Introduction
Malnutrition in children undergoing antineoplasic treatment has been much studied throughout the years. Childhood cancer patients are at risk of weight loss, particularly lean body mass loss, in part due to cancer-related cachexia. Dysfunctional metabolic reactions including increased lipolysis, glucose resistance and muscle wasting can occur during cancer(Reference Corkins1–Reference Dwyer, Erdman, Macdonald and Zeisel3). In parallel, treatment side effects such as nausea, vomiting and dysgeusia can reduce appetite and contribute to deteriorate nutritional status(Reference Corkins1). Malnutrition may lead to a suboptimal response to treatment and worsen side effects. At the moment, most literature on nutrition in childhood cancer is related to those difficulties. However, excessive weight gain during certain phases of cancer treatment is common. A meta-analysis of growth patterns in children with acute lymphoblastic leukaemia (ALL) showed an unhealthy increase in BMI Z-score in the early phase of their treatment and during the maintenance phase(Reference Zhang, Liu and Chung4). Importantly, that weight gain was maintained during survivorship(Reference Zhang, Liu and Chung4). In young adult survivors of childhood cancer, inactivity and unhealthy food habits have been identified as contributors to the development of health complications(Reference Hudson, Ness and Gurney5). Therefore, nutritional intervention during treatment could help prevent these problems. Not only could it contribute to decrease the risk of late sequelae, but it could also benefit patients’ nutritional status that is associated with treatment side effects and risk of infections(Reference Corkins1). In the present critical review, we aim to describe the literature on children’s food intake during cancer treatment, to explore the determinants contributing to their food habits and to assess the nutritional educational intervention developed for this clientele.
Methodology
For this critical review, literature was searched through MEDLINE and PubMed and included articles from January 1995 to January 2018 (Fig. 1). Search terms were: Children: children or child or infant or adoles* or teen* or kid or kids or toddler or youth or pediatric* or paediatric*; Childhood cancer: leukaem* or leukem* or hematological malignanc* or haematological malignanc* or cancer or childhood cancer or lymphom* or neoplasm* or tumor or sarcoma* or chemotherapy or radiotherapy or pediatric oncology or paediatric oncology or pediatric cancer or paediatric cancer; Nutritional status/eating habits: diet* or food habit* or nutrition* or feeding or dietary intake or nutritional status or micronutrient intake or macronutrient intake or energy intake or protein intake or eating* or inadequate food intake or food intake or pleasure from food. Reviews were excluded from the results of the search strategy. Google Scholar was also screened to find additional articles.
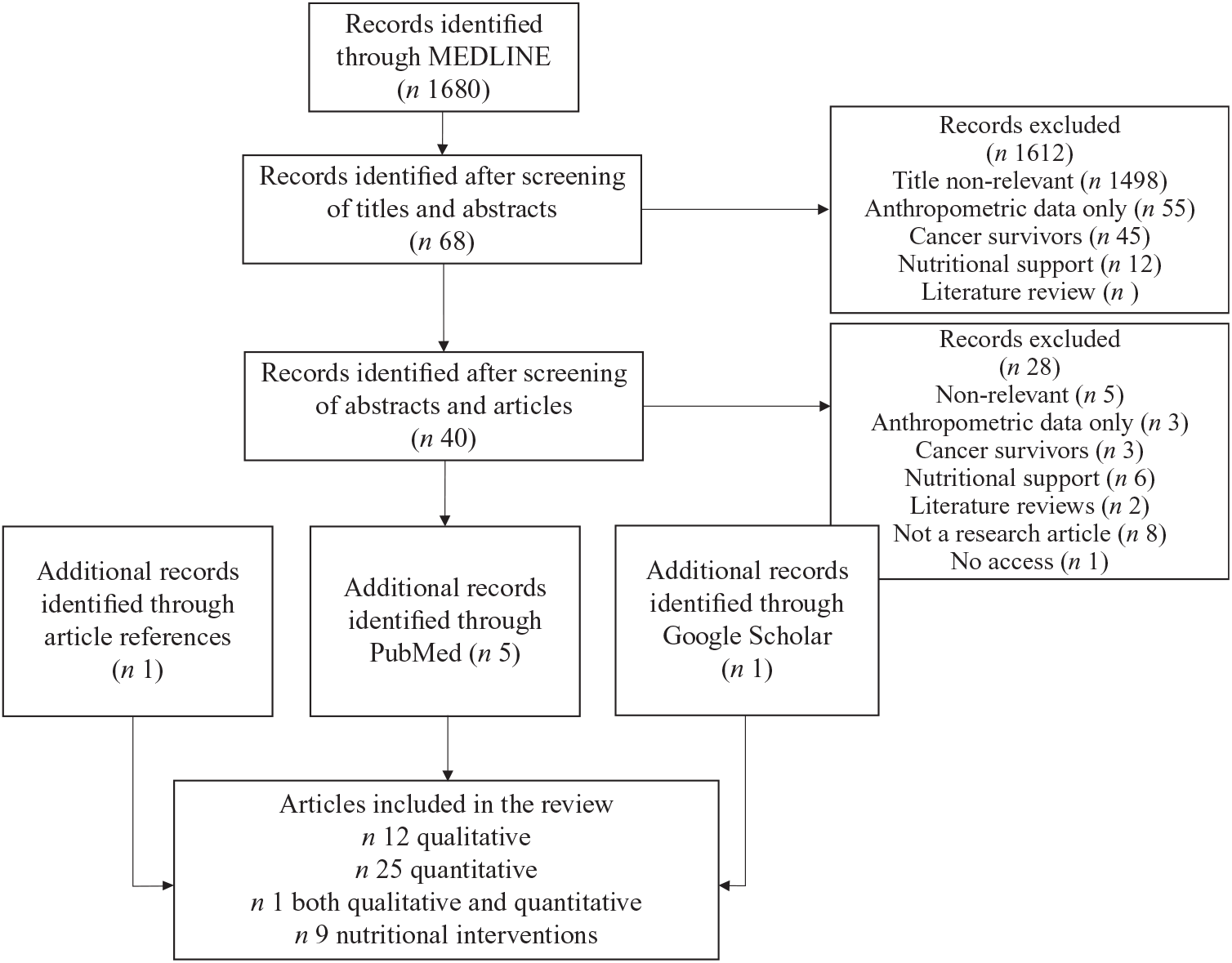
Fig. 1. Flow diagram describing search strategy of the critical review of the literature.
Articles were included if they were published in English in a peer-reviewed journal. Reviews and case reports were excluded. Studies were excluded if they addressed exclusively childhood cancer survivors, cancer prevention, screening evaluation or nutritional support. They were also excluded if the outcomes only included blood tests, anthropometric or body composition data or only compared with energy requirements without reporting nutrient or food intake. For articles reporting behaviours towards food, papers without a food-related thematic were excluded. Finally, for articles related to nutritional interventions, we excluded those without an educational aim, such as papers reporting medication-related interventions or framework development. After screening titles and abstracts, forty-seven articles were selected; thirty-eight studies were related to food intake and parental practices including twenty-five quantitative studies, twelve qualitative, and one both quantitative and qualitative. Nine studies were related to nutritional interventions.
