRI.Reference Colombo, Salamon and Raybaud9 Tassi et al., however, reported MRI to be uninformative in 22/66 (33%) cases of histologically confirmed FCD Type 1.Reference Tassi, Garbelli and Colombo10 MRI features of FCD Type 2 include cortical thickening, blurring of the gray–white matter junction, gyration anomalies, subcortical white matter signal change, or the transmantle sign.Reference Colombo, Salamon and Raybaud9 Results vary according to the subtype of FCD with 23/34 (68%) of FCD IIA and 61/66 (93%) of FCD IIB, correctly diagnosed on MRI.Reference Tassi, Garbelli and Colombo11 Another study from the same group reported a normal MRI in 19/37 (49%) of FCD IIA and 7 (9%) of FCD IIB.Reference Colombo, Tassi and Deleo12 A transmantle sign was significantly associated with FCD Type IIB.Reference Colombo, Salamon and Raybaud9, Reference Colombo, Tassi and Deleo12, Reference Barkovich, Kuzniecky and Bollen13 FCD Type III is a combination of abnormal cortical lamination with hippocampal sclerosis (Type IIIa), tumors (Type IIIb), vascular malformations (Type IIIc), or other acquired lesions (Type IIId). The dual pathology may be unappreciated on MRI, as the cortical lamination abnormality is often undetected.Reference Guerrini, Duchowny and Jayakar14
FCD has characteristic interictal EEG features. Rhythmic epileptiform discharges (REDs), rhythmic spikes or polyspikes, and repetitive bursts of fast waves (brushes) on scalp EEG correlate with continuous epileptogenic discharges (CEDs), rhythmic spikes or polyspikes and repetitive bursts of fast waves (brushes), observed during invasive monitoring using stereo-electroencephalograph (SEEG) or electrocorticography (ECoG), in patients with cortical dysplasia on histopathology.Reference Tassi, Garbelli and Colombo11, Reference Chassoux, Devaux and Landre15–Reference Palmini, Gambardella and Andermann17 Gambardella reported REDs in 15/34 (44%) and CEDs in 23/34 (67%) patients with cortical dysplasia compared with REDs in 0/44 (0%) and CEDs in 1/40 (2.5%) patients with non-dysplastic lesions.Reference Gambardella, Palmini and Andermann16
Brushes are significantly associated with the balloon cells of FCD IIB.Reference Tassi, Garbelli and Colombo11 Regional polyspikes on interictal scalp EEG were more often associated with FCD on MRI, than other regional interictal discharges.Reference Noachtar, Bilgin and Remi18
EEG biomarkers for FCD are specific but not very sensitive. The sensitivity of REDs to detect FCD compared to histopathology was 15/34 (44%) with a specificity of 40/40 (100%), and the sensitivity of regional polyspikes to detect FCD compared to MRI was 10/34 (29%) with a specificity of 460/479 (96%).Reference Gambardella, Palmini and Andermann16, Reference Noachtar, Bilgin and Remi18 The low sensitivity FCD findings on scalp EEG are probably explained by the well-known phenomenon of attenuation or distortion of cortical potentials by the tissues overlying the brain.Reference Gambardella, Palmini and Andermann16
Knowing the strengths and limitations of the tests of FCD is important when considering patients with focal onset epilepsy for surgical referral.
Objectives
The goal of this study was to assess whether detection of FCD on MRI or prolonged interictal scalp EEG in children with focal onset epilepsy was associated with referral to a CPEP. Our hypothesis was that interictal focal polyspikes, fast waves, or REDs detected on prolonged scalp EEG and FCD on MRI are recognized by referring physicians as biomarkers of FCD, and that their detection is associated with referral to a CPEP for surgical evaluation. This study also aimed to identify other diagnostic predictors of referral to a CPEP.
Methods
We performed a single-center, retrospective health record review of children referred for prolonged scalp video-EEG monitoring at McMaster Children’s Hospital. The pediatric neurologists who read and reported the EEGs participated in the study. The Hamilton Integrated Research Ethics Board approved the study (REB Application Number 2018-0860).
Inclusion Criteria
The study included children with focal onset epilepsy, between 1 month and 18 years of age, who completed a prolonged (>1 h) scalp EEG and had a brain MRI performed after 2 years of age at McMaster Children’s Hospital between 2008 and 2015.
