INTRODUCTION
The Nordic Seas, a collective geographic name usually referring to the Greenland, Iceland and Norwegian Seas (Drange et al., Reference Drange, Dokken, Furevik, Gerdes, Berger, Nesje, Orvik, Skagseth, Skjelvan, Østerhus, Drange, Dokken, Furevik, Gerdes and Berger2005; Korablev et al., Reference Korablev, Smirnov, Baranova, Seidov and Parsons2014), but here extended to also include the Barents Sea, constitute the main gateway where the relatively warm and saline North Atlantic Current enters the cold and subsaline waters of the Arctic Ocean (Hansen & Østerhus, Reference Hansen and Østerhus2000; Blindheim & Østerhus, Reference Blindheim, Østerhus, Drange, Dokken, Furevik, Gerdes and Berger2005) and, consequently, the Atlantic and Arctic biotas interchange (Loeng & Drinkwater, Reference Loeng and Drinkwater2007). Hence the biodiversity studies of the Nordic Seas are of great significance, especially in regard to a possible shutdown of thermohaline circulation. Furthermore, this region is characterized by a quite variable geomorphology with steep slopes and deep fjords along the coastline and seamounts, ridges and trenches in the offshore areas (Blindheim & Østerhus, Reference Blindheim, Østerhus, Drange, Dokken, Furevik, Gerdes and Berger2005). Due to such varying environments, the Nordic Seas host a highly diverse sessile invertebrate fauna (Shields & Hughes, Reference Shields and Hughes2009; Anisimova et al., Reference Anisimova, Jørgensen, Lyubin and Manushin2010; Schander et al., Reference Schander, Rapp, Kongsrud, Bakken, Berge, Cochrane, Oug, Byrkjedal, Cedhagen, Fosshagen, Gebruk, Larsen, Nygren, Obst, Plejel, Stöhr, Todt, Warén, Handler-Jacobsen, Kuening, Levin, Mikkelsen, Petersen, Thorseth and Pedersen2010; Kędra et al., Reference Kędra, Renaud, Andrade, Goszczko and Ambrose2013). One of the most prominent groups is the phylum Porifera, the sponges, which, in some areas, form dense aggregations known as ‘sponge grounds’ and play a crucial role in the functioning of bottom ecosystems (Klitgaard et al., Reference Klitgaard, Tendal, Westerberg, Hawkins, Hutchinson, Jensen, Sheader and Williams1997; Klitgaard & Tendal, Reference Klitgaard and Tendal2004; Cárdenas et al., Reference Cárdenas, Rapp, Klitgaard, Best, Thollesson and Tendal2013; Maldonado et al., Reference Maldonado, Aguilar, Bannister, Bell, Conway, Dayton, Diaz, Gutt, Kelly, Kenchington, Leys, Pomponi, Rapp, Rützler, Tendal, Vacelet, Young, Rossi, Bramanti, Gori and Orejas2016). The Nordic sponge fauna was extensively studied during more than 200 years, starting with the classical survey by Müller (Reference Müller1806) and followed by Schmidt (Reference Schmidt1870, Reference Schmidt1875), Sars (Reference Sars1872), von Marenzeller (Reference von Marenzeller1878), Sollas (Reference Sollas1882), Vosmaer (Reference Vosmaer1882, Reference Vosmaer1885), Hansen (Reference Hansen1885), Fristedt (Reference Fristedt1887), Arnesen (Reference Arnesen1900, Reference Arnesen1903, Reference Arnesen1920), Lundbeck (Reference Lundbeck1902, Reference Lundbeck1905, Reference Lundbeck, Gerlache de Gomery and Orléans1907, Reference Lundbeck1909, Reference Lundbeck1910), Breitfuss (Reference Breitfuss1911, Reference Breitfuss1912, Reference Breitfuss1930), Topsent (Reference Topsent1913), Rezvoj (Reference Rezvoj1924, Reference Rezvoj1928), Brøndsted (Reference Brøndsted1914, Reference Brøndsted, Jensen, Lundbeck and Ragnar Spärck1932, Reference Brøndsted1933), Hentschel (Reference Hentschel, Théel and Lönnberg1916, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929), Burton (Reference Burton1930a, Reference Burton, Fridriksson and Tuxen1959a), Arndt (Reference Arndt, Grimpe and Wagler1935), Koltun (Reference Koltun1964, Reference Koltun1966) and Ereskovsky (Reference Ereskovsky1993a, Reference Ereskovsky1994a, Reference Ereskovskyb, Reference Ereskovsky1995a, Reference Ereskovskyb, Reference Ereskovskyc). Although these studies have provided us with thorough descriptions of species and a comprehensive knowledge on their distribution, their data are nowadays seriously re-considered based on the novel material from poorly studied areas (e.g. Rapp, Reference Rapp2006, Reference Rapp2015 on calcareous sponges) and the molecular approaches in sponge taxonomy (e.g. Cárdenas et al., Reference Cárdenas, Rapp, Klitgaard, Best, Thollesson and Tendal2013 on geodiid sponges).
