INTRODUCTION
Enteric protozoa are important causes of infectious diseases affecting people in developing as well as developed countries (WHO, 2008). Compared with developing countries, relatively few enteric protozoa are included in operational surveillance systems in developed countries. Where these are included, they are mainly seen as indicators of foodborne and waterborne diseases outbreaks (Cretikos et al. Reference Cretikos, Telfer and Mcanulty2008b ; WHO, 2008; Stark et al. Reference Stark, Barratt, Van Hal, Marriott, Harkness and Ellis2009; Kucerova et al. Reference Kucerova, Sokolova, Demyanov, Kvac, Sak, Kvetonova and Secor2010; Dixon et al. Reference Dixon, Parrington, Cook, Pintar, Pollari, Kelton and Farber2011; Sokolova et al. Reference Sokolova, Demyanov, Bowers, Didier, Yakovlev, Skarlato and Sokolova2011). However, evidence suggests that while some enteric protozoa such as Entamoeba spp., Cryptosporidium spp. and Giardia intestinalis are more frequently identified in diarrhoeal cases in developing regions, like Asia and sub-Saharan Africa (Nair et al. Reference Nair, Ramamurthy, Bhattacharya, Krishnan, Ganguly, Saha, Rajendran, Manna, Ghosh, Okamoto and Takeda2010; Fletcher et al. Reference Fletcher, Stark and Ellis2011); others like Blastocystis spp. and Dientamoeba fragilis appear to be more prevalent in the developed countries (Roberts et al. Reference Roberts, Barratt, Harkness, Ellis and Stark2011; Fletcher et al. Reference Fletcher, Stark, Harkness and Ellis2012). In developed settings, however, enteric protozoa are often ignored as a cause of diarrhoea due to the often mistaken belief that better hygiene practices are occurring.
In developed settings, bacterial cultures are usually considered initially in the diagnosis for acute diarrhoeal illnesses, while parasitic infections are more likely to be considered in patients with chronic symptoms, appropriate travel histories or other risk factors (Ribes et al. Reference Ribes, Seabolt and Overman2004). However, laboratory-based surveillance has been used as an important tool for estimating the burden of infectious diseases in several countries worldwide (Flint et al. Reference Flint, Van Duynhoven, Angulo, Delong, Braun, Kirk, Scallan, Fitzgerald, Adak, Sockett, Ellis, Hall, Gargouri, Walke and Braam2005). In Australia, for example, Cryptosporidiosis and Giardiasis are the only parasitic gastrointestinal diseases included in the infectious disease surveillance (Cretikos et al. Reference Cretikos, Telfer and Mcanulty2008a , Costello et al. Reference Costello, Abbas, Allen, Ball, Bell, Bellamy, Friel, Groce, Johnson, Kett, Lee, Levy, Maslin, Mccoy, Mcguire, Montgomery, Napier, Pagel, Patel, De Oliveira, Redclift, Rees, Rogger, Scott, Stephenson, Twigg, Wolff and Patterson2009). Estimates of the actual prevalence of enteric protozoa in industrialized countries is often affected by: (i) the lack of routine testing for these parasites and (ii) the lack of sensitive diagnostic techniques to detect them in clinical specimens, while carrier stages and sub-clinical infections are often not diagnosed (Ng et al. Reference Ng, Yang, Whiffin, Cox and Ryan2011).
The actual burden of parasitic infections affecting Australians is relatively unknown. Anecdotal evidence suggests that the prevalence is relatively low; however, some individuals are at increased risk of infection. Parasitic infections are considered to be common among Aboriginal communities especially in children under 5 years of age (Commonwealth of Australia, 2000, Currie and Brewster, Reference Currie and Brewster2001). Reports indicate that men who have sex with men are at increased risk of infection (Stark, Reference Stark2007; Stark et al. Reference Stark, Van Hal, Matthews, Marriott and Harkness2008). However, it is assumed that the estimated prevalence of protozoon infections is relatively similar regardless of the testing protocol employed. However, a scientific assessment of this has neither been done; nor has a gold standard approach been determined for diagnosis of enteric protozoan disease. This information is needed for early and accurate diagnosis to aid in the optimal management of parasitic diseases. This not only allows initiation of appropriate therapy, but also implementation of health and hygiene education and control measures in the patients’ home and community.
