Protein digestion rates in human nutrition have received attention( Reference Dangin, Boirie and Garcia-Rodenas 1 – Reference Tang, Moore and Kujbida 3 ); however, the likelihood is that the focus on protein digestion rates in the formulation of least-cost diets for rapidly growing broiler chickens has been insufficient. Here the current emphasis is more on static, ileal amino acid digestibility coefficients rather than kinetics of protein and amino acid digestion. Moreover, it has been proposed that the rates of digestion of protein and absorption of amino acids are surpassed by the rates of starch digestion and glucose absorption and that this asynchrony in digestive dynamics compromises the performance of broiler chickens( Reference Liu and Selle 4 ). If so, it follows that the provision of slowly digestible starch will enhance broiler performance, and this concept has been validated to some extent( Reference Weurding, Enting and Verstegen 5 ). However, it also follows that the provision of rapidly digestible protein should enhance broiler performance in a reciprocal manner. Thus, the objective of this study was to examine the hypothesis that the dietary provision of rapidly digestible protein enhances the performance of broiler chickens.
To this end a conventional, maize–soya ‘slow-protein’ foundation diet and a ‘rapid-protein’ summit diet were formulated and an equal blend of these diets constituted an intermediate dietary treatment. The rationale was that the partial replacement of soyabean meal with casein and additional crystalline amino acids in the summit diet would accelerate protein digestion rates. Decades ago, it was demonstrated in rats that concentrations of lysine and methionine derived from casein were higher in the portal blood in comparison with those from soya flour( Reference Guggenheim, Halevy and Friedmann 6 , Reference Goldberg and Guggenheim 7 ). Both studies indicated that casein is a more rapidly digested and absorbed source of protein than soya and casein generates higher concentrations of amino acids in the portal circulation. Crystalline (or synthetic) amino acids do not undergo digestion and are directly available for absorption in the upper small intestine and appear in the portal circulation more rapidly than protein-bound amino acids( Reference Wu 8 ). Thus, crystalline amino acids are, axiomatically, rapid sources of ‘protein’.
The absorption of nutrients is considered to be a more important rate-limiting factor on the growth performance of broiler chickens than their digestion( Reference Croom, Brake and Coles 9 ). However, following their absorption, amino acids may be subject to catabolism in small intestinal enterocytes for energy provision to the gut( Reference Wu 10 ) and this is a partial determinant of their entry into the portal circulation and their post-enteral availability for protein accretion. Therefore, plasma concentrations of free amino acids in the portal (anterior mesenteric vein) and systemic (brachial vein) circulations were determined in birds offered the foundation and summit diets. It has been demonstrated that concentrations of six essential and three non-essential amino acids in plasma taken from the anterior mesenteric vein were significantly correlated (P<0·05) with feed conversion ratios (FCR) in broiler chicks from 7 to 27 d post-hatch( Reference Selle, Truong and McQuade 11 ). Therefore, it was considered that amino acid concentrations in the portal and systemic circulations would be instructive in the present study.
Methods
Diet preparation
A ‘slow-protein’ foundation and a ‘rapid-protein’ summit diet were formulated to be iso-energetic (12·97 MJ/kg) and to meet recommended nutrient specifications as shown in Table 1. Protein was derived from soyabean and rapeseed meals, maize and limited quantities of crystalline lysine, methionine and threonine in the ‘slow’ 220·0 g/kg protein foundation diet. The ‘rapid’ 208·6 g/kg protein summit diet contained considerably less soyabean meal, which was replaced by 50 g/kg casein and a total of 11·75 g/kg crystalline amino acids (arginine, isoleucine, lysine, methionine, threonine, tryptophan) so that digestible amino acid levels in the dietary treatments were comparable. An equal blend of the two diets (214·3 g/kg protein) was used as an intermediate dietary treatment so that any linear effects could be identified (Table 2).
Table 1 Composition and calculated nutrient specifications of foundation, intermediate and summit experimental diets
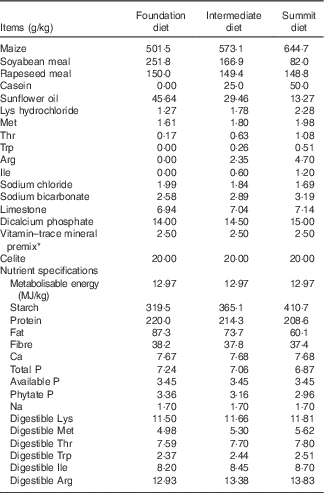
* The vitamin–mineral premix supplied per tonne of feed: (MIU): retinol, 12, cholecalciferol, 5; (g) tocopherol, 50; menadione, 3; thiamine, 3; riboflavin, 9; pyridoxine, 5; cobalamin, 0·025; niacin, 50; pantothenate, 18; folate, 2; biotin, 0·2; Cu, 20; Fe, 40; Mn, 110; Co, 0·25; iodine, 1, Mo, 2; Zn, 90; Se, 0·3.
Table 2 Analysed nutrient specifications of foundation, intermediate and summit experimental diets
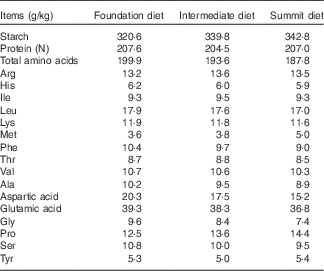
Maize and soyabean meal were ground through a 3·2-mm hammer-mill screen before being mixed into the diets. The experimental diets were steam-pelleted at a conditioning temperature of 80°C (14 s residence time) and crumbled after passing through a vertical cooler.
