Introduction
The genus Calicium Ach. in Caliciaceae Chevall. includes mazaediate fungi (Prieto et al. Reference Prieto, Baloch, Tehler and Wedin2013) with stalked ascomata and mostly 1-septate, dark, thick-walled spores with distinctive surface ornamentation. A recent molecular study supports a wider circumscription of the genus to also include some species with sessile ascomata that were previously placed in Cyphelium Ach. (Prieto & Wedin Reference Prieto and Wedin2017). However, Cyphelium sessile (Pers.) Trevis. and C. marcianum B. de Lesd., which are lichenicolous on species of Pertusaria and Lepra, were transferred to Acolium (Prieto & Wedin Reference Prieto and Wedin2017).
Regional revisions of Calicium species in Australasia (Tibell Reference Tibell1987), Europe (Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999a), North America (Selva et al. Reference Selva, Tibell, Gordon and McMullin2023a, Reference Selva, McMullin, Bell-Doyon, Henderson and Layb), South America (Tibell Reference Tibell1996, Reference Tibell1998) and the Himalayas (Tibell Reference Tibell2006) have contributed descriptions of numerous species, and Calicium has thus been rather well investigated on a global scale. With the new circumscription and the recent description of additional species, it is known to include 36 species (Tibell & Knutsson Reference Tibell and Knutsson2016; Prieto & Wedin Reference Prieto and Wedin2017; Selva et al. Reference Selva, Tibell, Gordon and McMullin2023a). Among these is the recently described Calicium episcalaris Tibell & Knutsson, parasitic on Hypocenomyce scalaris (Ach. ex Lilj.) M. Choisy (Tibell & Knutsson Reference Tibell and Knutsson2016; Selva et al. Reference Selva, McMullin, Bell-Doyon, Henderson and Lay2023b). All other described species of Calicium have a distinct, autonomous thallus that varies from thick and verrucose in some species to immersed in others.
Three of the authors were therefore surprised to encounter, independently of each other, what looked like a perfectly ‘classical’ small Calicium growing on the thallus of Ramboldia elabens (Fr.) Kantvilas & Elix in boreal and hemiboreal areas of northern Europe and north-eastern North America. The first collection was made by one of us (LT) in 1977 in Sweden. Since then, we have found the species at additional localities in Sweden, Norway and Canada, and we have also detected several other specimens in herbaria.
Material and Methods
Microscopy
Squash preparations and hand-cut sections of apothecia were mounted in water, 10% KOH (K), 1.5% Lugol's solution (I), and lactophenol cotton blue (LCB), and examined with light microscopy (LM). Measurements of ascospores and anatomical characters were made in water and are reported as (minimum–)a–b(–maximum) (n = number of measurements), where ‘a’ is the arithmetic mean minus 1 standard deviation (SD) and ‘b’ is the arithmetic mean plus 1 SD. Apothecial dimensions were measured in air-dried specimens examined with a stereomicroscope. For scanning electron microscopy (SEM), air-dried specimens were mounted on stubs, coated with gold in an Edwards S150A sputter coater, and examined at 15 kV in a JEOL2 JSM 6400 instrument at the University of New Brunswick.
