Post-weaning diarrhoea is a major problem in pig production( Reference McCracken, Gaskins and Ruwe-Kaiser 1 , Reference Brown, Maxwell and Erf 2 ), which can cause considerable loss due to mortality or production of non-marketable pigs( Reference Rose, Larour and Le Diguerher 3 ). As an effective strategy to solve this problem, reducing dietary protein level has been found to reduce the incidence of diarrhoea and the severity of digestive problems in piglets( Reference Nyachoti, Omogbenigun and Rademacher 4 – Reference Kong, Wu and Liao 6 ). Recent studies have shown that reducing dietary protein intake could improve gastrointestinal health and function after weaning( Reference Heo, Opapeju and Pluske 7 , Reference Rist, Weiss and Eklund 8 ). However, impairment of piglet growth begins to occur when crude protein (CP) content is reduced from the level recommended by the National Research Council (NRC)( 9 ) to 17 %, balanced with the first four limiting amino acids (AA) (lysine, methionine, threonine and tryptophan)( Reference Lordelo, Gaspar and Le Bellego 10 – Reference Le Bellego and Noblet 12 ). Thus, identifying the next-limiting AA that can maintain the growth performance of piglets fed reduced-protein diets is of enormous nutritional importance.
Numerous studies have suggested that branched-chain AA (BCAA), particularly valine and isoleucine, are the next-limiting AA for growth of piglets, and supplementation with these AA makes it possible to maintain growth performance of piglets fed reduced-protein diets( Reference Lordelo, Gaspar and Le Bellego 10 , Reference Le Bellego and Noblet 12 , Reference Nørgaard and Fernández 13 ). Moreover, it has been demonstrated that dietary deficiency of AA causes a rapid decline in animal feed intake, which is particularly the case for BCAA in pigs( Reference Gloaguen, Le Floc’h and Brossard 14 – Reference Gloaguen, Le Floc’h and Corrent 16 ) and mice( Reference Kamata, Yamamoto and Kamijo 17 ). In pigs, the suppressive effect of valine deficiency on feed intake is aggravated by an excess supply of leucine due to the shared enzymes in their catabolic pathways, which thereby increases the catabolism of valine( Reference Wiltafsky, Pfaffl and Roth 18 ). However, when valine and isoleucine requirement is fulfilled in the diet, an excess supply of leucine does not affect feed intake any more( Reference Soumeh, van Milgen and Sloth 19 , Reference Yin, Yao and Liu 20 ), but can act as a signalling molecule to increase muscle protein synthesis and growth performance in piglets( Reference Yin, Yao and Liu 20 – Reference Suryawan, Torrazza and Gazzaneo 22 ). In addition, as leucine is the most effective single AA in promoting muscle protein synthesis via stimulating mammalian target of rapamycin (mTOR) pathway activity, addition of leucine to a reduced-protein diet to regulate animal growth has been the focus of many previous studies( Reference Escobar, Frank and Suryawan 23 , Reference Escobar, Frank and Suryawan 24 ). Thus far, little has been reported regarding the enhancing effect of supplementation of all the three BCAA to reduced-protein diets on the growth performance of piglets.
Several AA have been known to act as signal molecules in regulating feed intake of rodents( Reference Cota, Proulx and Smith 25 – Reference Maurin, Benani and Lorsignol 27 ). However, the molecular mechanisms by which AA regulate the feed intake of pigs remain unknown. Hypothalamic neurons that express appetite-regulatory neuropeptides are considered as critical regulators of feeding behaviour and body weight (BW). Agouti-related peptide (Agrp) and co-express neuropeptide Y (NPY) stimulate feed intake, whereas pro-opiomelanocortin (POMC), melanocortin-4 receptor (MC4R) and cocaine- and amphetamine-regulated transcript (CART) inhibit feed intake( Reference Morton, Cummings and Baskin 28 ). Recently, two evolutionarily conserved signal transduction pathways, general AA control (GAAC) pathway in anterior piriform cortex (APC) and hypothalamus and mTOR pathway in hypothalamus, have been shown contribute to the regulation of feed intake in response to dietary AA changes( Reference Cota, Proulx and Smith 25 – Reference Maurin, Benani and Lorsignol 27 ).