Bird management
A total of 144 d-old, male Ross 308 chicks were procured from a commercial hatchery, housed in an environmentally controlled facility and were initially offered a proprietary starter ration. At 7 d post-hatch, birds were individually identified (wing-tags), weighed and allocated into twenty-four cages on the basis of body weights so that the means and standard deviation in each cage was almost identical. Each of the three dietary treatments was then offered to eight replicate cages (six birds per cage) from 7 to 28 d post-hatch. Birds had unlimited access to feed and water during the experimental feeding period under a ‘16-h-on’ lighting regimen, and room temperature was gradually reduced from 32°C at day 1 to 22°C at day 28. Body weights were again determined at 28 d post-hatch and feed intakes for each cage were recorded over the 21-d period feeding period to calculate FCR. These calculations were adjusted by the body weight of any dead or culled birds, which were monitored on a daily basis. Total excreta collection to determine parameters of nutrient utilisation was completed over a 48-h period from 25 d post-hatch. At 28 d post-hatch, the birds were euthanised (intravenous injection of sodium pentobarbitone) in order to collect various samples for analyses.
Sample collection and chemical analysis
Feed intake was monitored and total excreta collected from each cage over a 48-h period to determine apparent metabolisable energy (AME), metabolisable energy:gross energy (ME:GE) ratios, N retention and N-corrected AME (AMEn). Excreta were dried in a forced-air oven at 80°C for 24 h and the GE of excreta and diets were determined using an adiabatic bomb calorimeter. The AME values of the diets on a DM basis were calculated from the following equation:
ME:GE ratios were calculated by dividing AME by the GE of the appropriate diets. N contents of diets and excreta were determined using a N determinator (Leco Corporation) and N retentions calculated from the following equation:
AMEn (MJ/kg DM) values were calculated by correcting N retention to 0 using the factor of 36·54 kJ/g N retained in the body( Reference Hill and Anderson 12 ).
At day 28 following euthanasia, the abdominal cavities of the birds were opened and digesta were collected in their entirety from the proximal jejunum (PJ), distal jejunum (DJ), proximal ileum (PI) and distal ileum (DI) and pooled for each cage. The segments were demarcated by the mid-points between the end of the duodenal loop, Meckel’s diverticulum and the ileo-caecal junction. Digesta samples were freeze-dried to determine apparent digestibilities of starch, crude protein (N) and amino acids, using CeliteTM (World Minerals Inc.), a source of acid insoluble ash (AIA), as the inert dietary marker. Starch concentrations in diets and digesta were determined by a procedure based on dimethyl sulphoxide, α-amylase and amyloglucosidase as described by Mahasukhonthachat et al.( Reference Mahasukhonthachat, Sopade and Gidley 13 ). N content was obtained using an FP-428 determinator (Leco Corporation) and AIA concentrations were determined by the method of Siriwan et al.( Reference Siriwan, Bryden and Mollah 14 ). Amino acid concentrations of diets and digesta were determined following 24-h liquid hydrolysis at 110°C in 6 m HCl and then sixteen amino acids are analysed using the Waters AccQTag Ultra chemistry (Waters) on a Waters Acquity UPLC. Tryptophan and cysteine cannot be analysed accurately by this procedure. The apparent digestibility coefficients for starch and protein (N) in four small intestinal sites and the apparent digestibility coefficients of sixteen amino acids in the three posterior small intestinal segments (insufficient digesta in PJ) were calculated from the following equation:
Three birds at random were selected from each cage housing birds offered the foundation and summit diets and blood samples were taken from the brachial vein before euthanasia and the anterior mesenteric vein from the same three birds following euthanasia. Blood samples were then centrifuged and the decanted plasma samples were then kept at −80oC before analysis. Concentrations of twenty proteinogenic amino acids in plasma taken from the brachial and anterior mesenteric veins were determined using precolumn derivatisation amino acid analysis with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC; Waters™ AccQTag Ultra; www.waters.com) followed by separation of the derivatives and quantification by reversed phase ultra-performance liquid chromatography( Reference Cohen 15 ). All amino acids were detected by UV absorbance. This procedure is fully described in Selle et al.( Reference Selle, Truong and McQuade 11 ).
Statistical analysis
The experimental data derived from eight replicate cages for each of three dietary treatments were analysed as a one-way ANOVA using the IBM® SPSS® Statistics 20 program (IBM Corporation). Each replicate cage constituted an experimental unit and P<0·05 was considered to be statistically significant. Given a P value of <0·05 from the ANOVA, Fisher’s least significant difference (LSD) was calculated to compare mean values using the following equation:
Digestibility coefficients and disappearance rates for protein (N) and starch were determined in four small intestinal segments, but amino acid digestibility coefficients and disappearance rates were determined in three segments. Plasma concentrations of amino acids in the portal and systemic circulations in birds offered the foundation and summit diets were analysed as a 2 (foundation or summit diets)×2 (portal or systemic blood samples) factorial array of treatments. Pearson’s correlations, linear and quadratic regression equations were established for selected parameters where justified. The feeding study was conducted so as to comply with specific guidelines approved by the Animal Ethics Committee of the University of Sydney.
Results
The effects of dietary treatments on growth performance and parameters of nutrient utilisation are shown in Table 3. There were no significant treatment effects on growth performance from one-way ANOVAs; however, the summit diet supported a numerical increase in weight gain relative to the foundation diet by 4·48 % (1610 v. 1541 g/bird) and the linear effect closely approached significance (r 0·404; P=0·0501). The acceptable mortality/cull rate of 2·78 % was not related to treatment (P>0·75). Parameters of nutrient utilisation were significantly influenced (P<0·025–<0·001) by treatment. The summit diet supported superior nutrient utilisation in comparison with the foundation diet by 0·35 MJ (12·47 v. 12·12 MJ/kg) in AME, by 6·81 % (0·769 v. 0·720) in ME:GE ratios, by 4·32 percentage units (65·08 v. 60·76 %) in N retention and by 0·32 MJ (11·41 v. 11·09 MJ/kg) in AMEn. The transition from the foundation to summit diet linearly increased (P<0·01) all four nutrient utilisation parameters, where the impacts on ME:GE ratios (r 0·701; P<0·001) and N retention (r 0·762; P<0·001) were the most pronounced.
