Metabolic syndrome (MetS) is a common metabolic disorder resulting from a cluster of interrelated abnormalities: obesity, hypertension, hyperglycaemia and dyslipidaemia(1–Reference Alberti, Zimmet and Shaw3). There are currently several definitions for MetS provided by various expert groups and organizations including WHO, the European Group for the Study of Insulin Resistance, the National Cholesterol Education Program – Third Adult Treatment Panel and the International Diabetes Federation (IDF)(Reference Eckel, Grundy and Zimmet4). The aetiology of MetS includes obesity, which has been found to be responsible for the excess release of NEFA, cytokines and other pro-inflammatory factors, the development of insulin resistance and the development of hypertension and hyperlipidaemia(Reference Eckel, Grundy and Zimmet4).
The number of persons with MetS has been increasing intensively over the past two decades. The consequence of such an increase is a global epidemic of obesity and diabetes(Reference Zimmet, Alberti and Shaw5). There is also a high risk of CVD, highlighting an urgent need for a strategy of prevention and treatment(Reference Grundy, Hansen and Smith6).
Differences in lifestyle, nutrition and environmental conditions have an impact on health status, as well as on obesity, body fat distribution and predisposition for MetS. Obesity and higher body weight are strongly associated with sedentary lifestyle and lack of physical activity in the adult population of the European Union(Reference Martínez-González, Martínez and Hu7). Comparison of published MetS prevalences in different populations is difficult because such estimates have been obtained using varying diagnostic criteria(Reference Cameron, Shaw and Zimmet8). Despite differences in the study designs and diagnostic criteria used, certain interferences can be found(Reference Eckel, Grundy and Zimmet4). There is a wide variation with regard to prevalence in both sexes for the different populations studied, as well as for ethnic origin. MetS was also found to be highly age-dependent, with a higher prevalence in elderly people. MetS was significantly associated with age in populations from different Croatian island villages(Reference Kolčić, Vorko-Jović and Salzer9). It is already known that nutritional habits have a significant impact on MetS(Reference Giugliano, Ceriello and Esposito10). The Mediterranean diet may reduce the incidence of MetS(Reference Tortosa, Bes-Rastrollo and Sanchez-Villegas11). It includes a high consumption of fruit, vegetables, legumes and whole grains, moderate alcohol intake, moderate to low consumption of meat and meat products, and high MUFA:SFA ratio.
The aim of the present study was to investigate the prevalence of MetS in relatively healthy elderly Croatian populations from four different centres. The influence of different lifestyle habits between continental and Mediterranean–Adriatic centres, as well as between rural and urban centres, was examined. We also estimated age- and sex-specific prevalence of MetS and analysed the association between different biochemical markers and MetS.
Experimental methods
Study participants
The cohort was established in the late 1960s from the Voter Registry(Reference Pavlović and Čorović12–Reference Jazbec, Šimić and Čorović14). Initially (1969) the study was based on a random sample of the resident population, consisting of 4223 participants of both genders born between 1915 and 1934, from five communities representing two distinct climatic and cultural regions of Croatia. The cohort was re-examined in 1972 (n 3764), 1982 (n 2415) and 2006 (n 385) based on a registry of vital records. Continental Croatia was represented by two centres: the Virovitica municipality (mainly rural region, situated in the vicinity of the Hungarian border) and the City of Zagreb (Western District and Centre). The coastal region also included two centres: Omiš municipality and the City of Split. The Omiš municipality included a small proportion of an urban population (25 %) and the rest were from the surrounding rural and suburban area. The study centres were not directly affected by the 1991–1995 war in Croatia and the sample was selected as follows. During 2005, 2415 persons who had participated in the study in 1982 were identified, based on verification from: postal address in the Voter Registry, data on residential status, community health services, the Register of Deaths, nursing homes, and a short postal questionnaire or phone contact. In 2006, 907 survivors who had not moved away were invited to take part in the study. Of these, 317 could not be traced (they did not respond the invitation), seventy-seven refused to participate, and 128 were immobile, sick or lived in a nursing home and were consequently excluded from the study. The final sample included in the 2006 cross-sectional survey consisted of 385 volunteers aged more than 70 years. All participants were free-living, able to take care of themselves and to manage their everyday activities (inclusion criteria: non-institutionalized mobile residents, non-febrile at the time of medical check-up, cooperative for spirometry and with positive attitude towards participation in the study). Exclusion of sixty-five participants with missing anthropometric or questionnaire data, low blood sample volume for biochemical analyses or infectious and/or chronic liver diseases left a study population of 320 elderly persons among whom prevalence of MetS was investigated. The cohort was divided into continental v. coastal, rural v. urban and third (70–75 years) v. fourth (76–90 years) age subgroups.