Exclusion Criteria
Children with generalized onset epilepsy or generalized epilepsy syndromes and with EEG diagnoses of electrographical status epilepticus in sleep, childhood epilepsy with central temporal spikes, multiple independent spike foci, Lennox–Gastaut Syndrome, non-epileptic seizures, or MRI diagnosis of tuberous sclerosis complex were excluded from the study. Children with Lennox-Gastaut Syndrome were excluded from the study, even though some of these patients have focal onset epilepsy, due to the presence of paroxysmal fast activity seen on EEG in this epilepsy syndrome.
Each patient’s neurology clinic reports were reviewed for seizure and epilepsy types, epilepsy syndromes, etiology, CPEP referral, epilepsy surgery, and pathology. Prolonged (>1 h) scalp EEG reports were reviewed and categorized for interictal focal polyspikes, fast waves, or REDs. We elected to only assess the prolonged video-EEG studies, since the duration of recording has been shown to increase the yield of detecting interictal discharges.Reference Narayanan, Labar and Schaul 19
The MRI reports were reviewed for the presence or absence of FCD, malformations of cortical development other than FCD, tumor, stroke, vascular malformation, or mesial temporal sclerosis. All imaging sequences were obtained using a 1.5 Tesla (T) MRI.
EEG Definitions
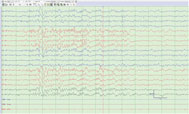
Focal polyspikes (Figure 1): Interictal polyphasic spikes occurring at one or a few neighboring electrodes on EEG.
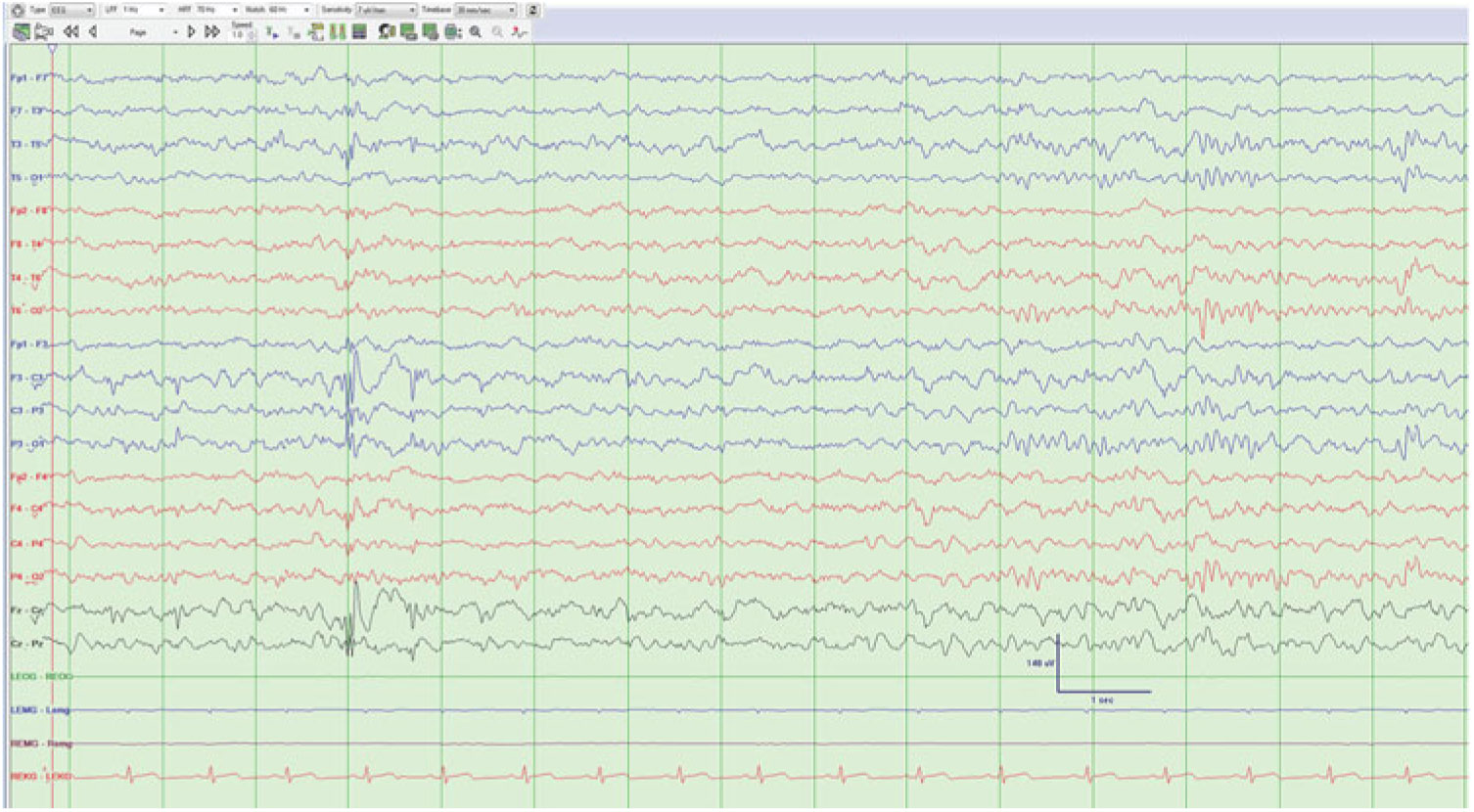
Figure 1: Left central and midline polyspike and wave complexes.
