Anaemia is a major public health problem, particularly in the developing countries(1). The problem is of more serious concern and magnitude in infants and children. Recent estimates from India documented an anaemia prevalence of approximately 80 % in children aged between 6 and 36 months(2). Among the various causes of anaemia, nutritional deficiencies are believed to be of foremost importance(Reference Cook, Skikne and Baynes3), the most common being Fe deficiency. The association between Fe deficiency and anaemia has long been considered so strong that one is often used as a surrogate for the other.
Combating Fe deficiency through either supplementation or fortification is, therefore, considered the most important component of the current global initiatives for reducing rates of anaemia in children. However, the benefits of Fe supplementation demonstrated through clinical trials have not translated into a substantial reduction in the prevalence of anaemia on a public health scale. The earlier assumption that Fe supplementation might control anaemia to a major extent is, therefore, now being increasingly questioned(Reference Beaton and McCabe4, Reference Scholl and Hediger5). In a recent meta-analysis evaluating the effects of Fe supplementation on anaemia in developing countries, the authors concluded that ‘there is a suggestion in the data, not well documented except in a couple of studies, that something other than Fe may be operating to limit hemoglobin response and anaemia control’(Reference Scholl and Hediger5). A recent systematic review evaluating the effect of Fe supplementation alone on Hb response in children estimated that on an average, in non-malarial endemic areas, between 38 % and 62 % of anaemia (Hb < 11 g/dl) is Fe responsive; the corresponding range for malarial hyperendemic regions was 6–32 %(Reference Gera, Sachdev, Nestel and Sachdev6).
A probable reason for this is that apart from Fe, several other micronutrient deficiencies can cause anaemia(Reference Hodges, Sauberlich, Canham, Wallace, Rucker, Mejia and Mohanram7–Reference Baker9). For example, it is now established that vitamin A deficiency is associated with anaemia. Riboflavin, folate, ascorbic acid, Zn and vitamin B12 deficiencies are known to impair Hb synthesis. In addition to nutrient deficiencies, infections and parasites also contribute to anaemia; these include malaria, HIV infection and helminth infections(Reference Menendez, Kahigwa and Hirt10–Reference Stephenson, Latham, Kurz, Kinoti, Oduori and Crompton12).
Thus public health intervention to control anaemia should attempt to address all other causes, in addition to the obvious problem of Fe deficiency. One possibility would be to supplement other micronutrients in addition to Fe. Such an approach on a public health scale may also have the benefit of addressing the problems of various micronutrient deficiencies with a unified programmatic approach. Although such an approach of simultaneous supplementation with multiple micronutrients may seem attractive, there is a strong possibility that there may not be any additional haematinic benefit to Fe supplementation alone because of the possible negative interactions they may have on each other in vivo. In order to address this complex issue, two systematic reviews of randomized controlled trials (RCT) in children were conducted to: (i) study the effect of combining multiple (two or more) micronutrients with Fe supplementation on Hb response when compared with placebo; and (ii) study the effect of combining multiple (two or more) micronutrients with Fe supplementation on Hb response when compared with Fe supplementation alone.
Methods
Searching
A Medline search (from 1966 until 6 February 2006) was conducted by using the search word ‘iron’ with limits pertaining to ‘English language’ and ‘human’ for both clinical trials and RCT; and by using the search words ‘iron fortif*’ and ‘iron supplement*’ with similar limits. A similar search of the COCHRANE controlled trials register using the search words ‘iron fortif*’ or ‘iron supplement*’ was also conducted. A search of the EMBASE database from 1982 to 29 January 2006 using the search words ‘iron supplement*’ or ‘iron fortif*’ limited to ‘human’ and ‘English’ was also made. Similar searches were also made using the IBIDS database and the Healthstar database. The title and abstract of the studies identified in the computerized search were scanned to exclude studies that were obviously irrelevant. The full text of the remaining studies was retrieved and relevant articles were identified. The reference lists of the identified articles were also reviewed to search for citations not listed in the computerized databases. These were supplemented by hand searches of reviews, bibliographies of books and abstracts and proceedings of international conferences or meetings. Finally donor agencies, ‘experts’ and authors of recent Fe and micronutrient supplementation trials were contacted for their knowledge of any additional or ongoing trials. To avoid publication bias, effort was made to include both published and unpublished trials.