Table 3 Effects of dietary treatments on growth performance from 7 to 28 d and nutrient utilisation at 25–27 d post-hatch
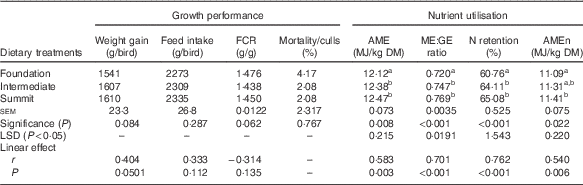
FCR, feed conversion ratios; AME, apparent metabolisable energy; AMEn, N-corrected AME; ME:GE, metabolisable energy:gross energy ratios; LSD, least significant difference.
a,b Mean values within column with unlike superscript letters were significantly different (P<0·05).
Table 4 shows the effects of dietary treatments on protein (N) and starch digestibility coefficients and disappearance rates in four small intestinal segments. The change from foundation to summit diets increased (P<0·001) protein (N) digestibility coefficients in the three posterior small intestinal segments by 10·4 % (0·740 v. 0·670) in DJ, 8·57 % (0·798 v. 0·735) in PI and 6·11 % (0·816 v. 0·769) in DI. Similarly, the dietary transition accelerated (P<0·001) protein (N) disappearance rates by 9·70 % (16·51 v. 15·05 g/bird per d) in DJ, 8·78 % (17·96 v. 16·51 g/bird per d) in PI and 6·48 % (18·41 v. 17·29 g/bird per d) in DI. The linear effects of diet type were highly significant for both protein (N) parameters in the three posterior small intestinal segments. The transition from the foundation to summit diets significantly increased starch digestibility coefficients by 3·96 % (0·946 v. 0·910) in PI and by 3·11 % (0·962 v. 0·933) in DI. Dietary treatments significantly influenced starch disappearance rates in all four segments; for example, the summit diet accelerated proximal ileal starch disappearance rates by 8·78 % (17·96 v. 16·51 g/bird per d). The linear effects of diet type were highly significant for starch digestibility coefficients in PI and DI and for starch disappearance rates in all four small intestinal segments.
Table 4 Effects of dietary treatments on apparent digestibility coefficients and apparent disappearance rates (g/bird per d) of protein (N) and starch in four small intestinal segments (proximal jejunum (PJ), distal jejunum (DJ), proximal ileum (PI), distal ileum (DI)) at 28 d post-hatch
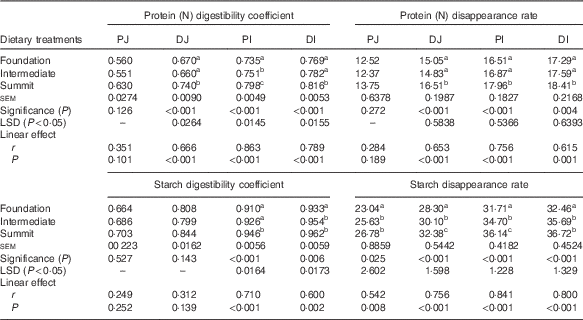
LSD, least significant difference.
a,b,c Mean values within column with unlike superscript letters were significantly different (P<0·05).
The effects of three dietary treatments on apparent digestibility coefficients of essential amino acids in three small intestinal segments are shown in Table 5. The transition from the foundation to the summit diet increased the digestibility of all essential amino acids in all three segments to highly significant (P<0·001) extents. The distal ileal digestibility coefficient of arginine was increased by 5·01 %, histidine by 4·88 %, isoleucine by 7·12 %, leucine by 7·10 %, lysine by 5·46 %, methionine by 3·66 %, phenylalanine by 6·06 %, threonine by 8·09 % and valine by 6·10 %. The three diets linearly increased digestibility coefficients of all amino acids in all three segments to significant extents with the sole exception of histidine in PJ.
Table 5 Effects of four dietary treatments on apparent digestibility coefficients of essential amino acids in three small intestinal segments (distal jejunum (DJ), proximal ileum (PI), distal ileum (DI)) at 28 d post-hatch
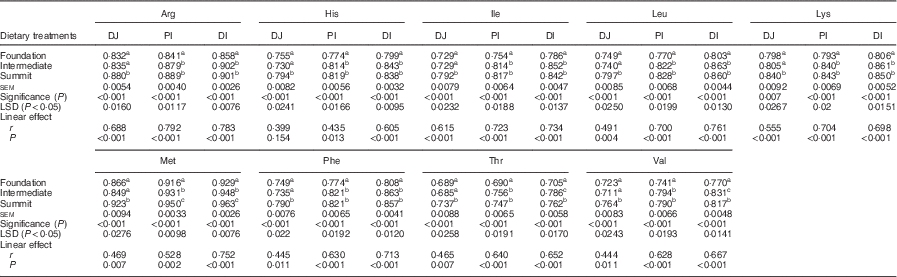
LSD, least significant difference.
a,b,c Mean values within column with unlike superscript letters were significantly different (P<0·05).
