INTRODUCTION
The burden associated with infectious acute gastrointestinal illness (AGI) is significant worldwide, whether in terms of morbidity and mortality, economic cost or social impact. In New Zealand, a country with 4·2 million people, the annual economic cost of the major potentially foodborne infectious intestinal illnesses alone has been estimated at NZ$156 million, which reinforces the importance of accurately quantifying the occurrence of AGI in the community [Reference Lake1].
There has traditionally been a reliance on routine notifiable disease surveillance data to describe the epidemiology of infectious enteric diseases. Notification of enteric pathogens in New Zealand currently requires an individual with AGI to first seek medical attention, a health professional to then request a faecal specimen, the affected individual to supply a specimen, a laboratory to successfully isolate a notifiable pathogen, and finally, a medical practitioner and/or laboratory to notify the regional public health service. Losses at each step in this process contribute to the under-ascertainment of the level of notifiable enteric diseases. This surveillance system is also unable to measure the substantial contribution of non-notifiable enteric pathogens to the national burden of AGI. During 2005, there were almost 19 000 notifications for enteric pathogens in New Zealand [2], although it has been estimated that the total number of AGI cases could be as high as 823 000 per year [Reference Lake3].
The AGI Community Survey is the first study to be conducted in New Zealand to directly quantify the occurrence of AGI in the general community using a representative sample of the national population recruited over a 12-month period. The objectives of this study were to estimate the occurrence, distribution and associated burden of AGI in New Zealand using a study methodology that allowed direct comparison of results with related overseas studies.
METHODS
Study design and data collection
The methodology of the AGI Community Study was based on studies conducted in Australia, Canada, Ireland and the USA, and guided by the International Collaboration on Enteric Disease ‘Burden of Illness’ Studies which is promoting inter-country comparisons through common definitions [Reference Scallan4, Reference Majowicz5]. This study was a representative, retrospective, cross-sectional telephone survey of the New Zealand community, conducted over a 12-month period from February 2006 to January 2007. A sample size of 3457 was calculated for this study based on 80% power, a significance level of 5%, an assumed 28-day period prevalence of 10% for AGI and a required precision of ±1%.
The study subjects consisted of a general sample, as well as a Maori booster sample to ensure adequate representation (at least 15%) of the Maori ethnic group. For the general sample, about 270 participants were recruited each calendar month, stratified by all 24 telephone directory regions to obtain a geographically representative sample. For the Maori booster sample, about 35 Maori participants were recruited each month from telephone directory regions with a high-density Maori population.
Private households were selected using random digit dialling based on telephone numbers randomly generated from all number ranges in the national telephone directory provider, allowing the capture of unlisted numbers. The individual with the last birthday in each household was selected as the study participant. For study participants aged <12 years, parental consent was acquired and an adult caregiver served as the interview respondent. Study participants aged between 12 and 16 years were directly interviewed (apart from personal and household information obtained from an adult caregiver) after parental consent was acquired. Interviews were conducted using computer-assisted telephone interviewing (CATI).
The survey questionnaire was based predominantly on questionnaire items used by the overseas ‘Burden of Illness’ studies. The survey questionnaire included items on the following: demographic and personal information, household information, occurrence of diarrhoea and/or vomiting in the previous 4 weeks, associated symptoms, medical consultation and treatment, and social and economic impact of illness. The questionnaire was pre-tested and piloted.
Ethics approval was obtained from the New Zealand Multi-region Ethics Committee.
Case definition
A case of AGI was defined as a study participant with ‘any’ diarrhoea, vomiting, or both, experienced in the previous 4 weeks, excluding non-infectious causes such as chronic illness, medication, medical treatment and pregnancy. Individuals experiencing more than one episode of diarrhoea, vomiting, or both, in the previous 4 weeks were considered as a single case of AGI only.
Other case definitions used were the international comparison of the prevalence of diarrhoea (⩾3 loose stools or bowel movements in any 24-h period) [Reference Scallan4], and a definition of AGI similar to that used in an Australian study (2 vomits or ⩾3 stools in any 24-h period) [Reference Hall6].