Taxon sampling
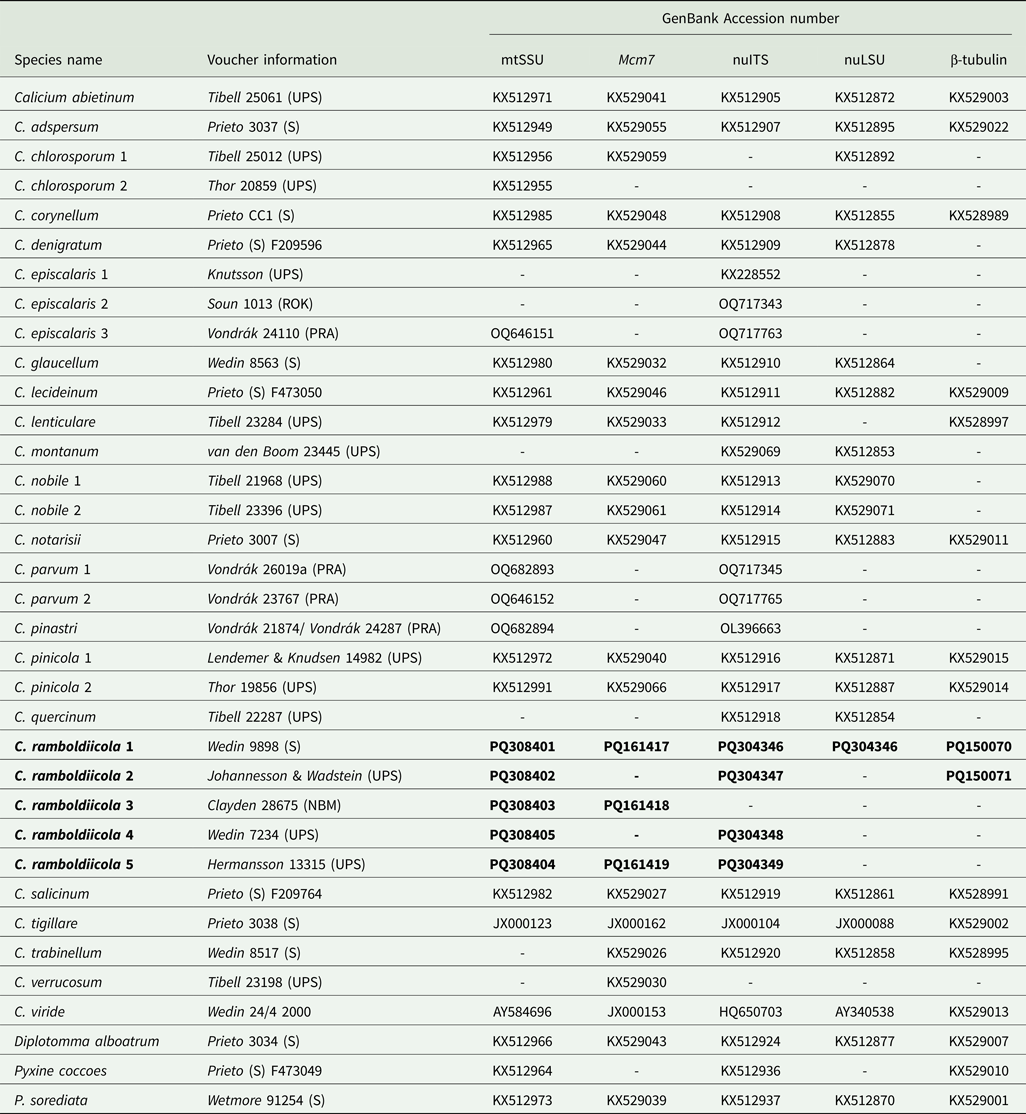
Five specimens of the new species and a wide selection of species of the genus Calicium were included in the phylogenetic analysis (Table 1). Based on previous studies (Prieto & Wedin Reference Prieto and Wedin2017), three members of the Caliciaceae (Diplotomma alboatrum (Hoffm.) Flot., Pyxine cocoes (Sw.) Nyl. and Pyxine sorediata (Ach.) Mont.) were used as outgroups.
Table 1. Details on taxa used in the phylogenetic analyses, including voucher information and GenBank Accession numbers. New species and sequences are in bold.
Molecular techniques
Sequences of Calicium were either downloaded from GenBank or produced in the laboratory for the new species. DNA was extracted using the DNeasy Plant Mini Kit (Qiagen), according to the manufacturer's instructions. Five regions were amplified: nuITS, nuLSU, mtSSU, β-tubulin and Mcm7. The nuITS was amplified with the primers ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990), and the nuLSU with LR0R (Rehner & Samuels Reference Rehner and Samuels1994), LR3, LR3R, LR6 and LR7 (Vilgalys & Hester Reference Vilgalys and Hester1990). We used the primers mtSSU1 and mtSSU3R for amplification of the mtSSU region (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999), Mcm7-709for and Mcm7-1348rev (Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelsen, Nelson, Rivas-Plata and Shimp2009) for amplification of the Mcm7 region and Bt3-LM and Bt10-LM for the protein coding β-tubulin (Myllys et al. Reference Myllys, Lohtander and Tehler2001).