The study was performed in accordance with the ethical principles of the Helsinki Declaration and was approved by a competent ethics committee. Informed consent was obtained from each examinee before inclusion in the study. Nurses and physicians were qualified and appropriately trained.
Tests were performed between 07.00 and 12.00 hours. The clinical interview comprised questions based on verified and coded history of chronic diseases classified by the International Classification of Diseases, IX Revision. The study also comprised a questionnaire related to eating habits: lard, olive oil, hard cheese, wine, spirits, pelagic fish (anchovy and/or mackerel and/or tuna) and freshwater fish (carp and/or catfish and/or trout). Regarding stated eating habits, participants were categorized into two subgroups. Those who reported daily consumption of lard, olive oil, spirits and wine and monthly (or more frequent) consumption of cheese and fish were assumed as one subgroup. The other subgroup consisted of participants who stated that they do not consume those foodstuffs at all or consume them rarely. Alcohol intake was measured by standard measures (1 drink = 200 ml wine or 30 ml spirits) and moderate drinking was defined as up to 3 drinks/d(Reference Milošević, Mustajbegović and Abdović15). Social circumstances, income, marital status and psychological instruments, including brief assessment of cognitive function, were also included in the questionnaire.
Arterial blood pressure measurements were obtained on two occasions in sitting position after 10–20 min rest, using a desk-type mercury sphygmomanometer (Reister, Jungingen, Germany).
Medical examination included anthropometric measurements, body height, waist circumference (WC) and body mass (portable field survey scale Korona 3516701; Niko Weiss Best, Korona, Germany) as well as BMI. BMI was calculated according to the formula: [mass (kg)]/[body height (m)]2. Height, weight and WC were measured according to the International Biological Programme Protocol.
Diagnosis of metabolic syndrome
MetS was diagnosed on the basis of anthropometric measurements, biochemical parameters (fasting glucose, TAG and HDL-cholesterol (HDL-C)) and arterial blood pressure. The IDF criteria for diagnosis of MetS were used. According to the IDF criteria, MetS was diagnosed if WC was ≥94 cm for males or ≥80 cm for females, plus any two of the following: fasting glucose ≥5·6 mmol/l (or previously diagnosed diabetes mellitus and antidiabetic therapy), TAG ≥1·7 mmol/l, HDL-C <1·03 mmol/l for males or <1·29 mmol/l for females, and blood pressure ≥130/85 mmHg.
Biochemical analyses
Blood samples were drawn from an antecubital vein after an overnight fast. Blood samples were centrifuged, divided into 1 ml aliquots and stored frozen. Glucose was measured using the enzymatic hexokinase method and the catalytic activity of alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT) by standardized methods. Analyses were performed on an Olympus AU400 selective autoanalyser (Olympus, Tokyo, Japan) using reagents from the same manufacturer (Olympus, Hamburg, Germany).
Lipoprotein parameters were measured on a Roche COBAS MIRA autoanalyser using enzymatic methods with reagents from Boehringer-Mannheim Diagnostics and Hoffmann-La Roche (Roche Diagnostics, Mannheim, Germany). LDL cholesterol (LDL-C) was calculated according to the Friedewald formula. To avoid participants with potential infectious diseases and also with liver diseases, we excluded those with C-reactive protein >10 mg/l, ALP >100 U/l and GGT >60 U/l.
Statistical analysis
Statistical analyses were performed with the STATISTICA 7 for Windows statistical software package (StatSoft, Inc., Tulsa, OK, USA). MetS group frequencies were calculated by counting. Categorical variables were compared using Pearson's χ 2 test. Binary logistic regression was used to assess the association between diet and MetS, with adjustment for centres. Continuous variables are expressed as mean and standard deviation or standard error. Group comparisons of continuous variables were performed by Student's t test. Multivariate analysis (MANOVA) with age and gender as covariates was used for testing the effects of dietary habits on continuous variables. The level of significance was set at 0·05.