Selection
The predefined criteria for inclusion of trials in the systematic review were: (i) randomized placebo-controlled efficacy trials involving Fe supplementation in combination with two or more other micronutrients; and (ii) Hb as one of the evaluated outcome measures.
Studies in which other drugs were also simultaneously administered were included if the only difference between the study and the control groups was supplementation with two or more micronutrients, for the second objective, and Fe plus two or more micronutrients for the first objective.
Validity assessment
We assessed the quality of trials using recommended criteria(Reference Clarke and Oxman13, Reference Juni, Altman and Egger14). Concealment of allocation was classed as adequate, unclear, inadequate, or not used. To assess attrition we classified studies by percentage of participants lost to follow-up (<3 %, 3–9·9 %, 10–19·9 % and ≥20 %). Blinding was classified as double blinding, single blinding, no blinding, and unclear.
Data abstraction
Data abstraction was done using preformed questionnaires. The data included in the review were derived from the published manuscript or were those provided by the authors (in cases of unpublished studies). Wherever possible, the authors were contacted for further clarifications, if required. T.G. abstracted all of the data.
Study characteristics
The studies were grouped and analysed for change in Hb before and after the supplementation period. To study the efficacy of multiple-micronutrient supplementation with Fe, one systematic review was done including those RCT which compared their haematinic effect with a placebo. To study the additive haematinic effect provided by the multiple micronutrients, a separate systematic review was done including those studies whose two treatment arms were Fe alone v. Fe and multiple micronutrients.
The contribution to heterogeneity of the variables in the pre-specified stratified analyses was also explored by meta-regression analysis using the METAREG command in STATA software with the restricted maximum likelihood option(Reference Sterne, Bradburn and Egger15).
Quantitative data synthesis
For computing the pooled estimates we were primarily interested in the extraction of sample size, mean change in Hb from the beginning to the end of intervention, and the sd of this change in the intervention and the control groups. Wherever available, the stated (or communicated/clarified) values were utilized for the computations.
In designs employing two or more different intervention groups (different dosage or administration regimens) and a single control group, to avoid multiple counting of the control group, the sample size of the control group was equally cleaved into the number of intervention groups while retaining the same value for the change and its sd (A Oxman, personal communication, 2003; J Deeks, personal communication, 2003). For an uneven split, a higher size in the cleaved control group was given to the intervention group with lower sample size or higher sd. In publications reporting such designs, each intervention subgroup was analysed separately for the purpose of meta-analysis. Thus, some trials contributed more than one ‘analytic component’ for the purpose of statistical analyses. This resulted in a greater number of ‘analytic components’ than the included trials.
The following principles were employed for derivations, if actual variables of interest were not stated: (i) in a group, the lower of the two stated sample sizes at the beginning or the end of a trial was assumed to be the sample size for the change; (ii) wherever feasible, sd were imputed (back-calculated) from the stated se, t or P values; (iii) wherever unstated, the mean age of subjects was computed as the average of the stated range; and (iv) wherever unstated, the mean change in Hb was computed as the difference of mean post- and pre-intervention levels.
The sd for the change in Hb could be extracted or imputed (from se, t or P values) in several but not all studies. In the remaining trials, these sd were computed using the following assumptions: (i) correlation of 0·5 between the pre-test and post-test variances(Reference Follmann, Elliott, Suh and Cutler16); and (ii) pre-test and post-test samples considered to be independent (no correlation). Considering the number of assumptions and imputations involved, for a confident interpretation, three types of pooled estimates were calculated. In two of these, the change sd for unstated or non-imputable values were computed with the assumptions of correlation (p) equal to 0·5 or of independence, while for the third the post-intervention levels and their respective sd were utilized. If the statistical significance was synchronous for all the three types of computations, the interpretation was obviously robust. However, for any discrepancy in significance computations by these three methods, the interpretation was considered to be statistically significant only if at least two of the three estimates had a probability value below 0·05.