Vast marine areas north of Russia, known as the Siberian Seas, are characterized by an inhospitable environment, with, for the most part, shallow depths, a strong influx of fresh water from the great Siberian rivers and considerable temperature fluctuations between winter, when the sea surface is covered with ice and the water temperature sinks below zero, and the warm season, when the surface water layers may be heated (Coachman & Aagaard, Reference Coachman, Aagaard and Herman1974). The impact of the freshwater inflow, the ice movements and the summer heating is especially severe in the large shallow-water areas along the coast. The bottom here comprises spacious plains covered with mud and clay (Herman, Reference Herman and Herman1974; Weber, Reference Weber and Herman1989). Due to the unstable environment, both in the water body and on the seabed, the biodiversity, especially the diversity of sessile macrobenthos, of these areas is considerably poorer than along the coasts of the Nordic Seas (Golikov & Scarlato, Reference Golikov, Scarlato and Herman1989). On the contrary, in the offshore areas of the Siberian Seas the salinity is more stable, a branch of the Atlantic current brings warm water to the deep (Coachman & Aagaard, Reference Coachman, Aagaard and Herman1974), and the northern coast of large offshore archipelagos, e.g. Severnaya Zemlya and New Siberian Islands, is characterized by the rock cliffs and steep slopes running to great depths (Herman, Reference Herman and Herman1974; Weber, Reference Weber and Herman1989). These areas are oases hosting a relatively rich bottom fauna, particularly some diverse sponge communities (Golikov et al., Reference Golikov, Scarlato, Averincev, Menshutkina, Novikov, Sheremetevsky and Golikov1990). The studies of the sponge fauna in the Siberian Seas were started by Fristedt (Reference Fristedt1887) and Levinsen (Reference Levinsen and Lütken1887) and continued by Rezvoj (Reference Rezvoj1924, Reference Rezvoj1928), Gorbunov (Reference Gorbunov1946) and Koltun (Reference Koltun1966). The latter study until now remains the most comprehensive description of the Arctic sponge species, although some data presented there are obviously out of date and need a serious revision based on the modern taxonomic concepts. Among the northern seas of Russia, the White Sea, a large semi-isolated, brackish gulf of the Barents Sea, stands out for its peculiar hydrological conditions affecting the biodiversity. The deep waters of the White Sea, where the temperature is below zero all the year round, are inhabited predominantly by Arctic species. Conversely, the shallow depths, where seasonal fluctuations of the water temperature are considerable, host opportunistic Atlantic species (Babkov & Golikov, Reference Babkov and Golikov1984). The exploration of the White Sea sponge communities begun by Merejkowsky (Reference Merejkowsky1878) was continued by Swarczewsky (Reference Swarczewsky1906), Koltun (Reference Koltun1966) and Ereskovsky (Reference Ereskovsky1993a, Reference Ereskovskyb, Reference Ereskovsky1994a, Reference Ereskovskyb, Reference Ereskovsky1995a, Reference Ereskovskyb, Reference Ereskovskyc). However, these unique communities still need further studies based on up-to-date approaches.
