Three cholinesterase inhibitors are available in the UK, donepezil, galantamine and rivastigmine, and are licensed for the treatment of mild-to-moderate Alzheimer's disease. Cholinesterase treatment is a new approach in the management of dementia and old age psychiatry services have struggled to develop ways of offering the service. New drug budgets and staffing levels had to be established and rationing has been necessary. The Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and MchughFolstein et al, 1975) cut-off point of 12 has been suggested by the National Institute for Clinical Excellence (NICE, 2001) which states that ‘the drug should normally only be continued while their MMSE score remains above 12 points…. When the MMSE score falls below 12 points, patients should not normally be prescribed any of these drugs’. In many areas this has been implemented firmly through clinical governance and management structures. However, this cut-off point is not based on evidence, in fact the pivotal trials included patients whose MMSE was down to 10 (Reference Corey-Bloom, Anand and VeachCorey-Bloom et al, 1998; Reference Rogers, Farlow and DoodyRogers et al, 1998; Reference Tariot, Solomon and MorrisTariot et al, 2000) and more recent studies have included patients with MMSE scores less than 10 (Reference Tariot, Farlow and GrossbergTariot et al, 2004). The old adage ‘no evidence of benefit does not equate to evidence of no benefit’ may well apply to severe Alzheimer's disease. The results of our audit have raised such concern that we felt an obligation and duty of care to report the findings nationally.
Method
The Alzheimer's Medication Service (AMS) in North Dorset, described in detail elsewhere (Reference Beavis and SimpsonBeavis & Simpson, 2003), has 45 000 elderly people living in its catchment area. It offers a treatment monitoring service and currently has funding to treat an active case-load of 126 at any one time. All patients are kept on the case-load of our AMS. The routine assessment used by our service includes the MMSE as an estimate of global cognitive function, the Neuropsychiatry Inventory (Reference Cummings, Mega and GrayCummings et al, 1994) to assess psychological complications, a carer stress scale (Reference Zarit, Reever and Bach-PetersonZarit et al, 1980) and the Bristol-Activities of Daily Living scale (B-ADL; Reference Bucks, Ashworth and WilcockBucks et al, 1996). Three consultants prescribe and act as responsible medical officers for the cholinesterase inhibitors; a nurse specialist provides the clinical input and monitoring, with support from the community mental health teams.
This article describes an audit of 325 consecutive new referrals to the AMS between 2000 and 2004. This was a 12 week prospective audit of acute responses to treatment with cholinesterase inhibitors and a 12 week follow-up after stopping the cholinesterase inhibitors of those patients who had been on them long term but had been withdrawn from treatment on the basis of NICE guidance when the MMSE score fell below 12.
Results
At the time of the audit, 31 patients had been treated as new patients outside of the NICE criteria, and 294 within the NICE criteria. Over the 12 weeks of acute treatment there was no significant change in the MMSE score for patients treated within the NICE criteria. However, there was a mean improvement of 1.4 points on the MMSE for patients treated outside the NICE criteria. The Neuropsychiatric Inventory showed a mean improvement of 5.3 for those treated outside the NICE criteria compared with only 1.4 for those treated within the NICE criteria. The mean improvement in carer stress was 5.8 points for those outside the NICE criteria and 1.2 points for those within the NICE criteria. The B-ADL scale showed a mean improvement of 6.5 for those outside the NICE criteria and only 0.8 for those within the NICE criteria (Table 1).
Table 1. The effect of treatment both inside and outside of NICE guidance
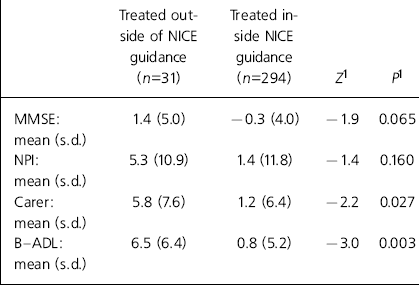
| Treated outside of NICE guidance (n=31) | Treated inside NICE guidance (n=294) | Z 1 | P 1 | |
|---|---|---|---|---|
| MMSE: mean (s.d.) | 1.4 (5.0) | -0.3 (4.0) | -1.9 | 0.065 |
| NPI: mean (s.d.) | 5.3 (10.9) | 1.4 (11.8) | -1.4 | 0.160 |
| Carer: mean (s.d.) | 5.8 (7.6) | 1.2 (6.4) | -2.2 | 0.027 |
| B—ADL: mean (s.d.) | 6.5 (6.4) | 0.8 (5.2) | -3.0 | 0.003 |
We also looked at the effect of stopping the medication when it was withdrawn on the basis of NICE guidance, i.e. the medication was withdrawn purely because of the stage of the dementia (n=25) rather than intercurrent illness or adherence issues. We followed all patients for 12 weeks after stopping the cholinesterase inhibitors and found that during this short period 17 (68%) had a poor outcome: 5 died and the remaining 12 experienced global deterioration. Overall, 7 (28%) patients displayed no change, with 1 (4%) displaying some level of improvement (Table 2).