Analysis
The cooperation rate was calculated as the number of completed interviews divided by the sum of the completed interviews, refusals before and after establishing contact with a suitable respondent including hang-ups, and those with language problems. The refusal rate was calculated as refusals before and after establishing contact with a suitable respondent including hang-ups, and those with language problems.
The 4-week period prevalence of AGI was calculated by dividing the number of AGI cases by the number of study participants. The incidence rate of AGI per person per year was determined by first dividing the 4-week period prevalence of AGI by 4 weeks and then multiplying by 52 weeks. Ninety-five percent confidence intervals (95% CI) for both period prevalence and incidence rates were calculated. The period prevalence and the incidence rate estimates were adjusted for differences between the survey sample and the national population by weighting for sex, age and indigenous status using the revised New Zealand 2006 population estimates as the reference population.
Log binomial regression was used to determine predictors of AGI by calculating relative risks (RR) for socio-demographic, geographic and seasonal variables with 95% CIs. To control for possible confounding, a multivariate model was used that included all variables (sex, age, indigenous status, geographical distribution, approximate season, household size, household income).
Statistical analysis including weighting adjustments was performed using SAS version 9.1 (SAS Institute Inc., USA).
Previously published clinical criteria were used to classify the severity of AGI as mild (⩾1 vomit or ⩾2 loose stools in 24 h), moderate (⩾2 vomits or ⩾3 loose stools in 24 h), or severe (at least 2 days of illness and ⩾5 loose stools or ⩾4 vomits in 24 h) [Reference Hall6].
RESULTS
Sample and response rates
There were 3220 study participants in the general sample and 435 in the Maori booster sample, giving a total survey sample of 3655. The cooperation rate was 25·3% in the general sample and 10·1% in the Maori booster sample with an overall cooperation rate of 21·4%. The refusal rate was 44% in the general sample and 40·7% in the Maori booster sample with an overall refusal rate of 43·1%.
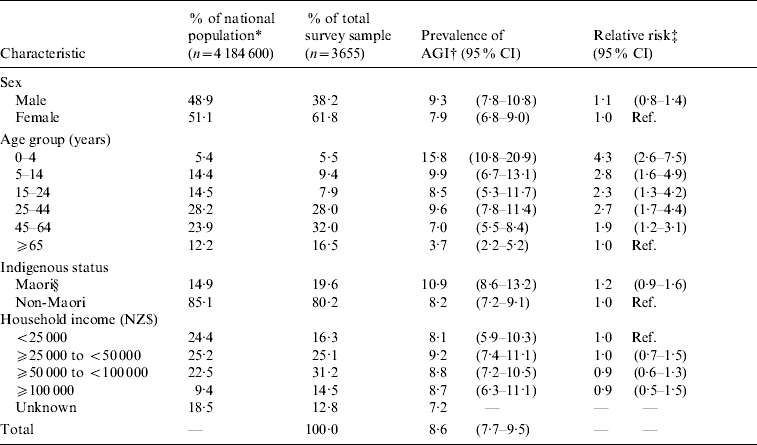
A summary of the socio-demographic characteristics of the study sample, compared to New Zealand 2006 census population statistics, is presented in Table 1. Statistically significant differences were seen in the higher percentage of female respondents, some under-representation in the 5–24 years age groups, and over-representation in those aged >45 years. Similarly, lower income households were significantly under-represented but those earning more than NZ$50000 were over-represented. Maori were over-represented by virtue of the Maori booster sampling.
Table 1. Socio-demographic characteristics of respondents to a national telephone survey on acute gastrointestinal illness (AGI) compared to the total population, along with 4-week period prevalence of AGI and relative risk of AGI in New Zealand 2006–2007
CI, Confidence interval.
* National population statistics for sex, age group and indigenous status based on the New Zealand 2006 Census of Population and Dwellings and national population statistics for household income based on the New Zealand 2001 Census of Population and Dwellings.
