Carotid endarterectomy (CEA) has historically been shown to be an effective tool in the management of high risk extracranial occlusive disease. 1 - 3 More recently, carotid artery angioplasty and stenting (CAS) has emerged as a less invasive alternative to CEA. The largest trial to date comparing CEA and CAS has been the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), which randomly assigned over two thousand patients to undergo either treatment.Reference Brott, Hobson and Howard 4 CREST’s results showed no statistical significant difference in the composite outcomes of death, stroke, or myocardial infarction (MI) between CEA and CAS, however during the periprocedural period (30 days after procedure) there was a higher risk of stroke with CAS.Reference Brott, Hobson and Howard 4 This conclusion was further supported in the recent meta-analysis by Yavin et al.Reference Yavin, Roberts, Tso, Sutherland, Eliasziw and Wong 5 Follow-up analysis of CREST results have shown that patients under the age of 70 have similar risks with CEA and CAS, however the risk increases in those patients over the age of 70 undergoing CAS; patient risk increases based on age.Reference Voeks, Howard and Roubin 6 A meta-analysis that pooled results from previous studies including the International Carotid Stenting Study, Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3s) and stent-protected angioplasty versus carotid endarterectomy (SPACE) has reached a similar conclusion that CAS might be as safe as CEA in younger patients (age <70).Reference Bonati, Dobson and Algra 7 These conclusions are reflected in the recently published American Heart Association/American Stroke Association (AHA/ASA) guidelines for prevention of stroke which state CEA is associated with improved outcomes in this age group.Reference Kernan, Ovbiagele and Black 8
In large studies such as CREST, randomized patients are medically fit to undergo either CEA or CAS, meaning these patients are in relatively good health.Reference Brott, Hobson and Howard 4 In clinical practice however, patients are pragmatically selected to CAS if they are ineligible (high risk) for CEA; a routine that clashes with the recent guidelines.Reference Kernan, Ovbiagele and Black 8 A historical cohort of CAS patients considered high risk for revascularization treatment provides an opportunity to study the most significant causes and consequences of adverse events.
Our primary goal was to determine the rates of periprocedural and long-term death, stroke, transient ischemic attach (TIA), or MI and identify the risk factors associated with complications after CAS in our high risk patients. Secondarily, we compared our rates of complications with those reported in the recent literature.
Methods
Patient Data Collection
We retrospectively collected patient data from patients who presented with carotid stenosis of >70% as determined by ultrasound, computed tomography angiography (CTA), or digital subtraction angiography (DSA), and were subsequently treated using CAS between September 2002 and August 2011. We reviewed the inpatient records, pre/post procedural notes, operative notes and radiologic reports to collect patient data including clinical, demographic, procedural technique, modified Rankin Score (mRS), and the procedure outcome data. Clinical data included history of systemic hypertension (blood pressure >140/90 or use of hypertensive medication at time of procedure), stroke, TIA, stages 3-5 chronic kidney disease (CKD – estimated glomerular filtration rate (eGFR) <60), dyslipidemia (low density lipoprotein >2.0), atrial fibrillation, diabetes mellitus (use of antidiabetic medication at time of procedure), chronic obstruction pulmonary disease, previous coronary artery disease or peripheral vascular disease (CAD/PVD), indications for CAS, contralateral internal carotid artery (ICA) stenosis, length of hospital stay, suitability for CEA, symptoms pre-CAS, and smoking status. Demographic data consists of age and sex. Procedure related data consisted of type of stent used and employment of pre or post stent angioplasty. We collected procedure outcome data including the events: death, stroke, TIA, and MI. Other periprocedural outcomes recorded were post-procedural hypotension, groin hematoma requiring vascular surgical repair, right external iliac thrombosis, and heparin induced thrombocytopenia syndrome during CAS procedure. We defined two time frames, periprocedural and long-term; periprocedural complication is defined as death, stroke, TIA or MI occurring within 30 days of the procedure, and long-term complication is defined as death, stroke, TIA, or MI occurring after 30 days of the procedure. The functional status of all patients was determined using the mRSReference Farrell, Godwin, Richards and Warlow 9 at both a pre-procedure and last clinical encounter dates by clinicians not involved in the CAS procedure. This study was approved by our institutional research ethics board.
CAS Technique
All patients received dual anti-platelet therapy for a minimum of five days before device implantation using aspirin (81 mg) and clopidogrel (75 mg) orally, once per day. We intended to position a distal embolic protection device in all cases. We performed a pre-stenting angioplasty when the stenosis was too severe to allow crossing of the protection device. We performed a post stenting angioplasty if residual stenosis was noted or brain perfusion was limited after positioning the stent. All patients underwent independent neurological evaluation before and after CAS and during follow-up at 30 days, six months, and annually.