Among all diverse sponge taxa inhabiting the Nordic and Siberian Seas the family Polymastiidae Gray, 1867 is one of the most common. Despite the polymastiids never reaching such large sizes as, for example, the astrophorid species do (Cárdenas et al., Reference Cárdenas, Rapp, Klitgaard, Best, Thollesson and Tendal2013), they are subdominants of shallow-water hard bottom communities in some Norwegian fjords (Svensen, personal communication), in the White Sea (Plotkin et al., Reference Plotkin, Railkin, Gerasimova, Pimenov and Sipenkova2005) and Laptev Sea (Golikov et al., Reference Golikov, Scarlato, Averincev, Menshutkina, Novikov, Sheremetevsky and Golikov1990). In the deep waters common polymastiids such as Tentorium semisuberites (Schmidt, Reference Schmidt1870) and Radiella spp. are often the most frequently recorded macrobenthic species (Barthel & Tendal, Reference Barthel and Tendal1993; Witte, Reference Witte1996). Polymastiidae were described in all studies on the Nordic and Russian sponge faunas (see the references above) and these records were summarized by Koltun (Reference Koltun1966), who listed eight polymastiid species for the Greenland Sea, 12 species for the Norwegian and Barents Sea, four species for the White Sea and eight species for the Siberian Seas and the Arctic Ocean. The White Sea list was appended by one more polymastiid by Ereskovsky (Reference Ereskovsky1993b), while Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) provided the re-descriptions of all these species and proposed some changes in their taxonomy.
Meanwhile, the records of most species presented by Koltun (Reference Koltun1966) and Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) were based on non-type material that may question their identification. Furthermore, the polymastiids of the Scandinavian Coast and Svalbard have been never properly revised. Additionally, rich sponge samples recently taken from the poorly studied underwater mountains and vents in the Greenland and Norwegian Sea must be examined. Finally, recently recovered sponge phylogenies based on molecular data (e.g. Cárdenas et al., Reference Cárdenas, Perez and Boury-Esnault2012; Morrow et al., Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012, Reference Morrow, Redmond, Picton, Thacker, Collins, Maggs, Sigwart and Allcock2013; Redmond et al., Reference Redmond, Morrow, Thacker, Diaz, Boury-Esnault, Cárdenas, Hajdu, Lôbo-Hajdu, Picton, Pomponi, Kayal and Collins2013; Morrow & Cárdenas, Reference Morrow and Cárdenas2015) challenge the traditional taxonomy based on morphology. Particularly they question the generally accepted concept of the relationships between the polymastiid genera (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002) as well as between the Polymastiidae and other families (Hooper & Van Soest, Reference Hooper and Van Soest2002). A monotypic order, Polymastiida Morrow & Cárdenas, Reference Morrow and Cárdenas2015, is established for the polymastiids, and a homoplasy of most morphological characters traditionally used in the taxonomy of this family and a non-monophyly of four genera from 15 polymastiid genera altogether known (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016) are revealed (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b).
The aim of the present study is to revise the polymastiid fauna of the Nordic and Siberian Seas based on morphological examination of the type material and other historical collections as well as on both morphological and molecular data from fresh material. We also provide a key for identification of the polymastiid species in the area of study (Appendix 1). The area covered by the study comprises the Scandinavian Coast from the Swedish Western Coast and Southern Norway to the Norwegian-Russian border, Russian Coasts (including the White Sea) from the border to the easternmost point, Icelandic Coast, Southern and Eastern Coasts of Greenland, offshore archipelagos Svalbard, Franz Josef Land, Novaya Zemlya, Nordenskjold and Severnaya Zemlya, offshore areas of the Greenland, Norwegian, Barents, Kara, Laptev, East-Siberian and Chukchi Seas and adjacent areas of the Arctic Ocean. We also compare the Nordic and Siberian sponges with individuals from the British Isles, Canadian Atlantic Coast and some other regions in order to explore the dispersal of the species.
MATERIALS AND METHODS
The study was based on historical and fresh material stored in 14 museums (Table 1). Altogether more than 1700 sponge individuals were studied (Online resource 1). The architecture of their skeletons was examined under light microscope on histological sections prepared on a precise saw with a diamond wafering blade after embedding of sponge fragments in epoxy resin as described by Boury-Esnault et al. (Reference Boury-Esnault, Marschal, Kornprobst and Barnathan2002), Vacelet (Reference Vacelet2006) and Boury-Esnault & Bézac (Reference Boury-Esnault, Bézac, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). Spicules were examined under light microscope and SEM after their isolation from organic matter in nitric acid following standard procedures. The number of specimens used for spicule measurements is given in the corresponding section of the description of each species. The number of spicules of each category measured in one specimen is indicated as N. Measurements are presented as minimum–mean–maximum, unless otherwise indicated.