† Adjusted for sex, age and indigenous status.
‡ Adjusted for sex, age, indigenous status, geographical distribution, approximate season, household size and household income.
§ Maori were oversampled to ensure adequate representation (at least 15%).
AGI occurrence
Of the 3655 study participants, 416 (11·4%) reported having experienced diarrhoea, vomiting, or both, in the previous 4 weeks. A total of 119 participants with reported diarrhoea and/or vomiting were excluded due to probable non-infectious causes. Overall, 297 participants were defined as AGI cases according to the case definition, giving a crude 4-week period prevalence for AGI of 8·1% (95% CI 7·2–9·0). After adjustment for age, sex and Maori/non-Maori ethnic status using the New Zealand population as the reference standard, the adjusted period prevalence was 8·6% (95% CI 7·6–9·6) and the incidence rate of AGI was 1·11 episodes/person per year (95% CI 1·00–1·23). Extrapolation of the adjusted estimates to the national population resulted in about 4·66 million episodes of AGI per year in New Zealand (95% CI 4·17–5·16). Of the 297 respondents who met the case definition for AGI, 82 (28%) reported they had experienced more than one episode of diarrhoea or vomiting separated by ⩾7 days in the 4 weeks prior to interview. It was not possible to apply any exclusion to these other episodes as questions regarding possible cause, symptoms and duration related to the last episode only.
If these cases are included then the upper estimate of all AGI episodes is 6·62 million (95% CI 6·03–7·21).
Calculation of the prevalence of diarrhoea according to the case definition used in the international comparative paper was complicated by the number of cases (49/297, 16·5%) who were unable to provide data on the number of loose stools in any 24-h period [Reference Scallan4]. Excluding these cases gave a weighted prevalence of 4·2% (95% CI 3·5–5·0; 2·3 million cases, 95% CI 1·9–2·7), while including them gave an upper estimate of 5·6% (95% CI 4·8–6·5; 3·1 million cases, 95% CI 2·6–3·5). Using the case definition similar to that used in Australia, the weighted incidence of AGI in New Zealand was 0·76 episodes/person per year (95% CI 0·65–0·88) excluding unknowns, or 0·90 episodes/person per year (95% CI 0·78–1·02) including unknowns.
AGI in relation to socio-demographic characteristics
The adjusted prevalence of AGI was highest in the 0–4 years age group (15·8%) with a general trend downwards as age increased, with the lowest prevalence observed in the ⩾65 years age group (3·7%) (Table 1). The prevalence of AGI in the 25–44 years age group (9·6%) did not follow the downward trend and this was the only age group where the prevalence in females was greater than in males (Fig. 1). Other differences by socio-demographic status were not significant (Table 1). Of note, study participants of Maori ethnicity tended to have a higher adjusted prevalence of AGI than non-Maori participants (10·9% vs. 8·2%), although the difference was not statistically significant (P=0·175). However, when the study participants were further segmented by ethnicity to exclude Pacific Island and Asian participants from the non-Maori category, the adjusted prevalence of AGI in Maori (10·8%) was significantly higher than European/Other participants (8·3%, P=0·04).