Statistical analysis
Patients with previous neck radiation were excluded. Clinical data included history of: CAD-PVD, stroke, TIA, ipsilateral past CEA, dyslipidemia, hypertension, congestive heart failure, atrial fibrillation, smoking, diabetes, Chronic obstructive pulmonary disease, chronic kidney disease, CEA suitability, and initial diagnosis. Demographics included age and sex. To compare clinical data within age subgroups, logistic regression analysis was performed. To detect risk factors associated with mRS increase logistic regression analysis was conducted after adjusting for pre-stent mRS (0-2 vs. 3-5). Bootstrap method was used for internal validation of the final multivariate logistic model. Logistic regression analysis was also used for detecting the association between clinical/demographic variables and the outcomes: periprocedural/long-term complications, hospital stay >2 days. R2 was used to measure the model fit. 10 Cox Proportional Hazard model was performed in the overall survival analysis. Fisher exact test was applied for detecting the relationship between periprocedural/long-term complications and contralateral ICA stenosis, between mRS increase and protection device, stent type, or post-CAS angiography. P-value <0.05 was considered statistically significant.
Results
Patient characteristics
We identified a total of 156 patients treated with CAS at our institution. Four of these patients had prior neck radiation and were excluded from the results and statistical analysis.
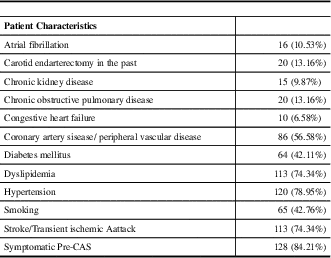
Our entire cohort used in the statistical analysis consisted of 152 patients of which 128 (84%) had symptomatic carotid stenosis. Patient characteristics are described in Table 1. The mean age was 71.4±10.0, with 114 (75%) male subjects. We had 67 (44%) patients younger than 70 years of age, eighty-five (55.9%) patients ≥70 years of age, and 38 (25%) patients ≥80 years of age. The most common indications for CAS were transient ischemic attack in 63 (41%) patients, stroke in 40 (27%) patients, ocular ischemia in 19 (13%) patients, and CEA restenosis in 10 (6%) patients. Based on interdisciplinary assessment, 16 (10%) patients were suitable for either CAS or CEA and opted to undergo CAS, whereas the remainder patients were considered not ideal for CEA.
Table 1 Patient characteristics represented by total patient number with percentage of total patient population in brackets
The relationships between the selected age subgroups and demographic/clinical data are displayed in Table 2. Those aged >70 were more likely to have atrial fibrillation (p=0.01) but less likely to have diabetes mellitus (p=0.02), smoking (p=0.002), history of CEA (p=0.049), or stroke/TIA (p=0.02). Those aged >80 were found to be less likely to have diabetes mellitus (p=0.03).
Table 2 Association of patient characteristics to age greater than 80. Bolded values are statistically significant

TIA=transient ischemic attack; OR=odds ratio; CI=confidence interval
In 131 (86%) procedures we employed the use of a distal embolic protection device. We placed a single stent device in 149 (98%) procedures. We performed post-CAS angioplasty in 81 (53%) procedures.
Primary Endpoints
The mean time to treatment was 87±186 days with a range of 0 to 1708 days. The median post-procedural length of hospital stay was two days (mean absolute difference =1). Pre-stent mRS 3-5 was significantly associated with length of hospital stay > two days (odds ration (OR) 2.933 confidence interval (CI)[1.269-6.886]).
The total follow-up time was 5,837 months with a mean follow-up of 38.4 months per patient. Figure 1 describes the distribution of frequencies of follow up time in the entire cohort.

Figure 1 Distribution of follow-up divided into four chronological categories with raw patient numbers in each group on x-axis and percentages located above each barn.
Pre-CAS mRS and mRS at last clinical encounter are shown in Figure 2. The majority of patients (143[94%]) had a pre-stent mRS ≤3. Thirty-nine (26%) patients had a mRS increase at the last clinical encounter after CAS. We found that CAD/PVD (p=0.03), dyslipidemia (p=0.02), CKD (p=0.01), and contralateral ICA stenosis (p=0.02) were non-modifiable risk factors associated with mRS increase after CAS. These factors remained significant when placed into multivariate analysis and were confirmed via bootstrapping. We found that age >70 (p=0.26), age >80 (p=0.23), initial diagnosis of stroke (p=0.27) or TIA (p=0.82), or sex (p=0.72), were not associated with an increase in mRS after CAS.

Figure 2 Modified Rankin Score distribution both pre-CAS and at last clinical assessment. Overlaid numbers represent percentage with mean follow up time for each group located in adjacent brackets. No patient received a mRS of 5 at last clinical assessment.