Nutritional intake in children during cancer treatment
Study findings: energy intake
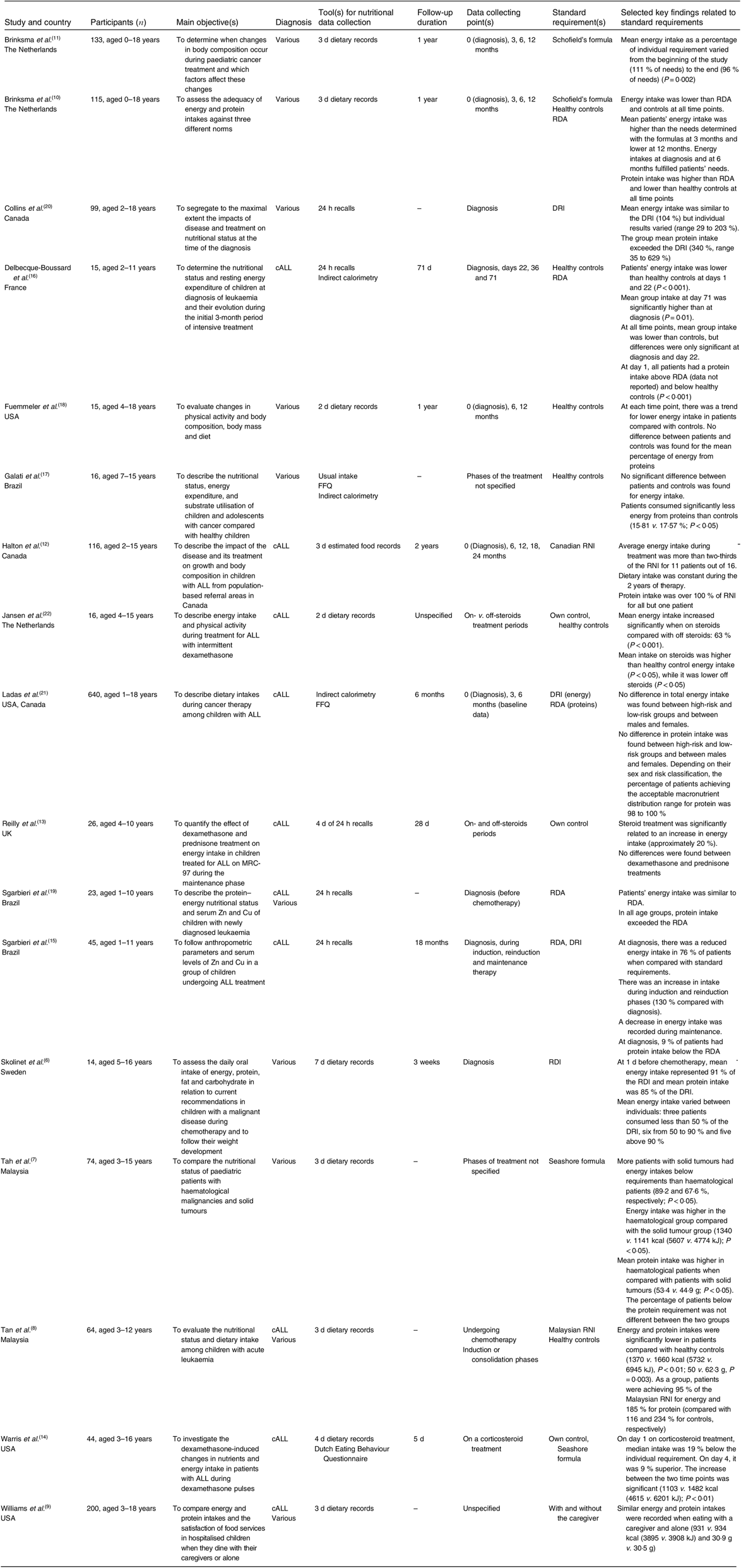
Seventeen articles reported quantitative data on patients’ energy intake. Table 1 presents the key findings. Nine of these studies used food records to assess patients’ energy intake(Reference Skolin, Axelsson and Ghannad6–Reference Warris, den Akker and Bierings14). Three studies compared intakes in subgroups of patients. Patients with haematological malignancies tended to have greater energy intake and were more likely to attain their energy requirements than those with solid tumours(Reference Tah, Nik Shanita and Poh7). Sgarbieri et al.(Reference Sgarbieri, Fisberg and Tone15) compared low- and high-risk ALL and showed no difference in energy intake. Williams et al.(Reference Williams, Hinds and Ke9) found that, in children aged from 3 to 18 years, energy intake did not differ in the presence or absence of the caregiver. Furthermore, using ANOVA, the authors showed that age and time since diagnosis were positively associated with energy intake.
Table 1. Key findings on energy and protein intakes compared with various standard requirements in cross-sectional and longitudinal studies

DRI, dietary reference intake; cALL, childhood acute lymphoblastic leukaemia; RNI, recommended nutrient intake; ALL, acute lymphoblastic leukaemia; MRC-97, Medical Research Council protocol 97; RDI, recommended daily intake.
Five studies have compared patients’ energy intakes with those of healthy controls. Typically, they consumed less energy than controls(Reference Tan, Poh and Nadrah8, Reference Brinksma, Roodbol and Sulkers10, Reference Delbecque-Boussard, Gottrand and Ategbo16) while the difference did not reach significance in two studies(Reference Galati, Resende and Salomao17, Reference Fuemmeler, Pendzich and Clark18). Galati et al.(Reference Galati, Resende and Salomao17) and Delbecque-Boussard et al.(Reference Delbecque-Boussard, Gottrand and Ategbo16) used indirect calorimetry to study energy expenditure in children with cancer(Reference Delbecque-Boussard, Gottrand and Ategbo16, Reference Galati, Resende and Salomao17). Both found no difference between patients’ and healthy controls’ energy expenditure. However, Galati et al.(Reference Galati, Resende and Salomao17) showed that patients’ metabolic rate was higher at diagnosis, but had decreased to be similar to controls after two cycles of chemotherapy.
Patients’ intakes were also evaluated in comparison with reference values or standard requirements. Results are inconsistent: recently diagnosed patients had either similar(Reference Halton, Atkinson and Barr12, Reference Sgarbieri, Fisberg and Tone19, Reference Collins, Collins and Nayiager20), lower(Reference Skolin, Axelsson and Ghannad6, Reference Brinksma, Roodbol and Sulkers10, Reference Sgarbieri, Fisberg and Tone15) or higher(Reference Brinksma, Roodbol and Sulkers10, Reference Brinksma, Roodbol and Sulkers11, Reference Ladas, Orjuela and Stevenson21) energy intake compared with standard requirements. Two studies showed that while mean energy intake was in line with requirements, results varied widely between individuals, especially at diagnosis(Reference Skolin, Axelsson and Ghannad6, Reference Collins, Collins and Nayiager20). Standards of energy intakes referred principally to dietary reference intakes (DRI) (n 2), RDA (n 2), recommended daily intakes (RDI) (n 1) and recommended nutrient intakes (RNI) (Canadian RNI, n 1; Malaysian RNI, n 1). Estimation equations such as Seashore and Schofield were used in three studies. Only a few studies mentioned the physical activity level used to determine patients’ individual energy requirement(Reference Brinksma, Roodbol and Sulkers10, Reference Brinksma, Roodbol and Sulkers11, Reference Ladas, Orjuela and Stevenson21). Delbecque-Boussard et al.(Reference Delbecque-Boussard, Gottrand and Ategbo16) raised the importance of assessing intake across time: ALL patients consumed less energy when compared with healthy peers at diagnosis and at day 22 of treatment, but this did not persist at days 36 and 72. Other prospective studies showed no significant variations across time(Reference Brinksma, Roodbol and Sulkers10, Reference Brinksma, Roodbol and Sulkers11, Reference Fuemmeler, Pendzich and Clark18), only presented baseline data(Reference Ladas, Orjuela and Stevenson21) or reported the mean intake of all the data collected during treatment(Reference Halton, Atkinson and Barr12). Ladas et al.(Reference Ladas, Orjuela and Stevenson21) presented a multicentric study on 640 patients with ALL and found no difference in the baseline energy intake in function of level of risk at diagnosis (standard and high-risk) and sex. More than 70 % of the patients had an energy intake that exceeded the recommendation(Reference Ladas, Orjuela and Stevenson21). Some studies assessed food intakes during different chemotherapy cycles, while others assessed them between. In children with various diagnoses (n 14), Skolin et al.(Reference Skolin, Axelsson and Ghannad6) found that median energy intake was 91 % of RDI at 1 d before the first chemotherapy and decreased weekly thereafter to 71, 66 and 54 % of RDI.