The effects of three dietary treatments on digestibility coefficients of non-essential amino acids in three small intestinal segments are shown in Table 6. Relative to the foundation diet, the summit diet significant increased distal ileal digestibility coefficients of alanine by 4·67 %, glutamic acid by 4·40 %, proline by 9·28 %, serine by 3·71 % and tyrosine by 7·61 %. Curiously, in the same comparison, diets had no impact on glycine and significantly decreased aspartic acid by 4·56 %. Dietary treatments generally linearly increased digestibility coefficients for non-essential amino acids in one or more segments to significant extents. However, this was not the case with glycine.
Table 6 Effects of four dietary treatments on apparent digestibility coefficients of non-essential amino acids in three small intestinal segments (distal jejunum (DJ), proximal ileum (PI), distal ileum (DI)) at 28 d post-hatch
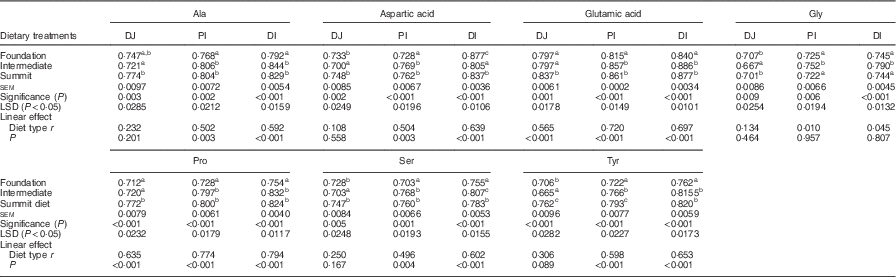
LSD, least significant difference.
a,b,c Mean values within column with unlike superscript letters were significantly different (P<0·05).
The effects of three dietary treatments on disappearance rates of essential amino acids in three small intestinal segments are shown in Table 7. The transition from the foundation to summit diets significantly accelerated disappearance rates of arginine, isoleucine, lysine, methionine, threonine and valine in all three segments. Alternatively, dietary treatments did not linearly influence the disappearance rates of histidine and leucine in any segment. Moreover, phenylalanine disappearance rates were linearly retarded by the transition from foundation to summit diet to subtle, but significant (P<0·005), extents in all three segments.
Table 7 Effects of four dietary treatments on apparent disappearance rates (g/bird per d) of essential amino acids in three small intestinal segments (distal jejunum (DJ), proximal ileum (PI), distal ileum (DI)) at 28 d post-hatch
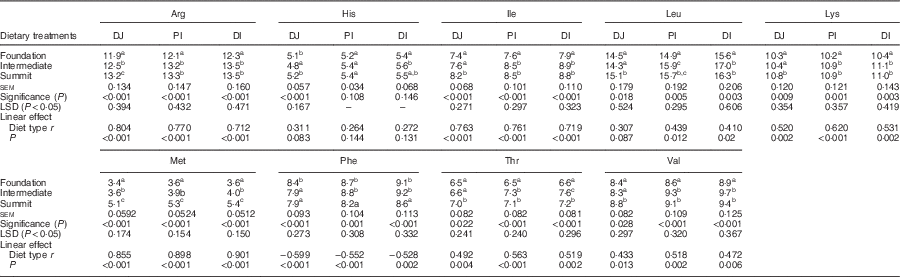
LSD, least significant difference.
a,b,c Mean values within column with unlike superscript letters were significantly different (P<0·05).
Table 8 records the effects of three dietary treatments on disappearance rates of non-essential amino acids. The transition from the foundation to summit diet linearly (P<0·001) retarded disappearance rates of alanine, aspartic acid and glycine in all three intestinal segments and retarded (P<0·01) serine in the PJ and DI. Conversely, the same transition accelerated (P<0·001) the disappearance of proline in all three segments. Treatment did not have any linear effects on the disappearance rates of glutamic acid and tyrosine.
Table 8 Effects of four dietary treatments on apparent disappearance rates (g/gird per d) of non-essential amino acids in three small intestinal segments (distal jejunum (DJ), proximal ileum (PI), distal ileum (DI)) at 28 d post-hatch
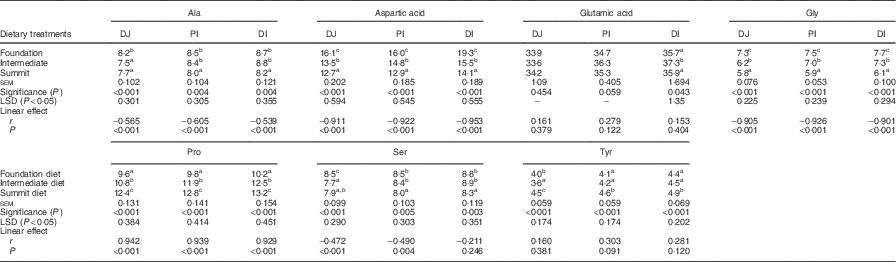
LSD, least significant difference.
a,b,c Mean values within column with unlike superscript letters were significantly different (P<0·05).
Concentrations of eighteen free amino acids in plasma taken from the anterior mesenteric or brachial veins in chicks offered the foundation or summit diets are shown in Table 9. There were no significant treatment interactions. As a main effect, the summit diet significantly increased concentrations of isoleucine by 12·8 % (14·9 to 16·8 µg/ml), methionine by 44·9 % (10·7–15·5 µg/ml), threonine by 42·1 % (57·0–81·0 µg/ml), proline by 22·9 % (52·5–64·5 µg/ml) and tyrosine by 16·6 % (36·2–42·2 µg/ml), relative to the foundation diet. Conversely, the same dietary change decreased concentrations of histidine by 18·8 % (14·9–12·1 µg/ml), aspartate/asparagine by 10·3 % (37·7–33·8 µg/ml), glycine by 23·2 % (59·0–45·3 µg/ml) and serine by 13·0 % (63·8–55·5 µg/ml), to significant extents. Concentrations of the remaining nine amino acids were not significantly influenced by the change in diets. Plasma concentrations of fourteen amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tryptophan, valine, alanine, cysteine, glutamate plus glutamine, glycine and proline) were significantly higher in the portal than systemic circulation. Plasma concentrations of the remaining four amino acids (arginine, threonine, serine, tyrosine) were numerically higher in the portal bloodstream.