Table 1. List of museums whose collections were used in the present study.
Genetic synapomorphies and autapomorphies of the species were defined in the 5′-end barcoding region of cytochrome oxidase subunit I (CO1) and the region coding the RNA of the large ribosomal subunit (28S rDNA) from helix B10 to helix E19. The sequences, the alignments and the respective phylogenies were presented by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b). GenBank accessions are indicated in Online resource 1, this study. Alignments and the respective phylogenetic trees are deposited in TreeBase and available at http://purl.org/phylo/treebase/phylows/study/TB2:S18487 (see Matrix M34248 and Tree Tr91844 for CO1, Matrix M34250 and Trees Tr91846–Tr91847 for 28S rDNA, complete dataset, and Matrix M34256 and Tree Tr91856 for 28S rDNA fragment D1–D19 demonstrating intragenomic polymorphism). Generalized phylogeny reconstructed from the concatenated dataset is presented in Figure 1, while the main apomorphies are indicated in Online resources 2 (for CO1) and 3 (for 28S rDNA), this study. Apomorphies in 28S rDNA were defined only within the unambiguously aligned parts of the matrix (positions 1–449, 492–577, 585–667, 685–940 and 949–2155 in the alignment). Based on the phylogenies recovered by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) we accept the abandonment of Radiella Schmidt, Reference Schmidt1870. However, we stick to the traditional taxonomy of other genera (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016) even, if they are not monophyletic in these phylogenies, until a new classification of Polymastiidae is built.
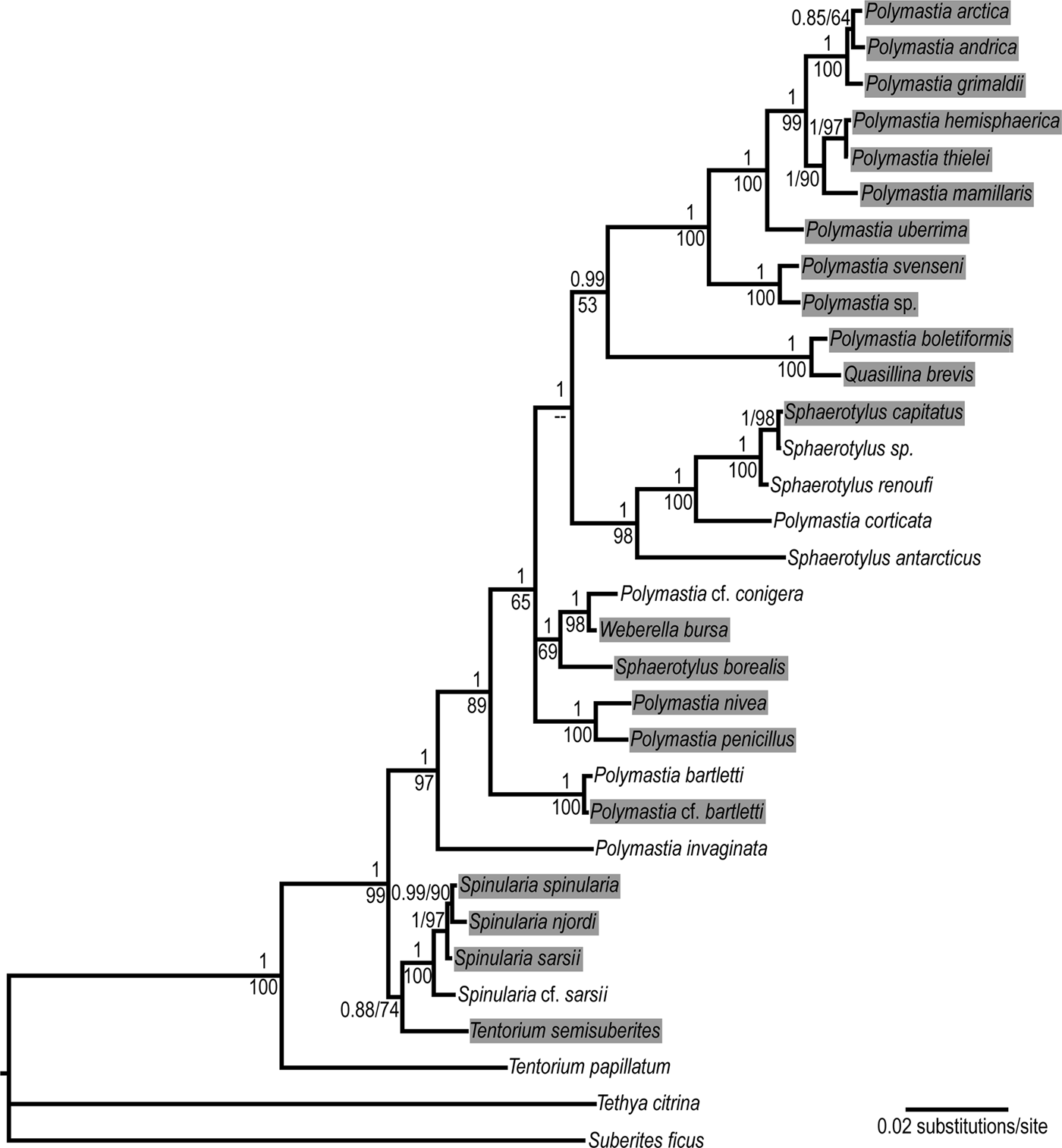