Here we summarize a multi-centre study to determine the relative prevalence of enteric protozoon infections from clinical specimens examined at four public hospitals in Sydney, and the comparison of the outcome of different testing algorithms for the detection of enteric protozoa. Finally, this study suggests that molecular methods should be employed as a gold standard approach for clinical diagnosis of enteric protozoa.
METHODS
Setting and study sites
Four hospitals, Liverpool Hospital (A), The Children's Hospital at Westmead (B), St. Vincent's Hospital (C), Sydney, Prince of Wales Hospital (D), all located in different geographic areas across Sydney were included in the study. These facilities were included based on the population served, and represented a cross-section of different socio-economic and cultural influences across the Sydney metropolitan region. Hospital A, is a tertiary referral hospital for Southwestern Sydney; hospital B is a stand-alone service dedicated to paediatrics attracting referrals on a State-wide basis; hospital C is a major public and a principal referral hospital attracting referrals on a State-wide and national basis; and hospital D is a major teaching hospital and one of 13 principal referral hospitals for adults based in Sydney's eastern suburb that also serves all of New South Wales. Each hospital provided a fully accredited laboratory service, providing comprehensive biomedical laboratory services.
Ethical approval for this study was received from the Human Research Ethics Committees of each hospital and the University of Technology, Sydney (UTS).
Microbiology methods
All four hospitals routinely tested for enteric organisms in persons who presented with gastrointestinal symptoms. On average, each laboratory tested one stool sample per patient, with between 45 and 89% of these specimens being loose – but not taking the shape of the container. Generally speaking, each laboratory used standard methods for the identification and isolation of enteric pathogens. Additionally, in all hospitals, stools were processed by a wet preparation in saline, and examined for white blood cells, red blood cells, cysts, ova and parasites (COP); bacteriological pathogens were identified using standard culturing methods and each hospital had specific criteria for the testing of viruses (Fletcher et al. Reference Fletcher, Sibbritt, Stark, Harkness, Rawlinson, Andresen, Van Hal, Merif and Ellis2015). A summary of the various tests done for parasitic agents is presented in Supplementary Table S1.
Parasitology
Hospital A
Stool specimens were routinely collected in sodium acetate acetic acid formalin (SAF) fixative (Oxoid Australia), and processed by a direct wet preparation. Light microscopy was routinely performed on all stool specimens. In the instances where no clinical information was received and the patient was an adult or age ≤10 years old, or the specimen was not received in SAF, then a Giardia/Cryptosporidium screen enzyme immunoassay (EIA) (ProSpecT™ Giardia/Cryptosporidium Microplate Assay) was performed. A 10% suspension of stool was prepared in 10% formalin (for G. intestinalis and Cryptosporidium) and the EIA was performed in accordance with the manufacturer's instructions and without modification. A full COP test was done on all positive microscopy and EIA results using an IHS with modified acid-fast stain.
Hospital B
Light microscopy of a direct saline preparation was performed on all stool specimens. Concentration techniques were performed routinely for persons with a history of overseas travel, prolonged diarrhoea illness (>7 days), attendees at refugee clinics and on specific requests for COP test by the clinician. When a COP test was requested and if any parasites were seen in the wet preparation, a sample of stool was placed into SAF fixative (Oxoid Australia) using a 1:5 ratio and processed for fecal concentration and stained using the fecal parasite concentrator (Evergreen Scientific, LA, CA), which uses centrifugation at 500 g for 10 min and examined for COP using oil immersion. Alternatively, the fixed smear was prepared for permanent staining by the iron haematoxylin technique. Additionally, each stool specimen had a Cryptosporidium smear done routinely using a Modified Kinyoun's acid-fast stain (Cold).
