The metabolic syndrome is a pattern of metabolic risk factors occurring concurrently, which is also named syndrome X or the insulin resistance syndrome(Reference Kendall, Josse and Esfahani1). Its main features consist of insulin resistance, hypertriacylglycerolaemia, high blood pressure and high fasting blood glucose, which is the common background of diabetes mellitus, fatty liver disease(Reference Watanabe, Yaginuma and Ikejima2) and CVD(Reference Egert, Bosy-Westphal and Seiberl3). Recently, there have been some data confirming the relationship between the high-fat diet-induced metabolic syndrome and renal injuries such as macrophage infiltration, mesangial proliferation and extracellular matrix accumulation(Reference Korantzopoulos, Elisaf and Milionis4, Reference Kume, Uzu and Araki5).
Although the exact mechanisms that link high-fat diet and renal injuries have not yet been elucidated completely, some experimental and clinical studies have suggested a correlation between renal lipid accumulation, lipotoxicity and the progression of renal injury(Reference Spencer, Mühlfeld and Segerer6, Reference Proctor, Jiang and Iwahashi7). Jiang et al. (Reference Jiang, Wang and Proctor8) found that hyperlipidaemia and obesity induced by a high-fat diet could lead to increased renal lipid accumulation and glomerulosclerosis in C57BL/6J mice via a sterol regulatory element-binding protein-1c-dependent pathway. In wild-type mice, Kume et al. (Reference Kume, Uzu and Araki5) also revealed that a high-fat diet induced core features of the metabolic syndrome, renal lipid accumulation and renal injuries including glomerulosclerosis, interstitial fibrosis and albuminuria. Renal tubular injury is always considered as an important event in many renal diseases such as acute renal failure(Reference Li, Hsu and Wei9), diabetic nephropathy(Reference Jun, Song and Chen10) and IgA nephropathy(Reference Lotan, Sheinberg and Kopolovic11). Renal tubular lipid accumulation has been reported to play an important role in the pathogenesis of renal injury in the metabolic syndrome(Reference Kume, Uzu and Araki5). However, its exact mechanisms have not been fully elucidated.
Sterol regulatory element binding protein-1 (SREBP-1) plays a critical role in regulating fatty acid synthesis and has two types of pattern: precursor segment and mature segment. SREBP-1 precursor segment is cleaved by proteases to release N-terminal segments, their mature and active forms, which can be transported into the nucleus and then regulate fatty acid metabolism(Reference Sakai, Duncan and Rawson12). Fatty acid synthase (FASN) is a key multifunctional enzyme that plays a central role in the endogenous biogenesis of fatty acid. Based on the intracellular localisation of FASN, two kinds of this lipogenic enzyme are classically recognised: cytosolic FASN (FASN I) and mitochondrial FASN (FASN II). Whereas cytosolic FASN is mainly responsible for de novo fatty acid biogenesis, mitochondrial FASN provides the octanoyl precursor required for the essential lipoylation pathway. This cytosolic enzyme catalyses the formation of long-chain fatty acids from acetyl-CoA, malonyl-CoA and NADPH, which may further be modified by elongases or desaturases to form more complex fatty acids that may be used for the synthesis of various cellular lipids. On the other hand, it may also produce smaller amounts of myristate, laureate and even shorter-chain fatty acids(Reference Menendez, Vazquez-Martin and Ortega13).
The purpose of the present study was to confirm renal tubular lipid accumulation in the high-fat diet-induced metabolic syndrome and explore the exact mechanisms of renal tubular lipid accumulation. Therefore, we first treated Wistar rats with a high-fat diet for 3 months and investigated the effect of a high-fat diet on renal SREBP-1 expression, tubular lipid metabolism, growth factor secretion and tubular interstitial fibrosis. Furthermore, a human renal proximal tubular epithelial cell line (HKC) was chosen to detect SREBP-1, FASN, transforming growth factor-β1 (TGF-β1) and fibronectin expressions in medium containing insulin and/or high glucose in order to ascertain the involved mechanisms.
Materials and methods
Ethics statement
All animals were managed according to the guidelines of Hebei Medical University. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Hebei Medical University (approval ID: HebMU 20080026).
Animals
Male Wistar rats (n 20, weighing 120–140 g) were purchased from the Experimental Animal Center of Hebei Medical University and randomly divided into two groups: normal diet group and high-fat diet (HFD) group. The animals were housed on an alternating 12 h light and dark cycle with a room temperature of 20 ± 3°C. Components of the high-fat diet were fat (59·8 %), protein (20·1 %) and carbohydrates (20·1 %), and components of the normal diet were fat (10·3 %), protein (24·2 %) and carbohydrates (65·5 %). This kind of a high-fat diet has been confirmed to cause marked insulin resistance by Wang et al. (Reference Wang, Jiang and Ma14). During the period of 3 months, two rats fed with the high-fat diet were killed. At the end of the experiment, rats were anaesthetised with chloral hydrate and blood samples were obtained from the abdominal aorta. The renal cortex was removed, cleaned, washed and immediately preserved in buffered neutral formalin for histopathological examination, or frozen in liquid N2 for extraction of protein.