Table 9 Plasma concentrations (µg/ml) of free amino acids (n 18) taken from the portal (anterior mesenteric vein) or systemic (brachial vein) circulation in chicks offered the foundation or summit diet
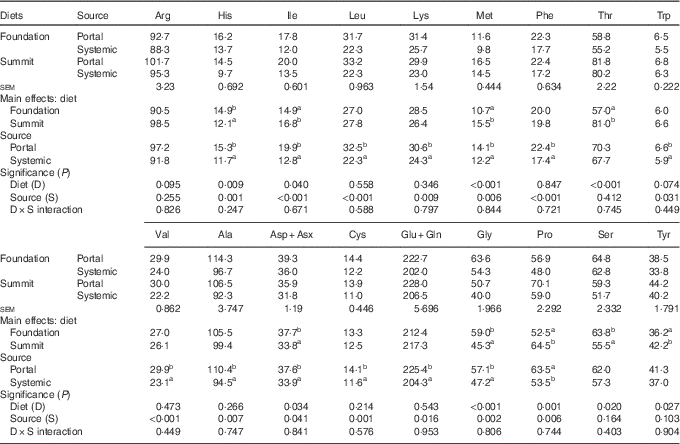
a,b Mean values within column with unlike superscript letters were significantly different (P<0·05).
Discussion
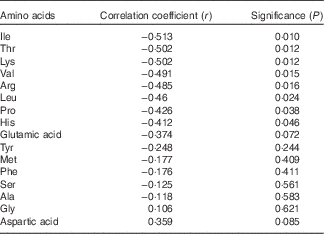
Dangin et al.( Reference Dangin, Boirie and Garcia-Rodenas 1 ) developed the concept of ‘slow’ and ‘fast’ protein and concluded that the rate of protein digestion independently modulates postprandial protein deposition in humans. In a following study, Dangin et al.( Reference Dangin, Boirie and Guillet 2 ) considered that ‘fast’ protein may be more beneficial than a ‘slow’ protein to limit body protein loss in elderly subjects. Subsequently, Tang et al.( Reference Tang, Moore and Kujbida 3 ) compared the effects of whey hydrolysate, casein and soya protein isolate on muscle protein synthesis in young men and proposed that the differences observed may be related to how quickly the proteins are digested. Interestingly, there were significant, negative correlations between the distal ileal disappearance rates of eight amino acids with FCR as shown in Table 10. Thus, the inference is that ‘fast’ protein, or the rapid disappearance/absorption of certain amino acids, benefits feed efficiency in poultry.
Table 10 Pearson’ correlations between distal ileal disappearance rates (g/bird per d) of sixteen amino acids and feed conversion ratios (ranked)
Growth performance
The overall performance of male broiler chicks from 7 to 28 d post-hatch in the present study was very acceptable in comparison with 2014 Ross 308 performance objectives (shown in parentheses). The overall weight gain was 1576 g/bird (1387 g/bird), feed intake was 2286 g/bird (2052 g/bird) and FCR was 1·451 (1·479). As expected, the transition from the foundation, via the intermediate, to the summit diet linearly accelerated protein (N) disappearance rates in the three posterior small intestinal segments by 6·48 to 9·70 %. The substitution of soyabean meal in the foundation diet with casein and crystalline amino acids in the summit diet had the desired impact of generating a ‘rapid protein’ diet. On the basis of a pair-wise comparison, birds offered the summit diet outperformed their foundation diet counterparts by 4·48 % (1610 v. 1541 g/bird; P=0·041) in weight gain. Interestingly, there was a positive association (r 0·706; P<0·001) between distal ileal protein (N) disappearance rates and weight gains (Fig. 1), which implies that ‘rapid protein’ was advantageous in this respect.
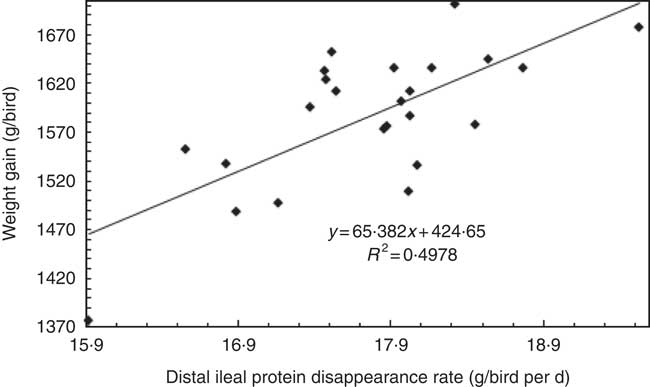
Fig. 1 Linear relationship between distal ileal protein (N) disappearance rates with 7 to 28 d weight gain (r 0·706; P<0·001).
Nutrient utilisation and starch digestibility
The transition from slow-protein foundation to rapid-protein summit diets had unequivocally positive effects on parameters of nutrient utilisation (Table 2). This is illustrated by the quadratic relationship between proximal ileal protein (N) disappearance rates with AME (r 0·530; P=0·032) and the linear relationship with N retention (r 0·491; P=0·015), as shown in Figs. 2 and 3. These relationships suggest that rapid protein disappearance rates enhanced energy utilisation, which may have stemmed from the positive influence the transition from slow- to rapid-protein diets had on apparent digestibly coefficients and disappearance rates of starch (Table 4). As illustrated in Fig. 4 and 5, there were linear relationships between proximal ileal protein (N) disappearance rates and proximal ileal starch digestibility coefficients (r 0·638; P<0·001) and proximal ileal starch disappearance rates (r 0·876; P<0·001).