Three studies described the changes in energy intake before and during the administration of corticosteroids(Reference Reilly, Brougham and Montgomery13, Reference Warris, den Akker and Bierings14, Reference Jansen, Postma and Stolk22). They all showed an elevated energy intake when on steroids compared with off-steroid periods. Jansen et al.(Reference Jansen, Postma and Stolk22) found that patients’ intakes were significantly lower than controls when off steroids and significantly higher when on steroids. Warris et al.(Reference Warris, den Akker and Bierings14) found similar results. When using Schofield formulas to calculate the energy requirement adjusted for disease, activity, growth and energy absorption factors (for enteral feeding), they found that, off steroids, the mean intakes represented 81 % of individual requirement, compared with 109 % when on steroids.
Study findings: macronutrient intake
Protein intake
In the general adult and paediatric population, a sufficient protein intake is necessary to maintain adequate lean body mass. Protein intake during childhood cancer has been examined in twelve studies (Table 1). Of the studies, seven found that, at diagnosis, children’s protein intake met(Reference Halton, Atkinson and Barr12, Reference Sgarbieri, Fisberg and Tone15, Reference Ladas, Orjuela and Stevenson21) or exceeded(Reference Tan, Poh and Nadrah8, Reference Brinksma, Roodbol and Sulkers10, Reference Delbecque-Boussard, Gottrand and Ategbo16, Reference Sgarbieri, Fisberg and Tone19, Reference Collins, Collins and Nayiager20) the recommendations. Skolin et al.(Reference Skolin, Axelsson and Ghannad6) found that the mean protein intake of fourteen patients was 85 % of the requirement, being the first study to show a mean intake below the standard requirement(Reference Skolin, Axelsson and Ghannad6). Only Tah et al.(Reference Tah, Nik Shanita and Poh7) used the Seashore formulas based on the N:energy ratio. Specifically, 65 % of those with solid tumours did not attain Seashore recommendations compared with 43 % of haematological patients(Reference Tah, Nik Shanita and Poh7). All other studies used general protein recommendations based on sex and age without correction for hospitalisation or disease. On the other hand, when compared with healthy peers, four studies found lower protein intake in children undergoing cancer treatment(Reference Tan, Poh and Nadrah8, Reference Brinksma, Roodbol and Sulkers10, Reference Delbecque-Boussard, Gottrand and Ategbo16, Reference Galati, Resende and Salomao17) and two did not observe a significant difference(Reference Fuemmeler, Pendzich and Clark18, Reference Sgarbieri, Fisberg and Tone19). In two studies conducted in Brazil, in which the majority of patients met or exceeded the RDA for proteins, the main sources varied from rice, beans and milk in one study(Reference Sgarbieri, Fisberg and Tone15) to milk, meat, eggs, pasta and cereals in the other(Reference Sgarbieri, Fisberg and Tone19).
Fat and carbohydrate intakes
Fat and carbohydrate intakes have been less studied in the context of paediatric cancer. In a prospective study, Delbecque-Boussard et al.(Reference Delbecque-Boussard, Gottrand and Ategbo16) found that the lipid intake of patients with ALL was lower than of healthy controls at diagnosis, 22 and 36 d post-diagnosis. The authors also recorded, compared with controls, lower carbohydrate intake at diagnosis and at day 22(Reference Delbecque-Boussard, Gottrand and Ategbo16). Another study showed no difference for these macronutrients between controls, children with solid tumours and with non-solid tumours(Reference Galati, Resende and Salomao17). Finally, Tan et al.(Reference Tan, Poh and Nadrah8) found that lipid intake was significantly lower in patients with ALL at induction or consolidation phases, compared with healthy controls. No further analysis was made to determine if the intake of these macronutrients was associated with treatment phase.
Micronutrient intake and deficiency
Several studies have reported intakes of Mg(Reference Atkinson, Halton and Bradley23), Ca(Reference Fuemmeler, Pendzich and Clark18), antioxidant vitamins (vitamins A, E and C)(Reference Tah, Nik Shanita and Poh7, Reference Ladas, Orjuela and Stevenson21, Reference Kennedy, Tucker and Ladas24–Reference Neyestani, Fereydouni and Hejazi27), Zn and Cu(Reference Sgarbieri, Fisberg and Tone15, Reference Sgarbieri, Fisberg and Tone19), Na(Reference Warris, den Akker and Bierings14) or of a variety of other micronutrients(Reference Tan, Poh and Nadrah8, Reference Galati, Resende and Salomao17) during childhood cancer. Considering these studies, vitamin C was the vitamin for which the RNI was the most frequently reached by patients(Reference Tah, Nik Shanita and Poh7, Reference Slegtenhorst, Visser and Burke25, Reference Neyestani, Fereydouni and Hejazi27). Conversely, one study conducted in Brazil found that, based on serum levels, 70 % of patients had vitamin C deficiency (serum levels below 0·2 mg/dl (11·4 μmol/l))(Reference Lima de Araújo, Maciel Barbosa and Gomes Ribeiro26). They also pointed out that patients who consumed less than three portions of food sources of vitamin C tended to be more deficient, without describing their intake. Neyastani et al.(Reference Neyestani, Fereydouni and Hejazi27) and Atkinson et al.(Reference Atkinson, Halton and Bradley23) showed that assessing micronutrient intake alone may not estimate status adequately: while patients attained their needs in vitamin C and Mg, blood concentrations of these nutrients were below the normal range for Mg and within the normal range for vitamin C(Reference Atkinson, Halton and Bradley23, Reference Neyestani, Fereydouni and Hejazi27). Compared with healthy controls, ALL patients consumed twice the vitamin C intake, but their mean serum levels were ten times lower(Reference Neyestani, Fereydouni and Hejazi27). Serum total antioxidant capacity was also lower in ALL patients than controls and the authors hypothesised a perturbed vitamin C utilisation and/or absorption(Reference Neyestani, Fereydouni and Hejazi27). Of note, in controls, vitamin C intake was obtained mostly from fresh fruits and vegetables while, in patients, fruit juices, fresh fruits and vitamin supplements were the main sources.
In turn, Sgarbieri et al.(Reference Sgarbieri, Fisberg and Tone15) found that Zn intake at diagnosis was below the RNI in 24 % of children, although the mean serum levels remained in the normal range. Compared with healthy controls, Galati et al.(Reference Galati, Resende and Salomao17) observed that patients had lower intakes of Zn, P, riboflavin and vitamin B12 and a higher intake of K. Conversely, Tan et al.(Reference Tan, Poh and Nadrah8) reported that patients’ micronutrient intake was not significantly different from controls. In general, regardless of time point or country, patients generally did not achieve the standard requirement (RDA and RNI) for vitamin E(Reference Ladas, Orjuela and Stevenson21, Reference Kennedy, Tucker and Ladas24), vitamin A or β-carotene(Reference Tah, Nik Shanita and Poh7, Reference Kennedy, Tucker and Ladas24), vitamin D(Reference Ladas, Orjuela and Stevenson21), Zn(Reference Sgarbieri, Fisberg and Tone19) and Cu(Reference Sgarbieri, Fisberg and Tone19).
