Animals may have target levels for body lipid and protein content which they try to maintain by feedback mechanisms that control growth, feed intake and body composition changes. Such stability is termed homeostasis, the maintenance of the internal body environment within tolerable limits. In farm animals, the amount of lipid stores induces feedback mechanisms increasing or decreasing intake and growth, to ensure animals maintain appropriate lipid levels(Reference Kuenzel1–Reference Richards5). This phenomenon is termed lipostatic regulation(Reference Kennedy6), and such responses are partly controlled by the leptin gene(Reference Friedman and Halaas7). Likewise, fish subjected to experimental depletion of lipid stores can subsequently fully or partially compensate for this and reach the level of lipid stores of non-treated fish(Reference Metcalfe and Thorpe8–Reference Álvarez and Nicieza13). Compared with lipid deposition, the role of body protein content in regulating subsequent intake and growth has received less attention (for a review, see Simpson & Raubenheimer(Reference Simpson and Raubenheimer14, Reference Simpson and Raubenheimer15)). However, it has been suggested that feedback mechanisms regulating excessive fat and carbohydrate intake may be in fact less stringent than those of protein intake(Reference Simpson and Raubenheimer15).
Previous studies in fish have increased our understanding of the regulation of body composition, especially in response to different diet and feeding treatments (cited above). It is of further interest to know whether naturally occurring within-population variation in fish body composition is related to subsequent feed utilisation and growth performance, and if such relationships are genetically determined. Such studies are fundamental to understanding the growth biology of fish and the genetic potential that exists for selective improvement of feed and biological efficiency, body composition and product quality in aquaculture breeding programmes.
We hypothesised that within-population variation in initial whole-body protein percentage, rather than lipid percentage, should be related to subsequent feed utilisation and growth in fish. The hypothesis was based on three observations. First, it is well established that fish maintain percentage body protein, but not lipid percentage, in homeostasis across a wide range of diets and feeding rations(Reference Simpson and Raubenheimer15–Reference Dumas, de Lange and France18). Second, previous quantitative genetic studies, including work on European whitefish (Coregonus lavaretus L.), show that body protein percentage displays a low amount of phenotypic and genetic variation compared with the variation in body lipid percentage(Reference Tobin, Kause and Mäntysaari19, Reference Quinton, Kause and Ruohonen20). Third, a study on rainbow trout (Oncorhynchus mykiss Walbaum) showed that genetic correlations of body protein percentage measured at different ages were near zero or negative(Reference Tobin, Kause and Mäntysaari19). In contrast, the respective correlations for body lipid percentage were highly positive, showing that families with low lipid percentage maintained their low rank during growth(Reference Tobin, Kause and Mäntysaari19). Thus, within populations there may be feedback mechanisms that constrain excess protein but not lipid growth.
In the present study, we examined body lipid and protein percentage regulation within a population of European whitefish. The European whitefish is a carnivorous salmonid that is farmed in Finland, and a breeding programme with individually marked fish and known family structure has been recently established(Reference Quinton, Kause and Ruohonen20, Reference Quinton, Kause and Koskela21). Due to the destructive nature of body composition analysis, the study was based on the analysis of sire-family means. Sire-mean correlations are broad-sense genetic correlations, i.e. approximations of true genetic correlations, suitable for small-scale physiological studies such as ours(Reference Roff22, Reference Astles, Moore and Preziosi23).
We tested here, first, whether initial body lipid percentage (Lipid%I) and initial protein percentage (Prot%I) could be differently related to later feed utilisation performance. Broad-sense genetic correlations of the initial body composition with subsequent body composition, feed intake, weight gain, feed efficiency (FE) and their respective lipid and protein components were calculated. The test was performed on two diets: a current fishmeal (FM)-based feed and a potential future soyabean-meal (SBM) feed. SBM use in farmed fish diets is increasing because of the global decline in FM availability(Reference Powell24). Nutrigenetic studies are needed to reveal how fish breeding programmes are affected by the change in the diet formulation, due to possible genotype × environment interactions for feed utilisation, in terms of heterogeneous variances and re-ranking of families across diets(Reference Lynch and Walsh25). Consequently, we tested in particular whether or not families with effective nutrient utilisation on traditional FM diets have high performance also on SBM-based diets. If genotype × environment interactions are weak, then current breeding programmes now selecting for increased fish performance on FM diets are indirectly improving the ability of fish to utilise SBM diets.