Table 2. Outcome in 25 patients 12 weeks after stopping cholinesterase inhibitors when MMSE score <12

| Outcome | n | % |
|---|---|---|
| Died | 5 | 20 |
| Global deterioration | 12 | 48 |
| No change | 7 | 28 |
| Improved | 1 | 4 |
Discussion
This is not a randomised controlled trial. However, it is a well-conducted prospective naturalistic audit of 325 consecutive patients who are likely to be representative of those seen in a routine old age psychiatry service. Therefore, the clinical application of the audit is likely to apply to other old age psychiatry services that offer similar Alzheimer's medication services (Reference Beavis and SimpsonBeavis & Simpson, 2003). With these limitations and strengths, the results reveal two main findings. First, patients treated outside NICE criteria had a better acute response to cholinesterase inhibitors and second, we found it to be clinically unsatisfactory to stop the medication when the MMSE score reached 12 and would like to suggest that until further evidence is available this is very questionable clinical practice.
This was not a randomised controlled trial and so we cannot conclude that patients with severe dementia should be prioritised, but perhaps clinicians should be allowed to use their clinical judgement more freely in selecting patients for treatment. The original randomised controlled trials included patients with MMSE scores from 10 onwards and more recent trials have been successfully completed on more severe cases of dementia with good response (Reference Tariot, Farlow and GrossbergTariot et al, 2004). Carer strain and activities of daily living seem to improve the most. The patients treated within the NICE criteria were less disabled. There may be a floor effect in that behavioural complications and carer strain are not as prominent as they are in the later stages of the illness. In simple terms, there may be more scope for improvement in patients with severe dementia. NICE criteria are based on randomised controlled trials of patients who are not likely to be representative of patients referred to old age psychiatry services in day-to-day practice. The AD2000 study (Reference Courtney, Farrell and GrayCourtney et al, 2004) found only a modest treatment effect in patients who are consistently within the NICE criteria. In the present study, it is possible that there was an audit sampling bias. Perhaps the consultants were biased in selecting patients outside the NICE criteria on the basis of more prominent behavioural disturbance and the targeting of the most deserving patients and families in their catchment area. Certainly there was benefit, but this was not placebo-controlled. Clearly, more randomised controlled trials are needed to establish how we use cholinesterase inhibitors in severe dementia.
The second main finding was the very poor outcomes when the medicines were stopped because of a low MMSE score. From a qualitative point of view we found great difficulty in getting families to agree to stop the medication. Many described deteriorations, the nature of which did not become completely apparent until the audit findings were looked at more systematically. It is possible that prolonged use of cholinesterase inhibitors leads to changes in receptor activity (Reference Volpicelli-Daley, Hrabovska and DuysemVolpicelli-Daley et al, 2003), which results in a rebound deterioration when the treatment is withdrawn. For example, Kemp et al (Reference Kemp, Holmes and Hoffman2003) used single photon emission computed tomography (SPECT) scans with an acetylcholine ligand called QNB and showed that patients on long-term cholinesterase inhibitors have biological changes in post-synaptic muscarinic M1 receptors. Therefore, when cholinesterase inhibitors are stopped there may be receptor changes in addition to simple loss of cholinergic tone attributable to enzyme blockage. Furthermore, there is clinical evidence to suggest that this deterioration is permanent if the medication is not recommenced within 6 weeks of being stopped (Reference Doody, Geldmacher and GordonDoody et al, 2001).
Other factors might have influenced the death of those with severe dementia. These patients are likely to be the most psychiatrically and physically ill and as such would have had other factors contributing towards their death. Therefore, it is possible that the high death rate reflects a sampling basis. However, this seems less likely because we only audited the 25 outcomes where patients had stopped their medication purely because of NICE guidelines and not because of intercurrent physical illness or adherence issues. Overall, our audit estimates that 68% of patients have adverse outcomes after stopping the medication. This poor outcome included a noticeable mortality, when otherwise at this stage of the illness the life expectancy would be for approximately 2 years to death (Reference Feldman, Gracon and GauthierFeldman & Gracon, 1996) and not the short 12 weeks that passed during this audit. Certainly, we found that patients did much worse than we had expected on stopping the medication. Clearly, more randomised controlled trials are needed to establish the benefits and disadvantages of stopping cholinesterase inhibitors safely.
Conclusion
The results of our audit were at such variance with what we expected given the confidence of NICE's recommendations, that we felt a strong duty of care to report these findings nationally. The results emphasise the need for new research on cholinesterase inhibitors in the severe stage of dementia and on how to change and withdraw medication safely in these patients. More neurobiological research is needed to determine the long-term effects on transmitter sensitivities as a result of taking cholinesterase inhibitors.
Declaration of interest
S.S. runs the clinical trials unit in Dorset. However, the present study was not funded by or had any input from any drug company.





eLetters
No eLetters have been published for this article.