Food groups
When considering food groups, the most studied has been fruit, vegetable and milk consumption. Milk consumption was not found different between patients and controls. Paediatric cancer patients had a lower(Reference Williams, Lamb and McCarthy28, Reference Carpentier, Mullins and Elkin29) or similar(Reference Galati, Resende and Salomao17, Reference Fuemmeler, Pendzich and Clark18) consumption of fruits and vegetables when compared with healthy peers. The consumption of fruits and vegetables ranged from no to two portions daily, which is drastically below the five portions recommended by the USDA (United States Department of Agriculture) guidelines(Reference Fuemmeler, Pendzich and Clark18). In the study of Galati et al.(Reference Galati, Resende and Salomao17), children with cancer and controls consumed fewer portions of vegetables and dairy products than recommended but ate enough fruits. A trend for higher meat consumption than recommended was seen in the cancer group. Fast food and salty snack consumption was similar between patients and controls, except for on-steroid patients who consumed more non-core foods compared with controls and with off-steroid groups(Reference Fuemmeler, Pendzich and Clark18, Reference Williams, Lamb and McCarthy28). In the study, parents were asked to report non-core foods, defined as non-healthy foods that include, for example, pizza, ice cream and hot dogs, chips and French fries(Reference Williams, Lamb and McCarthy28). Only one study assessed soft drinks and found limited intakes in both patients and controls (3 d mean intake of 50 ml in patients and 325 ml in controls)(Reference Fuemmeler, Pendzich and Clark18). Finally, So et al.(Reference So, Kim and Joo30) described three meal patterns in children with cancer, based on the consumption of meat, fish, fruits and vegetables, rice and fried chicken. They stratified the risk of being overweight at diagnosis according to the score of each pattern. Patients with high scores for fish, eggs, fruits and vegetables were less likely to be overweight at diagnosis and after 24 months. The group with a high score for fried meat and fish were more likely to be overweight at diagnosis and after 6 months. Also, patients included in the highest tertile of this group were at increased risk of premature death when compared with those in the lowest tertile. No correlation was established between other food patterns and all-cause mortality. This study stresses that the quality of food can influence patients’ weight gain patterns and survival.
Limitations
Energy intake
One of the difficulties in assessing the adequacy of intake in childhood cancer is to determine the appropriate standard reference value. It has been pointed out that, because of the lower lean mass and reduced physical activity during cancer treatment, comparing energy intake with those of healthy children is debatable even though differences in energy expenditure were not found(Reference Corkins1, Reference Delbecque-Boussard, Gottrand and Ategbo16, Reference Fuemmeler, Pendzich and Clark18, Reference Carpentier, Mullins and Elkin29). Also, with the exception of the DRI, all the reference values used in the reviewed articles were determined as ranges of values for a specific population of age and sex and did not take into account physical activity, weight or height. The RDA is defined as the average daily intake sufficient to meet the nutrient requirements of 97–98 % of a population(Reference Otten, Hellwig and Meyers31). It has been suggested that the RDA does not accurately define intakes of individuals or groups, because it could overestimate energy needs(Reference Dwyer, Erdman, Macdonald and Zeisel3, Reference Otten, Hellwig and Meyers31). In 1990, the RDA and Canadian RNI were replaced by the DRI in Canada and the USA. The DRI for energy are based on the estimated energy requirement, that is the need for energy to maintain health according to age, weight, height and level of physical activity and are adapted for children’s growth needs(Reference Otten, Hellwig and Meyers31). Assessing the adequacy of intakes of individuals or groups by comparing with the RDA or another reference value is not recommended by the Institute of Medicine of the National Academy of Sciences in the USA(Reference Otten, Hellwig and Meyers31). To conclude on the sufficiency of energy intake, it is rather essential to consider individual variations in weight since specific needs vary. Accordingly, one study using RDA as a reference concluded that children with cancer have reduced energy needs due to lower physical activity, lesser lean body mass and stagnation in growth(Reference Brinksma, Roodbol and Sulkers10). Also, interpretation of data is difficult because only a few studies have defined the thresholds of adequate intake. For example, a mean intake reaching 85 % of the reference value was classified as insufficient in a study(Reference Tah, Nik Shanita and Poh7), but other authors have not defined the minimum values that were considered sufficient(Reference Sgarbieri, Fisberg and Tone15, Reference Collins, Collins and Nayiager20).
In children with cancer, depending on their condition, adding activity and stress factors to energy needs has been proposed(Reference Sacks, Wallace, Desai and Corkins32). None of the studies reviewed measuring general energy intake has used an adjustment factor, with the exception of one that employed the Seashore equation which includes adjustment factors for hospitalisation, illness and growth(Reference Tah, Nik Shanita and Poh7). Conversely, Brinksma et al.(Reference Brinksma, Roodbol and Sulkers10) justified not using an illness adjustment factor by the lack of studies to support it.
The small sample sizes make difficult the interpretation of data relative to energy intake during childhood cancer. In many studies, differences did not reach statistical significance because of limited power. Sample sizes were too small to be stratified into subgroups of interest such as diagnosis, age, time since diagnosis, chemotherapy cycles and nutritional status.
Moreover, often the only data available were the mean group intake and/or individual mean intake gathered at selected time points during treatment. Studying mean energy intake in heterogeneous populations may not represent specific subpatterns of individuals. These limitations could perhaps mislead on patients’ actual nutritional intake or on intake patterns during treatment or among subgroups.
Macronutrient and micronutrient intake
It is difficult to establish a reference value for protein consumption in childhood cancer, knowing that healthy children typically eat more proteins than needed(Reference Butte, Fox and Briefel33, Reference Börnhorst, Huybrechts and Hebestreit34). The optimal protein requirement to support physiological functions according to age, sex and stage of the treatment remains unknown. As mentioned by some authors, it is important to detail the benefits and risks associated with an increased consumption of proteins(Reference Brinksma, Roodbol and Sulkers10), especially the impact on lean body mass and the immune system. Also, no detailed analysis has been made to assess the type of proteins, carbohydrates or lipids consumed. For example, the studies included in the present review were performed in six countries, which could result in regional and cultural differences affecting the type and quality of the macronutrient consumed.
Moreover, it is important to point out that micronutrient intake below the RDA does not indicate deficiency, which requires clinical and biochemical assessments. However, seven of the thirteen studies that have evaluated micronutrients in paediatric cancer only gathered data on nutritional intake without considering deficiency(Reference Tah, Nik Shanita and Poh7, Reference Tan, Poh and Nadrah8, Reference Warris, den Akker and Bierings14, Reference Galati, Resende and Salomao17, Reference Fuemmeler, Pendzich and Clark18, Reference Ladas, Orjuela and Stevenson21, Reference Slegtenhorst, Visser and Burke25).
Editorial comments
Considering the many factors that can influence dietary intake and requirements, such as phase of treatment, infections, chemotherapy agents(Reference Sgarbieri, Fisberg and Tone15, Reference Bauer, Jürgens and Frühwald35) and eating patterns, it would be ideal to consider these factors when collecting and analysing nutritional data. The methodology used has a great impact on the external validity of the data collected. Receiving chemotherapy or not at the moment of nutritional evaluation can contribute to explain the discrepancies between studies. For example, three studies on patients with ALL have confirmed the considerable impact of steroids on energy intake and highlighted the importance to carefully consider treatment when assessing intake or developing nutritional interventions(Reference Reilly, Brougham and Montgomery13, Reference Warris, den Akker and Bierings14, Reference Jansen, Postma and Stolk22). Also, a considerable number of studies had a cross-sectional design that does not provide a representative evolution of patients’ nutritional profile. A prospective evaluation of children’s intakes and needs could benefit the development of personalised interventions tailored for each phase of cancer treatment. Moreover, it is possible that the counselling provided during studies influences or modifies patients’ diet. In the study of Galati et al.(Reference Galati, Resende and Salomao17), patients tended to consume more portions of meat than recommended by the national guidelines, but if nutritional counselling addressed protein intake and quality was not documented in the study.