Materials and methods
Experimental design
Whitefish in the experiment originated from the breeding programme maintained at the Tervo station of the Finnish Game and Fisheries Research Institute (FGFRI) located inland. All procedures involving animals were approved by the animal care committee of the FGFRI.
To produce offspring for the present study, in October 2003, thirty-five sires and forty-two dams were mated during 2 d in a partial factorial design to create forty-eight families. Each sire was mated to one to two dams (average 1·4 dams) and each dam to one to two sires (average 1·1 sires). The original design included forty-five sires, fifty-two dams and seventy families but due to a human error during tissue sample preparation, only forty-eight out of the seventy full-sib families could be used in the study. The sires and dams belong to a base population and are assumed to be unrelated. At the eyed-egg stage, in January 2004, the families were transported to the Laukaa Research Station located inland.
From hatching until the start of the experiment, the families were held in separate indoor 150 litre fibreglass tanks (water 13–15°C) and fed commercial dry diets (larvae: AgloNorse; EWOS Ltd, Bergen, Norway) (juveniles: Nutra Parr and Royal Silver; Raisio Ltd, Raisio, Finland). To give all the fish the same initial nutritional environment, all fish were fed with a 1:1 mixture of the two experimental diets for 4 weeks before the diet trial.
The diet trial was conducted from July to October 2004. In July before initiation of the diet treatments, twenty randomly sampled fish from each of the forty-eight families were sampled for proximate body composition analysis. Then, an additional twenty-four fish were randomly sampled from each family and individually tagged for the growth trial. To set up a split-family design, each family was split into two groups to be reared with the two alternative diets. Fish on the FM and SBM diets were of similar initial weight (FM mean 40·9 (sd 11·2) g for 818 fish; SBM mean 40·4 (sd 10·4) g for 829 fish). Each family group was evenly distributed over six replicate indoor tanks per diet. The trial began with a total of 1680 fish, each replicate tank containing 140 fish (two fish from each family). Fish were fed 6 h per d using belt feeders. Feed was supplied in excess and rations were calculated by increasing the predicted feeding rates by 30 %(Reference Koskela26). Water temperature was held at 14·8–15·1°C (flow rate 8–16 litres/min; outlet water O2 level >80 % saturation) and a light period of 24 h was used.
After the 4-month growth trial, 1533 fish remained and these were slaughtered, and body weight and proximate body composition were individually measured. At the end of the trial, the full-sib family sizes on each diet were very similar; the mean family sizes were 10·9 (range 8–12) for the FM-fed fish and 10·8 (range 7–12) for the SBM-fed fish.
Diet formulations
To analyse the effect of future diet switch on breeding programmes, two isonitrogenous and isoenergetic (based on gross energy) pelleted diets that reflect practical industry-type diets were formulated. Because it was known that SBM contains less protein, certain amino acids, P and oil, and more carbohydrate than FM, these levels were adjusted as would be supposed to happen in practical future diet formulation. In nutritional experiments, it is also possible to change only one feedstuff at a time and analyse the effect. This practice was not used here because the resulting diets would have not been practical diets. A reader should also note that formulating diets on a crude basis, not digestible basis, does not guarantee that an SBM-based diet is nutritionally adequate for fish. The results of the present study might have been different if we had used diets formulated on a digestible basis.