Cell culture and groups
HKC was a gift from Dr Chen Xiang-mei, Division of Nephropathy, 301 Hospital, Beijing, China. The HKC cell line was originally established by Racusen et al. (Reference Racusen, Monteil and Sgrignoli15) who isolated human renal tubular epithelial cells and exposed them in culture to a hybrid immortalising virus, adenovirus 12-SV40. The HKC cell line expresses biochemical properties and differentiation markers of renal epithelium including cytokeratin. HKC cells were grown as described previously(Reference Jun, Song and Chen10). (1) In order to examine the time-dependent effect of insulin on SREBP-1, FASN, TGF-β1 and fibronectin expressions, cells were treated with 100 nm-insulin and were randomly divided into six groups: 0, 2, 4, 6, 12 and 24 h group. (2) To investigate the concentration-dependent effect of insulin, cells were randomly divided into five groups: 0, 1, 10, 100 and 200 nm-insulin group. At 6 h after the stimulation, cells were collected for SREBP-1, FASN, TGF-β1 and fibronectin detection. (3) In the RNA interference experiment, cells were randomly divided into three groups: untransfection group, negative control plasmid group (pGenesil-1-HK) and specific short-hairpin RNA plasmid aimed at human SREBP-1 gene group (pGenesil-1-SREBP1-2). The pGenesil-1-SREBP1-2 plasmid was constructed and used in our previous study(Reference Jun, Song and Chen10). (4) To determine the synergistic effect of insulin and glucose, cells were divided into four groups and respectively cultured with different media: normal control group, insulin group (100 nm-insulin), high glucose group (30 mm-glucose) and insulin+high glucose group (100 nm-insulin and 30 mm-glucose). At 6 h after the stimulation, HKC cells were collected and the relevant detections were performed.
Plasmid transient transfection
Lipofectamine 2000 was used for transfection according to the manufacturer's instruction. HKC cells were cultured in six-well plates and the medium was changed the following day until 80 % confluence was achieved. The cells were transfected with 4·0 μg plasmid DNA by 10 μl Lipofectamine 2000 in 2 ml serum-free Dulbecco's modified Eagle's medium (DMEM). At 6 h after the transfection, the medium was replaced by normal DMEM medium with 10 % fetal bovine serum. At 42 h after the transfection, the medium was changed again with DMEM containing 10 % fetal bovine serum and 100 nm-insulin. Stimulated by insulin for 6 h, HKC cells were collected for the extraction of protein and total RNA. All experiments were repeated three times.
Quantitative TAG detection
The lipid extraction of renal tissue was performed by the Folch method(Reference Folch, Lees and Sloane Stanley16). The quantitative TAG detection kit was purchased from Dongou Biotechnology Company (Wenzhou, Zhejiang, China) and used for the detection of TAG content.
Oil Red O staining and Masson staining
Accumulation of neutral fats in renal tubular cells was detected by Oil Red O (Sigma, St Louis, MO, USA) staining. Accumulation of the extracellular matrix is the feature of renal tubulointerstitial fibrosis and was investigated by the method of Masson staining. The stained sections were imaged with an Olympus microscope and examined in a blinded manner by a renal pathologist.
Immunohistochemistry and immunofluorescence
HKC cells were planted on cover slides in six-well plates. Cells were fixed with 10 % formalin at room temperature for 15 min. After pre-treatment of 0·3 % Triton X-100 for 20 min at 37°C, cells were blocked with goat serum for 30 min at 37°C. Cells were incubated with a rabbit anti-fibronectin polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After washing with PBS for three times, slides were incubated with a fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody (1:200; Santa Cruz Biotechnology) for 2 h at 37°C. Then, the slides were observed after rinsing with PBS for three times. Immunohistochemistry was performed to investigate the expression of fibronectin in the kidney of rats as described previously(Reference Jun, Song and Chen10). Antigen recovery was done by the method of microwave and the negative control was performed by replacing the primary antibody with PBS buffer.
Total protein extraction and Western blot
Total protein extracts from animal tissues and cultured cells were performed as described previously(Reference Jun, Song and Chen10). Protein was separated on 7·5 % SDS-PAGE gel. Polyvinylidene fluoride membrane (Millipore Corporation, Bedford, MA, USA) was used for transfer and then blocked for 1 h at room temperature with 5 % bovine serum albumin in Tris-buffered saline containing 0·05 % Tween 20 (TBST). Subsequently, blots were washed and incubated, respectively, overnight at 4°C in TBST containing 1 % bovine serum albumin with a 1:500 dilution of rabbit anti-SREBP-1 antibody (Santa Cruz Biotechnology), rabbit anti-TGF-β1 antibody and mouse anti-β-actin antibody. The rabbit anti-SREBP-1 antibody can detect both the precursor segment and mature segment of SREBP-1 protein. Membranes were washed three times with TBST, incubated with a goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000 dilutions in TBST containing 1 % bovine serum albumin; Santa Cruz Biotechnology) for 120 min at room temperature and then washed three times with TBST. After the chemiluminescence reaction (Pierce, Rockford, IL, USA), bands were detected by exposing blots to X-ray films for the appropriate time period. For quantitative analysis, bands were detected and evaluated densitometrically with LabWorks software (UVP Laboratory Products, Upland, CA, USA), normalised for β-actin density.