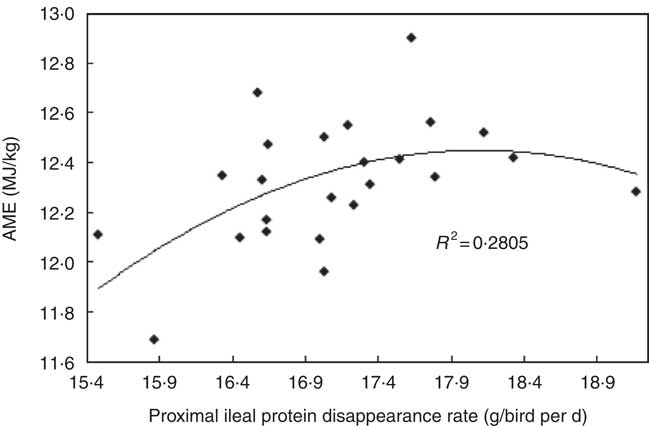
Fig. 2 Quadratic relationship between proximal ileal protein (N) disappearance rates with apparent metabolisable energy (AME) (r 0·530; P=0·032).
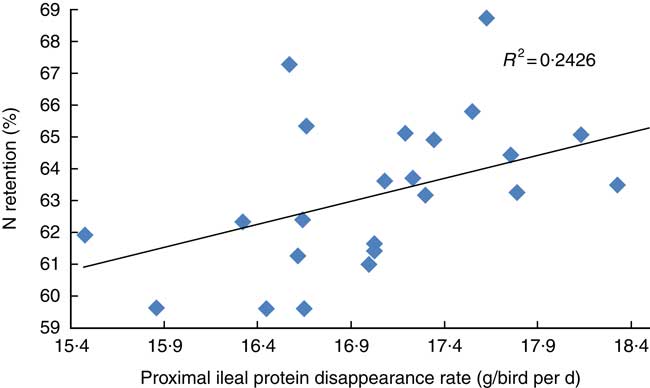
Fig. 3 Linear relationship between proximal ileal protein (N) disappearance rates with N retention (r 0·491; P=0·015).
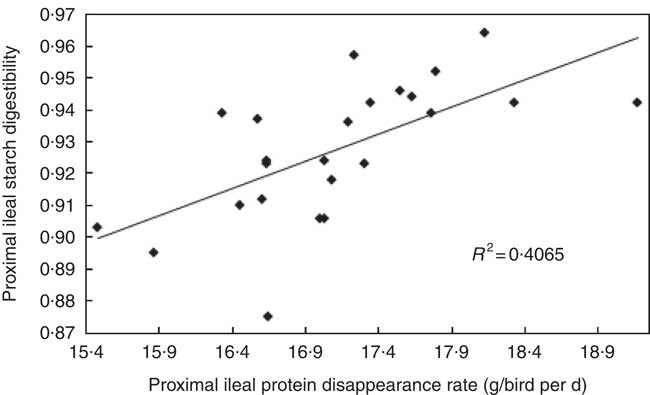
Fig. 4 Linear relationship between proximal ileal protein (N) disappearance rates and proximal ileal starch digestibility coefficients (r 0·638; P=0·001).
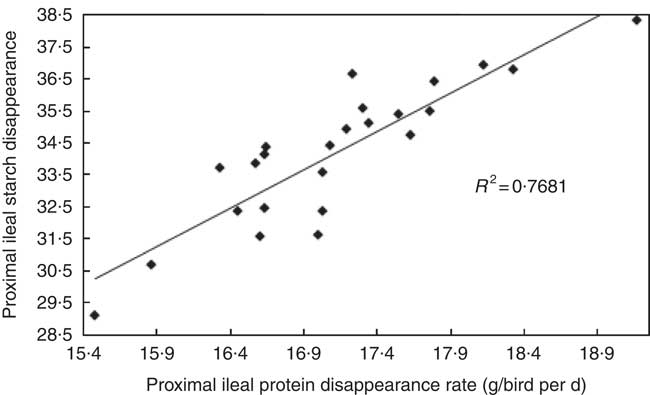
Fig. 5 Linear relationship between proximal ileal protein (N) disappearance rates and and proximal ileal starch disappearance rates (r 0·876; P<0·001).
The importance of biophysical and biochemical starch–protein interactions on energy utilisation in poultry is accepted, although these interactions have yet to be described precisely( Reference Truong, Liu and Selle 16 ). The relevant Rooney and Pflugfelder( Reference Rooney and Pflugfelder 17 ) review focuses on starch–protein interactions involving starch granules and prolamin proteins in the endosperm of feed grains. The dietary starch was mainly derived from maize in this study, and the interactions with protein from either casein or soyabean meal, rather than maize per se, appear to be influential. There is the possibility that negative interactions between maize starch and soya protein are being attenuated by the partial replacement of soyabean meal with casein. Soya protein has been shown to interact with wheat starch under in vitro conditions( Reference Rooney and Pflugfelder 18 ). Similar starch–protein interactions presumably could take place during steam-pelleting of the diets and/or in the avian digestive tract. Importantly, there is a precedent for different protein sources impacting on energy utilisation and starch digestibility. Sydenham et al.( Reference Sydenham, Truong and Moss 19 ) reported such outcomes following the partial substitution of soyabean meal with fishmeal in broiler diets. This substitution increased AME by 0·74 MJ (14·47 v. 13·73 MJ/kg; P<0·005) and ME:GE ratios by 8·03 % (0·767 v. 0·710; P<0·001). The same substitution significantly increased starch digestibility coefficients in four small intestinal segments culminating in an increase of 18·1 % (0·926 v. 0·784; P<0·001) in the DI.