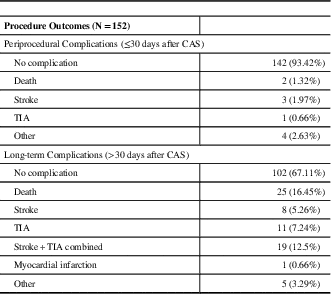
Periprocedural complications are listed in Table 3. We found two (1.32%) deaths, one (0.66%) transient ischemic attack, three (1.97%) strokes, and no cardiac complications. Characteristics of the three strokes are located in Table 4. Four (2.63%) patients incurred non-life threatening complications classified as “Other”. The two periprocedural deaths were due to multiple organ infarctions (due to embolic phenomenon) and immediate post procedural intracranial (intraventricular and subarachnoid) hemorrhages. The composite total of death, stroke, TIA, and MI rate was 3.95% (6/152). The presence of CKD was significantly associated with periprocedural complications (p=0.004 OR 10.763 CI [2.081-56.193]).
Table 3 Periprocedural and long-term complication rate. Total patient number with percentage of total patients in brackets
TIA=transient ischemic attack; CAS=carotid artery stenting
Table 4 Characteristic of three patients who suffered stroke in the periprocedural time frame
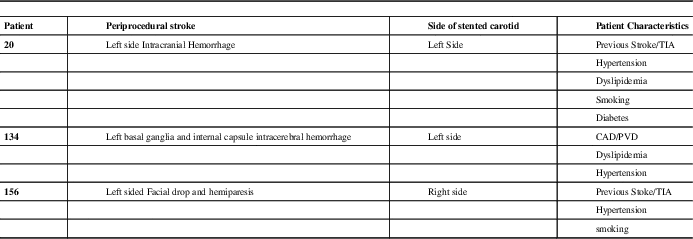
TIA=transient ischemic attack; CAD/PVD=coronary artery disease/peripheral vascular disease
Long-term complications are listed in Table 3. The mean time to mortality was 1131±762 days with a range of 1 to 3205. There were 25 (16%) deaths, 8 (5%) strokes, 11 (7%) TIAs, and 1 (0.66%) MI. The presence of CAD/PVD (p=0.009) and dyslipidemia (p=0.002) were significantly associated with long-term complications.
The Cox proportional hazard model analysis showed that CAD/PVD (p=0.004) along with CKD (p<0.001) was related to overall survival after CAS. These variables remained significant in multivariable analysis of overall survival. The Kaplan-Meier survival curves were created to examine the difference in survival between patients with either: CKD, CAD/PVD, symptomatic carotid stenosis or age ≥70 years. The graphs are shown Fig. 3 and depict that CKD and CAD/PVD were significantly related to survival by log rank test.

Figure 3 Kaplan-Meier Overall Survival curve with 95% CI in all patients. A) age ≥70 or age <70. B) with or without CAD/PVD. C) with or without CKD. D) symptomatic vs. asymptomatic
Contralateral ICA stenosis was not a risk factor for periprocedural (p=0.74) or long-term complications (p=0.28). As expected, suitability for CEA was not associated with mRS increase (p=0.07) or complications within (p=0.52) or after 30 days (p=0.68).
Discussion
The data we describe here reflects a practice where CEA is the first line of treatment for patients with symptomatic carotid stenosis. CAS is pragmatically chosen as an alternative when patients are unsuitable for or refuse CEA. These selection criteria imply that the inherent population of this study was deemed higher risk compared to the background of patients with stenosis. The decision to remove patients with prior neck radiation from the analysis was made because of the inherent lower longevity and higher risk of stroke in this patient population.Reference Plummer, Henderson, O'Sullivan and Read 11
Periprocedural Complication Rate
The periprocedural complication rate at our center, 3.95%, compares favorably with rates found in the recent literature. The CAS arm of CREST, which had an ideal patient population, reported a periprocedural rate of death, stroke, or MI of 5.9%.Reference Brott, Hobson and Howard 4 Case series reports in high risk patients report periprocedural rates range between 2.6-8.2%.Reference Gribar, Jiddou and Choksi 12 - Reference Alkins, Matouk, Cruz, Marotta, Montanera and Spears 14 Interim analysis of CREST data brought light to the idea that octogenarians have an increased risk of periprocedural complications.Reference Hobson, Howard and Roubin 15 A study further examining octogenarian CAS risk concluded that it was equal to CEA risk if good indications, meticulous techniques, and protection devices were used.Reference Henry, Henry, Polydorou and Hugel 16 Recently published AHA/ASA guidelines moved this age division down to 70 stating that CEA may be more appropriate for patients older than 70 but that CAS should be considered as equivalent for CEA in terms of periprocedural complications in those younger than 70.Reference Kernan, Ovbiagele and Black 8 This is supported by the recent case series by Alkins et al. that examined 159 patients who underwent CAS and concluded that CAS might be a safe alternative for those under 70 but in those over 70 careful patient selection is needed.Reference Alkins, Matouk, Cruz, Marotta, Montanera and Spears 14 Likely due to selection bias, our data is contrary to both of these conclusions as we found that age (using either the 70 or 80 cut off) was not significantly associated with periprocedural complication rate. Rather age, when using the >70 cut off, seemed to be protective for some of the patient characteristics we found, meaning that older patients actually had fewer comorbidities. From this we suggest that age might not play as important a factor in outcomes of CAS as previously believed.