Semi-quantitative RT-PCR
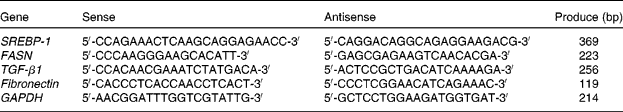
Total RNA was extracted from cultured cells with TRIzol reagent (Invitrogen Company, Carlsbad, CA, USA) and reverse-transcripted to complementary DNA using a Promega RT kit (Promega, Madison, WI, USA). Equal amounts of the product of the reverse transcription reaction were subjected to PCR amplification. The PCR amplification began with a 5 min denaturation at 95°C, followed by forty cycles of denaturation at 95°C for 45 s, annealing at 55°C for 45 s and extension at 72°C for 60 s. The final extension was set for 10 min at 72°C. After amplification, 5 μl of each PCR reaction product were electrophoresed on a 1·5 % (w/v) agarose gel containing ethidium bromide (0·5 μg/ml). The mRNA levels of SREBP-1, FASN, TGF-β1 and fibronectin were normalised with the glyceraldehyde-3-phosphate dehydrogenase mRNA level. All PCR primers were synthesised by Jierui Biotechnology (Shanghai, China). The sequences and the amplified lengths are shown in Table 1.
Table 1 Primers and produce for sterol regulatory element binding protein-1 (SREBP-1), fatty acid synthase (FASN), transforming growth factor-β1 (TGF-β1), fibronectin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
Statistical analysis
For all experiments, six individual experiments were performed in duplicate. All data are expressed as means and standard deviations and analysed using SPSS 11.0 for Windows. One-way ANOVA was used to determine statistically significant differences within and between groups, and means of every two different groups were detected with Student's t test. P < 0·05 was considered to be statistically significant.
Results
High-fat diet induced hyperinsulinaemia, hyperglycaemia and hypertriacylglycerolaemia in rats
After Wistar rats were fed with a high-fat diet for 3 months, body weight increased significantly. In addition, compared with rats fed a normal diet, serum glucose, TAG and insulin of rats fed a high-fat diet increased markedly (Table 2).
Table 2 Serum glucose, insulin and TAG in rats
(Mean values and standard deviations)
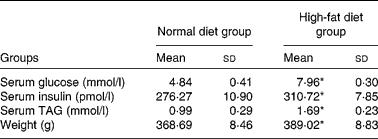
* Mean values were significantly different from those of the normal diet group (P < 0·05).
High-fat diet increased lipid droplet formation in renal tubular cells and renal tubulointerstitial extracellular matrix accumulation
The results showed that the concentration of TAG in the kidney of high-fat diet-fed rats was 6·90 (sd 0·22) g/l; however, that of normal control rats was 6·18 (sd 0·22) g/l. Compared with normal rats, high-fat diet-fed rats presented 1·12 times increase in TAG concentration. Oil Red O staining revealed that no clear lipid droplet was observed in the kidney of rats fed a normal diet; however, the renal tubular cells, not glomeruli, in rats fed a high-fat diet showed evident lipid deposits (Fig. 1(a) and (b)). The extracellular matrix was evaluated by the method of Masson staining and the results showed marked renal interstitial extracellular matrix protein accumulation in high-fat diet-treated rats in contrast to rats fed a normal diet (Fig. 1(c) and (d)). Fibronectin is a kind of important extracellular matrix protein and immunohistochemistry revealed that a high-fat diet significantly enhanced the expression of fibronectin in the renal proximal tubular cells of rats (Fig. 1(e) and (f)).

Fig. 1 Oil Red O staining, Masson staining and immunohistochemistry of fibronectin in the kidney of rats (400 × ). Oil Red O staining in the kidney of rats fed (a) a normal diet and (b) a high-fat diet. Images revealed the accumulation of neutral lipids in renal tubular cells of rats fed a high-fat diet. Representative photomicrographs of Masson-stained renal sections from rats fed (c) a normal diet and (d) a high-fat diet. Renal tubulointerstitium showed the accumulation of the extracellular matrix in high-fat diet-fed rats. Immunohistochemistry of fibronectin in the kidney of rats fed (e) a normal diet and (f) a high-fat diet.
High-fat diet up-regulated the expressions of sterol regulatory element binding protein-1 and transforming growth factor-β1 in the kidney of rats
Western blot revealed that a high-fat diet significantly increased renal expressions of the precursor segment and mature segment of SREBP-1 protein in comparison with rats fed a high-fat diet. TGF-β1 is an important cytokine for fibrosis that was detected by Western blot in order to reveal the mechanisms of extracellular matrix accumulation resulting from a high-fat diet. There was a 1·40 times increase in the kidney of high-fat diet-treated rats in contrast to rats fed a normal diet (Fig. 2).