Results
Using the IDF definition, MetS was identified in 206 (64·4 %) out of 320 elderly participants (aged 70–90 years) from four centres in continental and coastal Croatian regions. Frequency of MetS did not differ between the centres (Table 1). After pooling the two continental centres (Zagreb and Virovitica) and the two Adriatic coast centres (Split and Omiš), comparison of MetS frequencies between the continental region and the Adriatic coast did not show any significant difference. However, when we compared the large centres (Zagreb and Split) with the small centres (Virovitica and Omiš) there was a significant difference in prevalence of MetS (Table 2). Significantly lower MetS prevalence was found among participants from small urban centres in comparison to those from large urban centres (59·1 % v. 69·6 %; P = 0·051). No gender difference in MetS frequencies was found in the entire study group (P = 0·47), between participants from the continental centres (P = 0·20) or between those from the Adriatic coast (P = 0·68). The results were similar when the participants from the two large urban centres (P = 0·17) or the small urban centres (P = 0·75) were compared according to gender. Moreover, when participants were classified by age into two groups (70–75 years v. 76–90 years), no significant differences in MetS prevalence were found between the groups (P = 0·57).
Table 1 The frequency of metabolic syndrome in elderly participants (aged 70–90 years, n 320) from four centres in continental and coastal Croatia

MetS (−), without metabolic syndrome; MetS (+), with metabolic syndrome.
No significant difference in MetS prevalence by study centre: P = 0·272.
Table 2 The frequency of metabolic syndrome in elderly Croatian participants (aged 70–90 years, n 320), according to large and small urban centres

MetS (−), without metabolic syndrome; MetS (+), with metabolic syndrome.
Significant difference in MetS prevalence by size of urban centre: P = 0·05.
†Zagreb and Split.
‡Omiš and Virovitica.
Differences were observed in nutritional habits between participants from the continental centres and the Adriatic centres, as well as between participants from the large and small urban centres (Table 3). Participants from the Adriatic coast had higher consumption of olive oil, pelagic fish, hard cheese and wine, whereas the dietary pattern among those from the continental centres included higher consumption of lard, freshwater fish and spirits. Participants from the large urban centres consumed more olive oil and hard cheese, whereas those from the small urban centres consumed more lard, freshwater fish, spirits and wine. However, when the nutritional habits of the participants were compared with regard to the presence or absence of MetS (Table 4), a statistically significant difference was found only for wine (P = 0·05); that is, consumption of wine was less frequent among participants with MetS. Binary logistic regression analysis, with adjustments for centres (four different centres), showed similar results (data not presented).
Table 3 Comparison of nutritional habits in elderly Croatian participants (aged 70–90 years, n 320), according to continental and coastal centres, and large and small urban centres

Data represent the percentages of participants who reported ¶daily (for lard, olive oil, spirits and wine) or ††monthly or more frequent (for cheese and fish) intake of the nutrient.
Significance of the difference in the percentages of participants consuming the nutrient in continental v. coastal centres or large v. small urban centres: *P ≤ 0·05, **P < 0·01, ***P < 0·001.
†Zagreb and Virovitica.
‡Split and Omiš.
§Zagreb and Split.
||Omiš and Virovitica.
Table 4 Comparison of nutritional habits in elderly Croatian participants (aged 70–90 years, n 320) from different centres, according to the presence of metabolic syndrome

MetS (−), without metabolic syndrome; MetS (+), with metabolic syndrome.
Data represent the percentages of participants who reported †daily (for lard, olive oil, spirits and wine) or ‡monthly or more frequent (for cheese and fish) intake of the nutrient.
Significance of the difference in the percentages of participants with MetS v. without MetS consuming the nutrient: *P ≤ 0·05.
Table 5 shows the anthropometric and biochemical parameters in the groups of participants with or without MetS diagnosed, the differences between the groups being tested with Student's t test. The statistically significant differences between participants with and without MetS which were observed for BMI, WC, glucose, TAG and HDL-C confirm correct use of the diagnostic criteria for MetS. There were no significant differences for age, total cholesterol and LDL-C. In the male population, ALP and GGT activities were significantly higher in participants with MetS than in those without MetS. Relative consumption frequencies of lard, hard cheese, olive oil, pelagic fish and wine, with regard to cut-off levels for the biochemical components of MetS, are shown in Table 6. Compared with their peers with HDL-C <1·03 mmol/l, more male participants with HDL-C ≥1·03 mmol/l consumed wine (P = 0·04) or pelagic fish (P = 0·03). The prevalence of participants with TAG ≥1·7 mmol/l was significantly higher in wine non-consumers (P = 0·05) than in wine consumers. There were also statistically significant differences in wine consumption according to gender (60·7 % of men and 39·3 % of women; P < 0·001). Table 7 presents multivariate analysis of the biochemical parameters according to wine consumption, with age and gender as covariates. Results showed a significant inverse association of wine consumption with total cholesterol (P < 0·001), a significant positive association with HDL-C (P < 0·001) and a marginally inverse association with TAG (P = 0·06).