Taken together, we hypothesised that supplementation of BCAA to reduced-protein diets might increase feed intake and skeletal muscle mass in piglets, and these improvements are associated with changes in activities of related AA signalling pathways. Therefore, the aim of this study was to evaluate the effect of dietary BCAA supplementation on growth performance, feed intake and muscle mass in piglets fed reduced-protein diets with a CP content of 17 %. All diets were fortified with lysine, methionine, threonine and tryptophan to satisfy the standardised ileal digestible (SID) AA requirement. We further elucidated the roles of hypothalamic GAAC and mTOR signalling pathways in feed intake regulation as well as the role of mTOR signalling pathway involved in the control of muscle protein synthesis after dietary supplementation with BCAA.
Methods
Two experiments were conducted, and Huazhong Agriculture University Animal Care and Use Committee reviewed and approved the protocols for each experiment.
Animals and diets
In Expt 1, twenty-eight Large White×Landrace barrows weaned at 26 d of age were individually housed in metabolism cages (1·5×0·75 m) with ad libitum access to feed and water. The piglets continued to receive the same commercial starter diet they had received since weaning for a 7-d adaptation period. Piglets were assigned to one of four treatments in a completely randomised design based on BW and ancestry seven per group. Between 8 and 12 d after weaning, the starter diet was gradually replaced by the experimental diets, so that from 13 d after weaning piglets were fed the experimental diets only. The experiment began 13 d after weaning (a mean initial BW of 8·45 (sd 0·62) kg) and lasted for 28 d. Room temperature was initially set at 28°C and progressively decreased by 1°C/week. Piglets were weighed individually at the beginning and at the end of each week after an overnight fast. Feed intake was determined daily. Feed samples were collected weekly and pooled at the end of the experimental period for chemical analysis.
Four experimental diets based on maize and soyabean meal were formulated to differ in CP and BCAA contents (Table 1). Piglets were assigned randomly into one of the four diets, including a positive control (PC) diet, a reduced-protein negative control (NC) diet, a test 1 (T1) diet and a test 2 (T2) diet with CP contents of 19·5, 16·7, 16·7 and 17·2 %, respectively. The NC diet was formulated without considering SID valine, leucine or isoleucine concentrations, but was supplemented with 0·42 % Ala (isonitrogenous amount), and thus was deficient in valine and isoleucine compared with the recommendation by the NRC( 29 ). The T1 diet was supplemented with 0·17 % isoleucine, 0·24 % leucine and 0·16 % valine to provide SID AA equal to the levels in the PC diet. The T2 diet was supplemented with a 2-fold dose of each BCAA in T1 diet. All diets were fortified with lysine, methionine, threonine and tryptophan to satisfy the SID AA requirement as recommended by the NRC( 29 ).
Table 1 Composition of the experimental diets (as-fed basis)

PC, positive control; NC, reduced-protein negative control; T1, test 1; T2, test 2; CP, crude protein; SID, standardised ileal digestible; AA, amino acids; NE, net energy; BCAA, branched-chain AA; V, vitamin.
* PC diet and NC diet without considering SID Val, Leu or Ile concentration; T1 diet was formulated by adding 0·17 % Ile, 0 24 % Leu and 0·16 % Val to the NC diet to achieve the levels in the PC group. T2 diet was supplemented with a 2-fold dose of each BCAA in the T1 diet.
† Provided per kg of diet (as-fed basis): VA, 15 000 IU (4500 μg); VD3, 1500 IU (37.5 μg); VE, 30 mg; VB1, 3·2 mg; VB2, 8 mg; VB6, 4 mg; VB12, 0·03 mg; VK3, 3·2 mg; niacin, 34 mg; folate, 1·6 mg; pantothenic acid, 18 mg; biotin, 0·2 mg; choline chloride, 500 mg; Cu, 150 mg; Fe, 120 mg; Mn, 45 mg; Zn, 90 mg; I, 0·6 mg; Se, 0·3 mg; Co, 0·3 mg; chlortetracycline, 35 mg; tiamulin 12 mg.
‡ Values for SID AA concentration and NE were calculated according to the National Research Council( 29 ).
One piglet from the PC group and one from the NC group were excluded because of death due to acute respiratory disease during the period of gradual replacement of the starter diet. At the end of the experiment, six pigs were selected randomly from each group to be humanely euthanised by electrical stunning, coupled with exsanguination after 12 h of feed deprivation. The hypothalamus was extracted from the brain within 5–10 min, and subsequently the hypothalamus samples were rapidly removed, wrapped in foil and frozen in liquid N2 to be stored at −80°C until analyses.