Fig. 2 Sterol regulatory element binding protein-1 (SREBP-1) and transforming growth factor-β1 (TGF-β1) expressions were increased in the kidney of rats fed a high-fat diet. Cell lysates were subjected to SDS-PAGE and Western blot analysis of the expressions of SREBP-1 (both precursor segment (■) and mature segment (![]() )) and TGF-β1 (
)) and TGF-β1 (![]() ) protein in the kidney from rats fed a normal or high-fat diet. β-Actin staining revealed equivalent loading of total protein (rats fed a normal diet (N) and rats fed a high-fat diet (HFD)). SREBP-1 and TGF-β1 protein were quantitatively analysed using LabWorks software. Values are means of three separate experiments, with standard deviations represented by vertical bars. There was a significant increase in the precursor segment of SREBP-1, the mature segment of SREBP-1 and TGF-β1 protein in the renal cells of rats fed a high-fat diet compared with those fed a normal diet. * Mean values were significantly different from those of rats fed a normal diet (P < 0·05).
) protein in the kidney from rats fed a normal or high-fat diet. β-Actin staining revealed equivalent loading of total protein (rats fed a normal diet (N) and rats fed a high-fat diet (HFD)). SREBP-1 and TGF-β1 protein were quantitatively analysed using LabWorks software. Values are means of three separate experiments, with standard deviations represented by vertical bars. There was a significant increase in the precursor segment of SREBP-1, the mature segment of SREBP-1 and TGF-β1 protein in the renal cells of rats fed a high-fat diet compared with those fed a normal diet. * Mean values were significantly different from those of rats fed a normal diet (P < 0·05).
Time-dependent effect of insulin on sterol regulatory element binding protein-1, fatty acid synthase, transforming growth factor-β1 and fibronectin in vitro cultured human renal proximal tubular epithelial cell line cells
Time-kinetics experiment indicated that the maximum stimulatory effect of insulin occurred at 6 h. Enhanced mRNA levels of SREBP-1, FASN, TGF-β1 and fibronectin were demonstrated by the method of RT-PCR (Fig. 3). The expressions of SREBP-1 and TGF-β1 in protein level were determined by Western blot and the results showed that both the precursor segment and mature segment of SREBP-1 were higher in HKC cells after the 4, 6 and 12 h treatment with insulin than those in HKC cells after the 0, 2 and 24 h treatment with insulin, reaching the peak at 6 h (Fig. 4(a) and (b)). In addition, similar to the result of RT-PCR, TGF-β1 protein was also increased only in HKC cells after the 6 h treatment with insulin (Fig. 4(a) and (b)). Again, the result of Oil Red O staining revealed that lipid deposits were only seen in the 6 h group HKC cells and no lipid droplet was found in HKC cells of other groups (Fig. 4(c) and (d)).

Fig. 3 Time-dependent effect of insulin on sterol regulatory element binding protein-1 (SREBP-1, ■), fatty acid synthase (FASN, ![]() ), transforming growth factor-β1 (TGF-β1,
), transforming growth factor-β1 (TGF-β1, ![]() ) and fibronectin (
) and fibronectin (![]() ) mRNA expressions in human renal proximal tubular epithelial cell line (HKC) cells. Total RNA was isolated from the HKC cells using TRIzol, and complementary DNA was synthesised using reverse transcript reagents. All the data were calculated from triplicate reactions. HKC cells presented higher expressions of FASN mRNA at 4, 6, 12 and 24 h after the stimulation of 100 nm-insulin; however, in the 0 and 2 h groups, no difference was found. SREBP-1 mRNA expressions in HKC cells at 4, 6 and 12 h were stronger than at 0, 2 and 24 h, and there was no difference among the 0, 2 and 24 h groups. In addition, only the HKC cells at 6 h after the stimulation showed a significant up-regulation of TGF-β1 and fibronectin mRNA. * Mean values were significantly different from those of the 0 h group (P < 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
) mRNA expressions in human renal proximal tubular epithelial cell line (HKC) cells. Total RNA was isolated from the HKC cells using TRIzol, and complementary DNA was synthesised using reverse transcript reagents. All the data were calculated from triplicate reactions. HKC cells presented higher expressions of FASN mRNA at 4, 6, 12 and 24 h after the stimulation of 100 nm-insulin; however, in the 0 and 2 h groups, no difference was found. SREBP-1 mRNA expressions in HKC cells at 4, 6 and 12 h were stronger than at 0, 2 and 24 h, and there was no difference among the 0, 2 and 24 h groups. In addition, only the HKC cells at 6 h after the stimulation showed a significant up-regulation of TGF-β1 and fibronectin mRNA. * Mean values were significantly different from those of the 0 h group (P < 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.