In the FM diet, FM supplied 100 % of the dietary protein, whereas in the SBM diet, 50 % of the dietary protein was replaced with SBM-derived protein. To obtain similar crude lipid, protein and moisture content on both diets, wheat meal was added to be 267·5 and 34·0 g/kg in the FM and SBM diets, respectively. Amino acid (Evira, Helsinki, Finland) and P (Raisio Ltd, Raisio, Finland) compositions of the FM and SBM were analysed, and the SBM diet was supplemented with methionine, lysine and P to bring the amounts of these nutrients equal to those in the FM diet. FM and SBM diets contained 210 and 210 g lipid/kg (based on air-dry weight), 398 and 404 g protein/kg, 23·0 and 27·0 g water/kg, 10·0 and 22·0 g fibre/kg and 22·6 and 22·3 MJ gross energy/kg, respectively. Diet formulations and compositions have been detailed by Quinton et al. (Reference Quinton, Kause and Koskela21).
Proximate body composition analysis
For initial proximate body composition (lipid % and protein %, based on wet body weight), the twenty fish from each full-sib family were pooled within the family, and whole-body composition was analysed for the pooled sample. For final body composition, all fish were individually analysed for body composition at the end of the trial.
For both initial and final composition analyses, samples (pooled sample or individual fish) were minced and stored at − 20°C until chemical analysis. Each sample was homogenised (Losmixer; Miris AB, Uppsala, Sweden) in a standard solvent (Mirasolve; Miris AB). For each sample, two subsamples were analysed for lipid and protein percentages using mid-IR transmission spectroscopy (FMA2001 Milk Analyzer; Miris AB)(Reference Elvingsson and Sjauna27). Standard analytical methods for lipid(Reference Folch, Lees and Sloane Stanley28) and N(Reference Kjeldahl29) were used to construct the calibration curves between single wavelength absorption of IR light and concentration of the target substance. For each sample, the two subsample measurements were averaged to make one lipid percentage and one protein percentage observation.
Individual-level records
Individual wet body weights (g) of the tagged fish were recorded at the beginning and end of the trial. Individual daily weight gain (DG; g/d) was calculated as the difference between initial and final body weights, divided by the number of days in the trial. Individual final body lipid weight (LipBWF; g) and final protein body weight (ProtBWF; g) were calculated by multiplying final wet body weight by final lipid percentage (Lipid%F) and final protein percentage (Prot%F), respectively.
Individual daily feed intake (FI) was measured by X-radiography(Reference Talbot and Higgins30, Reference Jobling, Covès, Damsgård, Houlihan, Boujard and Jobling31) five times per individual during the course of the trial, with 2-week intervals between the measurements. Average individual FI (g/d) was calculated from the repeated measurements. Repeatability of the five FI records was moderate within both diets (r 0·28). By calculating the average of the five records, the repeatability of the average becomes 0·66, reflecting reasonable recording accuracy(Reference Kause, Tobin and Dobly32). Individual FE was calculated as the DG:FI ratio.
Calculation of sire-family means for traits analysed
All statistical analyses were based on paternal family means (thirty-five sires). The traits analysed were Lipid%I and Prot%I, Lipid%F and Prot%F, DG, FI, FE, lipid and protein components of DG (LipDG and ProtDG), FI (LipFI and ProtFI) and retention efficiency (LipRE and ProtRE).
Sire-means for initial proximate composition were calculated by averaging full-sib family observations for each sire. To calculate sire-means from individual data, full-sib family least-square means were first calculated separately for each diet with a model accounting for the experimental tank (six tanks per diet) as a random effect (Proc Mixed; SAS Institute, Inc., Cary, NC, USA). Then, sire-means on each diet were calculated from the full-sib family means.
The following traits were derived from the sire-means. Initial lipid weight (LipBWI; g) was calculated as Lipid%I × initial body weight (BWI). LipDG was calculated as LipBWF − LipBWI divided by the average number of days in the trial. LipFI was calculated as the sire-mean FI multiplied by the diet's lipid percentage. LipRE was calculated as LipDG/LipFI. Sire-family mean ProtDG, ProtFI and ProtRE were calculated in the same manner. Because proteins ingested can be used for lipid growth, LipRE can be substantially higher than unity. The division of lipid and protein intake provides novel information compared with wet weight intake only when comparing diet means because all feed intake observations within a diet are multiplied by the same constant value.
All trait sire-means were normally distributed.
Statistical analysis of sire-mean data
In all analyses, P < 0·05 was accepted as statistical significance.