In Expt 2, twenty-one Large White×Landrace barrows weaned at 28 d of age were individually housed in metabolism cages (1·5×0·75 m) with ad libitum access to water and were fed a commercial diet for a 3-d adaptation period. Subsequently, the piglets were assigned randomly to one of the three groups in a completely randomised design based on BW and ancestry seven per group, including NC, T1 and pair-fed T1 (P) groups. Gradual replacement of the starter diet by the experimental diets lasted for 5 d. The experiment began 9 d after weaning when the piglets obtained a mean initial BW of 9·21 (sd 0·70) kg, and were fed the experimental diets, which lasted for 28 d. NC and T1 diets were the same as in Expt 1 (Table 1). Piglets in the P group fed diet T1 were individually pair-fed with the piglets in the NC group as described by Swamy et al.( Reference Swamy, Smith and MacDonald 30 ). In brief, piglets in the NC group had free access to feed, whereas piglets in the P group were provided with the same amount of feed consumed by the NC group piglets on the previous day. Piglets were weighed individually at the beginning and at the end of the experiment, and the feed intake was determined daily. One piglet from the NC group was excluded because of sudden death due to unknown causes at the beginning of week 1. At the end of the experiment, all pigs were humanely slaughtered by electrical stunning, coupled with exsanguination after 12 h of feed deprivation. In all, twenty-three major skeletal muscles from the forequarter, midquarter and hindquarter of the left carcass side were separated entirely and weighed (Table 5). The longissimus dorsi (LD) muscle samples between the 10th and the last rib on the right carcass side were rapidly removed, wrapped in foil and frozen in liquid N2 to be stored at –80°C until analyses.
Chemical analyses
All diets were analysed for DM, CP (N×6·25), diethyl ether extract and crude fibre according to Association of Official Analytical Chemists( 31 ) procedures. Dietary AA contents were determined by ion-exchange chromatography using an Automatic Amino Acid Analyzer (Hitachi L-8800 Amino Acid Analyzer; Hitachi), and tryptophan content was analysed with an Agilent 1200 HPLC (Agilent Technologies) system using a C18 (4·6×150 mm) column after alkaline hydrolysis at 110°C for 20 h as previously described( Reference Wang, Wei and Cao 32 ). Feed samples were hydrolysed in 6 n-HCl at 110°C for 24 h under reflux, whereas sulphur AA content was measured after performic acid oxidation before acid hydrolysis.
Quantitative PCR
Tissue samples of the hypothalamus in Expt 1 were homogenised and total RNA (tRNA) was isolated and purified using TRIzol Reagent (100 mg tissue/1 ml Trizol; Life Technologies) according to the specifications of the manufacturer. The integrity of RNA was checked by 1 % agarose gel electrophoresis stained with 1 μg/ml of ethidium bromide. The RNA concentration and quality were determined using the Nanodrop 2000 spectrophotometer (NanoDrop Technologies). A sample of 2 μg of tRNA was reverse transcribed to synthesise complementary DNA (cDNA) for real-time PCR after treatment with TURBO™ DNase (2 U/μl; Life Technologies) for 50 min at 37°C to remove contaminating genomic DNA. The primers used for the real-time PCR detection of selected genes are listed in Table 2. Real-time quantitative PCR analyses were performed (CFX ConnectTM Real-time PCR Detection System; Bio-Rad) with a total volume of 10 μl containing 5 ng of cDNA, 5 μl iTaq™ Universal SYBR Green Supermix (Bio-Rad) and 0·3 μl of each of forward and reverse primers. The relative qualification of gene expression for each sample was adjusted with the control gene β-actin using the
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Livak and Schmittgen
33
) and normalised to the PC group.
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Livak and Schmittgen
33
) and normalised to the PC group.
Table 2 Primers used for real-time PCR analysis

AgRP, agouti-related peptide; NPY, co-express neuropeptide Y; POMC, pro-opiomelanocortin; MC4R, melanocortin-4 receptor; CART, cocaine- and amphetamine-regulated transcript.
Western blotting
The hypothalamus samples were used to detect the levels of p-mTOR, mTOR, p-ribosomal protein S6 kinase 1 (S6K1), S6K1, p-eukaryotic initiation factor 2α (eIF2α) and eIF2α proteins. The skeletal muscle samples were used to detect the levels of p-mTOR, mTOR, p-S6K1 and S6K1 proteins.