Describing nutrient and energy needs in paediatric cancer patients is complex because of the many factors that could make an impact on energy balance. This population is at risk of malnutrition, lean-mass deficit and weight loss and, on the other hand, of overnutrition, fat mass increase and obesity. It is still unknown how cancer and treatment could affect the absorption, metabolism and utilisation of various vitamins and elements, which could alter patients’ needs and modify normal range values for this population. It is our opinion that it might be inappropriate to use a value of requirement only based on weight and age to classify the energy and nutrient intake of a heterogeneous group of patients that are undergoing various stages of treatments and have different diagnoses. While the above-mentioned factors are poorly documented in most studies, they could contribute to explain the discrepancies in macronutrient and micronutrient status observed in the literature. Also, it would be relevant to examine in depth the quality of children’s diet. This could be studied with dietary patterns (for example, Mediterranean, prudent, Western) or with macronutrient content, food groups or diet types (for example, polyunsaturated fats, red meat, vegetarian diet).
Briefly, there is a need to harmonise the reference values for energy and nutrient requirements and to exhaustively document the collection methods used to assess and compare nutritional intake in this population. A multitude of factors appears to affect dietary intake and thus alter the accuracy of the mean intake within a group. It is our opinion that prospective studies should be prioritised. Moreover, it would be important to document the phase of treatment and the medication taken when collecting nutritional data. This would improve result interpretation and comparisons between studies. Besides, numerous standard reference values have been used to assess adequacy of intake. Providing data on absolute energy intake, and not only as percentage of a standard reference, could allow comparison between studies and consequently lead to new findings on the nutritional status of children with cancer. We believe that all these aspects should be documented when assessing nutritional intake and analysing data.
Principal determinants of behaviours towards food
Study findings
Here, we report the perceptions of patients, parents and nurses to describe the determinants of behaviours towards food in children with cancer. This section mostly refers to twelve qualitative studies(Reference Gibson, Shipway and Barry36–Reference Young, Dixon-Woods and Findlay47), to one study reporting parental practices using quantitative analysis(Reference Gerhardt, Baughcum and Johnston48) and to another one describing both qualitative and quantitative data(Reference Williams, Lamb and McCarthy28). Most of the studies reviewed have collected data with in-depth or semi-structured interviews, while one study used only focus groups(Reference Martinson and Yee43) and two used photovoice as stimuli for focus groups(Reference Kim, Yi and Sang44) or interviews(Reference Gibson, Shipway and Barry36).
Treatments and side effects
Qualitative studies raise possible causes that can explain the reduced intake reported in some quantitative studies. Parents, children and nurses reported that side effects including nausea, sore mouth, vomiting and altered smell or taste were associated with lower food intake and appetite(Reference Gibson, Shipway and Barry36–Reference Skolin, Wahlin and Broman38, Reference Klanjsek and Pajnkihar40–Reference Martinson and Yee43). Interviews with twenty-nine parents (including eight parent dyads) exposed that altered taste is the main disruptor of children’s eating habits, leading to food aversions(Reference Skolin, Wahlin and Broman38). In a study that aimed to better understand how children were coping with nausea and mucositis during chemotherapy, three of eight children mentioned having developed their own strategies to limit treatment side effects, such as choosing well-tolerated foods(Reference Green, Horn and Erickson37). Neutropenia and fear of infections were also reported as important factors that affect children’s food intake(Reference Gibson, Shipway and Barry36, Reference Klanjsek and Pajnkihar40, Reference Kim, Yi and Sang44, Reference Moody, Meyer and Mancuso45). Food restrictions were mentioned by the five mothers in a focus group as a cause of frustration related to their child’s food intake and affecting the pleasure of eating(Reference Kim, Yi and Sang44). These restrictions, combined with treatment side effects such as altered taste and smell, were also identified as lessening the pleasure of eating in interviews with thirty-one patients aged between 5 and 21 years(Reference Moody, Meyer and Mancuso45).
Regarding the administration of corticosteroids, parents have expressed their difficulties in managing their child’s cravings, urgency to eat and pickiness(Reference Gibson, Shipway and Barry36, Reference Skolin, Wahlin and Broman38, Reference Williams and McCarthy46), but studies have not assessed a specific time pattern for these behaviours. In one study, it was reported that cravings and unhealthy nutritional habits could persist weeks after active treatment(Reference Williams and McCarthy46). A study comparing forty-three parents of children with ALL and thirty of healthy controls showed that parental practices have a different impact on intake depending if the child is on steroid treatment or not. In the on-steroids group, parental overprotection and inconsistent discipline were associated with an increase in non-core food intake, but this was not observed in off-steroid and control children(Reference Williams, Lamb and McCarthy28).
Three studies have described parental concerns about their child’s weight and growth(Reference Skolin, Koivisto Hursti and Wahlin39, Reference Fleming, Cohen and Murphy41, Reference Gerhardt, Baughcum and Johnston48). Fleming et al.(Reference Fleming, Cohen and Murphy41) exposed that more than half of the parents (thirty-eight in total) expressed concerns about weight loss, whereas only a small proportion was preoccupied with weight gain. Parents were afraid that weight loss would affect treatment efficacy, a preoccupation that was confirmed by Skolin et al.(Reference Skolin, Koivisto Hursti and Wahlin39). In this study, parents of eleven children were interviewed. When asked about their perception of their child’s eating, six parents reported that the child was eating poorly. One parent explained that the prolonged reduced intake was stressful because of its potential to negatively affect treatment outcome. Three parents reported increased appetite during steroid treatments and two of them considered this as reassuring(Reference Skolin, Koivisto Hursti and Wahlin39). This was corroborated by another study that found that the majority of fifteen mothers interviewed were pleased when their child’s appetite was increased, as it could counterbalance the poor eating periods(Reference Williams and McCarthy46).
Parental feeding practices
Parental feeding practices influence eating patterns of young children and teenagers(Reference Patrick and Nicklas49, Reference Hughes, Patrick and Power50). Practices reported from the qualitative studies included in the present review are summarised in Table 2. Briefly, the majority of parents of a child with cancer have expressed frustration, anxiety or concerns over their child’s eating patterns(Reference Gibson, Shipway and Barry36, Reference Skolin, Koivisto Hursti and Wahlin39, Reference Fleming, Cohen and Murphy41–Reference Kim, Yi and Sang44). Using questionnaires, 37·5 % of nurses (n 24) reported that children with cancer might refuse to eat in order to prove their autonomy and to gain control, especially during hospitalisation(Reference Klanjsek and Pajnkihar40). This issue was reported in another study by 12 % of the nurses interviewed(Reference Skolin, Wahlin and Broman38).
Table 2. Strategies used to manage poor appetite as reported by parents or nurses

During cancer treatment, parents feel responsible for their child’s food intake but are rather powerless at facing some treatment-related difficulties such as cravings or increased risk of food poisoning(Reference Gibson, Shipway and Barry36, Reference Sari, Yilmaz and Ozsoy42, Reference Young, Dixon-Woods and Findlay47). Interviews with twelve parents revealed their high level of anxiety towards the risk of infections(Reference Sari, Yilmaz and Ozsoy42). Other parents reported to have changed their feeding approach and became laxer in regards to their eating rules(Reference Williams, Lamb and McCarthy28, Reference Williams and McCarthy46). In a study including forty-three parents of children from 2 to 6 years old treated for ALL, Williams et al.(Reference Williams, Lamb and McCarthy28) assessed parents’ discipline and feeding practices. They associated the different styles with children’s intake and compared the results with parents of healthy children. They found that parents of a child with cancer were more permissive for house and eating rules than controls. They concluded that, for healthy children, overprotection was associated with an increased consumption of fruits and vegetables whereas it had the opposite effect for children with cancer. In another qualitative study, most of the fifteen parents interviewed reported increased laxness in their parenting rules since the diagnosis, as they believed it could help them and their child overcome medical appointments and procedures(Reference Williams and McCarthy46).