The presence of publication bias in the extracted data was evaluated quasi-statistically using the funnel plot(Reference Sterne, Egger and Smith17). Statistical tests for funnel plot asymmetry, namely Begg’s and Egger’s methods, were conducted using the METABIAS command in the STATA software(Reference Sterne, Bradburn and Egger15). The pooled estimates of the weighted mean difference of the evaluated change in Hb between the control and treatment group were calculated by both fixed effects and random effects model assumptions using the METAN command in STATA software(Reference Sterne, Bradburn and Egger15). This program (STATA version 9·2; StataCorp LP, College Station, TX, USA) also computes the formal test of heterogeneity, the statistic Q. We report primarily random effects estimates because of frequent statistical heterogeneity in the pooled results.
Stratified analyses (specified in advance) were conducted for: (i) methodological quality; (ii) route of Fe and micronutrient administration (oral medicinal supplement or food fortification); (iii) duration of supplementation; (iv) baseline Hb of the supplemented group; (v) nature and total number of micronutrients given; (vi) nutritional status of the study population; and (vii) development status and malarial endemicity of the study area. The contribution of these variables to heterogeneity was also explored by meta-regression analysis(Reference Sterne, Bradburn and Egger15). A variable was considered to be an important explanatory factor if statistical significance was consistently documented in the stratified and in the meta-regression analyses. A greater credence was attached to the meta-regression results, particularly those controlling for all variables.
Results
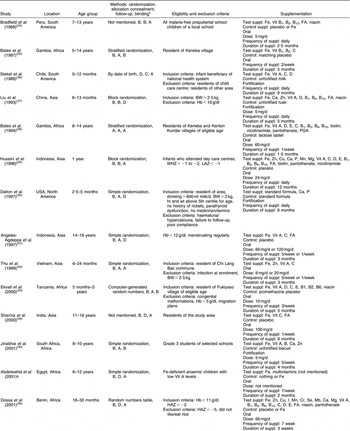
A total of thirty-six studies were identified as potentially eligible for inclusion in the systematic review. After thorough scrutiny, six of these trials were excluded for specific reasons (Fig. 1)(Reference Mahloudji, Reinhold, Haghshenass, Ronaghy, Fox and Halsted18–Reference Baqui, Walker, Zaman, Arifeen, Chowdhury, Wahed, Black and Caulfield23). Thus thirty trials (Table 1) were included in the systematic review, of which twenty-nine(Reference Bradfield, Jensen, Gonzales and Garrayar24–Reference Tielsch, Khatry, Stoltzfus, Katz, LeClerq, Adhikari, Mullany, Shresta and Black52) were published in various indexed journals whereas one was unpublished (HA Abdelwahid, MS Khattab, MAA Mostaffa, HF El-sayed and AE Saad, The effect of treatment with Vit. A alone or in combination with iron in iron deficient anemic children in Ismailia city, unpublished results). Table 1 depicts the characteristics of the analysed trials. To evaluate the additional haematinic effect of multiple micronutrients, separate analyses were done for studies that compared the effect of Fe and micronutrient supplementation with placebo; and those studies that compared the effect of Fe and micronutrient supplementation with Fe supplementation.

Fig. 1 Selection process for randomized controlled trials (RCT) included in the present meta-analysis
Table 1 Characteristics of randomized controlled trials (RCT) included in the present meta-analysis

BW, birth weight, WHZ, weight-for-height Z score; LAZ, length-for-age Z score; ht, height; wt, weight; Vit, vitamin(s); HAZ, height-for-age Z score; MUAC, mid upper-arm circumference; suppl, supplement/supplementation; PGA, pteroyl glutamic acid; FA, folic acid; SP, sulfadoxine-pyrimethamine.
*Allocation concealment: A, adequate; B, unclear; C, inadequate; D, not used. Follow up: A, <3% of participants excluded; B, 3% to 9·9% of participants excluded; C, 10% to 19·9% of participants excluded; D, 20% or more of participants excluded. Blinding: A, double blinding; B, single blinding; C, no blinding; D, unclear.