The frozen tissues samples were powdered under liquid N2, and the powdered tissue (100 mg) was dissolved in 1 ml protein lysis buffer containing 50 mm-Tris-HCl (pH 7·4), 150 mm-NaCl, 1 % Triton X-100, 1 % sodium deoxycholate, 0·1 % SDS and 1 mm-phenylmethylsulphonyl fluoride plus a 10 μl phosphatase inhibitors mixture (P1260; Applygen Technologies Inc.). After centrifugation at 10 000 g and 4°C for 15 min, the protein concentration in the supernatant fluid was determined by bicinchoninic acid assay (Beyotime Biotechnology) according to the manufacturer’s instructions. All the samples were adjusted to an equal protein concentration and then diluted with 5× loading buffer (1·25 ml of 1 m-Tris-HCl (pH 6·8), 2·5 ml glycerol, 0·5 g SDS, 0·25 ml β-mercaptoethanol, 25 mg bromophenol blue and 1 ml water to a final volume of 5 ml) to a final volume of 1 ml. The samples were boiled for 10 min and cooled on ice before being used for Western blot analysis. Aliquots of samples (50 μg protein) were separated by electrophoresis on a 10 % (S6K1), 12 % (eIF2α) or 8 % (mTOR) SDS-PAGE, and electrotransferred to poly vinylidene fluoride membranes (Millipore). The membranes were blocked in 5 % fat-free milk in Tris-buffered saline containing 0·1 % Tween-20 (TBST) for 3 h and then incubated with the following primary antibodies at 4°C overnight with gentle rocking: anti-S6K1 (1:1000; Santa Cruz Biotechnology), anti-p-S6K1 (1:500; Affinity Biosciences), anti-eIF2α (1:500; Affinity Biosciences), anti-p-eIF2α (1:1000; Cell Signaling Technology), anti-mTOR (1:1000; Cell Signaling Technology), anti-p-mTOR (1:1000; Cell Signaling Technology) and anti-β-actin (1:1000; Santa Cruz Biotechnology). After being washed three times with TBST, the membranes were incubated at room temperature for 3 h with horseradish peroxidase-linked secondary antibodies diluted 1:20 000 in 1 % bovine serum albumin TBST. Finally, the membranes were washed three times with TBST and three times with TBS, followed by development using the SuperSignal West Pico Chemiluminescent Substrate according to the manufacturer’s instructions (Pierce). Images were quantified using the Gel Logic Pro 2200 system (Carestream Molecular Imaging) and Image J software.
Statistical analyses
For all data analyses, the individual piglet was used as the experimental unit. The data were analysed by ANOVA according to a randomised complete block design using general linear model procedures. Significant ANOVA effects were further examined using Duncan’s multiple range test. Simple linear and quadratic relationships between feed intake and hypothalamic appetite regulatory gene expressions were determined using regression procedures. All analyses were performed using SAS 8.0. Results are presented as means with their standard errors. Probabilities <0·05 were considered as significant.
Results
Growth performance
The growth performance of piglets in Expt 1 is presented in Table 3. Compared with the PC group, the NC group showed a decrease (P<0·05) in final BW, average daily gain (ADG) during weeks 1–2, overall ADG (d 0–28) and gain:feed intake (G:F) during week 1. No differences in piglet growth were detected among PC, T1 and T2 groups, but final BW, ADG for all experimental periods, G:F during week 1 and overall G:F were higher (P<0·05) in T1 and T2 groups compared with the NC group. Interestingly, average daily feed intake (ADFI) for all experimental periods was decreased (P<0·05) in the NC group, whereas in T1 and T2 groups it was restored to the levels of the PC group. Thus, we conducted a pair-feeding experiment (Expt 2) to ensure similar feed intake among piglets between NC and P groups. In Expt 2, ADFI was higher (P<0·05) in the T1 group than in the NC group, which is consistent with the results of Expt 1. T1 and P groups had significantly higher (P<0·05) ADG and G:F compared with the NC group (Table 4).
Table 3 Effect of supplementing branched-chain amino acids (BCAA) to reduced-protein diets on growth performance of piglets (Expt 1) (Mean values with their pooled standard errors, n 6–7/group)

PC, positive control; NC, reduced-protein negative control; T1, test 1; T2, test 2; BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; G:F, gain:feed intake; SID, standardised ileal digestible.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* PC diet and NC diet without considering SID Val, Leu or Ile concentrations; T1 diet was formulated by adding 0·17 % Ile, 0·24 % Leu and 0·16 % Val to the NC diet to achieve the levels in the PC group. T2 diet was supplemented with a 2-fold dose of each BCAA in the T1 diet.