Diet differences in means
Diet differences in the means of LipDG, ProtDG, LipFI, ProtFI, LipRE and ProtRE were tested with parametric ANOVA (Proc Mixed; SAS Institute, Inc.). The model contained the fixed effect of diet. The residuals of the models were normally distributed and the variances did not differ significantly between the diets (log-likelihood ratio test in Proc Mixed). To standardise the traits to common body weight, additional analyses were performed in which BWI was included as a covariate.
The diet means and genetic analysis for individual records of DG, body weight, feed intake, FE and final composition have been reported previously(Reference Quinton, Kause and Ruohonen20, Reference Quinton, Kause and Koskela21). The trait means for these traits are given in Table 1, and the means for the newly reported traits in Table 2.
Table 1 Traits recorded before the diet trial and on fishmeal and soyabean-meal diets
(Mean values and standard deviations, sample size of thirty-five sires for all traits)
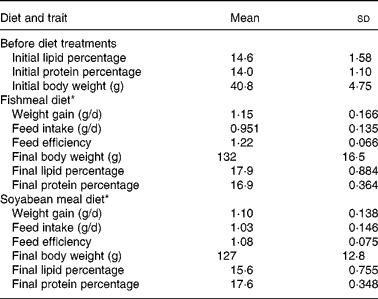
* Results of individual-based data for these traits have been reported by Quinton et al. (Reference Quinton, Kause and Ruohonen20, Reference Quinton, Kause and Koskela21).
Table 2 Diet differences in lipid and protein gain, intake and retention efficiency on fishmeal and soyabean-meal diets
(Mean values with their standard errors)
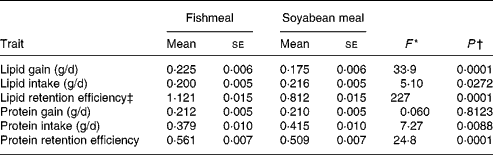
* For all traits, nominator df = 1 and denominator df = 68 for the F test of a one-way ANOVA.
† From one-way ANOVA, reflecting the probability that trait means differ between the diets.
‡ Lipid retention efficiency can be higher than unity because proteins ingested can be used for lipid growth.
Regulation of body lipid and protein percent
To assess the relationship of initial body composition with subsequent DG, FI, FE and their lipid and protein components, partial correlations were calculated between the traits within each diet.
For the partial correlations of Lipid%I, the effects of BWI and Prot%I were removed from the correlations. For the partial correlations of Prot%I, the effects of BWI and Lipid%I were removed. These partial correlations should be interpreted as relationships between the two traits studied (for example, Lipid%I and DG), given that the corrected traits (for example, Prot%I and BWI) would have been constant. Thus, we are statistically removing the correlated effects of the tertiary traits from the relationship between the two traits of interest. The number of dams for each sire was used as a weight variable when calculating the partial correlations, to give more weight on sires whose mean more accurately described their genetic value.
Sire-mean correlations are broad-sense genetic correlations that can be confounded by dominance effects, and by potential maternal and common environment effects(Reference Roff22).
Genotype×environment interactions
The degree of sire × diet interaction for LipDG, ProtDG, LipRE and ProtRE was quantified in two ways. First, the magnitude of sire family re-ranking across diets for a trait was assessed by calculating the correlation between the trait recorded on two diets. In contrast to the usual testing if a correlation differs from zero, here the subject of interest was to statistically test if the correlation was equal to one. This was performed by bootstrap resampling the correlations 10 000 times(Reference Efron and Tibshirani33). The obtained frequency distributions were used to calculate the mean correlations, and percentiles were used to identify 5, 1 and 0·1 % probabilities that the correlation differed from unity and zero(Reference Efron and Tibshirani33).