Hospital C
Direct wet preparation and light microscopy were performed routinely on all stool specimens. The wet preparation was examined under a low-power objective (10×) and then scanned under the high dry (40×) objective. All stool specimens were emulsified in SAF fixative (Oxoid Australia) using a 1:3 ratio; and then the samples were centrifuged at 500 g for 10 min. Samples were then processed for permanent staining by a modified iron haematoxylin staining technique (mIHS) technique incorporating a carbol fuschin step to stain for acid-fast organisms (Isospora, Cryptosporidium and Cyclospora). Stool samples also underwent direct DNA extraction using a QIAamP DNA stool minikit (Qiagen, Hilden, Germany) using a portion of fresh stools sample for the identification of Entamoeba spp. These methods have been previously described by Stark and colleagues (Stark et al. Reference Stark, Schuller, Sloots, James, Halliday and Carter2010c ; Banik et al. Reference Banik, Barratt, Marriott, Harkness, Ellis and Stark2011; Roberts et al. Reference Roberts, Barratt, Harkness, Ellis and Stark2011).
Hospital D
Direct wet preparation microscopy was only conducted on patients at risk to parasitic infection as indicated in the clinical history and for patients with recent overseas travel or on request by the clinicians. The wet preparation was examined by light microscopy under low-power objective (10×) and then scanned under the high dry (40×) objective. A sample of stool was also placed into SAF fixative (Meridian Bioscience, Inc., Cincinnati, Ohio followed by fecal concentration using the Mini Parasep®SF concentration kit (DiaSys Europe LTD, Laboratory Diagnostics PTY LTD). In addition, an EIA was performed routinely as a screening test for the detection of G. intestinalis and Cryptosporidium (ProSpecT™ Giardia/Cryptosporidium Microplate Assay) and the detection of Entamoeba histolytica/dispar (ProSpecT™ Entamoeba histolytica, Remel). A 10% suspension of stool was prepared in 10% formalin (for G. intestinalis and Cryptosporidium) and in specimen buffer provided in kit (for E. histolytica) and the EIA was performed in accordance with the manufacturer's instructions and without modification. All positive findings from the EIAs were confirmed by microscopy (i.e. iodine preparation and acid-fast stain). Samples testing positive on the E. histolytica EIA that could not be confirmed by direct microscopy (i.e. iodine preparation) were sent to a reference laboratory for permanent stain preparation and examination. In order to detect Cryptosporidium oocysts, smears were made directly from feces and stained by the Ziehl–Neilsen based on the procedures described elsewhere (Collins and Lyne, Reference Collins and Lyne1984).
Data extraction and analysis
Each hospital provided a spread sheet containing de-identified microbiology test results for the period January 2007 to December 2010 (hospital C's data were for 2008–2010). The data were then arranged by medical record number, and date of service/stool request in ascending order. For each hospital, the testing protocols were consulted to determine the number of specimens tested for COP/intestinal parasites. The laboratory data were placed into a Statistical Package for the Social Science (SPSS) database and duplicate results removed to avoid duplicate counting of specimens. Duplicates were considered to be any stool specimen from the same individual that was collected on the same date and had the same request number. For the purposes of this analysis, each individual stool sample and results were counted. Positivity was calculated on the basis of one organism per specimen. The percentage positivity rate was calculated as the total number of stool samples positive for an enteric organism divided by the total number of specimens tested. Odds ratios were calculated for each specific test conducted to measure the association between the detection of pathogens (outcome) at different hospitals (exposure). A laboratory survey identified laboratory procedures and information captured on laboratory request forms.
RESULTS
The laboratory survey identified laboratory procedures and information captured on laboratory request forms in the four hospitals. The date of sample collection, age and gender were reported routinely on all requests in all hospitals. Differential diagnosis was reported only sometimes in two hospitals and rarely in the other two. Signs and symptoms were only reported sometimes at all sites, and date of onset of illness was rarely reported except by hospital D, where it was routinely done. Across all hospitals, between 1 and 10% of stool specimens received were formed, while 45–89% were unformed (loose but not taking the shape of the container). Only 10–50% of specimens were considered liquid (taking the shape of the container).