Table 5 Anthropometric and biochemical parameters, and the differences between groups with or without metabolic syndrome, in elderly Croatian participants (aged 70–90 years, n 320) from different centres

MetS (−), without metabolic syndrome; MetS (+), with metabolic syndrome; WC, waist circumference; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase; T, total; M, male; F, female.
Mean value was significantly different in participants with MetS v. without MetS: *P ≤ 0·05, ***P < 0·001.
Table 6 Relative consumption frequencies of lard, cheese, olive oil, pelagic fish and wine with regard to cut-off levels for biochemical components of the metabolic syndrome in elderly Croatian participants (aged 70–90 years, n 320) from different centres
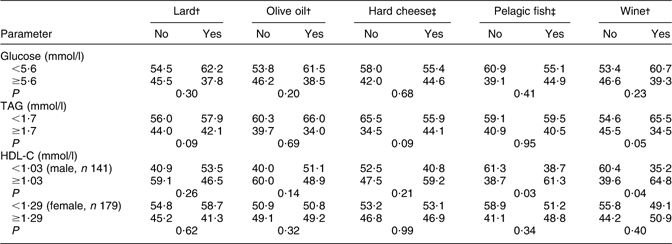
HDL-C, HDL cholesterol.
Data represent the percentages of participants who declined (no) or reported (yes) †daily (for lard, olive oil and wine) or ‡monthly or more frequent (for cheese and fish) intake of the nutrient.
Table 7 The influence of wine consumption on relevant biochemical parameters in elderly Croatian participants (aged 70–90 years, n 320) from different centres

HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
†Multivariate analysis; covariates: age and gender.
Discussion
In most countries worldwide between 20 % and 30 % of the adult population can be characterized as having MetS, although in certain populations or population segments MetS prevalence is even higher. Populations in which young adults predominate have lower MetS prevalence, but MetS prevalence increases with increasing affluence and ageing of the population(Reference Grundy16). Our study was conducted among a relatively healthy, free-living elderly Croatian population with age more than 70 years. The prevalence of MetS in this population ranged between 60 % and 70 % according to the IDF diagnostic criteria. Epidemiological data on MetS prevalence in elderly populations are rare. In an earlier study of regional prevalence of MetS in 9070 Croatian subjects(Reference Vuletić, Kern and Ivanković17) the prevalence was found to be 15·2 % for males and 22·5 % for females. However, in that study the diagnostic criteria for MetS differed from the IDF criteria (WC ≥102 cm for males and ≥88 cm for females, and at least two of the following: arterial blood pressure ≥140/90 mm Hg, self-reported elevated blood lipids and self-reported elevated blood sugar). Increased prevalence of MetS in elderly populations was also observed in several European population studies. Estimated MetS prevalence (according to IDF criteria) in the population-based KORA Survey 2000 (age group 55–74 years) was 46 % for women and 57 % for men(Reference Rathmann, Haastert and Icks18). The MetS-Greece Multicentre Study showed a 14·7-fold increased risk for having MetS in the age group >70 years old(Reference Athyros, Bouloukos and Pehlivanidis19).