Focal fast waves (Figure 2): Interictal beta waves occurring at one or a few neighboring electrodes on EEG.
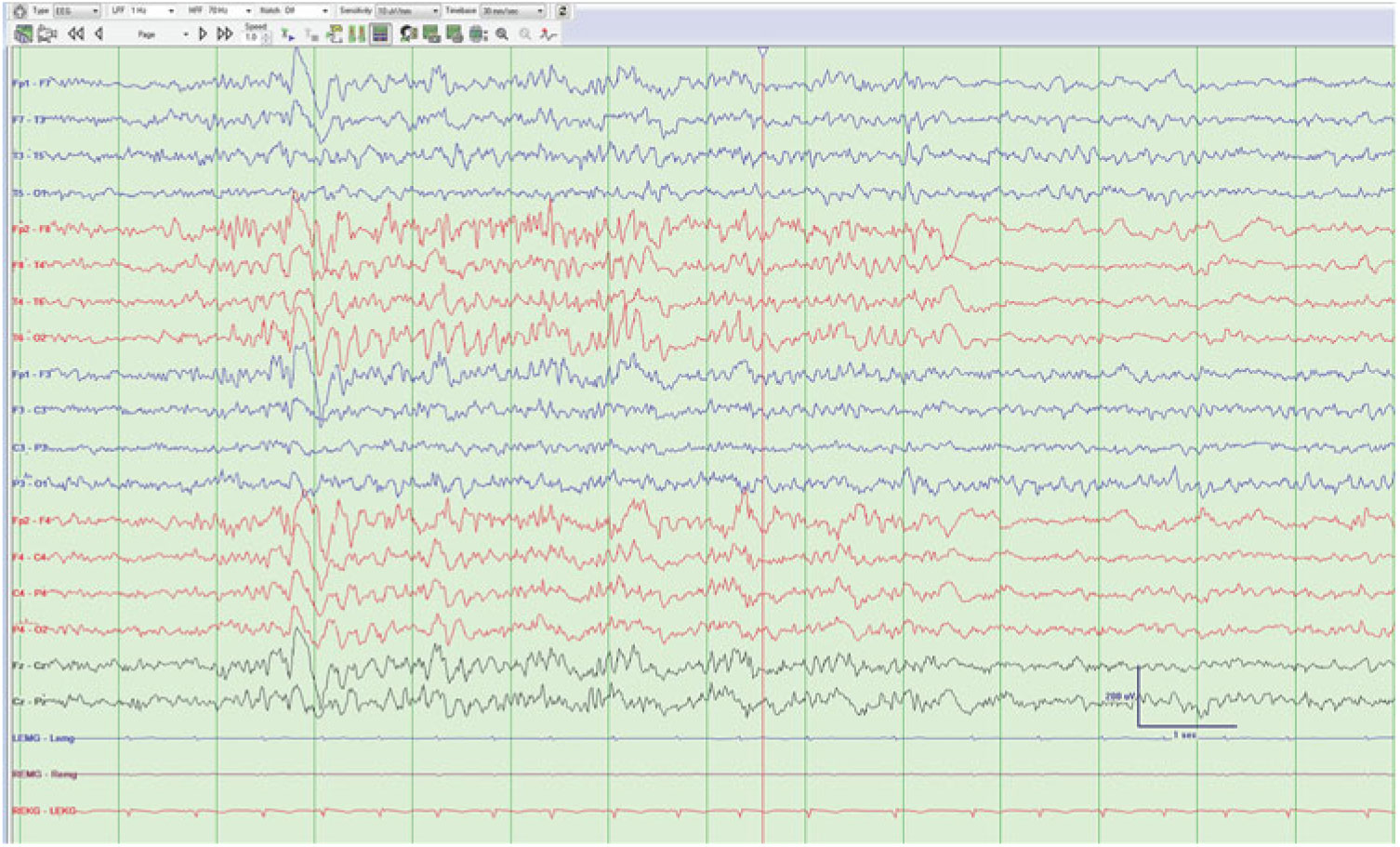
Figure 2: Bursts of fast waves from the right frontal region.