PCR amplifications were carried out using Illustra™ Hot Start Mix RTG PCR beads (GE Healthcare, UK) in 25 μl, adding 3–6 μl of diluted genomic DNA, 10 μM of each primer and distilled water. The following amplification programme was used: initial denaturation at 95 °C for 15 min, 35 cycles of 95 °C for 45 s, 54–56 °C for 50 s, 72 °C for 1 min, and a final extension at 72 °C for 5 min. PCR products were purified with ExoSAP-IT (USB Corporation, Santa Clara, California, USA) and sequenced using the same amplification primers.
Sequences were assembled and edited using Sequencher v. 4.10.1. (Genes Codes Corporation, Ann Arbor, Michigan, USA) and subsequently aligned using MacClade v. 4.01 (Maddison & Maddison Reference Maddison and Maddison2001). Ambiguous regions (sensu Lutzoni et al. Reference Lutzoni, Wagner, Reeb and Zoller2000) and introns were delimited manually and excluded from phylogenetic analyses.
Phylogenetic analyses
Each individual gene region was analyzed using maximum likelihood (ML) implemented in RAxML v. 8.2.12 (Stamatakis Reference Stamatakis2014) with a GTRGAMMA model for tree inference and bootstrapping with a GTRCAT model and 1000 replicates. Gene-tree incongruence was checked by comparing maximum likelihood bootstrap values (ML-BS) between the individual gene trees. After checking there was no conflict among clades, data were combined into a single concatenated data matrix. The combined maximum likelihood (ML) analysis was run with seven distinct partitions (nuITS, nuLSU, mtSSU, first and second codon positions of the Mcm7 and β-tubulin and the third codon position of the Mcm7 and β-tubulin) as individual analyses. All analyses were run on the CIPRES Science Gateway v. 3.3 (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). The resulting trees were visualized and edited in Figtree v. 1.4.4. (Rambaut Reference Rambaut2010).
Results
The concatenated data matrix consisted of 25 taxa, 22 being Calicium species, and three outgroups. Calicium was represented by 109 sequences, of which 15 are new. In the analysis (Fig. 1), Calicium was strongly supported, and within the genus two major clades are apparent. Calicium ramboldiicola, together with C. abietinum and C. verrucosum Tibell, belongs to a strongly supported subclade. The other lichenicolous Calicium species, C. episcalaris, belongs to the same major clade as C. ramboldiicola.

Figure 1. Phylogeny of Calicium using a combination of five loci (nuITS, nuLSU, mtSSU, Mcm7 and β-tubulin) resulting from maximum likelihood (ML) in RAxML, with bootstrap values shown above nodes. Strongly supported branches (ML ≥ 70) and C. ramboldiicola are shown in bold.
Taxonomy
Calicium ramboldiicola Tibell, S. R. Clayden & Wedin
MycoBank No.: MB 856766
Resembling Calicium abietinum but occurring on Ramboldia elabens, lacking a distinct thallus, and with smaller apothecia, shorter asci, and ascospores with more coarsely and irregularly cracked ornamentation.
Type: Sweden, Lule Lappmark, Jokkmokk parish, River Pärlälven, W of Lake Nårvejaure, valley of the brook Laddonbäcken, on decorticated fallen Pinus, 66°36ʹ22ʺN, 19°16ʹ54ʺE, alt. c. 360 m, 2021, Wedin 9898 (S—holotype; CANL—isotype). The type has a full representation of all five DNA regions used in this study, and was chosen for this reason.
(Fig. 2)

Figure 2. Calicium ramboldiicola. A, apothecia arising from thallus and apothecia of Ramboldia elabens (Clayden 21343). B, longitudinal section of mature apothecium, LM (Clayden 21343). C, immature and mature apothecia, arising from thin to endoxylic area of thallus of R. elabens, SEM (Clayden 24619). D, mature ascospores, liberated from asci, LM (Clayden 28675). E, capitulum in oblique view, with wall rupturing apically and exposing the mazaedium, SEM (Clayden 24619). F, mature ascospore with surface ornamentation formed by irregular cracking, SEM (Clayden 21343). G, mature ascospore constricted at septum and with coarsely ornamented surface, SEM (Clayden 21343). LM = light microscopy; SEM = scanning electron microscopy. Scales: A = 1 mm; B & C = 100 μm; D = 10 μm; E = 50 μm; F & G = 5 μm. In colour online.