Table 4 Effect of supplementing branched-chain amino acids (BCAA) to reduced-protein diets on growth performance of piglets fed ad libitum or at a similar level in the group without supplemental BCAA (Expt 2) (Mean values with their pooled standard errors, n 6–7/group)
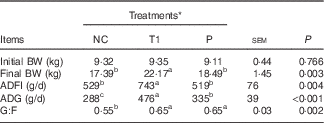
NC, reduced-protein negative control; T1, test 1; P, pair-fed T1 group; BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; G:F, gain:feed intake; SID, standardised ileal digestible.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* NC diet without considering SID Val, Leu or Ile concentrations; T1=test 1 diet, which was formulated by adding 0·17 % Ile, 0·24 % Leu and 0·16 % Val to the NC diet; P, pair-fed T1 group, in which piglets fed the T1 diet were provided with the same amount of feed consumed by the NC group piglets.
Skeletal muscle mass
In total, twenty-three major skeletal muscles of piglets in NC, T1 and P groups were separated entirely and weighed in Expt 2. The results showed that the absolute (Table 5) and relative (expressed as a percentage of the BW) (Table 6) weights of skeletal muscles in piglets were affected by the treatments. Compared with the NC group, the absolute weights of most muscles in the forequarter and midquarter were enhanced (P<0·05) in the T1 group, except for infraspinatus, teres major, brachii and psoas major muscles. Moreover, the absolute weights of three muscles with large mass (biceps femoris, semitendinosus and quadriceps femoris muscles) in the hindquarter were also increased (P<0·05) in the T1 group. When the piglets were provided with the same amount of feed, the P group had increased (P<0·05) weights of LD, trapezius, supraspinatus, deltoids and latissimus dorsi muscles compared with the NC group, particularly LD muscle.
Table 5 Effect of supplementing branched-chain amino acids (BCAA) to reduced-protein diets on the absolute skeletal muscle mass of piglets fed ad libitum or at a similar level in the group without supplemental BCAA (Expt 2) (Mean values with their pooled standard errors, n 6–7/group)

NC, reduced-protein negative control; T1, test 1; P, pair-fed T1 group; SID, standardised ileal digestible.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* NC diet without considering SID Val, Leu or Ile concentration; T1 was formulated by adding 0·17 % Ile, 0·24 % Leu and 0·16 % Val to NC diet; P-fed T1 group, in which piglets fed the T1 diet were provided with the same amount of feed consumed by the NC group piglets.
Table 6 Effect of supplementing branched-chain amino acids (BCAA) to reduced-protein diets on relative skeletal muscle mass of piglets fed ad libitum or at a similar level in the group without supplemental BCAA (Expt 2) (Mean values with their pooled standard errors, n 6–7/group)

NC, reduced-protein negative control; T1, test 1; P, pair-fed T1 group; SID, standardised ileal digestible.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* NC diet without considering SID Val, Leu or Ile concentrations; T1 was formulated by adding 0·17 % Ile, 0·24 % Leu and 0·16 % Val to NC diet; P-fed T1 group, in which piglets fed T1 diet were provided with the same amount of feed consumed by NC group piglets.
† Relative muscle mass is expressed as a percentage of the body weight.
The P group showed higher (P<0·05) relative weights of trapezius, deltoids, latissimus dorsi, pectoralis profundus, LD and biceps femoris muscles compared with the NC group. The relative weights of these muscles in the T1 group were intermediate between those in the other two groups, but only two muscles (deltoids and LD) showed statistically different weights (P<0·05) compared with the NC group. There were no differences in the relative weights of other muscles among all the three groups.
mRNA expressions of appetite regulatory genes in the hypothalamus
Agrp and NPY are classified as orexigenic genes, but POMC, MC4R and CART are classified as anorexigenic genes. We detected the mRNA expressions of these appetite regulatory genes in the hypothalamus in Expt 1, and the results are shown in Fig. 1. Hypothalamic Agrp and NPY mRNA levels were higher (P<0·05) in the T2 group compared with the NC group (Fig. 1(A)), whereas MC4R mRNA level in the T2 group and CART mRNA level in the T1 group were lower (P<0·05) compared with the NC group (Fig. 1(B)). However, POMC mRNA level showed no differences among the four groups. Interestingly, there were no differences in hypothalamic Agrp, NPY, MC4R and CART mRNA levels among T1, T2 and PC groups, except for the higher (P<0·05) NPY mRNA level in the T2 group compared with the PC group. Further regression analysis showed that ADFI was positively (P<0·05) associated with NPY mRNA levels ranging from 0·32 to 2·94, whereas it was not affected by further increase in NPY mRNA levels (Fig. 2(a)). ADFI linearly decreased (P<0·05) with an increase in MC4R mRNA levels (Fig. 2(b)), whereas there were no significant quadratic or linear relationships between ADFI and the mRNA levels of Agrp and CART (data not shown).