Rhythmic epileptiform discharges (Figure 3): Interictal stereotyped rhythmic sequences of repetitive sharp waves or spikes, lasting more than 1 s without clinical signs.Reference Gambardella, Palmini and Andermann 16
Figure 3: Rhythmic sharp waves from the left subtemporal and midtemporal regions without evolution.
MRI Definitions
Focal cortical dysplasia: Cortical thickening, blurring of the gray–white matter junction, gyration anomalies, subcortical white matter signal change, or a transmantle sign on MRI.Reference Colombo, Salamon and Raybaud 9 , Reference Colombo, Tassi and Deleo 12
Outcome Measures
The primary outcome measure was referral to a CPEP by a pediatric neurologist or pediatrician, and the secondary outcome was epilepsy surgery. The following variables were included in the analysis: sex, age of seizure onset, age at first prolonged scalp EEG and first MRI after 2 years of age, duration of prolonged scalp EEG, response to medication, signs of FCD on interictal scalp EEG, MRI diagnosis, and etiology. MRI diagnosis included FCD, malformations of cortical development other than FCD, tumor, stroke, vascular malformation, or mesial temporal sclerosis.
Statistical Analysis
Categorical and continuous variables were analyzed with 95% confidence intervals, using the chi-squared and student’s t-test, respectively, with SPSS Statistics, Version 25. The variables were also analyzed using a logistic regression model.
Results
Two hundred and seventy children who completed a prolonged scalp video-EEG between 2008 and 2015 were evaluated for enrollment. Of these, 68 were included in the study.
Of the study cohort, there were 15/68 (22%) with FCD on interictal scalp EEG, 22/68 (32%) with FCD on MRI, and 7/68 (10%) with FCD on both. The MRI reports for EEG-only-positive patients were not suggestive of FCD at any time on 1.5 T MRI, even though several patients had multiple MRI scans. For those with MRI evidence of FCD only, the interictal EEGs typically reported rare, intermittent, or frequent focal sharp waves, sharp and slow waves, or spike and waves. Focal delta or theta waves were also occasionally reported. When EEG findings of FCD were evident, they were reported in the body of the report. The report impressions focused on localization of the ictal onset and irritative zones. The EEG findings of FCD were correlated in the report with either a structural etiology or FCD in 6/15 cases.
Treatment resistant epilepsy was present in 12/15 (80%) patients with EEG markers of FCD, 18/22 (81%) with FCD on MRI, and 7/7 (100%) of those with both markers. There was no difference in response to epilepsy treatment between those with the EEG marker and those with MRI findings of FCD. Differences in seizure frequencies between the groups were not recorded in the study, as this was not consistently reported in the medical charts.
Thirty-seven of the 68 (54%) cases were referred to a CPEP. The referring physicians were pediatric neurologists or pediatricians. Of these, 9/37 (24%) had signs of FCD on interictal scalp EEG, 19/37 (51%) on MRI, and 6/37 (16%) with FCD on both. For patients with both findings, they were discussed at seizure conference after the MRI was positive in five cases. One case was not referred.
All nine patients with FCD markers on EEG, referred for epilepsy surgery evaluation, had treatment-resistant epilepsy. Of the six patients with FCD markers on EEG not referred for epilepsy surgery evaluation, three had treatment-resistant epilepsy and the other three had treatment-responsive epilepsy.
Of the nine patients with FCD markers on EEG, referred for surgical evaluation, six included EEG findings of FCD or a structural etiology, in the impression of the EEG report, while in three, only the focal pattern of the EEG was discussed. Of the six patients with FCD markers of EEG, not referred for surgical evaluation, one included EEG findings of FCD or a structural etiology, while five only discussed the focal pattern of the EEG in the impression of the report.
The mean age of seizure onset was 84 months (SD 46 months) for the EEG-only patients, and 81 months (SD 49 months) for the MRI-only-positive patients (p = 0.88). These were not significantly different.