Thallus not evident, immersed in the thallus of Ramboldia elabens, not causing obvious necrosis or discoloration of the host lichen.
Apothecia on the areoles and rarely the apothecia of R. elabens, stalked to nearly sessile, with a black capitulum and dark olive-brown stalk, shiny except for the black mazaedium, lacking pruina, (0.25–)0.30–0.44(–0.55) mm tall (n = 30), all parts K−, I−. Stalk 0.08–0.20 mm diam., consisting of densely interwoven thick-walled hyphae with lumina 0.7‒2 μm wide, central part dark olive-brown, grading to pale brown to hyaline in outer part, with an outermost clear gelatinous layer 5–10 μm wide. Capitulum initially globose to obovoid, becoming flat-topped-cupulate, (0.15–)0.17–0.27(–0.35) mm diam. (n = 30). Excipulum to c. 50 μm thick at base, narrowing to c. 20 μm laterally, structure continuous with that of the stalk, of ±isodiametric to somewhat elongated cells with strongly thickened walls, inner part dark olive-brown, outermost hyaline gelatinous layer thinner than on the stalk. Hypothecium c. 30–60 μm tall, slightly concave at top, upper part concolorous with inner part of exciple and stalk, lower part hyaline to very pale brown. Asci cylindrical to narrowly clavate, (34–)35.8–41.8(–43) × (4–)4.2–5.4(–6) μm (n = 10), with uniseriate but somewhat irregularly orientated spores. Ascospores initially greenish grey, soon becoming dark brown, ellipsoid, 1-septate, constricted or not at septum, surface initially smooth to finely roughened, developing coarse irregular cracks, (9.5–)10.8–13.9(–16.5) × (5–)5.7–7.2(–8) μm (n = 73).
Etymology
Named after the genus of the host lichen, Ramboldia elabens.
Distribution
Calicium ramboldiicola is currently known from Norway and Sweden in northern Europe, and from the eastern Canadian provinces of New Brunswick and Québec in north-eastern North America. The occurrences in Canada are in intermediate oceanic–continental and continental (OC and C1) sectors of the hemiboreal bioclimatic zone (sensu Tuhkanen Reference Tuhkanen1984; Clayden Reference Clayden, McAlpine and Smith2010). In Scandinavia, C. ramboldiicola has a more distinctly boreal distribution. To date, it has been found at eight localities, spanning c. 10° of latitude, from the southern to northern sub-zones of the boreal zone and their elevational counterparts as defined by Ahti et al. (Reference Ahti, Hämet-Ahti and Jalas1968). These occurrences are in areas with intermediate oceanic–continental (OC, O1) climates, not the more highly oceanic sectors (O2, O3) of westernmost Scandinavia.
Further study is needed to determine whether the distribution of C. ramboldiicola conforms more fully to that of its host lichen. Ramboldia elabens occurs widely in boreal and temperate-montane areas of the Northern Hemisphere (Kantvilas & Elix Reference Kantvilas and Elix2007). In North America, it has a mainly Appalachian–Great Lakes distribution, with outliers as far west as the Black Hills of South Dakota (Wetmore Reference Wetmore1967). Reports of R. elabens from areas of North America west of the Black Hills are based on misidentifications of other lichens (Pérez-Ortega et al. Reference Pérez-Ortega, Spribille, Palice, Elix and Printzen2010). In Eurasia, it occurs discontinuously from Scandinavia and subalpine western and central Europe (Hafellner Reference Hafellner1993) to Japan (Inoue Reference Inoue1982) and the Russian Far East (e.g. Konoreva et al. Reference Konoreva, Tchabanenko, Ezhkin, Schumm and Chesnokov2018).
Ecology
Calicium ramboldiicola has been found exclusively on the crustose lichen Ramboldia elabens growing on hard exposed wood (‘lignum’) of conifers, including standing snags and fallen trees. Ramboldia elabens also occurs less frequently on hard, weathered wood of built structures such as old barns or fences, but no occurrences of C. ramboldiicola are known on host thalli occupying such substrata. In Scandinavia, all samples are from very old Pinus sylvestris L. snags in old-growth forest localities. In New Brunswick, the habitat at one of the localities is an open, south-west-facing, granitic boulder talus slope with patches of Picea rubens Sarg. and Pinus strobus L. At the other, the habitat is an open, wet, ombrotrophic bog, with scattered low thickets of Larix laricina (Du Roi) K. Koch and Picea mariana (Mill.) Britton et al. Associated lignicolous lichens at one or both localities in New Brunswick include Imshaugia aleurites (Ach.) S. L. F. Mey., Mycoblastus alpinus (Fr.) Kernst., M. sanguinarius (L.) Norman, Ochrolechia mahluensis Räsänen, O. pseudopallescens Brodo, Pertusaria sulcata Dibben, Xylographa disseminata Willey, and X. vitiligo (Ach.) J. R. Laundon.