As we took into account populations from four different Croatian centres (two continental: Zagreb and Virovitica; two coastal: Split and Omiš) it was interesting to analyse the differences between them. Regional differences in the Croatian population have been observed before, particularly regarding risk factors for CHD(Reference Bergovec, Reiner and Miličić20). Hospitalized patients in the continental regions had higher prevalence of hypertension and decreased HDL-C(Reference Reiner, Mihatov and Miličić21). The present results did not show any significant differences in the frequency of MetS between the Adriatic coastal centres and the continental centres. However, we observed significantly higher MetS prevalence in the large urban centres compared with the small centres, with the emphasis on rural lifestyle habits. This difference might be attributable to diet. The HALE project on elderly European men and women showed that Mediterranean diet, moderate alcohol consumption, moderate to high physical activity levels and non-smoking were associated with lower mortality rates from all causes, CHD, CVD, cancer and other causes during a 10-year follow-up period(Reference Knoops, de Groot and Kromhout22). An optimal diet for the prevention of weight gain, obesity, MetS and type 2 diabetes is reported to be a fat-reduced diet without any industrially produced trans fatty acids, fibre-rich, high in low-energy-density carbohydrates (fruit, vegetables and whole-grain products) and with a restricted intake of energy-containing drinks(Reference Astrup, Dyerberg and Selleck23). FFQ data previously showed significant differences in nutritional habits, including greater amounts of pelagic fish (rich in PUFA), olive oil (rich in MUFA and polyphenols) and wine (rich in polyphenols), in subjects of Mediterranean origin(Reference Esposito, Ciotola and Giugliano24). Although no data are available, it is generally known that nutritional habits in the Mediterranean have changed greatly over the past two decades, mainly because of socio-economic factors. For example, consumption of poor-quality carbohydrates and meat has increased in the Mediterranean region, especially in the urban areas. Such data were confirmed by investigations on the prevalence of chronic diseases, such as obesity, diabetes and cardiovascular disorders in the southern Mediterranean (African) countries(Reference Belahsen and Rguibi25). Data reported from this region showed that there has been a shift in dietary habits from a traditional Mediterranean diet to industrial food, including high consumption of SFA and refined carbohydrates, low fibre consumption as well as sedentary behaviour(Reference Belahsen and Rguibi25). A multicentre study of the Mediterranean Group for the Study of Diabetes showed that MetS is not related to the Mediterranean type of diet and its prevalence varied greatly among five Mediterranean countries(Reference Thanopoulou, Karamanos and Angelico26). The prevalence of MetS in an isolated population on the Croatian island of Hvar was significantly high in spite of adherence to a relatively traditional lifestyle pattern, together with a Mediterranean diet and rural habitat(Reference Deka, Narančić and Xip27).
This exaggerated rate and risks for CVD and diabetes could be attributed to increasing urbanization with effective changes in lifestyles. Higher prevalence of MetS in urban rather than rural areas has been observed for several different populations(Reference Ramírez-Vargas, Arnaud-Viñas Mdel and Delisle28–Reference Abdul-Rahim, Holmboe-Ottesen and Stene30). The reasons for the difference between urban and rural prevalences of MetS and other chronic diseases lie in the characteristics of urbanization(Reference Athyros, Liberopoulos and Mikhailidis31). Urbanization includes access to energy-dense industrialized foods, technological changes in both work and transportation, and reduced physical activities in the workplace and at leisure time(Reference Ramírez-Vargas, Arnaud-Viñas Mdel and Delisle28). Our questionnaire data showed higher prevalences of different kinds of daily physical activity in participants from the rural area. Persons who live in a rural area practise livestock breeding, horticulture, fishing, etc. Epidemiological studies suggest that regular physical activity prevents MetS, type 2 diabetes, CVD and premature mortality(Reference Lakka and Laaksonen32). In a study among employees of a large manufacturing company, men with MetS who reported higher levels of anxiety, anger and work stress had higher blood pressure than men without MetS, who reported lower levels of these mental health indicators(Reference Capizzi, Allen and Murphy33).
A number of studies have focused on the relationship between alcohol consumption and prevalence of MetS. Nutritional habits of our study participants with and without MetS did not differ significantly apart from the consumption of wine. The results indicated that wine might have a protective effect against MetS. A trend for lower MetS prevalence with alcohol consumption was observed in a Mediterranean Greek population study, which also showed that wine consumption was associated with a slightly better effect than beer or spirits consumption on the prevalence of CVD(Reference Athyros, Liberopoulos and Mikhailidis31). A Quebec cardiovascular study showed that moderate daily alcohol consumption has cardioprotective properties, especially in persons with a deteriorated risk profile, such as those with MetS(Reference Gigleux, Gagnon and St-Pierre34). However alcohol consumption might show opposite effects on manifestation of MetS. This depends primarily on the amounts and types of alcohol consumed(Reference Goude, Fagerberg and Hulthe35). Moderate alcohol consumption is associated with a decreased incidence of MetS and beneficial effects on plasma lipid levels (increase in plasma HDL-C levels), WC and fasting plasma glucose(Reference Jelski and Szmitkowski36). Moderate wine consumption in our study (mean wine intake 0·33 (95 % CI 0·15, 0·55) l/d; i.e. 1·65 standard drinks daily) confirmed a beneficial effect on TAG, total cholesterol, HDL-C and LDL-C concentrations in men. The relationship between alcohol consumption and incidence of MetS is more pronounced among red wine consumers because the polyphenols contained in red wine increase the activity of endothelial nitric oxide synthase that plays a key role in the pathogenesis of MetS as well as in the transport of HDL molecules(Reference Leighton, Miranda-Rottmann and Urquiaga37).