Thirty-one of the 68 cases (46%) were not referred. Of the patients not referred for epilepsy surgery, 17/31 (54%) had treatment-resistant epilepsy. There were 6/31 (19%) with signs of FCD on interictal scalp EEG (3 had treatment-resistant epilepsy and 3 had treatment-responsive epilepsy), 3/31 (10%) on MRI (1 with treatment-resistant epilepsy and 2 without), and 1/31 (3%) with signs of FCD on both (with treatment-resistant epilepsy). Signs of FCD on MRI were significantly associated with referral to a CPEP (p < 0.05), while signs of FCD on EEG were not.
Seventeen of the 37 (46%) referred to CPEP had epilepsy surgery. There were 3/17 (18%) with FCD on interictal scalp EEG and 7/17 (41%) with FCD on MRI who had epilepsy surgery, compared with 6/20 (30%) with FCD on interictal scalp EEG and 12/20 (60%) with FCD on MRI who did not. Neither signs of FCD on interictal EEG nor MRI were significantly associated with epilepsy surgery.
Of the six patients with FCD on both interictal scalp EEG and MRI referred for surgical evaluation, two underwent surgery. Pathology was reported as ganglioglioma in one patient and not available in the other. Of these patients who did not undergo surgery, one declined surgery, one was unable to tolerate intracranial monitoring, and two were not surgical candidates due to the inclusion of eloquent cortex within the epileptogenic zone. Of the eight patients with FCD on EEG only, three were referred for surgical evaluation and one had surgery, which was found to be a dysembryoplastic neuroepithelial tumor on pathology. One was not a surgical candidate and the other required further evaluation. Out of the 15 patients with FCD on MRI, only 10 were referred for surgical evaluation. Epilepsy surgery was undertaken in five, with one case of FCD, one of subcortical gray matter heterotopia, and two with ganglioglioma on pathology. Epilepsy surgery was not performed due to response to medication in four, eloquent cortex within the epileptogenic zone in one, inability to capture the ictal onset zone in one, and an unknown reason in another case. Of those with FCD neither detected on EEG nor on MRI, 15 were referred for surgical evaluation and 8 underwent surgery. Histopathology included hippocampal sclerosis (1), oligodendrogliosis (1), FCD IIA (1), gliosis (2), and non-specific pathology (1).
The cohort was evaluated for predictors of referral to a CPEP. The results are tabulated in Table 1. Fifty-two of 68 (76%) had medical refractory epilepsy. Of these, 35 out of 52 (67%) were referred to a CPEP. Medically refractory epilepsy was significantly associated with referral to a CPEP (p < 0.05).
Table 1: Patient characteristics and referral predictors
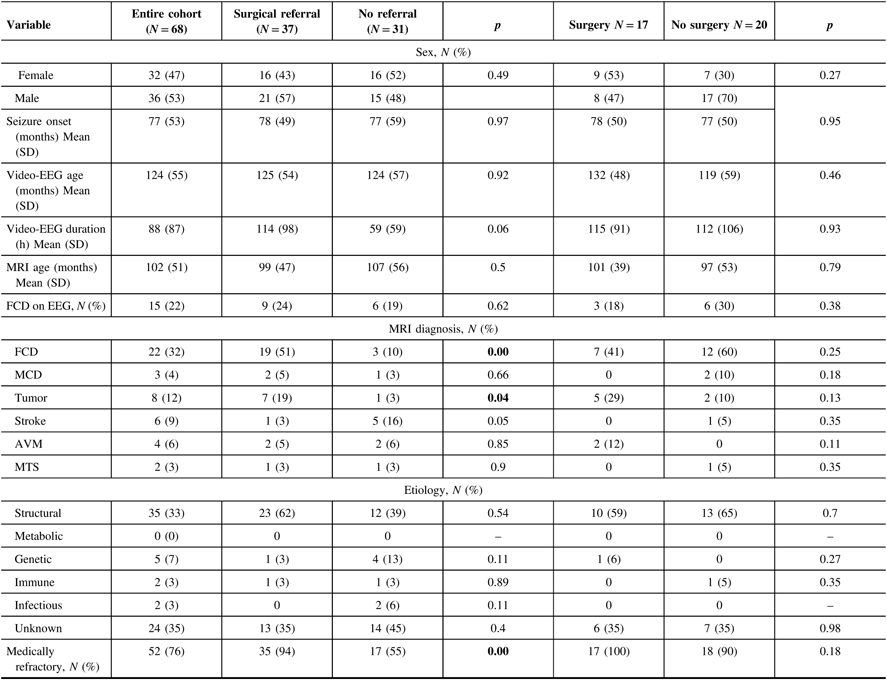
Children with focal onset epilepsy were tabulated according to epilepsy surgery referrals and nonreferrals, surgery vs. no surgery. The results were compared with respect to gender, age at seizure onset, age at first prolonged scalp EEG, EEG duration, FCD biomarkers on EEG and MRI, age at MRI, MRI diagnosis, etiology, and medication failure (AVM – arteriovenous malformation, MCD – malformations of cortical development, MTS – mesial temporal sclerosis, N – number, SD – standard deviation). Bold values show p values of < 0.05.