Tests for enteric parasites were conducted on 2138 individual specimens from 1518 persons at hospital A; 11097 specimens from 5229 persons at hospital B; 8613 specimens tested from 6273 persons at hospital C; and 6078 specimens tested from 3772 persons at hospital D.
Enteric parasites summary
Enteric protozoa were identified in an average of 3·6% (95% CI 1·1–11·2%) of specimens from the four hospitals. Across the four hospitals, the most common enteric protozoon detect was Blastocystis spp., identified in an average of 5·4% (95% CI 5·0–5·7%) of cases, followed by G. intestinalis 1·1% (95% CI 1·0–1·2%), D. fragilis in 0·8% (95% CI 0·7–1·0 %), E. histolytica/dispar in 0·5% (95% CI 0·4–0·6%), Cryptosporidium spp. 0·3% (95% CI 0·3–0·4%), Cyclospora 0·1% (95% CI 0·02–0·1%). Non-pathogenic protozoa, including Entamoeba spp., Enteromonas hominis and Iodamoeba butschlli were found in <1% of cases, respectively.
At hospital A, 29% of patients submitted multiple specimens, with an enteric protozoon found in 8%. At hospital B, 48% of patients submitted multiple specimens, with enteric protozoa detected in <1%. At hospital C, 38% of patients submitted multiple specimens and protozoa were detected in 8·5%. At hospital D, 38% of patients submitted multiple specimens and 0·5% tested positive.
The results for hospital A are summarized in Table 1. A total 9% (187/2138) of stool specimens examined had an enteric protozoon identified. Overall, Blastocystis spp. (5·71% or 122/2138) and G. intestinalis (1·17% or 25/2138) were most frequently detected. At hospital B, an enteric protozoon was detected in 1% (70/ 10123) of stools (Table 2); G. intestinalis (0·5% or 48/10123) and Blastocystis spp. (0·4% or 40/10123) were most frequently identified. One or more protozoa were found in 12% (1003/8613) specimens at hospital C (Table 3). Blastocystis spp., 7% (571/8613), Giardia in 2% (141/8613) and D. fragilis in 1% (100/8613) were most frequently detected. At hospital D, 1% (78/6078) of stool specimens tested positive for one or more enteric protozoa (Table 4). A total of 1% (77/3772) tested positive for the Giardia/Cryptosporidium coproantigen test (by EIA). However, only 56% (43) of these were confirmed by microscopy of wet preparation to be G. intestinalis and 20 (26·0%) confirmed to be Cryptosporidium spp.
Table 1. Overall prevalence of enteric protozoa from Cyst, Ova and Parasite test, Hospital A, 2007–2010

Table 2. Overall prevalence of enteric protozoa from cyst, ova and parasite test, hospital B 2007–2010
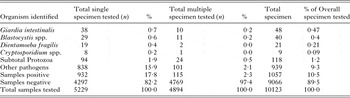
Table 3. Overall prevalence of enteric protozoa from cyst, ova and parasite test, hospital C for 2008–2010
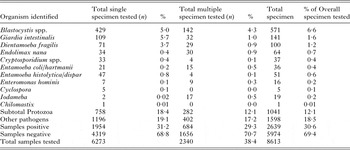
Table 4. Overall prevalence of enteric protozoa from cyst, ova and parasite test, hospital D for 2007–2010
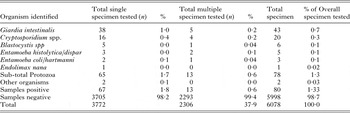
Comparison of results based on testing protocols
Approximately 2·5% (95% CI 2·3–2·7%) of protozoon infections was detected by permanent staining (IHS or mIHS), 1·1% (95% CI 1·0–1·2%) by microscopy of wet preparations and 0·6% (95% CI 0·5–0·7%) by EIA combined with microscopy.