Fig. 1. Bayesian consensus tree reconstructed from the concatenated dataset CO1 + 28S rDNA of 30 polymastiid species. Nodal supports: upper values – Bayesian posterior probabilities, lower values – ML bootstrap supports in percentages. Data are taken from Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b). Complete 28S rDNA alignment is used. The original trees are available at http://purl.org/phylo/treebase/phylows/study/TB2:S18487. Branches corresponding to different individuals of the same species are collapsed. The species from the Nordic and Siberian Seas are highlighted. The following species from Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) are renamed according to the classification accepted in the present study: Polymastia sp. 1 as Polymastia svenseni, Radiella hemisphaerica as Polymastia hemisphaerica, Radiella sarsii as Spinularia sarsii, Radiella sp. as Spinularia njordi and Sphaerotylus sp. 2 as Sphaerotylus renoufi.
SYSTEMATICS
Systematic index
Class DEMOSPONGIAE Sollas, Reference Sollas1885
Suborder HETEROSCLEROMORPHA Cárdenas, Perez & Boury-Esnault, Reference Cárdenas, Perez and Boury-Esnault2012
Order POLYMASTIIDA Morrow & Cárdenas, Reference Morrow and Cárdenas2015
Family POLYMASTIIDAE Gray, Reference Gray1867
Genus Polymastia Bowerbank, Reference Bowerbank1862
P. andrica de Laubenfels, Reference de Laubenfels1949
P. arctica (Merejkowsky, Reference Merejkowsky1878)
P. cf. bartletti de Laubenfels, Reference de Laubenfels1942
P. boletiformis (Lamarck, Reference Lamarck1815)
P. grimaldii (Topsent, Reference Topsent1913)
P. hemisphaerica (Sars, Reference Sars1872)
P. mamillaris (Müller, Reference Müller1806)
P. nivea (Hansen, Reference Hansen1885)
P. penicillus (Montagu, Reference Montagu1814)
P. svenseni sp. nov. P. thielei Koltun, Reference Koltun1964
P. uberrima (Schmidt, Reference Schmidt1870)
Polymastia sp.