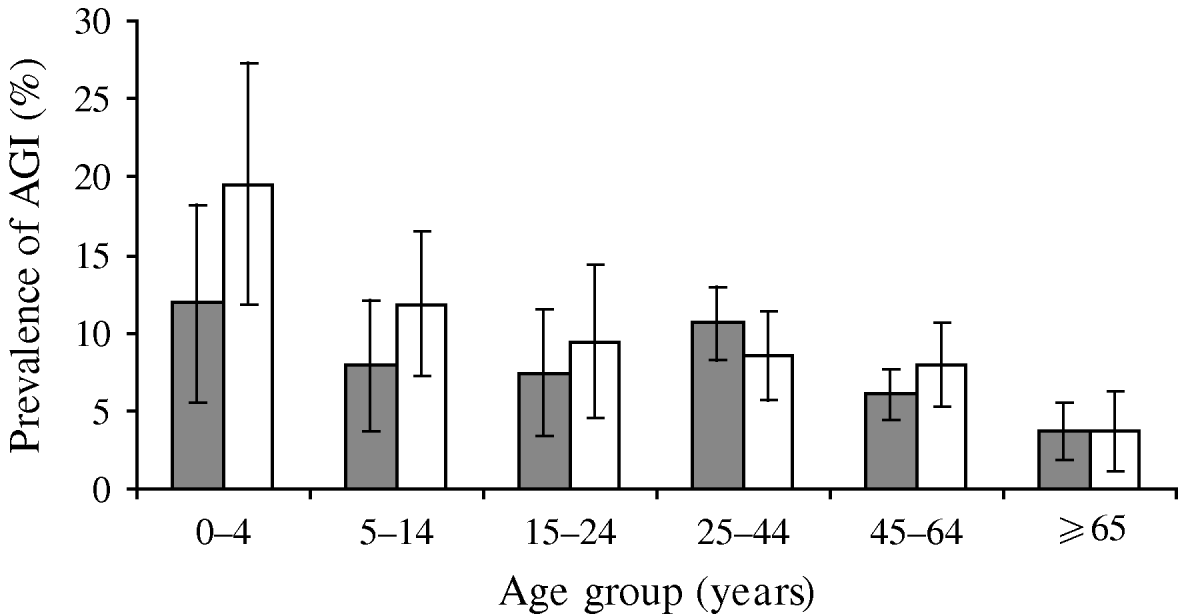
Fig. 1. Prevalence of acute gastrointestinal illness (AGI) by age and sex, New Zealand 2006–2007. ![]() , Female; □, male.
, Female; □, male.
Using log binomial regression, all age-group categories were significantly associated with higher risk of AGI compared to the reference category (⩾65 years) (Table 1). The age group at the highest risk was the 0–4 years age group (RR 4·3, 95% CI 2·5–7·5), followed by the 5–14 years age group (RR 2·8, 95% CI 1·6- 4·9) and the 25–44 years age group (RR 2·7, 95% CI 1·7–4·4). Indigenous status was not a significant predictor of AGI, with a RR of 1·2 (95% CI 0·9–1·6) for Maori. Sex and household income did not have a statistically significant association with AGI.
AGI symptoms, healthcare and impact
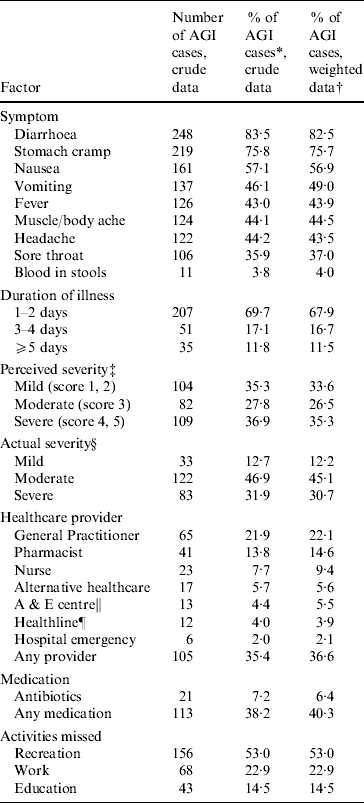
The most common symptoms in AGI cases were diarrhoea (82·5%), stomach cramps (75·7%), nausea (56·9%) and vomiting (49·0%) (Table 2). The least frequent symptom experienced by AGI cases was blood in stool (4·0%). Of the 276 cases whose illness had resolved at the time of interview, 49 (17·8%) had vomiting only, 146 (52·9%) had diarrhoea only, while the remaining 81 (29·3%) had both.
Table 2. Symptoms, healthcare and illness impact reported by a sample of acute gastrointestinal illness (AGI) cases, New Zealand 2006–2007
A & E, Accident and emergency.