The mean difference between tests conducted at each hospital is presented in Table 5. Blastocystis spp. was more frequently detected at hospital A when compared with hospitals B and D (mean difference 5·3 and 5·6%, respectively; P = 0·0002), and at hospital C when compared with hospitals B and D (mean difference >6% each; P < 0·0001). A higher rate of detection was also observed for Giardia at hospital A when compared with hospitals B and D (mean diff. 0·7%; P < 0·0002 and 0·5%, P < 0·05, respectively) and hospital C when compared with hospitals B and D (mean different 1·2 and 0·93%; respectively; P < 0·0001). In addition, hospital C diagnosed significantly more E. histolytica/dispar when compared with hospital D (mean diff. 0·5%; P < 0·0002).
Table 5. Mean difference in protozoa detection rates amongst four Sydney hospitals

N/C, not calculated. Cells contained fewer than 5, hence estimates could not be calculated. NT, test not done. Total specimen tested hospital A, 2138; hospital B, 10123; hospital C, 8163; and hospital D, 6078.
The combination of microscopy of wet preparation and EIA detected the prevalence of Cryptosporidium spp., in an average of 0·4% [95% CI 0·3–0·5%; OR 1·4 (0·7–3·0)] and G. intestinalis in 0·9% [95% CI 0·7–1·1%; OR 1·7 (1·0–2·7)] with no significant differences in detection between hospitals A and D (P > 0·05). Microscopy of wet preparation detected Blastocystis spp., in an average of 2·9% (95% CI 2·5–3·4%) of cases, with significantly higher detection rates at hospital A compared with hospital B (OR 14·4; 95% CI 10·1–20·7; P < 0·0001). Permanent staining with IHS or mIHS detected D. fragilis in an average of 1·1% (95% CI 0·9–1·4%) of cases between hospitals A and C; with hospital C detecting significantly higher rates by employing a mIHS (OR 3·6; 95% CI 1·7–7·7; P < 0·001).
DISCUSSION
We present the prevalence of enteric protozoa amongst persons seeking care for gastrointestinal illnesses in Sydney across four major public hospitals. The study reveals that while all four laboratories performed direct microscopy on stool specimens for the detection of cyst, ova and parasites, different approaches are used for different species and tests for some protozoa are not routinely done. We found that, while the prevalence of enteric protozoa species is relatively low in this population, widespread variability in the testing protocols as well as individual and population characteristics may influence protozoon detection rates in this population. Progress towards development of a gold standard approach for diagnosis of disease is warranted.
The rate of detection of enteric parasites between the four hospitals varied. Generally, Blastocystis spp. and G. intestinalis were the most common enteric protozoa identified in patients. An age relationship was observed with Giardia prevalence, with higher rates detected in the 0–5 age group, compared with higher rates of Blastocystis spp. in the over 5 age group. This age relationship has been previously described in this population (Fletcher et al. Reference Fletcher, Caprarelli, Merif, Andresen, Hal, Stark and Ellis2014) and may indicate that children are more frequently exposed to giardiasis risk factors in this setting, as described previously (Fletcher et al. Reference Fletcher, Stark, Harkness and Ellis2012; Yoder et al. Reference Yoder, Gargano, Wallace and Beach2012).