Fig. 4 Time-dependent effect of insulin on sterol regulatory element binding protein-1 (SREBP-1) and transforming growth factor-β1 (TGF-β1) protein expressions. (a) Human renal proximal tubular epithelial cell line (HKC) cells were homogenised and total protein extracts were used for Western blotting as described in the ‘Materials and methods’ section. The blots revealed that the precursor segment (■) and mature segment (![]() ) of SREBP-1 were higher in HKC cells of the 4, 6 and 12 h groups than those in HKC cells of the 0, 2 and 24 h groups. TGF-β1 (
) of SREBP-1 were higher in HKC cells of the 4, 6 and 12 h groups than those in HKC cells of the 0, 2 and 24 h groups. TGF-β1 (![]() ) protein was increased only in HKC cells at 6 h after the stimulation of 100 nm-insulin. (b) The precursor segment and mature segment of SREBP-1 and TGF-β1 proteins were quantitatively analysed. * Mean values were significantly different from those of the 0 h group (P < 0·05). Oil Red O staining of HKC cells stimulated by 100 nm-insulin for different time (400 × ): (c) 0 h group and (d) 6 h group. The evident lipid droplets were only found in HKC cells treated by 100 nm-insulin for 6 h.
) protein was increased only in HKC cells at 6 h after the stimulation of 100 nm-insulin. (b) The precursor segment and mature segment of SREBP-1 and TGF-β1 proteins were quantitatively analysed. * Mean values were significantly different from those of the 0 h group (P < 0·05). Oil Red O staining of HKC cells stimulated by 100 nm-insulin for different time (400 × ): (c) 0 h group and (d) 6 h group. The evident lipid droplets were only found in HKC cells treated by 100 nm-insulin for 6 h.
Concentration-dependent effect of insulin on sterol regulatory element binding protein-1, fatty acid synthase, transforming growth factor-β1 and fibronectin in vitro cultured human renal proximal tubular epithelial cell line cells
HKC cells treated with insulin at concentrations of 10, 100 or 200 nmol/l showed a significant up-regulation of SREBP-1, FASN, TGF-β1 and fibronectin mRNA, presenting the peak in the 100 nm-insulin-treated cells (Fig. 5). SREBP-1 and TGF-β1 proteins were further investigated by the method of Western blot and similar results were revealed. Both the precursor segment and mature segment of SREBP-1 were significantly up-regulated in the cells treated with insulin at concentrations of 10, 100 or 200 nmol/l (Fig. 6(a) and (b)). As shown in Fig. 6(a) and (b), TGF-β1 protein was also increased by the addition of insulin and presented the strongest expression in cells treated with 100 nm-insulin. Oil Red O staining demonstrated that the markedly deposited lipid droplets were only observed in the 100 nm-insulin-treated HKC cells (Fig. 6(c) and (d)).

Fig. 5 Concentration-dependent effect of insulin on sterol regulatory element binding protein-1 (SREBP-1, ■), fatty acid synthase (FASN, ![]() ), transforming growth factor-β1 (TGF-β1,
), transforming growth factor-β1 (TGF-β1, ![]() ) and fibronectin (
) and fibronectin (![]() ) mRNA expressions in human renal proximal tubular epithelial cell line (HKC) cells. Total cellular RNA from HKC cells under the stimulation of different concentrations of insulin was subjected to RT-PCR analyses. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. Values are means of three separate experiments, with standard deviations represented by vertical bars. (1) 0 nm-insulin group; (2) 1 nm-insulin group; (3) 10 nm-insulin group; (4) 100 nm-insulin group; (5) 200 nm-insulin group. * Mean values were significantly different from those of the 0 nm-insulin group (P < 0·05).
) mRNA expressions in human renal proximal tubular epithelial cell line (HKC) cells. Total cellular RNA from HKC cells under the stimulation of different concentrations of insulin was subjected to RT-PCR analyses. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. Values are means of three separate experiments, with standard deviations represented by vertical bars. (1) 0 nm-insulin group; (2) 1 nm-insulin group; (3) 10 nm-insulin group; (4) 100 nm-insulin group; (5) 200 nm-insulin group. * Mean values were significantly different from those of the 0 nm-insulin group (P < 0·05).

Fig. 6 Concentration-dependent effect of insulin on sterol regulatory element binding protein-1 (SREBP-1; both precursor segment (■) and mature segment (![]() )), transforming growth factor-β1 (TGF-β1,
)), transforming growth factor-β1 (TGF-β1, ![]() ) protein expression and lipid droplet formation. (a, b) The lysates of human renal proximal tubular epithelial cell line (HKC) cells were subjected to SDS-PAGE and Western blot analysis using antibodies against SREBP-1 and TGF-β1. β-Actin staining of blots after transfer revealed equivalent loading of total protein. Values are means, with standard deviations represented by vertical bars. (1) 0 nm-insulin group; (2) 1 nm-insulin group; (3) 10 nm-insulin group; (4) 100 nm-insulin group; (5) 200 nm-insulin group. * Mean values were significantly different from those of the 0 nm-insulin group (P < 0·05). Oil Red O staining of HKC cells stimulated by insulin at different concentrations (400 × ): (c) 0 nm-insulin group and (d) 100 nm-insulin group.