* Unknowns excluded, therefore denominators not always 297 cases.
† Adjusted for sex, age and indigenous status.
‡ Perceived severity is based on a self-reported scale.
§ Actual severity is based on published clinical criteria.
∥ A & E centres include non-hospital community-based accident and emergency centres only.
¶ Healthline is a national free 24-h telephone health advice service.
The duration of illness for most AGI cases (67·9%) was 1–2 days, with a median duration of 2·0 days and a mean duration of 2·5 days. Based on a self-reported scale from 1 to 5, where 1 is very mild and 5 is very severe, 33·6% of AGI cases perceived the severity of their illness as mild (score 1–2), 26·5% as moderate (score 3), and 35·3% as severe (score 4–5). Using previously published clinical criteria, 12·2% of AGI cases had an actual severity of illness classified as mild, 45·1% as moderate, and 30·7% as severe [Reference Hall6].
About a third (36·6%) of all AGI cases sought a form of professional healthcare advice or treatment (Table 2). General practitioners (GPs) were the health professional seen most frequently (22·1%), followed by pharmacists (14·6%) and nurses (9·4%). Based on the extrapolated national estimate of 4·66 million episodes of AGI per year, adjusted for sex, age and indigenous status, the estimated burden of AGI on healthcare providers equated to 1·52 million patient/client visits per year, of which 920 000 visits were made to GPs. Multivariate analysis of symptoms associated with a GP visit demonstrated that diarrhoea was not a predictor of AGI cases visiting a GP (RR 0·6), while vomiting (RR 2·0, 95% CI 1·3–3·1), blood in stool (RR 2·3, 95% CI 1·2–4·3), headache (RR 2·1, 95% CI 1·3–3·3), fever (RR 1·7, 95% CI 1·1–2·6), muscle/body ache (RR 1·6, 95% CI 1·0–2·5), and sore throat (RR 1·7, 95% CI 1·1–2·5) were predictors of a visit to a GP, and these observations were statistically significant. As the duration of illness increased, the association with a GP visit strengthened, with a RR of 2·3 (95% CI 1·3–3·9) for a duration of 3–4 days and a RR of 5·1 (95% CI 3·3–7·8) for a duration of ⩾5 days. Of the 297 cases of gastroenteritis in the total survey sample, 248 had diarrhoea and of these, 49 attended a GP. Stool samples for laboratory pathogen testing were requested in 40·8% of these cases (20/49).
There was no difference between the sexes in terms of the likelihood of an AGI case visiting a GP. Cases aged 0–4 years were more likely to visit a GP (39·5% of cases, P=0·013) than other age groups. Maori cases were more likely to visit a GP (31·9% of cases) than non-Maori (19·8% of cases, P=0·045). Maori cases visiting a GP were more likely to be asked for a stool sample (9/17, 52·9%) than non-Maori (11/32, 34·4%) but this difference was not statistically significant (after weighting, P=0·32).
Of all AGI cases, 40·3% used at least one type of medication to treat or relieve symptoms and 6·4% of AGI cases took antibiotics (Table 2). Based on the extrapolated national estimate of 4·66 million episodes of AGI per year, adjusted for sex, age and indigenous status, the number of antibiotic courses equated to about 313 000 courses per year (95% CI 179 000–447 000).
Of the 297 AGI cases, 267 (90%) reported loss of time at work, school or recreation. A third of all cases (36·4%) reported missed work time for either themselves (22·9%) or another person acting as caregiver (13·5%) for a mean of 2·9 days. When extrapolated to the New Zealand population, 4·52 million (95% CI 3·15–5·90) days of paid work were missed by either the AGI cases themselves (2·85 million, 95% CI 1·84–3·87) or their caregiver(s) (1·67 million, 95% CI 1·04–2·29). School/preschool/other educational activity was missed by 14·0% of cases (mean 3·3 days).