Second, the magnitude of the scaling effect for a trait was assessed by testing whether scaled variation in sire-means differed between diets. To do this, sire-means of LipDG, ProtDG, LipRE and ProtRE were first log-transformed. Log-transformation scales trait variances similar to the calculation of CV(Reference Sokal and Rohlf34). Then, for each trait, a one-way ANOVA with diet as a fixed effect was fitted to the data, and a model with one common variance was compared with a model with separate variances for each diet using a log-likelihood ratio test (Proc Mixed). Because sire-means approximate sire genetic values(Reference Roff22), this is an approximate test of whether genetic variation was different on the two diets.
Results
Diet differences in trait means
LipDG, LipRE and ProtRE were significantly higher on the FM diet compared with the SBM diet (Table 2). In contrast, diet had no significant effect on ProtDG. On the other hand, LipFI and ProtFI were significantly higher on the SBM diet. Including BWI as a covariate in these analyses produced similar results (data not shown).
Previous studies showed that individual DG and FE were significantly higher on the FM diet, but individual FI was higher on the SBM diet(Reference Quinton, Kause and Koskela21). Individual Lipid%F was significantly higher on the FM diet, whereas individual Prot%F did not differ significantly between the diets(Reference Quinton, Kause and Ruohonen20).
Correlation between initial and final composition
The moderate negative correlation between Prot%I and Prot%F was marginally significant on the FM diet and significant on the SBM diet (Table 3). Correlation between Lipid%I and Lipid%F, in turn, was NS on the FM diet and significantly positive on the SBM diet. Accordingly, ranking of families during growth was strongly reversed for protein percentage but not for lipid percentage.
Table 3 Sire-mean correlations between initial and final body composition on fishmeal and soyabean-meal diets

* Pearson correlation coefficient.
† Probability that correlation differs from zero. Sample size is thirty-five sires for all correlations.
Correlations of initial composition with subsequent performance
Using correlations between initial body composition and subsequent growth performance, mechanisms for the negative correlation between Prot%I and Prot%F were explored. Prot%I but not Lipid%I was significantly correlated to subsequent growth and feed utilisation (Table 4). None of the partial correlations of Lipid%I with the subsequent performance traits was statistically significant, whereas seven out of the fourteen correlations of Prot%I were significant (Table 4).
Table 4 Partial sire-mean correlations of initial body lipid percentage and protein percentage with weight gain, feed intake, feed efficiency and their lipid and protein components on fishmeal and soyabean-meal diets

* Partial correlation coefficient from which the impacts of initial body weight and initial protein percentage were removed.
† Probability that correlation differs from zero. Sample size is thirty-five sires for all correlations.
‡ Partial correlation coefficient from which the impacts of initial body weight and initial lipid percentage were removed.
Correlations of initial protein percentage with subsequent performance
Correlation of Prot%I with DG was significantly negative on the SBM diet, and moderately negative but NS on the FM diet (Table 4). The stronger relationship on the SBM diet is consistent with the observation that the negative correlation between Prot%I and Prot%F was stronger on the SBM diet (Table 3). The negative correlation between Prot%I with DG resulted because both FI and FE, the two underlying components of DG, were negatively but NS correlated with Prot%I on both diets (Table 4). These results reflect that lower Prot%I was related to faster weight gain especially on the SBM diet.
Prot%I displayed significant negative correlations with ProtDG and ProtRE on both diets (Table 4). Thus, the sire families with low Prot%I had higher ProtDG and higher ProtRE compared with the families with high Prot%I. This implies that the families with low Prot%I were catching up with the families with higher Prot%I. Correlations of Prot%I with LipDG and LipRE were significantly negative on the SBM diet, implying that low Prot%I was also accompanied by increased lipid growth (Table 4). On the FM diet, this relationship was NS.
Genotype×environment interactions
The sire-means of LipDG, ProtDG, LipRE and ProtRE were positively correlated across diets (Table 5). The correlations differed significantly from both zero and one. The component daily gains were more strongly correlated than the nutrient retention efficiencies, indicating stronger re-ranking of families for the efficiency traits. When the effect of variation in BWI was removed from these correlations, using partial correlations, the results did not change (data not shown). Log-likelihood ratio tests showed that scaled variances of LipDG, ProtDG, LipRE and ProtRE did not differ significantly between diets (Table 6).