†Unpublished study (HA Abdelwahid, MS Khattab, MAA Mostaffa, HF El-sayed and AE Saad, The effect of treatment with Vit. A alone or in combination with iron in iron deficient anemic children in Ismailia city, unpublished results).
Iron and multiple micronutrient supplementation v. placebo
Study characteristics
A total of twenty-five studies (contributing thirty-five analytic components) were included in this systematic review. All of the studies were from developing countries (eleven trials were from Asia, three were from South America and eleven were from Africa). The studies were equally distributed with respect to the age group of the study population; thirteen were conducted in infants and pre-school children (0–5 years) and twelve trials included older children (>5 years of age). In three studies, evaluation was done for 2 months or less, while twenty-two investigators followed up the subjects for longer. In almost half of the studies (13/25) a medicinal supplement was used, the rest (12/25) used fortified foods. In the thirty-five analytic components, Fe and micronutrient supplementation was done daily in fifteen and intermittently in the rest.
Quantitative data synthesis
The funnel plot (Fig. 2) was symmetrical, indicating the probable absence of publication bias which was confirmed using Egger’s (weighted regression) method (P for bias = 0·16) and Begg’s (rank correlation) method (continuity corrected P = 0·94).

Fig. 2 Funnel plot with pseudo 95 % confidence limits of weighted mean difference (WMD) in Hb concentration for iron and multiple micronutrients v. placebo, with unknown sd derived with assumption p = 0·5
Data were available on 4981 subjects, 2675 of whom received Fe with other micronutrients and 2316 a placebo. The pooled weighted mean difference (WMD) of the Hb change (pre- to post-test difference) following Fe and micronutrient supplementation was 0·65 g/dl (95 % CI 0·50, 0·80, P < 0·001; test for heterogeneity Q = 306·47, I 2 = 89·6 %, P < 0·001) (Fig. 3). The results were similar when the sd were calculated by assuming p = 0·5 (depicted in previous sentence), by independence assumption (WMD = 0·65 g/dl, 95 % CI 0·5, 0·8, P < 0·001; test for heterogeneity Q = 291·92, I 2 = 89·0 %, P < 0·001) and with post-test scores (Table 2). The effect size also was similar when the analysis was restricted to studies with available (or imputed) Hb change sd scores (WMD = 0·65 g/dl, 95 % CI 0·47, 0·82, P < 0·001; test for heterogeneity Q = 274·03, I 2 = 92·0 %, P < 0·001).

Fig. 3 Forest plot for iron and multiple micronutrient v. placebo with unknown sd derived with assumption p = 0·5. Weighted mean difference (WMD) in Hb concentration, 95 % confidence interval and weights from random effects analysis are given; see Table 1 for details of the studies
Table 2 Sensitivity and subgroup analyses of pooled estimates of Hb weighted mean difference (WMD) for studies comparing iron and micronutrient supplementation v. placebo

Except for the all category, calculations performed by sd calculated with the assumption p = 0·5.
Not done for country development status, as all studies were from developing countries.
*Number of analytic components.
Sensitivity and stratified analyses suggested that a higher Hb response was seen in studies with an attrition rate over 10 %, non double-blinded studies, in malarial non-endemic regions, with medicinal supplementation, with increasing dose, frequency and duration of supplementation, and in children with lower weight-for-age, weight-for-height and height-for-age Z scores. Also, subjects receiving ‘other micronutrients’ (micronutrients other than Zn, vitamin A, riboflavin, B12, folic acid and ascorbic acid) had a significantly lower Hb response (Table 2).
Combined scrutiny of stratified and meta-regression analyses indicated that lower baseline Hb, lower height-for-age Z score and no intake of ‘other micronutrients’ (micronutrients other than Zn, vitamin A, riboflavin, B12, folic acid and ascorbic acid) were significant predictors of a positive effect of Fe and multiple-micronutrient supplementation, whereas residence in a malarial non-endemic area was close to statistical significance (P = 0·06) on meta-regression with univariable analysis for a positive haematinic effect of the supplement (Table 3).