It is apparent in our series that CKD is a risk factor associated with periprocedural complications and with long-term disability and death in patients undergoing CAS. Previous case reports similarly demonstrated that CKD was associated with higher rates of complications after CAS.Reference Saw, Gurm and Fathi 17 This is in contrast to the recent Care Registry analysis which showed, after risk factor adjustment, that CKD was not an independent predictor 30-day outcome.Reference Gruberg, Jeremias and Rundback 18 A recent study done by AbuRahma et al. sought to understand what role CKD plays in outcomes of CEA and found that patients with moderate to severe CKD (GFR <60) had an significant increased odds ratio for early stroke or death.Reference AbuRahma, Srivastava, Chong, Dean, Stone and Koszewski 19 His data suggested that CEA would not be a suitable alternative to CAS in patients with CKD.
Long-term Study
In contrast to previous studies evaluating long-term data in CAS, our study population was unique in that it provided reliable outcome data beyond 30 days. Less than one third of our patients had long term complications of stroke or MI. Our long-term mortality rate, 16%, is lower than that previously reported. The SAPHIRE study showed at three-year follow up the combined death, stroke, or MI rate was 24.6%.Reference Gurm, Yadav and Fayad 20 CREST showed at four-year follow up the combined stroke, MI, or death rate was 27.7%.Reference Brott, Hobson and Howard 4 Recent cases series report all-cause mortality rates to range from 16.8-47.5% within five years.Reference Gribar, Jiddou and Choksi 12 , Reference Lago, Parkhutik and Tembl 21 , Reference Eskandari, Usman and Garcia-Toca 22 Brooks et al. conducted a randomized trial to compare the ten-year efficacy of CAS versus CEA and found 50% mortality in those who achieved follow up of ten years, and most commonly of nonvascular causes.Reference Brooks, Jones and Gisler 23 This wide range found in the literature likely implies that the patient populations within these various studies were heterogeneous. Additionally, CKD was a risk factor for long-term mortality placing further emphasis on the potential need for more rigorous surveillance.
Modified Rankin Score Analysis
We examined changes in mRS in patients undergoing CAS. This allowed the identification of factors relating to functional decline as opposed to solely examining death, stroke, TIA, or MI rates. A majority of our patients gained a benefit in functioning post CAS. The previously described age categories (>70 or >80 years of age) were not related to mRS increase. The risk factors related to long-term mRS increase after CAS (CAD/PVD, dyslipidemia, CKD, contralateral ICA stenosis) suggest that atherosclerosis-related diseases contribute to the decline in patients functioning over time. Because of the sparse data published in this area, it is not possible to compare our rates. However, based on our data pre-CAS mRS could serve in determining which patients will have a longer length of hospital stay and a more complicated clinical course after CAS.
Limitations
We cannot exclude the possibility of residual confounding factors related to the retrospectively analyzed data. Given the pragmatic limitations of our practice we do not have a large low-risk CAS group for comparison. Although a regular clinical follow up schedule was desired, it was not achieved in all patients; this might have interfered with timing of detection of complications. Because we observed so few periprocedural complications, the power to statistically evaluate the factors contributing to these outcomes could have been insufficient. Since our mRS assessment was done at the last clinical encounter the improvement cannot solely be attributed to CAS.
Conclusion
Survival analysis in this retrospective cohort with long-term follow up data found that our periprocedural and long-term complication rates in a high risk population compare favorably to large scale trials that have ideal patients. We identified CKD as a significant risk factor for periprocedural complications and long-term mRS increase. Age was not associated with periprocedural outcomes, increase in mRS, long-term complications and long-term mortality. We identified mRS as a possible predictive tool and reported rates than can be used for comparison in future studies. This data encourages the consideration of CAS for patients considered to be high risk for CEA who need therapy for carotid atherosclerotic burden and provides possible patient characteristics (CKD) to help with risk stratification.
Acknowledgements
We would like to thank Dr. Liying Zhang PhD, Senior Statistician MacroStat Inc., for her help with statistical analysis. We thank Mrs. Christina Tsoukanas for research and administrative assistance.
Statement of Authorship
VD and ACMR made an equal contribution to this work and should be considered joint first authors.
Disclosures
We declare that we have no conflicts of interest.