Table 5 Sire-mean correlations between diets for lipid and protein gains and retention efficiencies

* Pearson correlation coefficient reflecting the consistency of sire-family performance across the two diets. Sample size is thirty-five sires for all correlations.
† Probability that correlation differs from 0, obtained from 10 000 bootstrap estimates.
‡ Probability that correlation differs from 1, obtained from 10 000 bootstrap estimates.
Table 6 Log-likelihood ratio test for diet differences in scaled variance for lipid and protein gains and retention efficiencies
(Mean values with their standard errors)
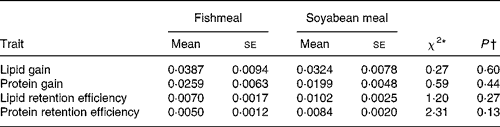
* The χ2 statistic was obtained by comparing a one-way ANOVA model with one common variance with a model with separate variances for each diet.
† From χ2 test, probability that scaled variances differ between the diets.
Discussion
The implementation of effective feed intake-recording technology has only recently allowed large-scale genetic studies on feed intake and FE in fish(Reference Quinton, Kause and Ruohonen20, Reference Quinton, Kause and Koskela21, Reference Kause, Tobin and Dobly32, Reference Silverstein, Bosworth and Waldbieser35, Reference Kause, Tobin and Houlihan36). In the present study, we showed that Prot%I, but not Lipid%I, was related to subsequent growth and nutrient utilisation. Decreasing Prot%I was linked to growth performance that increased subsequent protein percentage. This indicates that the mechanisms of protein homeostasis overrode those of lipid stasis. The present study was based on the analysis of sire-family means, and thus the correlations estimated here are broad-sense genetic correlations that approximate true genetic correlations. The present results provide evidence that body protein percentage in particular, not lipid, may be genetically regulated by feed utilisation and growth responses during fish growth. These results explain why cascades of lipid deposition occur during growth within farmed fish populations, leading to high amounts of phenotypic and genetic variation for body lipid percentage, whereas body protein percentage exhibits low phenotypic and genetic variation(Reference Tobin, Kause and Mäntysaari19).
Regulation of body protein percentage
In European whitefish, the mechanisms of protein homeostasis were more effective than those of lipostatic regulation. The results showed that variation in future growth was more strongly related to the among-sire variation in Prot%I than to the variation in Lipid%I. The sire families with low Prot%I had increased weight gain, protein growth and ProtRE, while the families with high Prot%I had decreased performance. Therefore, the families with low Prot%I were able to catch up with the other families, leading to the reversed ranking of families during growth for body protein percentage. Such responses should maintain low variation in percentage body protein during growth.
Indeed, using the same fish, Quinton et al. (Reference Quinton, Kause and Ruohonen20) showed that the coefficient of phenotypic variation and heritability for Prot%F was clearly lower (h 2 0·07–0·06; CV 6·9–7·4 %) than for Lipid%F (h 2 0·18–0·21; CV 13·1–14·3 %). These results are in line with other genetic studies on salmonids(Reference Tobin, Kause and Mäntysaari19). Likewise, in a study on rainbow trout, Tobin et al. (Reference Tobin, Kause and Mäntysaari19) found that correlations of body protein percentage recorded at different ages during growth up to 2·5 kg were weak or negative, but for lipid percentage strongly positive, maintaining low variation in protein percentage during growth. The low variability of percentage body protein, but not lipid percentage, across diets and feeding rations is also well established(Reference Simpson and Raubenheimer15–Reference Dumas, de Lange and France18).