Fig. 1 mRNA levels of (A) orexigenic genes (agouti-related peptide (AgRP) and co-express neuropeptide Y (NPY)) and (B) anorectic genes (pro-opiomelanocortin (POMC), melanocortin-4 receptor (MC4R) and cocaine- and amphetamine-regulated transcript (CART)) in the hypothalamus of piglets fed positive control (PC, ![]() ) diet, reduced-protein negative control (NC,
) diet, reduced-protein negative control (NC, ![]() ) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (
) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (![]() ) and test 2 (
) and test 2 (![]() ), respectively) in Expt 1. Values were normalised using β-actin as an internal control. Data are expressed relative to expression in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
), respectively) in Expt 1. Values were normalised using β-actin as an internal control. Data are expressed relative to expression in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).

Fig. 2 Regression relationships between average daily feed intake (ADFI) in week 4 and the mRNA levels of (a) co-express neuropeptide Y (NPY) (y=−7·84x 2+101·81x+819·23; R 2 0·39; P=0·02) and (b) melanocortin-4 receptor (MC4R) (y=−98·59x+1113·68; R 2 0·24; P=0·02) genes in the hypothalamus of piglets.
Protein abundance of general amino acids control signalling pathway in the hypothalamus
Stimulation of the GAAC pathway in the hypothalamus has been shown to inhibit feed intake of animals. We detected the abundance of phosphorylated and total eIF2α protein in the hypothalamus in Expt 1 by Western blotting. Compared with the PC group, the NC group had increased (P<0·05) abundance of phosphorylated eIF2α protein (expressed as a ratio to total eIF2α or β-actin), which was restored to the that of the PC group in T1 and T2 groups (Fig. 3). The changes in the abundance of phosphorylated eIF2α protein were opposite to the changes in feed intake. However, there was no difference in the abundance of total eIF2α protein among the four groups.

Fig. 3 Abundance of phosphorylated and total eukaryotic translation initiation factor 2α (eIF2α) protein in the hypothalamus of piglets fed positive control (PC, ![]() ) diet, reduced-protein negative control (NC,
) diet, reduced-protein negative control (NC, ![]() ) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (
) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (![]() ) and test 2 (
) and test 2 (![]() ), respectively) in Expt 1. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
), respectively) in Expt 1. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
Protein abundance of mammalian target of rapamycin signalling pathway in the hypothalamus
The protein abundance of mTOR signalling pathway in the hypothalamus in Expt 1 is shown in Fig. 4. The abundance of total S6K1 and mTOR proteins was not different among the four groups. However, compared with the NC group, PC, T1 and T2 groups had enhanced (P<0·05) abundance of phosphorylated S6K1 protein (expressed as a ratio to total S6K1 or β-actin) (Fig. 4(A)). Moreover, the amount of phosphorylated mTOR protein (expressed as a ratio to total mTOR) was higher in the T1 group (P<0·05) than in the NC group (Fig. 4(B)). There were no differences in the abundance of phosphorylated S6K1 and mTOR proteins among PC, T1 and T2 groups.

Fig. 4 Abundance of phosphorylated and total (A) ribosomal protein S6 kinase 1 (S6K1) and (B) mammalian target of rapamycin (mTOR) proteins in the hypothalamus of piglets fed positive control (PC, ![]() ) diet, reduced-protein negative control (NC,
) diet, reduced-protein negative control (NC, ![]() ) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (
) diet or two NC diets supplemented with a 1- or 2-fold dose of BCAA (test 1 (![]() ) and test 2 (
) and test 2 (![]() ), respectively) in Expt 1. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
), respectively) in Expt 1. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the PC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
Protein abundance of mammalian target of rapamycin signalling pathway in longissimus dorsi muscle
In Expt 2, compared with T1 and P groups, the NC group had reduced (P<0·05) abundance of phosphorylated S6K1 (Fig. 5(A)) and mTOR (Fig. 5(B)) proteins in LD muscle, whereas no differences were detected between T1 and P groups. Besides, the abundance of total S6K1 and mTOR proteins was not different among the three groups.