Starch–protein interactions in wheat can influence glycaemic responses in humans( Reference Jenkins, Thorne and Wolever 20 ) or intestinal uptakes of glucose. Intestinal uptakes of amino acids and sugars are complex and interactive, as reviewed by Stevens et al.( Reference Stevens, Kaunitz and Wright 21 ) and amino acids and glucose may effectively compete for absorption along the small intestine( Reference Alvarado and Robinson 22 , Reference Murer, Sigristnelson and Hopfer 23 ). Certainly, Vinardell( Reference Vinardell 24 ) concluded that intestinal uptakes of amino acids and sugars are subject to mutual inhibition, which may particularly apply to competition for co-absorption with Na via their respective Na+-dependent transport systems. Perhaps a better comprehension of starch–protein interactions would be gained by focusing on their constituent sugars and amino acids. Starch pasting profiles of feed grains determined by rapid visco-analysis (RVA) may be indicative of their nutritive value in poultry( Reference Truong, Khoddami and Moss 25 ); therefore, it is interesting that amino acids have been shown to influence RVA starch pasting profiles in several studies including Ito et al.( Reference Ito, Hattori and Yoshida 26 ). The starch–protein interaction is an intriguing target for future research as one speculative interpretation is that the dietary provision of ‘rapid protein’ may have favourably manipulated the competition between glucose and amino acids for intestinal uptakes both in the Sydenham et al.( Reference Sydenham, Truong and Moss 19 ) study and the present study.
Apparent protein and amino acid digestibility
Apparent protein (N) digestibility coefficients in the three caudal small intestinal segments of the birds offered the summit diet were significantly higher than their foundation diet counterparts, and this translated to significant linear increases in protein (N) disappearance rates in the corresponding segments. The improvements in apparent protein (N) digestibility coefficients may be attributed partially to the inherently better digestibility of casein and crystalline amino acids in comparison with soya protein. From data generated by Bryden et al.( Reference Bryden, Li and Ravindran 27 ), the transition from summit to foundation diets would improve ileal protein digestibility by an estimated 3·72 % (0·837 v. 0·807). Thus, the 6·11 % improvement observed in the present study is somewhat greater than could be expected from the manipulation of the diets.
The transition from foundation to summit diets linearly increased (P<0·001) the average apparent digestibility coefficients of the total of 16 amino acids by 5·42 % (0·797 v. 0·756) in the DJ, by 6·36 % (0·819 v. 0·770) in the PI and by 5·53 % (0·839 v. 0·795) in the DI. The average apparent jejunal digestibility of eleven protein-bound amino acids (0·771) in the summit diet was surpassed by the average apparent digestibility of five amino acids (0·834) that were present in the diet as both crystalline and protein-bound amino acids by 8·17 %. This advantage diminished to 6·13 and 4·60 % in the proximal and DI, respectively. These outcomes reflect the accepted view that crystalline amino acids are rapidly ‘digested’ and absorbed in the anterior small intestine and are fully bioavailable in poultry( Reference Izquierdo, Parsons and Baker 28 ).
Threonine and proline are two of the most abundant amino acids in porcine mucin( Reference Lien, Sauer and Fenton 29 ) and the transition from foundation to summit diets improved the apparent distal ileal digestibility of these amino acids by 8·09 and 9·28 %, respectively. Given that the average improvement for the balance of fourteen amino acids was 4·36 %, there is the implication that increasing dietary casein and crystalline synthetic amino acids reduced mucin secretion and endogenous amino acid flows, thereby enhancing apparent amino acid digestibility coefficients.
Amino acid disappearance rates
Essentially, the transition from the foundation to summit diet linearly accelerated amino acid disappearance rates in three small intestinal segments (Tables 7 and 8). The effects of the dietary transition were highly significant (P<0·001) in all three segments for three essential amino acids – arginine (32·6 %), isoleucine (12·9 %) and methionine (34·1 %) – which were present in the summit diet as both crystalline (proportions shown in parentheses) and protein-bound amino acids. The effects of the dietary transition were highly significant (P<0·001) in all three segments for four non-essential amino acids (alanine, aspartic acid, glycine and proline). Conversely, significant linear effects were not observed for histidine, glutamic acid and tyrosine in any segment. This is the case as following the transition from foundation to summit diet there is a highly significant linear relationship (r 0·822; P<0·001) between percentage increases in distal ileal amino acid disappearance rates and percentage increases in free amino acid concentrations in blood taken from the anterior mesenteric vein.
Concentrations of amino acids in portal systemic circulations
Intestinal uptakes of nutrients, including amino acids, are crucial to bird performance( Reference Croom, Brake and Coles 9 ); nevertheless, absorbed amino acids do not necessarily enter the portal circulation to become available for protein accretion. As reviewed by Wu et al.( Reference Wu, Knabe and Flynn 30 ), this subject is complex because a large proportion of absorbed amino acids become involved in anabolic and catabolic pathways in enterocytes. Moreover, amino acids that do enter the portal circulation may have entered the gut mucosa from either the gut lumen or the arterial circulation and are not necessarily amino acids of dietary origin. Amino acids in enterocytes may be synthesised into proteins to maintain gut integrity or serve as precursors for digestive enzymes, mucin, nucleotides, polyamines and amino acids( Reference Wu 10 ). In addition, amino acids are subject to catabolism in the gut mucosa, and Reeds et al.( Reference Reeds, Burrin and Stoll 31 ) concluded that amino acids are critical energy sources for the intestinal mucosa, with the caveat that whether they are subject to nutritional regulation requires further investigation. Either glucose or amino acids, especially glutamate/glutamine, are catabolised in avian enterocytes for the provision of energy( Reference Watford, Lund and Krebs 32 ). Glucose and glutamine provide energy to the gut mucosa in rats to approximately equal extents, but it appears that energy is derived more efficiently from glucose( Reference Fleming, Zambell and Fitch 33 ). Thus, there is a ‘catabolic ratio’ between amino acids and glucose within enterocytes and it should be advantageous to manipulate this ratio so that more glucose and less amino acids are catabolised. If achieved, amino acids would be spared from catabolism to enter the portal circulation and become available for protein accretion, and the copious energy requirement of the gut would be met more efficiently.