Most of the previous studies on the control of body composition have focused on lipid deposition(Reference Kuenzel1–Reference Álvarez and Nicieza13). However, there is recent evidence in animals and human subjects that feed intake may also be controlled to reach a protein intake target rather than a fat intake target(Reference Simpson and Raubenheimer15). Increased feed intake, in turn, has been shown to induce elevated protein synthesis in fish, providing a mechanism for compensatory protein growth(Reference Houlihan, Hall and Gray37–Reference McCarthy, Houlihan and Carter39). However, when an animal increases its feed intake to reach the protein intake target, excess lipid and carbohydrates are ingested. Consequently, high feed intakes of individuals or families typically elevate their lipid deposition(Reference Simpson and Raubenheimer15), as also shown in European whitefish and rainbow trout(Reference Ruohonen, Simpson and Raubenheimer17, Reference Quinton, Kause and Ruohonen20, Reference Kause, Tobin and Mäntysaari40). Thus, control of body protein content can occur at the expense of increased lipid deposition, and this may again boost lipid deposition(Reference Simpson and Raubenheimer15, Reference Ruohonen, Simpson and Raubenheimer17).
The low phenotypic and genetic variation in body protein percentage can be regarded as a fundamental genetic constraint for selective breeding. Breeders would especially like to increase body protein percentage, but there are genetically determined constraints that hamper selection efforts to increase body protein percentage. It should be noted, however, that moderate heritability for body protein percentage (h 2 0·39) has been found for large (2·5 kg) but not for smaller rainbow trout ( < 800 g) (h 2 < 0·15)(Reference Tobin, Kause and Mäntysaari19), which may be due to the extensive lipid deposition and the associated increase in differences for lipid content between families at the latter ages.
Regulation of body lipid percentage
Compared with protein, lipid deposition in European whitefish was less strongly associated with subsequent growth performance that would have maintained lipid percentage invariable. This leads to cascades of lipid deposition creating positive rather than negative correlations between lipid body percentage in differently aged fish, as show by the present study and a previous study(Reference Tobin, Kause and Mäntysaari19). That excess lipid deposition induces only weak or no feedback mechanisms has been found to occur in fish and other animals(Reference Simpson and Raubenheimer15, Reference Regost, Arzel and Cardinal41, Reference Jobling, Larsen and Andreassen42). Because there are weaker feedback mechanisms for excess lipid growth, fish tend to store large amounts of lipid. Yet, there is a bulk of evidence that when lipid body stores or body weight are experimentally depleted, fish tend to fully or partially compensate for this(Reference Metcalfe and Thorpe8–Reference Johansen, Ekli and Jobling11, Reference Álvarez and Nicieza13).
At first glance, these previous studies and the present study seem to produce inconsistent results. However, we were studying naturally occurring within-population variation where both lipid and protein stasis could be potentially occurring simultaneously. Such an approach does not need to produce the same result as extensive experimental manipulation of fish lipid stores. For instance, Quinton et al. (Reference Quinton, Kause and Ruohonen20) showed that on the SBM diet, feed intake was higher but simultaneously Lipid%F was lower compared with fish fed the FM diet. In contrast, within a diet, increased feed intake was both phenotypically and genetically related to increased Lipid%F(Reference Quinton, Kause and Ruohonen20). The present results emphasise that both lipostatic regulation and protein homeostasis should be studied simultaneously to judge their relative importance in regulating fish growth and feed utilisation.
LipDG, ProtDG, LipRE and ProtRE were calculated using the values of Lipid%I and Prot%I. This may lead to autocorrelative effects generating negative correlations between initial component percentage and subsequent component gain. However, at least three findings suggest that our main results were not generated by computational autocorrelative effects. First, correlations of initial composition with wet-weight gain, FI and FE are free of autocorrelative effects. The results for these wet weight-based traits were in agreement with the results of their component traits. Second, both lipid and protein should be equally influenced by autocorrelations, but Lipid%I and Prot%I displayed different correlations with their respective component gains and efficiencies. Third, the negative correlations found between Prot%I and Prot%F are not influenced by an autocorrelation, and they call for feedback mechanisms to be understood.