) protein expression and lipid droplet formation. (a, b) The lysates of human renal proximal tubular epithelial cell line (HKC) cells were subjected to SDS-PAGE and Western blot analysis using antibodies against SREBP-1 and TGF-β1. β-Actin staining of blots after transfer revealed equivalent loading of total protein. Values are means, with standard deviations represented by vertical bars. (1) 0 nm-insulin group; (2) 1 nm-insulin group; (3) 10 nm-insulin group; (4) 100 nm-insulin group; (5) 200 nm-insulin group. * Mean values were significantly different from those of the 0 nm-insulin group (P < 0·05). Oil Red O staining of HKC cells stimulated by insulin at different concentrations (400 × ): (c) 0 nm-insulin group and (d) 100 nm-insulin group.
Specific RNA interference plasmid aimed at sterol regulatory element binding protein-1 lessened the effect of insulin on fatty acid synthase, transforming growth factor-β1 and fibronectin in human renal proximal tubular epithelial cell line cells
pGenesil-1-SREBP1-2, a short-hairpin RNA plasmid aimed at human SREBP-1 gene, was transfected into HKC cells that were then stimulated by 100 nm-insulin for 6 h. Western blot and RT-PCR were used for the detection of SREBP protein and mRNA and the results showed that pGenesil-1-SREBP1-2 plasmid exhibited 47·56 % inhibition of SREBP-1 mRNA up-regulated by insulin. FASN, TGF-β1 and fibronectin mRNA were further detected, and it was found that they were all suppressed by the transfection of pGenesil-1-SREBP1-2 (Fig. 7). As illustrated in Fig. 8(a)–(c), fibronectin protein was detected by immunofluorescence, and it could be significantly shown that the specific short-hairpin RNA plasmid, pGenesil-1-SREBP1-2, decreased fibronectin expression. Oil Red O staining revealed some red-stained lipid droplets in untransfected cells and pGenesil-1-HK-transfected cells. However, no clear lipid droplet can be seen in pGenesil-1-SREBP1-2-transfected cells (Fig. 8(d)–(f)).

Fig. 7 Specific pGenesil-1-SREBP1-2 plasmid decreased sterol regulatory element binding protein-1 (SREBP-1), fatty acid synthase (FASN), transforming growth factor-β1 (TGF-β1) and fibronectin expression in human renal proximal tubular epithelial cell line (HKC) cells. (a, b) The abundance of the precursor segment (■) and mature segment (![]() ) of SREBP-1 were measured by Western analysis and the results showed that transient transfection of HKC cells with recombinant short-hairpin RNA plasmids (pGenesil-1-SREBP1-2) significantly decreased SREBP-1 protein. These data were from a representative experiment. (c, d) Shown are the results of semi-quantitative RT-PCR. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Values are means of three determinations, with standard deviations represented by vertical bars. (1) Untransfection HKC cells; (2) negative control plasmid (pGenesil-1-HK)-transfected HKC cells; (3) pGenesil-1-SREBP1-2-transfected HKC cells. ■, SREBP-1;
) of SREBP-1 were measured by Western analysis and the results showed that transient transfection of HKC cells with recombinant short-hairpin RNA plasmids (pGenesil-1-SREBP1-2) significantly decreased SREBP-1 protein. These data were from a representative experiment. (c, d) Shown are the results of semi-quantitative RT-PCR. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Values are means of three determinations, with standard deviations represented by vertical bars. (1) Untransfection HKC cells; (2) negative control plasmid (pGenesil-1-HK)-transfected HKC cells; (3) pGenesil-1-SREBP1-2-transfected HKC cells. ■, SREBP-1; ![]() , FASN;
, FASN; ![]() , TGF-β1;
, TGF-β1; ![]() , fibronectin. * Mean values were significantly different from those of pGenesil-1-HK group HKC cells (P < 0·01).
, fibronectin. * Mean values were significantly different from those of pGenesil-1-HK group HKC cells (P < 0·01).

Fig. 8 Immunofluorescence of fibronectin and Oil Red O staining in human renal proximal tubular epithelial cell line (HKC) cells (400 × ). Immunofluorescence of fibronectin in HKC cells: (a) untransfection HKC cells; (b) negative control plasmid (pGenesil-1-HK)-transfected HKC cells; (c) specific short-hairpin RNA plasmid (pGenesil-1-SREBP1-2)-transfected HKC cells. Oil Red O staining: (d) untransfection HKC cells; (e) pGenesil-1-HK-transfected HKC cells; (f) pGenesil-1-SREBP1-2-transfected HKC cells. SREBP-1, sterol regulatory element binding protein-1.