Genus Quasillina Norman, Reference Norman1869
Q. brevis Bowerbank, Reference Bowerbank1866
Genus Sphaerotylus Topsent, Reference Topsent1898
S. borealis (Swarczewsky, Reference Swarczewsky1906)
S. capitatus (Vosmaer, Reference Vosmaer1885)
Genus Spinularia Gray, Reference Gray1867
S. njordi sp. nov.
S. sarsii (Ridley & Dendy, Reference Ridley and Dendy1886) comb. nov.
S. spinularia (Bowerbank, Reference Bowerbank1866)
Genus Tentorium Vosmaer, Reference Vosmaer and Bronn1887
T. semisuberites (Schmidt, Reference Schmidt1870)
Genus Weberella Vosmaer, Reference Vosmaer1885
W. bursa (Müller, Reference Müller1806)
Description of taxa
Family POLYMASTIIDAE Gray, Reference Gray1867
DIAGNOSIS
Sponges of encrusting, massive, globular, hemispherical, discoid, columnar or pedunculate body shape. Oscula are often located at the summits of papillae or, sometimes, directly on the surface of the main body. Assortment of spicules comprises at least two size categories of smooth monactines. Tracts of principal monactines radiating from the sponge base or forming a reticulation constitute the main choanosomal skeleton or the innermost layer of the cortex. Auxiliary choanosomal skeleton comprises smaller spicules, free-scattered or grouped in little bundles, which may be smooth monactines, smooth or acanthose oxeas, raphides in trichodragmata or astrotylostyles. A complex specialized cortical skeleton is developed to a greater or lesser degree, composed of at least a palisade of smooth tylostyles, subtylostyles, or oxeas and/or exotyles. A fringe of extra-long monactines may be present at the edge of the body where it is in contact with the substrate.
Genus Polymastia Bowerbank, Reference Bowerbank1862
Original description: Polymastia Bowerbank, Reference Bowerbank1862, p. 1104.
SYNONYMS
Pencillaria Gray, Reference Gray1867, p. 527.
Polymastica Gray, Reference Gray1867, p. 527.
Rinalda Schmidt, Reference Schmidt1870, p. 51.
Trichostemma Sars, Reference Sars1872, p. 62.
TYPE SPECIES
Halichondria mamillaris Johnston, Reference Johnston1842 (= Spongia mamillaris Müller, Reference Müller1806) (by original designation).
DIAGNOSIS
Polymastiidae of encrusting, massive, globular, hemispherical or discoid body shape, always bearing papillae with oscula at the summits. Main choanosomal skeleton composed of tracts of principal monactines radiating from the sponge base or forming a reticulation. Auxiliary choanosomal skeleton comprises smaller monactines, free-scattered or grouped in little bundles. Cortical skeleton constituted at least by a superficial palisade of small smooth tylostyles or subtylostyles and an internal layer of larger monactines lying obliquely to the surface and may include middle layers. A fringe of extra-long monactines may be present at the edge of the body.
DISCUSSION
Polymastia Bowerbank, Reference Bowerbank1862, with its currently accepted assortment of species (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016), is not monophyletic as was suggested by Plotkin et al. (Reference Plotkin, Gerasimova and Rapp2012) based on morphological data and confirmed by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) based on the CO1 and 28S rDNA phylogenies (see also Figure 1, this study). In both phylogenies the type species of Polymastia, P. mamillaris (Müller, Reference Müller1806), formed a strongly supported clade with only five other species of this genus, P. andrica de Laubenfels, Reference de Laubenfels1949, P. arctica (Merejkowsky, Reference Merejkowsky1878), P. grimaldii (Topsent, Reference Topsent1913), P. uberrima (Schmidt, Reference Schmidt1870) and P. thielei Koltun, Reference Koltun1964, along with Trichostemma hemisphaericum Sars, Reference Sars1872, which was in fact the type species of Trichostemma Sars, Reference Sars1872 accepted as Radiella hemisphaerica at the time of the study by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b). However, no morphological synapomorphies of this clade could be defined. In the 28S rDNA tree a pair of unidentified species Polymastia sp. 1 and Polymastia sp. 2 was the sister to the Polymastia-clade with a strong Bayesian support. In the CO1 tree a trio of unidentified species Polymastia sp. 1, Polymastia sp. 2 and Polymastia sp. 3 was the sister to the Polymastia-clade, although with a weak support. All other Polymastia spp. including four species described in the present study, P. boletiformis (Lamarck, Reference Lamarck1815), P. bartletti de Laubenfels, Reference de Laubenfels1942, P. nivea (Hansen, Reference Hansen1885) and P. penicillus (Montagu, Reference Montagu1814), fell in the clades with the species of other genera in both molecular trees.