To better handle the difficulties during treatment, parents have reported adapting their strategies according to side effects and the child’s behaviour. For example, parents typically fed their child certain types of foods when he feels nauseated or is on steroids(Reference Green, Horn and Erickson37). Coercion, pressure and threatening to use nutritional support are strategies that are utilised by parents(Reference Skolin, Koivisto Hursti and Wahlin39, Reference Fleming, Cohen and Murphy41, Reference Young, Dixon-Woods and Findlay47), but they were not associated with increased intake in healthy children(Reference Birch51, Reference Galloway, Fiorito and Francis52).
Parents have reported that a positive ambiance and the presence of family members during mealtime encourage the child to eat during periods of poor appetite(Reference Gibson, Shipway and Barry36, Reference Skolin, Koivisto Hursti and Wahlin39). One mother stated that the ambiance was more important than the food itself to make her child eat(Reference Gibson, Shipway and Barry36). A parent reported that a child who has developed a trusting relationship with a nurse will, in his or her presence, better accept hospital food(Reference Skolin, Koivisto Hursti and Wahlin39). Via questionnaire, 41 % of nurses (n 24) reported that feelings of loneliness or boredom negatively affect children’s eating(Reference Klanjsek and Pajnkihar40). Having parents going through a divorce or conflicts during mealtimes were also identified as factors negatively affecting food intake(Reference Fleming, Cohen and Murphy41).
Beliefs and culture can also influence how parents cope with their child’s disease: those who believe that healthy habits can improve the efficacy of treatment will be more prompt to offer foods with higher nutritional value(Reference Sari, Yilmaz and Ozsoy42, Reference Martinson and Yee43). Focus groups highlighted strategies to provide adequate nutrition including to stimulate the child’s appetite, to provide food supplements, to restrict some forbidden foods and to prepare soup(Reference Martinson and Yee43). Other strategies reported by parents include adjusting nutrition according to blood values and preferring organic foods(Reference Sari, Yilmaz and Ozsoy42). In some countries, the use of alternative therapies such as Chinese herbs or soups is also a reassuring strategy for parents(Reference Martinson and Yee43, Reference Young, Dixon-Woods and Findlay47). Generally, when asked about nutrition appointments during treatments, parents only remember the pieces of advice related to food enrichment or to limit weight gain(Reference Green, Horn and Erickson37). Sari et al.(Reference Sari, Yilmaz and Ozsoy42) found that, globally, most parents do not understand or recall information provided by the nurse or the doctor before hospital discharge. Also, parents have reported being hesitant to ask for nutrition advice from health professionals(Reference Gibson, Shipway and Barry36, Reference Sari, Yilmaz and Ozsoy42). In fact, their principal sources of information are the Internet, magazines and other parents(Reference Gibson, Shipway and Barry36).
Food preferences during cancer treatment
Children’s food preferences during cancer treatment are not well studied or understood. Table 3 lists the food preferences reported in qualitative studies. Children and their parents stated that savoury and salty foods are preferred to sweet foods(Reference Gibson, Shipway and Barry36, Reference Skolin, Wahlin and Broman38, Reference Klanjsek and Pajnkihar40), which was confirmed by nurses(Reference Klanjsek and Pajnkihar40). Bland and light foods, such as pasta and yogurt, are preferred and well tolerated during periods of poor appetite or nausea, while strong-flavoured and fried foods are favoured when on steroids(Reference Green, Horn and Erickson37, Reference Skolin, Koivisto Hursti and Wahlin39). Familiar foods were also reported as well tolerated, especially compared with hospital food or during periods of poor appetite(Reference Skolin, Koivisto Hursti and Wahlin39). Some parents stated that they constantly offer a variety of foods in order to tempt their child to eat even though they found that practice burdensome(Reference Gibson, Shipway and Barry36). Likewise, more than the half of the eight parents in the study of Green et al.(Reference Green, Horn and Erickson37) stated this tactic as an effective strategy to get their child to eat.
Table 3. Food preferences during cancer treatment reported by children, parents and nurses

Meat, energy-dense commercial drinks and hospital foods were mostly disliked by patients(Reference Gibson, Shipway and Barry36, Reference Skolin, Wahlin and Broman38, Reference Skolin, Koivisto Hursti and Wahlin39, Reference Moody, Meyer and Mancuso45). For example, interviews with twenty-one children revealed that 38, 33 and 29 % disliked meat, hospital food and sweets, respectively(Reference Skolin, Wahlin and Broman38). In this study, a teenager reported consuming energy-dense commercial drinks only to avoid enteral feeding(Reference Skolin, Wahlin and Broman38). Skolin et al.(Reference Skolin, Koivisto Hursti and Wahlin39) proposed that energy drinks are perceived by children as medication rather than food, which could explain their poor appreciation.
Refusing to eat hospital foods has been reported in almost all studies included in the present review. This was observed in children of all ages(Reference Gibson, Shipway and Barry36–Reference Skolin, Koivisto Hursti and Wahlin39, Reference Moody, Meyer and Mancuso45), but was more frequent in teenagers(Reference Skolin, Wahlin and Broman38, Reference Klanjsek and Pajnkihar40). The look, taste and smell of food were the main reasons evocated by children not to eat. Moody et al.(Reference Moody, Meyer and Mancuso45) reported that 33 % of teenagers aged 8–17 years disapproved of the preparation and the selection of meals, and sometimes their smell. Gibson et al.(Reference Gibson, Shipway and Barry36) described the same complaint in twenty-three of the twenty-four children interviewed who also criticised the unfamiliar taste and repulsing aspect of foods. Parents also often criticised the hospital meal schedule, describing it as inflexible and non-favourable to optimise the child’s intake(Reference Gibson, Shipway and Barry36–Reference Skolin, Wahlin and Broman38, Reference Klanjsek and Pajnkihar40, Reference Young, Dixon-Woods and Findlay47). While nurses emphasised the importance of a flexible meal schedule, they also stated that the majority of parents had a favourable opinion of the hospital food service, but the authors did not explain this positive perception(Reference Klanjsek and Pajnkihar40).
Children have reported that foods purveyed by their parents and familiar foods were better tolerated(Reference Skolin, Wahlin and Broman38, Reference Skolin, Koivisto Hursti and Wahlin39). Accordingly, six of eleven parents reported bringing food to their child during hospitalisation(Reference Skolin, Koivisto Hursti and Wahlin39). The majority of the seventeen nurses interviewed mentioned that most of the food consumed by the children on the ward was purveyed by parents(Reference Skolin, Wahlin and Broman38). Besides, a quantitative study comparing in-home with hospital daily intake showed a higher intake at home(Reference Skolin, Axelsson and Ghannad6).
Limitations
Qualitative studies allow to better comprehend the motivations and difficulties that parents experience in the context of childhood cancer, but these perceptions cannot be generalised to all families. For instance, some parents were interviewed after the acute phase of treatment, so their perception could be different from parents whose children are undergoing treatment at interview(Reference Green, Horn and Erickson37, Reference Skolin, Koivisto Hursti and Wahlin39, Reference Fleming, Cohen and Murphy41).