Fig. 5 Abundance of phosphorylated and total (A) ribosomal protein S6 kinase 1 (S6K1) and (B) mammalian target of rapamycin (mTOR) proteins in longissimus dorsi muscle of piglets in reduced-protein negative control (NC, ![]() ) group, NC group supplemented with a 1-fold dose of BCAA (test 1(T1,
) group, NC group supplemented with a 1-fold dose of BCAA (test 1(T1, ![]() ) or pair-fed T1 (P) (
) or pair-fed T1 (P) (![]() ) group in Expt 2. Piglets in P group were fed the same amount of T1 diet as the NC group piglets. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the NC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
) group in Expt 2. Piglets in P group were fed the same amount of T1 diet as the NC group piglets. Western blotting method was used. Values were normalised using β-actin or relative total protein as an internal control. Data are expressed relative to expressions in the NC group; values are means (n 4–6/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
Discussion
The results of the present study showed that reducing CP content from 19·5 to 16·7 % without balancing the BCAA levels impaired piglet growth, which was restored to the level of the PC group after supplementation with BCAA. However, the reduced-protein diet supplemented with a 2-fold dose of each BCAA did not further increase piglet growth. The positive effect of dietary BCAA supplementation on growth of piglets fed reduced-protein diets was also previously observed( Reference Zhang, Qiao and Ren 11 ). A novel and important finding of this study is that the increased feed intake of piglets is associated with the enhanced mTOR and reduced GAAC pathway activities in the hypothalamus after BCAA supplementation. Furthermore, we also indicate that BCAA may act as signalling molecules to increase local mTOR pathway activity, which is independent of change in feed intake, and thus might partly contribute to the elevated muscle mass after the supplementation of BCAA to the reduced-protein diet.
Dietary branched-chain amino acids supplementation increases feed intake of piglets fed reduced-protein diets
There are growing evidences showing that many species are able to sense a dietary AA imbalance and respond to it by reducing feed intake( Reference Gietzen, Hao and Anthony 26 ). In growing pigs, the suppressive effect on growth after valine- or tryptophan-deficiency treatment is mainly associated with a reduction in feed intake( Reference Gloaguen, Le Floc’h and Brossard 14 – Reference Gloaguen, Le Floc’h and Corrent 16 , Reference Ettle and Roth 34 ). Recent studies with mice have shown that dietary deficiency in isoleucine, valine and leucine causes the most dramatic decrease in feed intake( Reference Kamata, Yamamoto and Kamijo 17 ). In this study, we reported that the deficiency of valine and isoleucine in diet (NC group) for 4 weeks reduced the feed intake of piglets, which was restored to the level of the PC group after dietary supplementation with BCAA (Tables 3 and 4). In contrast, intracerebroventricular administration of leucine inhibited feed intake( Reference Cota, Proulx and Smith 25 , Reference Blouet, Jo and Li 35 ). This paradoxical effect of leucine on feed intake is likely due to an extremely higher treatment level than the level under physiological conditions, which may lead to an imbalance among BCAA in the brain. Noteworthily, dietary BCAA imbalance (deficiency of valine and excess supply of leucine) results in subsequent reduction of feed intake in pigs( Reference Gloaguen, Le Floc’h and Brossard 14 – Reference Gloaguen, Le Floc’h and Corrent 16 , Reference Wiltafsky, Pfaffl and Roth 18 ).
There have been a number of speculations about the mechanism responsible for the effect of dietary AA imbalance on the inhibition of feed intake in animals. In mice and rats, an AA imbalance is detected by the APC and the hypothalamus to inhibit feed intake( Reference Maurin, Benani and Lorsignol 27 , Reference Maurin, Jousse and Averous 36 , Reference Hao, Sharp and Ross-Inta 37 ). AA deficiency stimulates the GAAC pathway via an accumulation of its uncharged tRNA, which induces phosphorylation of eIF2α via the general control nonderepressible kinase 2 (GCN2). In addition, mTOR is a cellular fuel and AA sensor (leucine particularly), whose hypothalamic activity is directly related to the regulation of feed intake. A previous study showed that intracerebroventricular administration of leucine decreased the feed intake of rats by stimulating the mTOR pathway activity( Reference Cota, Proulx and Smith 25 ). In contrast, central ghrelin administration promoted feed intake by stimulating hypothalamic mTOR pathway activity( Reference Martins, Fernández-Mallo and Novelle 38 ). Further studies are needed to explain this seemingly paradoxical role of hypothalamic mTOR.