DISCUSSION
Using the case definition chosen for international comparisons gave a lower prevalence of diarrhoea in New Zealand (up to 5·6%) than found in studies from Ontario, Canada (7·6%), British Columbia, Canada (8·8%), the USA (7·6%), and Australia (6·4%), but higher than found for Ireland (3·4%) [Reference Scallan4, Reference Thomas7]. It may be that the exclusion criteria applied in the New Zealand study were more restrictive; 28·6% of cases were excluded for non-infectious causes in New Zealand whereas 19·2% and 16·0% of cases were excluded in the British Columbia and Ontario studies, respectively [Reference Thomas7, Reference Sargeant8].
The prevalence of AGI in New Zealand using the case definition similar to that used in the Australian study (up to 0·90 episodes/person per year), is comparable to the 0·92 episodes/person per year found in Australia, although in that study individuals reporting respiratory symptoms as well as gastrointestinal symptoms required a higher threshold of gastrointestinal symptoms to be included [Reference Hall6].
As found by all overseas studies, the highest prevalence and incidence of AGI was in children aged <5 years (P⩽0·0001), with a decreasing prevalence and incidence with increasing age, so that the lowest rates were observed in people aged >65 years.
The notification rates of most enteric diseases, such as campylobacteriosis [Reference Baker, Sneyd and Wilson9], are significantly lower in the Maori population. Rates of AGI in this study were at least as high in Maori as those reported by the non-Maori population, and Maori cases were more likely to visit a GP than non-Maori. These apparently conflicting findings suggest that the Maori components of this study should be treated with caution, given the low cooperation rate.
The proportion of AGI cases visiting a GP in New Zealand is similar to those reported visiting a medical health professional in overseas studies (~20%) [Reference Hall6]. The percentage of AGI cases taking medication in New Zealand (38·2%) is similar to Australia, but lower than reported for Canada and Ireland [Reference Scallan4]. The percentage of cases taking antibiotics (7·2%) was within the range for other developed countries (3·6–8·3) [Reference Roy, Scallan and Beach10].
Limitations of this study are common to those in comparable international studies, in particular falling response rates. The cooperation rate to telephone-based surveys is now typically in the 20–30% range, and the refusal rate in this study is higher than found in earlier studies, e.g. the 2001–2002 Australian study found a refusal rate of 28·0% [Reference Hall11]. This raises issues of representativeness, and supports the use of a Maori booster sample. Nevertheless, for this reason it may not be appropriate to draw firm conclusions from some of the relatively small differences in AGI occurrence across socio-demographic groups within the sample (such as between Maori and non-Maori).
Information from this study helps to define the surveillance pyramid for AGI in New Zealand, i.e. quantitative under-ascertainment of cases at each step of the pathway (GP, clinical laboratory, notifiable disease system) leading to an AGI-related notified illness [Reference Lake12]. On its own, such a study cannot estimate the pyramids for any specific cause of AGI. It would be useful to carry out further work in New Zealand to measure pathogen-specific rates of AGI in the community. Such work would require resource intensive community cohort studies, or modelling approaches to produce plausible estimates [Reference Wheeler13–Reference Voetsch16]. Periodic repeated community surveys could be considered as ways of monitoring AGI incidence, with notifiable disease data as an indicator of disease trends.
CONCLUSION
These results are consistent with studies in other countries using similar methodologies in determining the prevalence of AGI. Extrapolation of the adjusted estimates to New Zealand's national population indicates there are about 4·66 million episodes of AGI per year in New Zealand, nearly a million visits to the GP, and in excess of 300 000 courses of antibiotics being taken. It was estimated more than 4·5 million days of paid work were missed due to AGI and this represents a significant burden of disease. Although the illness is generally of short duration, its high frequency means that AGI has a large societal impact in terms of days lost to disabling illness, lost productivity, and healthcare visits.
ACKNOWLEDGEMENTS
The New Zealand Food Safety Authority funded this study. The Computer Assisted Telephone Interview (CATI) survey was undertaken by UMR Consulting.
DECLARATION OF INTEREST
None.