Considering the large number of strategies reported by parents, it is possible that they do not understand which one could be helpful or not. Also, parents may not be prepared to respond to sudden changes in their child’s food preferences and selectivity. The sickness and schedule of treatments increase the burden of daily responsibilities. Like every parent, they have to manage the child’s normal eating behaviour development such as neophobia, need for familiarity and routine(Reference Birch and Fisher53). Expressing the desire for autonomy at preschool age is a normal behaviour(Reference Birch and Fisher53), but frontiers between normal behaviour and cancer- or side effect-related comportment are unclear. While the increased laxness is not well accepted by the parents themselves, the disease of their child is an emotional challenge. Parents understand that it will not facilitate the development of healthy lifestyle habits, but the need to protect their child surpasses this consideration(Reference Williams and McCarthy46).
Studies reveal that the sources of information used by parents are variable and that they do not always understand the advice from health professionals. Thus, parents use a variety of strategies to make the child eat without knowing if they are beneficial to the long-term development of healthy nutritional habits(Reference Fleming, Cohen and Murphy41) Additionally, little to no guidelines are available to guide health professionals on how to inform parents on the strategies to use during treatment.
In summary, results and perceptions presented in this review allow the identification of some general behaviours and certain types of foods preferred and disliked, but do not permit clarification of the impact of treatments and side effects on food preferences.
Editorial comments
A variety of factors can affect how parents manage their child’s food intake and behaviours during treatments. For example, periods of poor intakes are difficult for the parents as they valorise weight gain that is perceived as a sign of wellness. Besides, the neutropenic diet is a source of frustration for many parents. As a matter of fact, it can be restrictive and requires, for example, avoiding raw fruits and vegetables in order to reduce the risk of foodborne infections. It is promoted in some hospitals, but not in others, as there is a lack of proof of its efficacy in the paediatric oncology population(Reference Tramsen, Salzmann-Manrique and Bochennek54, Reference van Dalen, Mank and Leclercq55).
As the importance of family mealtime has already been established for the quality of intake in healthy children(Reference Christian, Evans and Hancock56, Reference Berge, Rowley and Trofholz57), perceptions of parents and nurses tend to demonstrate that it could have the same positive impact during cancer. In fact, conflicts related to food intake can create a negative ambiance resulting in the child limiting or avoiding mealtime. Also, finding the right timing to promote eating seems to be an important factor: apart from choosing a moment when the child is rested, it is crucial to respect his hunger.
Besides, children typically avoided hospital food and oral supplements. It is our opinion that parental and professional pressure related to the consumption of these types of foods negatively affects their likeability. Finally, children’s food preferences are known to vary during the course of cancer treatment as some chemotherapeutic agents’ side effects can influence tastes. As pointed out in several studies, treatment-related changes in taste affect children differently. Thus, professionals should advise parents to adapt their feeding strategies to their child’s condition and to seize the opportunity to introduce new types of foods.
Interventions on nutritional education
We report nutritional interventions (n 9) (Table 4) that have an educational aim to improve nutritional or cooking knowledge or eating habits in patients with cancer and their families. Almost half (n 4) of the studies were conducted with ALL patients(Reference Gibson, August and Greene58–Reference Hill, Hamby and Bashore61) and, in three studies interventions were performed during the maintenance phase(Reference Li, Donnella and Knouse59–Reference Hill, Hamby and Bashore61) when patients are prone to weight gain.
Table 4. Summary of interventions on nutritional interventions
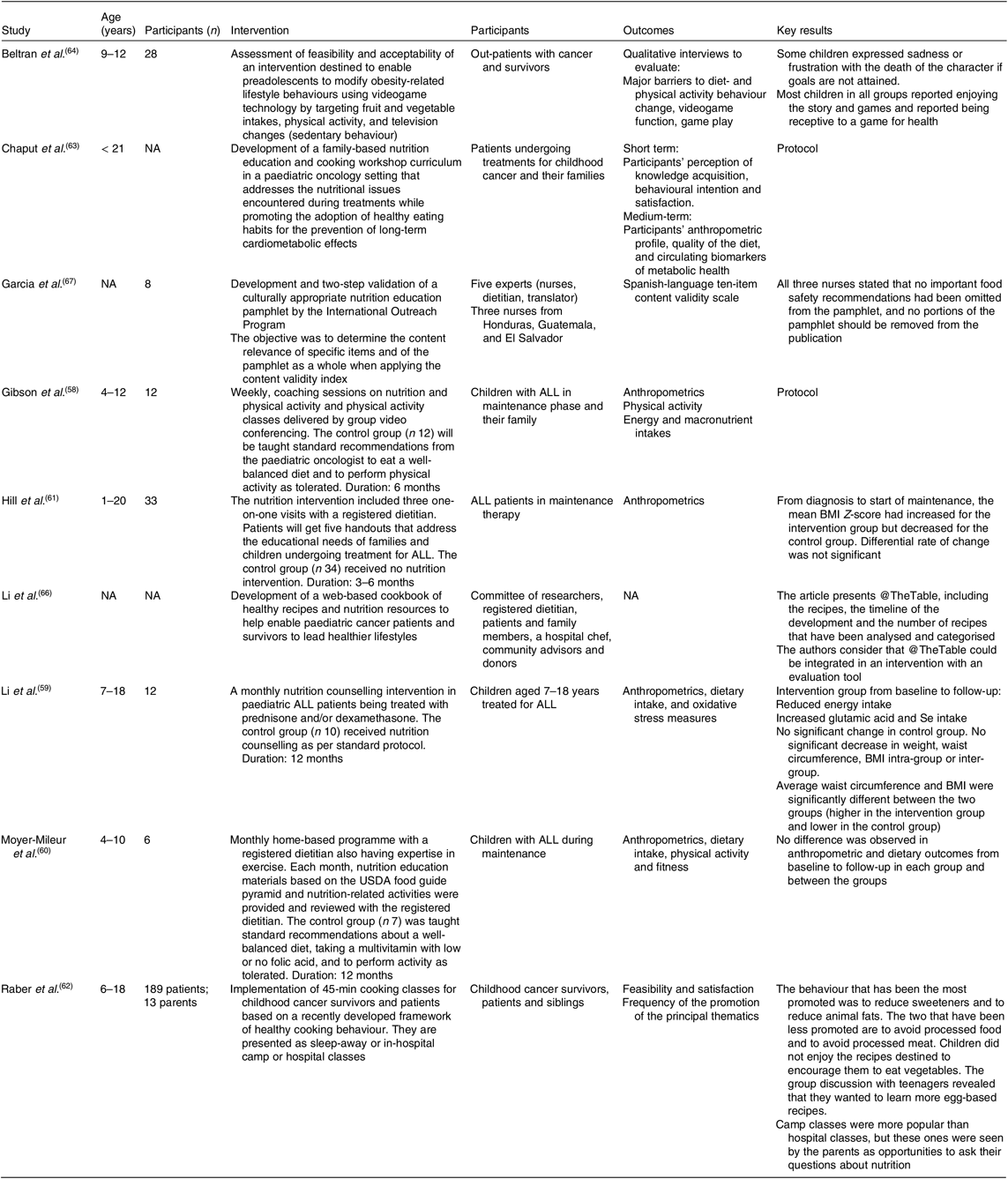
NA, non-applicable; ALL, acute lymphoblastic leukaemia; USDA, United States Department of Agriculture.
Study findings
In a randomised study, the intervention consisted of monthly nutritional counselling for 1 year(Reference Li, Donnella and Knouse59). The goal was to limit weight gain in children treated with corticosteroids. The counselling sessions included a motivation component to help families achieve their objectives. The control group received standard nutritional care. The intervention resulted in lower energy intake from baseline to follow-up for the intervention group (25·9 %; P = 0·0522), but the difference in intake between groups was not statistically different. The intervention also led to a significant increase in Se and glutamic acid intakes. Anthropometric data, such as BMI and waist circumference, were not different between groups.