Although the thallus of R. elabens is often moderately thick, with contiguous, somewhat lumpy areoles contrasting with the black, shiny apothecia, it can also be thin and even to mostly endoxylic towards the thallus margin or more extensively. Apothecia of Calicium ramboldiicola are present mainly on well-developed areoles of R. elabens (Fig. 2A), but also on more or less endoxylic portions of some thalli (Fig. 2C), and less commonly on the host apothecia. Because of the small amount or age of the material available for study, we have not examined areoles histologically to assess whether the hyphae of C. ramboldiicola are distinguishable from those of R. elabens, or whether there are differences in the abundance or appearance of photobiont cells in areoles of R. elabens with or without apothecia of C. ramboldiicola. Older apothecia of R. elabens sometimes develop uneven or wrinkled discs, but these changes occur in thalli with or without C. ramboldiicola. Apothecia of C. ramboldiicola are sporadically present on eroded or senescent areoles of the host lichen, but these occurrences may be accidental. That is, the deterioration of such areoles could have been caused by agents other than the Calicium.
Additional specimens examined
Canada: New Brunswick: Kings Co., Canadian Forces Base Gagetown, Nerepis Hills, 1 km NE of confluence of Askwith Brook with Nerepis River, 45.442°N, 66.298°W, alt. 140 m, on decorticated snag of Picea rubens, 2010, Clayden 21343 (NBM); ibid., 2022, Clayden 28675 (NBM); Sunbury Co., Bull Pasture Bog Protected Natural Area, 46.0524°N, 66.3113°W, alt. 100 m, lignicolous on snag of Larix laricina, 2014, Clayden 24619 (NBM). Québec: Parc de la Vérendrye, Réservoir Cabonga, Îles Bruyères, 47°14ʹ30ʺN, 76°34ʹ30ʺW, sur un vieux tronc de conifère en bordure du réservoir, 1991, Bastien 789 (QFA).—Norway: Troms: Storfjord, 13.5 km SSE of Skibotn, along Skibotnelva, above Gustavsvingen, 69°16ʹN, 20°28ʹE, alt. 120–200 m, 2003, Wedin 7234 (UPS). Buskerud: Nore of Uvdal, Viken, Godfarfoss S, 60.4312°N, 8.605°E, 2021, Reiso (O L-2289081).—Sweden: Dalarna: Los parish, Gönhammaren, on dry, still standing Pinus, Forsslund (UPS); Ore parish, Jässelåsen, Rärmyren, 61°14ʹN, 15°16ʹE, alt. 370 m, on dry, still standing Pinus snags, 2003, Hermansson 13315 (UPS); Svärdsjö parish, E of Orrtjärnen, on dry snag in a bog, 2024, Skog (S). Jämtland: Undersåker parish c. 15.5 km S of Undersåker church, south side of Lake Håckrenmagasinet, c. 570 m E of Furubergskojan, 63.19080°N, 13.42355°E, 2020, Johannesson & Wadstein (UPS). Lule Lappmark: Gällivare parish, Linaåive Nature Reserve, E of Parajaure, 67°16ʹN, 20°26ʹE, alt. 490 m, on decorticated branches of fallen, dead Pinus, 1977, Tibell 6916 (UPS); ibid., Jokkmokk par., Karats area, SW of Mt Luspevarasj, 66.62713°N, 18.81745°E, elev. 440 m, 2021, Westberg SCNB150 (UPS).