In the present study based on these phylogenies Trichostemma is regarded as a junior synonym of Polymastia, Polymastia sp. 1 is described as P. svenseni sp. nov., Polymastia sp. 2 is described as an unidentified species and Polymastia sp. 3 is not considered because it occurs outside the area covered by the study. Meanwhile, for the sake of taxonomic stability until a new classification of Polymastiidae is built, we retain the allocation of P. boletiformis, P. bartletti, P. nivea and P. penicillus to Polymastia, though it contradicts the molecular phylogenies. Consequently, the diagnosis of Polymastia (see above) is emended accordingly.
Polymastia andrica de Laubenfels, Reference de Laubenfels1949
(Figure 2)
Original description: Polymastia andrica de Laubenfels, Reference de Laubenfels1949, p. 22, figures 19 & 20.
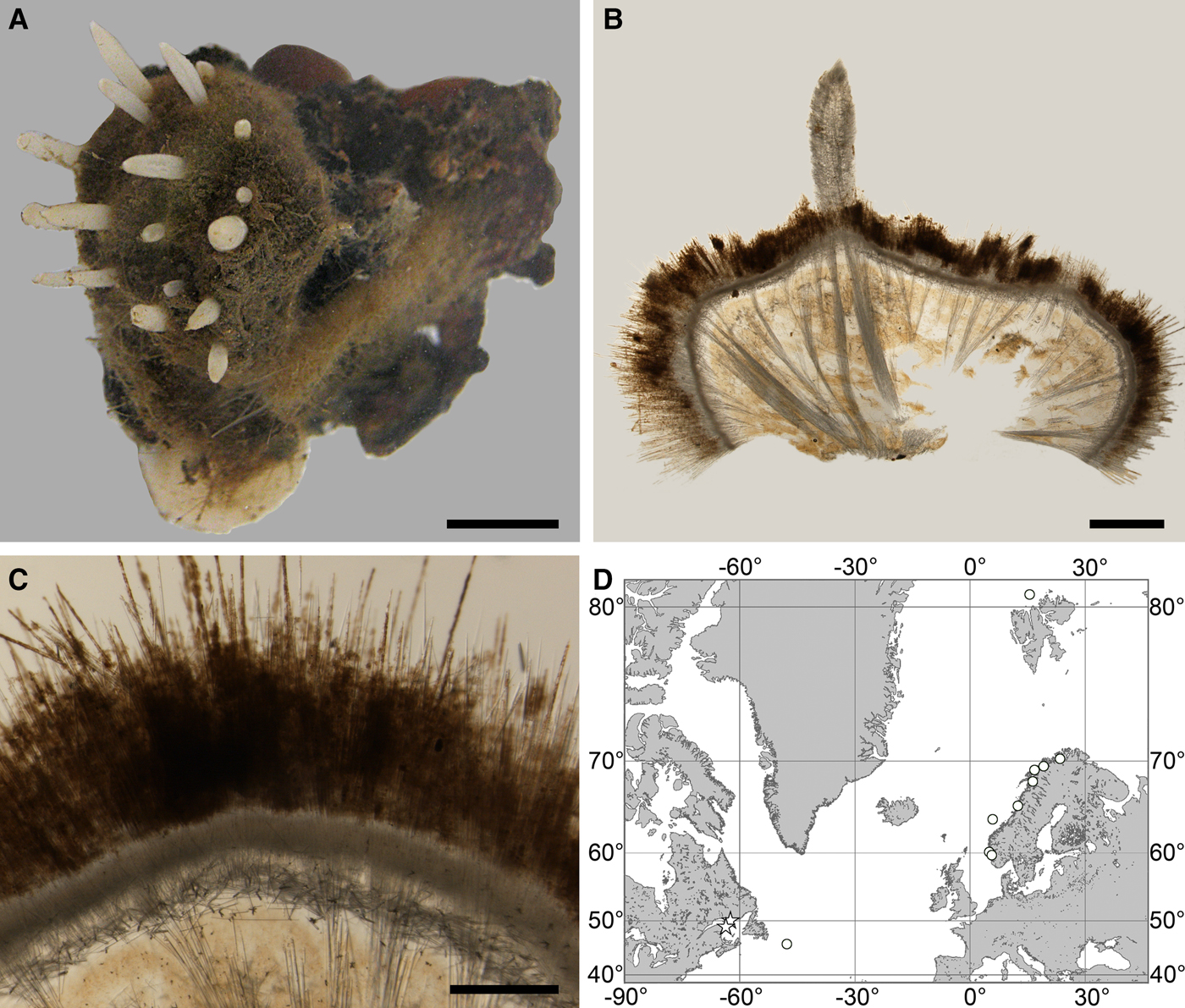
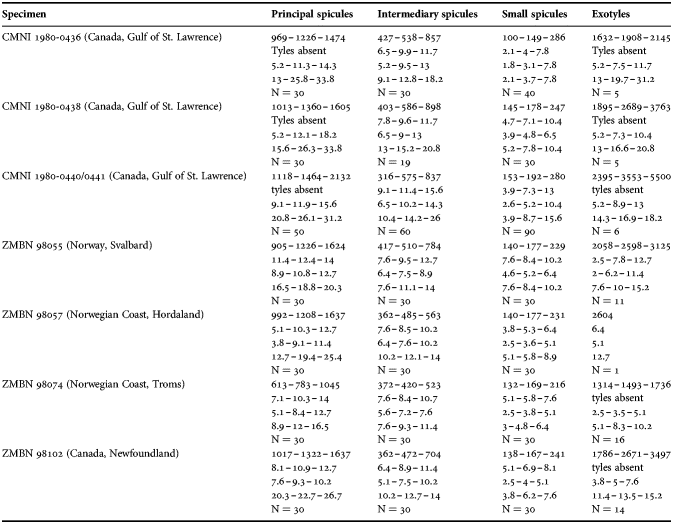
Fig. 2. Polymastia andrica: (A) ZMBN 098057, habitus; (B) the same individual, longitudinal section through the body, general view; (C) the same section, detail of cortex; (D) distribution: white stars, type localities; white circles, our data. Scale bars: A, 1 cm; B, 2 mm; C, 0.5 mm.
SYNONYMS AND CITATIONS
Polymastia mamillaris (Whiteaves, Reference Whiteaves1874, p. 184; Lambe, Reference Lambe1896, p. 196, pl. III figure 1; Whiteaves, Reference Whiteaves1901, p. 13).
TYPE MATERIAL
Holotype (lost?): Gulf of St. Lawrence, Canada, coll. Whiteaves.