Hill et al.(Reference Hill, Hamby and Bashore61) compared weight gain between a control group (n 34) recruited before the initiation of the intervention and an intervention group (n 33) who received three nutrition sessions with a dietitian during the first 6 months of the maintenance phase. The counselling was standardised and included goal setting for the family. BMI Z-score at diagnosis was documented from files. The authors showed that both the BMI Z-score at diagnosis and its variation from diagnosis to maintenance were associated with BMI during the maintenance phase. When controlling for these factors, multivariate analysis showed a lower increase in BMI Z-score in the intervention group compared with the control group. Since weight gain before the maintenance phase appears to be a determinant for the success of a nutritional intervention, the authors raised the importance of intervening early in the process of cancer treatments.
Moyer-Mileur et al.(Reference Moyer-Mileur, Ransdell and Bruggers60) conducted a 12-month randomised home-based nutritional intervention that included a physical activity component and took place during the maintenance phase. The physical activity and nutrition programme consisted of monthly assessments in which families (n 6) were provided with information and recorded their nutritional achievements. The control families (n 7) received standard nutrition counselling. Energy and nutrient intakes were assessed every 3 months. No intra- or intergroup difference in dietary intake was found at any time point of the study. Level of physical activity improved with time: at 12 months, the total minutes of physical activity was significantly greater in the intervention than in the control group. Also, the total minutes of physical activity were negatively correlated with weight, BMI and lean body mass, although the correlation with lean body mass did not persist after 6 months of intervention.
Similarly, Gibson et al.(Reference Gibson, August and Greene58) described the protocol of a randomised technology-based nutrition and physical activity intervention destined to childhood ALL patients during the maintenance phase. The study will consist of a weekly intervention with coaching in nutrition and in physical activity with video-conferencing technology. The authors aim to assess the feasibility, adherence and efficacy of the programme. Food group, energy and nutrient intakes collected from food records will be used to assess the efficacy of the intervention, in addition to BMI, physical activity level and weight.
Two studies have included a culinary element in their intervention. One reported the feasibility of a culinary intervention in a summer camp for patients, survivors and their siblings(Reference Raber, Crawford and Chandra62). A total of twenty-four cooking classes were presented and 189 children attended the classes. The intervention also included in-hospital cooking classes for children and parents who preferred not to travel (twenty-four children and thirteen parents). The cooking classes aimed to improve children’s cooking skills and to discover new foods in a summer camp ambiance. The intervention was based on the promotion of twelve behaviours such as ‘adding fruits and vegetables’ and ‘reduce sweeteners’. Results were mostly obtained from the facilitators’ field notes who recorded how frequently thematic behaviours were promoted. According to the authors, some behaviours were difficult to promote (for example, adding vegetables) due to the lack of interest in this type of food. They concluded that the development of future culinary interventions with this population is a promising avenue. Likewise, Chaput et al.(Reference Chaput, Beaulieu-Gagnon and Bélanger63) described the development of six culinary and educational workshops aimed at paediatric oncology patients and their families. The themes of the workshops address acute difficulties during treatment and general healthy eating messages to prevent long-term side effects. Each workshop comprises of key messages based on scientific evidence and clinical practice and includes a culinary demonstration with thematic recipes. The study will assess the feasibility of a nutrition education programme coupled with a culinary component. Participants’ perception of knowledge acquisition will be measured after each workshop as the principal outcome. Biochemical, anthropometric and nutritional data will also be analysed in relation to workshop attendance.
Another study investigated the acceptability of a videogame-centred programme on healthful nutritional habits in children with cancer(Reference Beltran, Li and Ater64). The game had already been validated with healthy children and resulted in an increased consumption of fruits and vegetables in the intervention group compared with the control group(Reference Baranowski, Baranowski and Thompson65). Interviews with patients revealed that the videogame was acceptable and enjoyable, but had components that were less appreciated (for example, sadness linked to the death of a character). However, the impact of the videogame on children’s behaviour or nutrition knowledge was not measured.
Two studies described the development of educational tools for parents to improve children’s eating habits. Li et al.(Reference Li, Raber and Chandra66) developed a web-based cookbook to address difficulties during treatment and to propose healthy recipes to patients and survivors. Website traffic will be used to evaluate the utility of the tool. Moreover, Garcia et al.(Reference Garcia, Chismark and Mosby67) presented the development and validation of an educational pamphlet about healthy eating and the prevention of foodborne infections to fit the need for this type of resource, as expressed by paediatric oncology nurses. None of these studies has described the intention to evaluate their impact on behaviours or knowledge.
Limitations
Determining the general efficacy of nutrition intervention during treatment is a complex task, especially because different outcomes have been measured in the literature. Also, different means were used to promote a healthy lifestyle including culinary demonstrations, summer-camp cooking classes, videogames and technology-based interventions. In some studies, the impact of the intervention on food intake, behaviour or knowledge was not assessed. Some authors have proposed that proving the efficacy of a nutrition education programme is difficult because the interventions are often fairly similar to the standard nutrition treatment(Reference Li, Donnella and Knouse59, Reference Moyer-Mileur, Ransdell and Bruggers60).
The two studies including a culinary dimension to their intervention involve different methods to present the information to participants: one with hands-on cooking classes(Reference Raber, Crawford and Chandra62), the other with cooking demonstrations combined with nutritional messages(Reference Chaput, Beaulieu-Gagnon and Bélanger63). Cooking lessons allow the concrete delivery of nutritional knowledge(Reference Condrasky, Griffin and Catalano68). Studies performed in various populations tend to show that hands-on culinary activities are more promising to improve culinary skills and competency(Reference Condrasky, Graham and Kamp69, Reference Dixon, Condrasky and Sharp70), but this has not been explored in the context of paediatric oncology.
Editorial comments
Interventions on nutritional support have not been included in the present review since they have been comprehensively reviewed elsewhere(Reference Ward, Henry and Friend71, Reference Trimpe, Shaw and Wilson72). Briefly, it was concluded that nutritional support is safe and efficient to increase weight in malnourished children with cancer. The choice of the route of administration (enteral or parenteral) needs to be determined after evaluation of the patient’s individual condition by a dietitian experienced in paediatric oncology.
To this day, most nutritional education studies have been published as protocols or have only assessed feasibility. Although this remains relevant, future studies should also appraise their utility, appreciation and impact on participants’ eating habits.
Conclusion
Globally, we conclude on the crucial importance for health professionals to consider the multiple aspects of the patient’s condition when developing nutritional evaluation and intervention, including the disease, phase of treatment, food preferences, family’s knowledge and beliefs. Therefore, patients could benefit from simple nutrition guidance to improve their dietary habits, which could contribute to a reduction in the risk of unhealthy weight gain and premature mortality during treatment. A better understanding of parents’ and caregivers’ perceptions could help health professionals to personalise their approach in paediatric oncology. Also, the large variability among study designs makes it difficult to assess the impact of nutritional interventions on dietary intake, nutritional status and other health outcomes. To facilitate interpretation of results, future nutritional studies should document the different factors that influence patients’ intake. Finally, it is important to assess the utility and impact of the interventions on patient nutrition knowledge or behaviour after evaluating their feasibility in this population.
Author ORCIDs
Sabrina Beaulieu Gagnon 0000-0002-1344-8029
Acknowledgements
The present review is funded by The Fondation Centre de cancérologie Charles-Bruneau, The Joy of Eating Better Foundation, the Fonds de Recherche du Québec en Santé, the Canadian Institutes of Health Research and by a Transition Grant from the Cole Foundation.
S. B.-G. performed the literature research. S. B.-G. performed the literature research. S. B.-G, V. B. and V. M. wrote the manuscript and approved the final version.
The authors reported no conflict of interest.