There were 8/64 (12%) cases diagnosed with tumor on MRI. Seven of eight (88%) were referred to a CPEP, while 1/8 (12%) was not. Tumor on MRI was significantly associated with referral to a CPEP (p = 0.04). There was no significant difference in referral to a CPEP or epilepsy surgery when sex, age of seizure onset, age at first prolonged scalp EEG, age at first MRI, duration of scalp EEG, etiology and MRI diagnosis of malformations of cortical development, stroke, vascular malformation, or mesial temporal sclerosis were evaluated.
Logistical regression analysis was performed. FCD on MRI was significantly associated with referral to a CPEP with an odds ratio of 7 (CI 2.04–24.06, p < 0.01).
Discussion
This study found that children with focal onset epilepsy were more likely to be referred for surgical evaluation if they were medically refractory, or were diagnosed with FCD or tumor on MRI. Referring physicians are probably familiar with these indications for referral. Children with focal onset epilepsy and signs of FCD on prolonged scalp EEG, other structural lesions on MRI, or non-lesional medically refractory epilepsy were not more likely to be selected. This suggests that there is less awareness among physicians of the scalp EEG signs of FCD and the fact that FCD I, IIA, and III may be missed by MRI alone. A free online tool developed by the CASES expert panelists, using evidence and consensus opinions, is a useful guide for physicians in identifying children with focal epilepsy who may benefit from epilepsy surgery evaluation.Reference Jette, Quan and Tellez-Zenteno 20
Signs of FCD on interictal EEG or MRI were not significantly associated with epilepsy surgery. This suggests that other factors play a role in determining surgical candidacy. These include further investigations with high resolution, epilepsy protocolled MRI, fluorodeoxyglucose positron emission tomography, magnetoencephalography, ictal single-photon emission computed tomography, invasive monitoring with ECoG or SEEG, and high-frequency oscillation analysis, as well as neuropsychological testing and localization of eloquent cortex with functional MRI, and discussion at seizure conference in a CPEP.Reference Guerrini, Duchowny and Jayakar 14 The potential epileptogenic zone may include eloquent cortex, which excludes epilepsy surgery as an option for some patients.
This study is limited by its retrospective, single-center design, relatively small sample size, use of 1.5 T MRI, and the number of subjects without MRI or EEG markers of FCD. The MRI and EEG data were collected from the patient’s medical records without being reviewed objectively by the research physicians. The detection of FCD on MRI may therefore have varied between radiologists. The EEGs were originally read by one of the two study neurologists; however, the report impressions tended to emphasize the location of the interictal discharges rather than correlating clinically with a structural etiology or FCD. This may have limited the recognition of FCD and decision for referral by the primary neurologist. Referral paths also changed during the course of the study. The Hospital for Sick Children (Sickkids) in Toronto was the epilepsy surgery center for children in the Hamilton region between 2008 and May 2014. McMaster Children’s Hospital was designated a District Epilepsy Centre in May 2014 with Sickkids as the Regional Epilepsy Centre. These changes may have affected the referral rates for children with FCD.
Knowing how to detect FCD, and the criteria for epilepsy surgery referral in children with focal onset epilepsy is essential to limit inappropriate referrals and underutilization of epilepsy surgery in potential candidates. The use of the free online tool developed by the CASES expert panelists is a useful adjunct for primary physicians caring for children with epilepsy.
Acknowledgements
The authors thank the physicians, nurses and EEG technologists at McMaster Children's Hospital who cared for the patients presented in this study.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Statement of Authorship
MSF reviewed patient health records, collected data, identified patients for inclusion, interpreted the data, drafted the initial manuscript, reviewed, and revised the manuscript.
Dr. BN performed the statistical analysis, interpreted the data, reviewed, and revised the manuscript.
Dr. MAN reviewed and revised the manuscript.
Dr. RR reviewed and revised the manuscript.
Dr. KCJ reviewed the patient data and revised the manuscript.