This is the rationale for the determination of concentrations of free amino acids in the portal and systemic blood samples (Table 9). Overall, the sum concentrations of eighteen amino acids tabulated was 929·5 μg/ml in the portal circulation, which declined by 12·7 % to 811·0 μg/ml in the systemic circulation. All amino acids declined, to significant or numerical extents, which is indicative of their utilisation in the body. The four largest relative reductions were observed for the branched-chain amino acids (isoleucine 35·7 %, leucine 31·4 %, valine 22·7 %) and histidine (23·5 %). As a main effect, the change from the ‘slow’ summit diet to the ‘rapid’ protein diet significantly increased plasma concentrations of isoleucine, methionine, threonine, proline and tyrosine but significantly decreased concentrations of histidine, aspartate/asparagine, glycine and serine. It is probably relevant that isoleucine, methionine and threonine were present as crystalline amino acids at higher inclusions in the summit diet.
A direct comparison of the impact of the ‘slow’ summit diet in comparison with the ‘rapid’ protein foundation diet on amino acid concentrations in the portal circulation per se is of interest. Of relevance, is that crystalline amino acids represented 8·8 % of lysine, 30·8 % of methionine and 1·9 % of threonine of total dietary amino acids in the foundation diet. In contrast, in the summit diet, crystalline amino acids comprised 14·3 % of total lysine, 34·1 % methionine, 12·3 % threonine, 18·6 % tryptophan, 32·6 % arginine and 12·9 % isoleucine. Birds offered the summit diet had significantly increased concentrations of methionine by 42·2 % (16·5 v. 11·6 μg/ml; P<0·001), threonine by 39·1 % (81·8 v. 58·8 μg/ml; P<0·001) and proline by 23·2 % (70·1 v. 56·9 μg/ml; P=0·01) relative to their counterparts on foundation diet on the basis of pair-wise comparisons. It should be noted that methionine and threonine are two of the first three limiting amino acids in poultry diets. On the other hand, glycine concentrations were decreased by 20·3 % (P=0·004) and the balance of amino acids were not influenced (P>0·125) by the transition from foundation to summit diets. Six amino acids were present in the summit diet in both crystalline and protein-bound forms, and the transition from slow to rapid protein diets increased their collective concentrations in the portal circulation by 17·4 % from 219 to 257 μg/ml. In contrast, there were twelve protein-bound amino acids in the summit diet, and the transition fractionally decreased their concentrations by 0·84 % (709 v. 715 μg/ml). Although not conclusive, this outcome appears to be consistent with the possibility that crystalline amino acids are less prone to be catabolised in the gut mucosa than their protein-bound counterparts. The assertion here is that because crystalline amino acids are rapidly absorbed in the anterior small intestine, where more glucose is available as an alternative energy substrate, they may be spared from catabolism. Thus, this preliminary investigation into free amino acid plasma concentrations in poultry suggests that accelerating protein digestion rates may be a dietary means of manipulating the catabolic ratio between amino acids and glucose. Reductions in ammonia concentrations in the portal circulation could provide more specific indications of reductions in amino acid catabolism. Stoll et al.( Reference Stoll, Henry and Reeds 34 ) reported that net portal outflows of ammonia accounted for 18 % of total amino acid nitrogen intake in pigs, and thus any dietary strategy that reduces concentrations of ammonia in the anterior mesenteric vein has probably suppressed amino acid catabolism in the gut mucosa. This will be the subject of future investigations.
Summary
In the present study, as defined, ‘rapid protein’ advantaged weight gain and parameters of nutrient utilisation and influenced post-enteral availability of amino acids in broiler chickens. The dietary provision of ‘rapid protein’ increased starch digestibility coefficients in both ileal segments and starch disappearance rates in four small intestinal segments, which appeared to be associated with linear increases in parameters of nutrient utilisation (AME, ME:GE ratios, N retention, AMEn). In addition, the present study provided indications that plasma concentrations of free amino acids in the portal circulation can be influenced by dietary manipulation. These outcomes indicate that future research into the relevance of protein and starch digestive dynamics to chicken-meat production is justified.
Acknowledgements
The authors would like to acknowledge Professor Mingan Choct and the Poultry CRC for their support of both Dr Ha Truong’s PhD candidature and this particular study. The authors would also like to thank the indefatigable Ms Joy Gill and her team in the Poultry Research Foundation, and Mr Bernie McInerney, Dr Leon McQuade and their team at Australian Proteome Analytical Facility (Macquarie University) for their invaluable contributions.
The funders had no role in the design, analysis or writing of this article.
The authors’ contributions were as follows: H. H. T. contributed to the study design and data analyses; P. V. C. contributed to the study design, diet formulation and manuscript writing; H. H. T. and A. F. M contributed to data collection; H. H. T., A. F. M, P. H. S. and S. Y. L. contributed to manuscript writing, P. H. S. and S. Y. L. contributed to experimental design and data analysis. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.


