However, the roles of hypothalamic GAAC and mTOR signalling pathways, which are involved in the feed intake regulation by dietary AA, have not been identified in pigs. In our study, we reported for the first time that the increase in feed intake of piglets after dietary BCAA supplementation is related to the elevation/reduction of hypothalamic mTOR/GAAC pathway activity, which supports the speculation that the mTOR or GAAC pathway may play an important role in the positive effect of BCAA on the feed intake of piglets. Moreover, the expressions of hypothalamic appetite regulatory genes (Agrp and NPY) that stimulate feed intake were up-regulated, but that of the genes inhibiting feed intake (MC4R and CART) were down-regulated after dietary BCAA supplementation (Fig. 1). Further assessment of the correlation between feed intake and expression of these appetite regulatory genes (Fig. 2) indicates that the expressions of NPY and MC4R genes appear to be more responsive to BCAA supplementation compared with the other two genes. In addition, it has been demonstrated that hypothalamic mTOR plays an important role in regulating the expressions of appetite regulatory genes( Reference Cota, Proulx and Smith 25 , Reference Martins, Fernández-Mallo and Novelle 38 ). Morrison et al.( Reference Morrison, Xi and White 39 ) suggested that AA can act within the brain to inhibit feed intake of rats and that the direct mTOR-dependent inhibition of Agrp gene expression may contribute to this effect. These observations indicate that mTOR or GAAC may function as an AA sensor to regulate the expressions of hypothalamic appetite regulatory genes and feed intake in piglets. However, dietary supplementation with a 2-fold dose of each BCAA in the present study did not further increase the feed intake of piglets (Table 3). As no changes in hypothalamic GAAC and mTOR pathway activities were observed between T1 and T2 groups (Fig. 3 & 4), it is possible that supplementing a 1-fold dose of BCAA to the reduced-protein diet is sufficient to activate these hypothalamic appetite regulatory signalling pathways.
Dietary branched-chain amino acids supplementation increases muscle growth of piglets fed reduced-protein diets
Skeletal muscle accounts for approximately 50 % of the body mass. In the present study, the absolute weights of nearly half of the muscles (Table 5) and relative weights of some muscles (Table 6) were greater in both BCAA-supplemented T1 and P groups compared with the NC group. The increased muscle growth in response to BCAA supplementation may be due to, at least in part, the activation of translation initiation factors, which is supported by the stimulation of mTOR signalling pathway activity in LD muscle, independent of change in feed intake after dietary BCAA supplementation observed in the present study (Fig. 5). mTOR has been known to stimulate the activation of translation initiation factors induced by AA( Reference Meijer and Dubbelhuis 40 , Reference Columbus, Fiorotto and Davis 41 ). The effect of triggering the activation of translation initiation factors appears to be unique to BCAA, especially leucine, both in vivo ( Reference Escobar, Frank and Suryawan 23 , Reference Escobar, Frank and Suryawan 24 ) and in intro ( Reference Atherton, Smith and Etheridge 42 , Reference Hara, Yonezawa and Weng 43 ). It has been shown that supplementing excess leucine to reduced-protein diets increases the muscle protein synthesis of piglets by activating the mTOR signalling pathway to stimulate translation initiation( Reference Yin, Yao and Liu 20 – Reference Suryawan, Torrazza and Gazzaneo 22 ).
On the other hand, even though BCAA-supplemented T1 and P groups had similar muscular mTOR activity, the absolute weight of LD muscle in the P group did not reach the level of the T1 group (Table 5). Interestingly, a previous study has reported that protein synthesis in skeletal muscle was not stimulated by infusion of leucine for 2 h despite the continuous activation of translation initiation signals( Reference Escobar, Frank and Suryawan 23 ). These results indicate that stimulation of muscle protein synthesis needs both the availability of sufficient substrates for synthesis and the activation of translation initiation factors.
In conclusion, the present study shows that supplementing BCAA to reduced-protein diets can increase growth performance of piglets, and the increase in feed intake and direct skeletal muscle growth-promoting effect contribute to this improvement. Our results for the first time indicate the possible important role of hypothalamic GAAC or mTOR pathway in the feed intake regulation of piglets after dietary BCAA supplementation. The muscle growth-promoting effect induced by BCAA supplementation may be mediated by the mTOR signalling pathway. However, supplementing a 2-fold dose of each BCAA to the reduced-protein diet in the present study did not further increase growth performance. Thus, further studies are needed to reveal the appropriate dose of each BCAA to be supplemented to the reduced-protein diet for maximum growth in piglets.
Acknowledgements
The authors are grateful for English revision from Z. Liu.
This study was jointly supported by the National Basic Research Programme of China (no. 2013CB127305), the Natural Science Foundation of Hubei Province of China (no. 2013CFA010) and the Fundamental Research Funds for the Central Universities (no. 2013PY047).
The authors’ contributions are as follows: J. Peng, L. Z. and H. W. designed the research; L. Z., C. C., Q. X and J. Pang conducted the research; L. Z. analysed the data; L. Z., H. W. and J. Peng wrote the manuscript.
The authors have no conflicts of interest to declare.