The proportion of stool specimens positive for an enteric parasite between hospitals ranged from a low of 1% in hospitals B and D, to a high of 11·6% at hospital C. The difference in detection rates between Hospitals may be due to various factors. Individual hospitals had different testing criteria for enteric protozoa, hence not all stool specimen were tested for enteric protozoa. Both hospitals A and C tested for a wider range of pathogens routinely (see Tables 1 and 3), including non-pathogenic species, which may be driven by the high-risk populations served including recent migrants (A), men who have sex with men, and HIV/AIDs infected persons (C). Secondly, the composition of the population seen at each hospital could have influenced test results if risk factors were unequally distributed in the population (Mohr and Mohr, Reference Mohr and Mohr1992). Previously published data on a randomly selected subset of this population indicated that there were minor differences in the sex distribution between hospitals except for hospital C, where there was slightly more males. However, there were significant differences between the age distribution, particularly in the under 5 years age group between hospitals; based on the population served by hospitals. Enteric protozoa were more prevalent in children under 5 years of age in this population (Fletcher et al. Reference Fletcher, Sibbritt, Stark, Harkness, Rawlinson, Andresen, Van Hal, Merif and Ellis2015). Age associated risk factors influencing the underlying prevalence of the condition in this population may therefore account for some variations in detection rates between hospitals. On the other hand, some hospitals did not routinely test for COP if the prevalence of protozoa was relatively low in the population, and was likely to generate many false negatives. The testing protocols may therefore be secondary to a perceived prevalence within high-risk groups in the wider population (e.g. men who have sex with men, recent immigrants and lower socio-economic groups). Both hospitals A and C had specific clinics that catered to high-risk groups.
The differences in detection rates could also be associated with the different diagnostic techniques and handling practices between hospitals. According to Libman et al. (Reference Libman, Gyorkos, Kokoskin and Maclean2008), significant variations in specimen handling and processing practices between laboratories can affect the assessment of the diagnostic processes. This study found that where wet preparation microscopy of fresh or fixed stool (SAF) specimens, detected a lower prevalence of protozoa. One disadvantage of using the microscopy method only is its low sensitivity to detect protozoa, which lead to false-negative results (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a ; Roberts et al. Reference Roberts, Barratt, Harkness, Ellis and Stark2011). This is particularly true for protozoon such as D. fragilis that requires special staining techniques to detect its nuclear structure (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b ; Stark et al. Reference Stark, Al-Qassab, Barratt, Stanley, Roberts, Marriott, Harkness and Ellis2011).
The mIHS method consistently detected significantly higher rates of Blastocystis spp. and G. intestinalis at hospital C and significantly higher rates of D. fragilis when compared with the IHS-only method at hospital A and wet preparation microscopy at hospital B. Microscopy remains a widely used tool for protozoan detection, even in low prevalence settings such as Sydney (Bruijnesteijn Van Coppenraet et al. Reference Bruijnesteijn Van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009; Ghoshal et al. Reference Ghoshal, Dey, Ranjan, Khanduja, Agarwal and Ghoshal2016). While molecular methods are more sensitive for pathogen detection, these tools are still not widely available or routinely employed even in developed settings (Fletcher et al. Reference Fletcher, Stark, Harkness and Ellis2012; Ghoshal et al. Reference Ghoshal, Dey, Ranjan, Khanduja, Agarwal and Ghoshal2016).
This study has various limitations. The data were collected retrospectively since approval was not obtained for prospective data collection. The authors are mindful that the incidence figures for each hospital should be compared with caution based on the differences between the testing protocols, and their ability to detect protozoa. The authors cannot exclude that some of the differences found between the four hospitals can be explained by actual differences in parasite prevalence in the underlying populations tested. One potential bias of this study is that the hospitals that followed specific criteria for stool testing for protozoa, identified higher rates of protozoa, regardless of the test used as evidenced by Blastocystis and Giardia rates at hospital A. This is further impacted by the relatively low prevalence of some organisms in the population, small proportion of persons who seek medical attention and even fewer who get tested.
Notwithstanding, these results are useful to local and state health authorities to guide disease surveillance activities for these organisms, aid in the understanding of the epidemiology of protozoon infections in Sydney, and provide the basis for setting research priorities and planning interventions. The development of a gold standard approach for diagnosis of enteric protozoa, which addresses issues such as relatively low incidence of some species such as Cryptosporidium and Cyclospora; the difficulties in diagnosis (e.g. D. fragilis) and differentiation (e.g. Entamoeba spp.) of some species, is therefore warranted.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/pao.2016.7.
ACKNOWLEDGEMENTS
We acknowledge the medical records and laboratory staff that extracted the data for each hospital.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