Discussion
Fungi with stalked ascomata resembling those of Calicium are a heterogeneous group from a phylogenetic standpoint, but they are often studied together and thus form a defined research area (Temu et al. Reference Temu, Tibell, Tibuhwa and Tibell2024). Although lichenicolous species occur in many calicioid genera, especially Chaenothecopsis and Sphinctrina (both in the Mycocaliciales; Tibell & Wedin Reference Tibell and Wedin2000), Calicium ramboldiicola is one of rather few lichenicolous species discovered in Caliciaceae. In this family, Acolium marcianum (B. de Lesd.) M. Prieto & Wedin and A. sessile (Pers.) Arnold are associated with Pertusaria and Lepra species (Pertusariaceae and Variolariaceae, respectively; both Pertusariales), whereas the species described here is found with Ramboldia (Ramboldiaceae, Lecanorales; Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014). This is only the second Calicium species known to occur on Lecanoromycetes, the other being Calicium episcalaris, on Hypocenomyce (Ophioparmaceae, Umbilicariales). Other genera of Caliciaceae including small numbers of lichenicolous species are Diplotomma and Tetramelas (Cannon et al. Reference Cannon, Prieto, Coppins, Sanderson, Scheidegger and Simkin2021).
The ascomata of C. ramboldiicola are small but otherwise similar to those of other species in Calicium, such as C. glaucellum. In our analysis (Fig. 1), C. ramboldiicola is sister to C. abietinum, and indeed resembles a miniature of that species, with much smaller ascomata but different spore ornamentation: C. ramboldiicola has an irregularly cracked spore surface, while that of C. abietinum presents with minute, distinct warts. The clade including C. abietinum, C. ramboldiicola and C. verrucosum has high support (Fig. 1), but apart from a general similarity in ascoma morphology, we have not been able to find any specific morphological synapomorphies.
Calicium ramboldiicola also resembles C. pinastri in its small size, lack of pruina, short cylindrical asci, and spore ornamentation. However, the apothecial stalks in C. pinastri are black, lacking the brown hue that is usually present in C. ramboldiicola. Also, C. pinastri is a mainly corticolous species, in Europe occurring especially on trunks of Pinus sylvestris (Tibell Reference Tibell, Ahti, Jørgensen, Kristinsson, Moberg, Søchting and Thor1999a, Reference Tibellb). Our phylogenetic analysis (Fig. 1) indicates that these two species are not closely related to one another within Calicium.
Ramboldia elabens is currently red-listed in Sweden and categorized as near-threatened (SLU Artdatabanken 2020). As C. ramboldiicola is known only from this host, it makes the new lichenicolous species extremely rare per se. Similar to R. elabens, it probably occurs only in localities of very high conservation value. Thus, it should be given consideration as a threatened species and probably placed in a higher category than its host.
Acknowledgements
SRC is grateful to Steven Selva for his encouragement of the studies reported here, and to Claude Roy and Philip Bell-Doyon for their generous help at the Herbier Louis-Marie (QFA), Université Laval. Permission to enter and make collections in CFB Gagetown, New Brunswick (NB), Canada, was arranged by Deanna McCullum, Range Biologist with the Canadian Department of National Defence. Collecting in Bull Pasture Bog PNA, NB, was carried out through the BiotaNB program of the New Brunswick Museum, with the support of the NB Wildlife Trust Fund and a research permit from the NB Department of Natural Resources and Energy Development. SRC also thanks Roger Smith of Fredericton, NB, for macrophotography and preparation of Fig. 2, and Nhu Trieu and Steven Cogswell for SEM work in the Microscopy and Microanalysis Facility at the University of New Brunswick. MW was supported by funds from the Swedish Royal Academy of Sciences (KVA). We thank the Molecular Systematic Laboratory (MSL) at the Swedish Museum of Natural History, in particular Bodil Cronholm, for skilful laboratory assistance. We are also indebted to Associate Editor, Paul Diederich, and to two anonymous reviewers for their helpful comments on the manuscript.
Author Contribution
LT made the initial discovery, conceived and directed the study, and prepared the original draft of the manuscript. SRC carried out field and morphological studies. MP performed the phylogenetic analyses. MW carried out field, laboratory and morphological studies. All four authors contributed to writing and revising the manuscript.
Author ORCIDs
Leif Tibell, 0000-0002-8629-7989; Stephen R. Clayden, 0000-0004-9031-2151; María Prieto, 0000-0002-1692-9821; Mats Wedin, 0000-0002-8295-5198.