Insulin and high glucose synergistically increased sterol regulatory element binding protein-1, fatty acid synthase, transforming growth factor-β1 and fibronectin in human renal proximal tubular epithelial cell line cells
HKC cells were cultured under the stimulation of 100 nm-insulin and/or 30 mm-glucose for 6 h to explore the synergistic effect. RT-PCR and Western blot revealed that SREBP-1 protein was significantly up-regulated in HKC cells that received a treatment of either a single factor (insulin or high glucose) or a combination of the two. Furthermore, it was seen that compared with HKC cells simply stimulated with insulin, expressions of SREBP-1, FASN, TGF-β1 and fibronectin mRNA were, respectively, increased by 1·690, 1·279, 1·770 and 1·407 times in HKC cells treated with insulin plus high glucose, followed by up-regulation of SREBP-1 and TGF-β1 protein (Fig. 9). As shown in Fig. 10, cells treated with insulin plus high glucose presented much more lipid droplets than those treated only with insulin.

Fig. 9 Insulin and high glucose synergistically increased sterol regulatory element binding protein-1 (SREBP-1), fatty acid synthase (FASN), transforming growth factor-β1 (TGF-β1) and fibronectin expression in human renal proximal tubular epithelial cell line (HKC) cells. (a, b) Total RNA was isolated using TRIzol from HKC cells and quantified for the indicated genes: (1) normal control group; (2) insulin group; (3) high glucose; (4) insulin plus high glucose group. ■, Precursor segment of SREBP-1; ![]() , mature segment of SREBP-1;
, mature segment of SREBP-1; ![]() , TGF-β1. (c, d) Cell lysates were subjected to SDS-PAGE and Western blot analysis using SREBP-1 and TGF-β1 antibodies. Western blots showed the precursor and mature segment of SREBP-1 and TGF-β1 protein levels for the different cultured conditions: (1) normal control group; (2) insulin group; (3) high glucose; (4) insulin plus high glucose group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. * Mean values were significantly different from those of the insulin group (P < 0·05). ■, SREBP-1;
, TGF-β1. (c, d) Cell lysates were subjected to SDS-PAGE and Western blot analysis using SREBP-1 and TGF-β1 antibodies. Western blots showed the precursor and mature segment of SREBP-1 and TGF-β1 protein levels for the different cultured conditions: (1) normal control group; (2) insulin group; (3) high glucose; (4) insulin plus high glucose group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. * Mean values were significantly different from those of the insulin group (P < 0·05). ■, SREBP-1; ![]() , FASN;
, FASN; ![]() , TGF-β1;
, TGF-β1; ![]() , fibronectin.
, fibronectin.

Fig. 10 Oil Red O staining in human renal proximal tubular epithelial cell line (HKC) cells treated with insulin plus high glucose (400 × ). Insulin and high glucose markedly increased lipid droplets formation in HKC cells. (a) Insulin-treated cells and (b) insulin plus high glucose-treated cells.
Discussion
A high-fat diet means a diet in which energy is derived mainly from fat, which has been suggested to contribute to a number of serious conditions. Many studies have reported that a high-fat diet could cause hepatic steatosis(Reference Hsu and Yen17), adipocyte hypertrophy and hyperplasia(Reference Moraes, Blondet and Birkenkamp-Demtroeder18), accumulation of intramyocellular lipids(Reference Dobbins, Szczepaniak and Bentley19) and insulin resistance, which is a physiological condition where the natural hormone, insulin, becomes less effective at lowering blood sugars. The resulting increase in blood glucose may raise levels outside the normal range and cause adverse health effects(Reference Afonso, Lautt and Schafer20). The present results showed that a high-fat diet induced systemic metabolic abnormalities such as obesity, hyperglycaemia, hyperinsulinaemia and hypertriacylglycerolaemia, which is consistent with other studies which found that a high-fat diet significantly induced hyperglycaemia, hyperinsulinaemia and high insulin resistance in comparison with the control group(Reference Bourgoin, Bachelard and Badeau21), together with renal tubular lipid accumulation and renal tubular interstitial extracellular matrix accumulation. Furthermore, we detected SREBP-1 expression and determined that a high-fat diet enhanced renal SREBP-1 protein in rats. The present study showed similar results to Jiang's study, which suggested that diet-induced obesity caused increased renal lipid accumulation and glomerulosclerosis in C57BL/6J mice via an SREBP-1c-dependent pathway(Reference Jiang, Wang and Proctor8). Therefore, the above observations provided evidence that a high-fat diet increased SREBP-1 protein in kidney that might contribute to the development of renal tubular lipid accumulation. TGF-β1 is a cytokine that can stimulate renal tubular cells and fibroblast cells to produce the extracellular matrix, furthermore promoting renal tubular cell injury, transformation and fibrosis(Reference Santoso and Rohman22). Recently, Kohli et al. (Reference Kohli, Kirby and Xanthakos23) revealed that histological hepatic fibrosis, collagen 1 mRNA, TGF-β1 mRNA and α-smooth muscle actin mRNA levels were significantly increased in mice fed a high-fat high-carbohydrate diet. A similar relationship between a high-fat diet and TGF-β1 up-regulation, as well as fibrosis has also been reported in heart(Reference Carroll and Tyagi24). Taken together, the present results suggested that a high-fat diet might induce renal tubulointerstitial extracellular matrix accumulation via enhancing the expression of TGF-β1 in renal cells. However, the exact factors implicated in high-fat diet-induced renal up-regulation of SREBP-1 and TGF-β1, as well as the direct correlation between SREBP-1, TGF-β1 expression and high-fat diet-induced renal tubular lipid and extracellular matrix metabolism abnormality both need to be fully elucidated.