Non-alcoholic fatty liver disease may be considered an additional feature of MetS(Reference Marchesini, Brizi and Bianchi38). The increasing prevalences of obesity, diabetes, dyslipidaemia and hypertension, characteristics of MetS, put a very large population at risk of forthcoming liver failure(Reference Marchesini, Bugianesi and Forlani39). People with non-alcoholic fatty liver disease typically have elevated circulating concentrations of markers of liver injury, including aspartate aminotransferase, alanine aminotransferase, GGT and ALP(Reference Hanley, Williams and Festa40). We noticed slightly higher activity of ALP and GGT in participants with MetS, although only in men, who consume more wine than women (Table 5). Thus we cannot conclude that increased markers of liver injury are due only to MetS, and thereby the consumption of wine might have opposite effects on a person's health. Several population studies have shown the influence of obesity and alcohol consumption on increased serum GGT activity(Reference Niemelä41). Obesity and moderate drinking seem to have synergistic effect on increasing the risk of liver injury(Reference Puukka, Hietala and Koivisto42). It is known that alcohol induces hepatic microsomal production of GGT, or it may cause the leakage of liver enzymes, including GGT(Reference Barouki, Chobert and Finidori43). Although ALP is not primary indicator of liver injury, taking into account increased GGT in the current population of older and relatively healthy people (and the known relationship between ALP and GGT), slightly increased ALP in men with MetS might be due to moderate liver injury.
In the present study there was evidence that the prevalence of men with HDL-C ≥1·03 mmol/l (cut-off for MetS) was higher in those who consumed pelagic fish. It is well known that pelagic fish are rich in PUFA(Reference Connor, DeFrancesco and Connor44). n-3 PUFA have been shown to reduce total cholesterol and LDL-C, and to increase HDL-C(Reference Zuliani, Galvani and Leitersdorf45). However, the beneficial effects of n-3 fatty acids in lowering TAG levels and increasing HDL-C levels are not entirely understood(Reference Bitzur, Cohen and Kamari46). Studies on animal models showed that a higher dietary ratio of n-6:n-3 PUFA increases the expression of several genes (apoA1, ABCA1) involved in the metabolism of HDL-C and cholesterol reverse transport(Reference Zhang, Geng and Xiao47). The influence of PUFA in modulating the effect of apoA1 polymorphism and on HDL-C concentrations was also observed in the Framingham Offspring Study(Reference Ordovas, Corella and Cupples48). This might explain the possible effect of PUFA dietary intake on the expression of genes involved in cholesterol metabolism.
Conclusions
High MetS prevalence was observed in an elderly Croatian population. Consumption of poor-quality carbohydrates and meat has increased in the Mediterranean area, especially in the urban area, and thus the prevalence of MetS was similar in the Mediterranean area to that in the continental area. The difference in lifestyle habits between the rural and urban centres might be responsible for the significantly lower MetS frequency in rural centres. The nutritional habits of the participants in our multicentre study confirmed that wine and pelagic fish might have a protective effect against MetS. The number of people with MetS is increasing and the consequence is a global epidemic of obesity and diabetes, as well as high risk of CVD and liver injury.
Acknowledgements
Sources of funding: The work was supported by Project Grants 022-0222411-2407, 277-2770966-0965, 108-1080316-0298 and 022-0222411-2408 of the Croatian Ministry of Science, Education and Sport. Conflict of interest declaration: There are no conflicts of interest. Authorship responsibilities: The study was designed by M.P. D.P. and M.P. wrote the manuscript. M.P. and N.Č. were responsible for the medical check-ups and D.P., S.D. and J.J. for the biochemical analyses. D.P. and A.P. supervised the statistical analysis. D.P. was responsible for review and revision of the manuscript, with assistance from M.P. and J.J.