Diet differences in trait means and genotype×environment interactions
Due to the decline and fluctuation of wild fish stocks harvested for FM production and the predicted increases in FM prices, feed manufacturers are replacing FM with alternatives such as SBM(Reference Powell24). Alternative plant-based protein sources are still inferior compared with FM-based diets(Reference Francis, Makkar and Becker43, Reference Gatlin, Barrows and Brown44), as also shown by the present study. Whitefish on the SBM diet had higher LipFI and ProtFI as well as lower LipRE and ProtRE compared with fish on the FM diet. The likely explanation is that fish on the SBM diet had a higher intake to reach their nutritional demand. The two diets were isoenergetic on a gross basis, but SBM typically contains anti-nutritional factors that limit the availability of some amino acids, fatty acids and other essential nutrients on a digestible basis(Reference Francis, Makkar and Becker43, Reference Gatlin, Barrows and Brown44). Restricted or poor-quality dietary nutrients and reduced lipid deposits are known to increase feed intake in salmonids(Reference Jobling and Johansen9–Reference Johansen, Ekli and Jobling11, Reference Jobling and Koskela45). Formulating the diets on a digestible basis would have reduced the difference between the diets, and yielded different results. In contrast to LipDG and body lipid percentage, ProtDG and body protein percentage did not significantly differ between the diet treatments (present study; Quinton et al. (Reference Quinton, Kause and Ruohonen20)). This again shows that percentage protein is less influenced by the environment compared with the respective lipid traits.
The present study revealed weak but existent sire × diet interactions for LipDG, ProtDG, LipRE and ProtRE. First, there was moderate re-ranking of family-means across diets, especially for LipRE and ProtRE. Yet, these positive correlations between the diets (r 0·43–0·78) mean that the families having a high ability to use the FM diet also had a high ability to use the SBM diet, and thus selection on the FM diet will also improve fish performance on the SBM diet. It should be noted that these family-mean correlations across diets are likely to be underestimates of the true genetic correlations(Reference Astles, Moore and Preziosi23), and therefore the degree of re-ranking may be overestimated here. Previous studies have shown even lower degrees of re-ranking for growth(Reference Palti, Silverstein and Wieman46), FI, FE and body composition(Reference Quinton, Kause and Ruohonen20, Reference Quinton, Kause and Koskela21) across FM- and plant-protein-based diets. Second, the within-diet correlations tended to be higher on the SBM diet, but it is unclear why this should be so. It is possible, yet speculative, that the elevated lipid deposition induced by the FM diet erodes the connection between initial and final body composition and masks strong relationships between growth, feed and nutrient utilisation(Reference Kause, Tobin and Houlihan36, Reference Kause, Tobin and Mäntysaari40). Third, the genotype × diet interaction did not induce scaling effects, i.e. the novel diet did not induce increased family differences that would have allowed more effective detection of family differences. Such effects have been observed among wild animals tested on novel or stressful environments(Reference Charmantier and Garant47). All in all, these results together show that genotype × diet interactions are weak enough that current fish-breeding programmes selecting fish on current FM diets are improving the ability of the fish to utilise novel soyabean-based diets.
Conclusions
To conclude, the present study provided evidence that protein stores may be more strongly regulated than lipid stores in a salmonid fish. So far, most studies on fish have focused solely on the regulation of lipid deposition. This is because percentage body protein remains invariable across diets, individuals and families in fish. Extensive variability in a trait typically fuels research to explain the causes of the variability. In contrast, the lack of variability in protein traits seems to have reduced the interest of researchers. Moreover, the fact that diet treatments have only minor impact on percentage body protein reduces possibilities to experimentally alter fish protein content and to follow the subsequent growth and feed utilisation. Here we showed that the invariability of body protein percentage is related to the highly variable underlying responses in growth performance. Homeostasis of percentage body protein is recognised as a major constraint for selective breeding and farmed fish nutrition. A soyabean-based protein source, in turn, was shown to induce only weak genotype × diet interactions, aiding in the genetic improvement of farmed fish.
Acknowledgements
The authors acknowledge O. Ritola, T. Paananen, S. Airaksinen, J. Bomberg, T. Jokelainen, and J. Kämäräinen for their work in fish management and trait recording, and A. Dumas and three anonymous referees for comments on the earlier text versions. The study was funded by the Employment and Economic Development Centre of Central Finland and the Ministry of Agriculture and Forestry. There are no conflicts of interest. All authors contributed to the planning, writing and development of the ideas; C. Q. and A. K. analysed the data.