Table 3 Meta-regression analyses for Hb weighted mean difference (WMD) (restricted maximum likelihood method) for studies comparing iron and micronutrient v. placebo supplementation

Unless specified separately, the number of analytic components is 33.
In the multivariate model, the number of analytic components is 32 and the proportion of residual variation due to heterogeneity (I 2) is 0·75. Some variables could not be included in the model because of insufficient observations and limit to the number of modelled variables.
Iron and multiple-micronutrient supplementation v. iron supplementation
Study characteristics
A total of thirteen studies (contributing fifteen analytic components) were included in this systematic review. All studies, except one, were from developing countries (three in Asia, three in South and Latin America, one in North America and six in Africa). Most (11/13) of the trials were conducted in infants and pre-school children (0–5 years) and only two trials included older children (>5 years of age). In two studies, evaluation was done over 2 months or less, while eleven investigators followed the subjects for more than 2 months. In almost half of the studies the subjects received Fe and multiple-micronutrient supplementation in the form of oral medicine (7/13); in six studies fortified foods were ingested. In the fifteen analytic components, Fe and micronutrient supplementation was done daily in eleven and intermittently in the rest.
Quantitative data synthesis
The funnel plot (Fig. 4) was symmetrical, indicating the probable absence of publication bias, which was confirmed using Egger’s (weighted regression) method (P for bias = 0·62) and Begg’s (rank correlation) method (continuity corrected P = 0·92).

Fig. 4 Funnel plot with pseudo 95 % confidence limits of weighted mean difference (WMD) in Hb concentration for iron and multiple-micronutrient supplementation v. iron supplementation with unknown sd derived with assumption p = 0·5
This systematic review provides pooled data on 1483 subjects, 707 received Fe and multiple micronutrients while 776 received Fe alone (Table 4). The pooled WMD of the Hb change (pre- to post-test difference) following Fe with micronutrient supplementation compared with Fe alone was 0·14 g/dl (95 % CI 0·00, 0·28, P = 0·044; test for heterogeneity Q = 58·41, I 2 = 76·0 %, P < 0·001) (Fig. 5). The results were almost the same when the sd were calculated by assuming p = 0·5 (depicted in previous sentence) and by independence assumption but not significant when using post-test scores. The effect size was higher when the analysis was restricted to those studies with available (or imputed) Hb change sd scores (WMD = 0·22 g/dl, 95 % CI 0·05, 0·39, P = 0·014; test for heterogeneity Q = 46·42, I 2 = 87·1 %, P < 0·001) (Table 4).

Fig. 5 Forest plot for iron and multiple-micronutrient supplementation v. iron supplementation with unknown sd derived with assumption p = 0·5. Weighted mean difference (WMD) in Hb concentration, 95 % confidence interval and weights from random effects analysis are given; see Table 1 for details of the studies. *Unpublished study (HA Abdelwahid, MS Khattab, MAA Mostaffa, HF El-sayed and AE Saad, The effect of treatment with Vit. A alone or in combination with iron in iron deficient anemic children in Ismailia city, unpublished results)
On combined scrutiny of stratified and meta-regression analyses, none of the variables was found to be a significant predictor (Tables 4 and 5).
Table 4 Sensitivity and subgroup analyses of pooled estimates of Hb weighted mean difference (WMD) for studies comparing iron and micronutrient supplementation v. iron

Except for the all category, calculations performed by sd calculated with the assumption p = 0·5.
Not done for country development status as, except one, all studies were from developing countries.
*Number of analytic components.
Table 5 Meta-regression analyses for Hb weighted mean difference (WMD) (restricted maximum likelihood method) for studies comparing iron and micronutrient v. iron supplementation

Unless specified separately, the number of analytic components is 15.
In the multivariate model, the number of analytic components is 14 and the proportion of residual variation due to heterogeneity (I 2) is 0·87. Some variables could not be included in the model because of insufficient observations and limit to the number of modelled variables.