Many previous studies have demonstrated that insulin had an important role in the regulation of lipid metabolism in liver(Reference Lara-Castro and Garvey25), adipose tissue(Reference van der Kallen, Voors-Pette and Bouwman26) and kidney(Reference Hoffler, Hobbie and Wilson27). To determine whether high serum insulin per se induced lipid accumulation in renal tubular cells, we incubated human proximal tubular cells with medium containing insulin. Consistent with previous studies revealing that insulin might stimulate lipid synthesis in the liver by selectively inducing transcription of the SREBP-1c gene(Reference Shimomura, Bashmakov and Ikemoto28, Reference Chen, Liang and Ou29), the present results also indicated that insulin per se could directly induce lipid accumulation in renal tubular cells through up-regulating the transcription factor SREBP-1 and FASN. In addition, the present data also supported the conclusion that insulin indeed induced extracellular matrix accumulation in renal tubular cells through up-regulating TGF-β1, which was similar to several previous studies demonstrating that insulin can also mediate extracellular matrix metabolism in rat mesangial cells(Reference Anderson, Zhang and Tian30), HepG2 cells(Reference Olsson, Egnell and Lee31) and airway smooth muscle cells(Reference Dekkers, Schaafsma and Tran32).
We next performed studies in HKC cells using RNA interference technique to determine whether increased TGF-β1 and fibronectin were also resulted from up-regulation of SREBP-1 in insulin-treated HKC cells. pGenesil-1-SREBP1-2 is a specific short-hairpin RNA plasmid that had already been used in our previous study and had significant inhibition of SREBP-1 expression. We found that pGenesil-1-SREBP1-2 plasmid effectively silenced insulin-induced up-regulation of SREBP-1 mRNA and protein simultaneously accompanied by attenuated FASN mRNA expression; interestingly, increased TGF-β1 and fibronectin in response to insulin were also prevented. Previous studies in renal mesangial and tubular cells have also shown that high glucose causes a marked up-regulation of SREBP-1, resulting in the increased expressions of TGF-β1 and fibronectin(Reference Jun, Song and Chen10, Reference Sun, Halaihel and Zhang33). Therefore, the above findings supported the conclusion that SREBP-1 could mediate TGF-β1 and fibronectin expressions in insulin-induced HKC cells and might simultaneously play an important role in regulating lipid metabolism and extracellular matrix accumulation.
The present results also revealed that HKC cells stimulated by insulin plus high glucose presented higher expressions of SREBP-1, FASN, TGF-β1 and fibronectin than HKC cells treated with high glucose or insulin alone. These were similar to O'Callaghan's(Reference O'Callaghan, Koo and Wu34) finding in rat primary cultured hepatocytes revealing that glucose and insulin synergistically activated the expression of acetyl-CoA carboxylase mRNA transcribed from the PI promoter. Several other studies have also demonstrated that the presence of elevated concentrations of both glucose and insulin was necessary to induce the expressions of genes involved in fatty acid synthesis in the liver, namely, the l-pyruvate kinase, FASN, acetyl-CoA carboxylase and S14 genes(Reference Dentin, Girard and Postic35). In contrast, the present results suggested that a high-fat diet caused increased serum insulin and glucose and then they synergistically resulted in increased SREBP-1 and TGF-β1, lipid accumulation and extracellular matrix produce in renal tubular cells. However, the glucose and lipid metabolism is a very complex network involved in many regulating factors including carbohydrate response element binding protein(Reference Dentin, Pégorier and Benhamed36), PPAR and liver X receptor(Reference Proctor, Jiang and Iwahashi7) besides SREBP-1. Again, the SREBP-1 gene gives rise to two different isoforms, SREBP-1c and SREBP-1a. In the present study, we can say that this general pathway is involved; however, we are not completely clear as to which pathway (SREBP-1c or SREBP-1a) is being affected. Therefore, to fully elucidate this network, much more fundamental study is needed.
Acknowledgements
The present study was supported by a grant from the Public Health Department of Hebei Province of China (no. 20090050). We thank Dr Chen Xiang-mei for HKC. None of the authors had a personal or financial conflict of interest. The authors' responsibilities were as follows: J. H. and H.-j. D. contributed to the conception and design of the study; J. H., S.-x. L., S. Z., Q.-j. L. and W. L. were involved in the analysis and interpretation of the data; J. H. and S.-x. L. contributed to writing of the manuscript.