Discussion
The results from the present largely heterogeneous data set derived from randomized controlled efficacy trials reveal that combined Fe and micronutrient supplementation in comparison to placebo alone resulted in a significant increase in Hb in children (WMD = 0·65 g/dl, 95 % CI 0·50, 0·80, P < 0·001). The rise was greater in initially anaemic subjects and children with lower height-for-age Z scores; and was lower in malarial hyperendemic areas and with addition of micronutrients other than vitamin A, riboflavin, Zn, vitamin B12, folic acid and ascorbic acid. These parameters were also significant predictors for heterogeneity on meta-regression analysis. On pooled analyses of studies comparing combined Fe and micronutrients v. Fe supplementation alone, the addition of multiple micronutrients to Fe resulted in a small but significant increase in Hb (WMD = 0·14 g/dl, 95 % CI 0·00, 0·28, P = 0·04). On stratified and meta-regression analyses, none of the variables emerged as a significant predictor.
It is prudent to examine the strengths and limitations of these analyses before drawing operational inferences. The main conclusion in relation to the rise in Hb concentration remained stable over the large spectrum of sensitivity analyses performed. Influence analyses, i.e. the effect of omitting one study at a time (data not depicted), did not reveal an overwhelming effect of any single trial.
However, the following limitations merit consideration. First, anaemia is a disease with multifactorial aetiology. Most of the included trials did not identify the cause of anaemia or the relative contribution from Fe and micronutrient deficiencies. This is important because lower Hb levels have often been attributed to other potential confounding factors such as poverty, undernutrition, maternal hypoferraemia, haemoglobinopathies, worm infestation, malaria and other coexistent infections. Second, we could not confidently differentiate between the therapeutic and preventive effects of Fe and multiple-micronutrient supplementation. This was because very few studies provided relevant data or they were not conducted with the objective and sample sizes to evaluate a preventive role. Third, in the absence of actually stated data on the variability of the change in outcome scores, several imputations had to be done on the basis of pre-specified assumptions. The sensitivity analyses suggest these imputations were robust because the interpretation and quantification with various assumptions were invariably synchronous. Finally, multiple subgroup and meta-regression analyses increased the possibility of false positive results and, because of the relatively small number of trials, it is also possible while analysing individual micronutrients that similar data sets may sometimes be getting compared. The identified significant predictors of response should, therefore, be considered as exploratory in nature, rather than definitive.
A few interesting observations have emerged from the present systematic review, which have programmatic implications and can provide direction for future research.
In a previously conducted meta-analysis(Reference Gera, Sachdev, Nestel and Sachdev6), the pooled estimate (random effects model) from fifty-six trials for the change in Hb concentration following Fe supplementation v. placebo (WMD) was 0·74 g/dl (95 % CI 0·61, 0·87, P < 0·001; P < 0·001 for heterogeneity). Lower baseline Hb level, oral medicinal Fe supplementation and non-hyperendemic malarial region were significant predictors of greater Hb response and heterogeneity. Projections suggested that between 38 % and 62 % of baseline anaemia (Hb < 11 g/dl) was responsive to Fe supplementation alone among children under 6 years old; the corresponding range for malarial hyperendemic regions was 5–31 %. The pooled estimate (random effects model) from the current thirty-six cohorts for change in Hb with Fe and multiple micronutrients v. placebo was 0·65 g/dl (95 % CI 0·50, 0·80, P < 0·001). Lower baseline Hb level and non-hyperendemic malarial region were significant predictors of greater Hb response, whereas ‘other micronutrients’ were a significant predictor of lower Hb increment on meta-regression analysis. From these two analyses it may be postulated that, compared with placebo, the effect size of the change in Hb concentration is not likely to alter much with the addition of multiple micronutrients to Fe supplementation. This postulation is strengthened by the second analysis, which showed that the difference in Hb levels in groups receiving Fe or Fe and multiple micronutrients was statistically significant but small (0·14 g/dl, 95 % CI 0·00, 0·28, P = 0·04). These analyses suggest that addition of multiple micronutrients to Fe supplementation may only marginally improve the Hb response in comparison to Fe supplementation alone.
The basic assumption behind giving many micronutrients as a supplement is that nutrients either act synergistically or in isolation. However, the interaction of many of these micronutrients is still poorly understood and under investigation(Reference Zimmermann, Biebinger, Rohner, Dip, Zeder, Hurrel and Chauki53, Reference Lind, Lonnerdal, Stenlund, Ismail, Seswandhana, Ekstrom and Persson54). The possibility of negative and unpredictable interactions with co-administration of several micronutrients is, therefore, real. One noteworthy finding is the possibility of a decremental Hb response with the addition of ‘other micronutrients’ in the first analysis. This supports the fact that micronutrients are often added to supplements without understanding of the possible complex interactions that may lead to a poorer Hb response than Fe supplementation alone and strengthens the case for a careful and judicious selection of micronutrients to be added for such purpose in health programmes.
Frequently scientists and public health professionals state that deficiencies of various micronutrients like Fe, vitamin A, Zn and I occur simultaneously in the developing countries. Multiple-micronutrient supplementation is therefore considered a cost-effective panacea for this problem(Reference Alnwick55). However, the contribution of ecological factors to multiple deficiencies has been ignored. For instance, seasonality limits the availability of year-round access to micronutrient-rich foods in developing countries(Reference Latham56). Similarly, poverty is an important factor that limits the access to and choice of food, leading to various deficiencies and chronic malnutrition(Reference Baker, Schootman, Barnidge and Kelly57). A lower rise in Hb with Fe and multiple micronutrients in children with lower height-for-age Z scores, an indicator of chronic undernutrition, can be considered as a stimulus to generate more information on the role of food insecurity and chronic deprivation in the high prevalence of anaemia.
Parasitic infections or infestations also contribute to anaemia and are unlikely to respond to micronutrient supplementation. Malaria is an important cause of anaemia(Reference Fleming and Werblinska58). The lower Hb response to Fe and micronutrient supplementation in malaria hyperendemic areas (−0·15 g/dl on multivariable meta-regression analysis) confirms that these micronutrients alone cannot address the problem of anaemia in such settings. Evidence thus supports the current recommendations, which stress integrated strategies to control Fe deficiency and malaria where these conditions coexist(59). Similarly, worm infestations lead to blood loss, reduced appetite and poor absorption of nutrients. Therefore, deworming along with haematinic supplementation has been suggested as an effective means to control anaemia(Reference Ahluwalia60, Reference Gulani, Nagpal, Osmond and Sachdev61).
In conclusion, synthesized evidence alone indicates that a judicious addition of multiple micronutrients to Fe supplementation alone is not likely to impair the Hb response to Fe supplementation in children and may even have marginal benefits. However, there is a suggestion that the addition of micronutrients other than Zn, vitamin A, riboflavin, B12, folic acid and ascorbic acid may have a negative effect on Hb response. Routine addition of unselected multiple micronutrients to Fe therefore appears unjustified for nutritional anaemia control programmes.
Acknowledgements
Contributors: T.G. prepared the protocol, applied the search strategy, performed the retrieval of articles, and extracted the data from the included studies. H.P.S.S. and P.N. developed the idea for the review, finalized the protocol and search strategy. H.P.S.S. performed the statistical analysis. All authors contributed to the drafting of the final version of the paper. H.P.S.S. and T.G. are the guarantors.
Funding: The work was funded by the United States Agency for International Development through its cooperative agreement (no. HRN-A-00-98-00027-00) with the Human Nutrition Institute of the International Life Sciences Institute (ILSI) Research Foundation. The funding source had no influence on the study design, analysis and interpretation, and the decision to submit for publication.
Conflict of interest: H.P.S.S. was an honorary member of the International Nutritional Anaemia Consultative Group (INACG) Steering Committee that was managed by ILSI. T.G. was supported by ILSI for travel to Hanoi (Vietnam), Marrakech (Morocco) and Lima (Peru) for presenting research work at the annual INACG symposiums. P.N. was a consultant to ILSI.