Several individuals sampled by Whiteaves from various localities in the Gulf of St. Lawrence in 1871–1872 were identified by him (Whiteaves, Reference Whiteaves1874, Reference Whiteaves1901) as Polymastia mamillaris. Lambe (Reference Lambe1896) studied four of these individuals and confirmed the identification. De Laubenfels (Reference de Laubenfels1949) designated one of these sponges, with field number 8, as the holotype of his new species Polymastia andrica. Only three individuals of the four described by Lambe (Reference Lambe1896) are now available in the Canadian Museum of Nature (Online resource 1), but none of them bear field number 8. We have examined histological sections and spicules from these sponges and found that they fit with the descriptions by Lambe (Reference Lambe1896) and de Laubenfels (Reference de Laubenfels1949).
MATERIAL EXAMINED
(see Online resource 1 for details)
Canada: Quebec, Gulf of St. Lawrence: CMNI 1980-0436 (spicule slide from one specimen), CMNI 1980-0437/0440/0441 (histological sections and spicule slides from one specimen), CMNI 1980-0438 (spicule slide from one specimen), Newfoundland: ZMBN 098102 (one specimen).
Norway: Hordaland: ZMBN 098057 and ZMBN 107572 (two specimens), Nordland: NTNU-VM-54990, NTNU-VM-55034 and NTNU-VM-72533 (three specimens), Troms: NTNU-VM-55603 and ZMBN 098074 (two specimens), Finnmark: NTNU-VM-54850 (one specimen), Svalbard: ZMBN 098055 (one specimen).
Norwegian Sea, offshore: ZMBN 098108 (one specimen).
DESCRIPTION
External morphology
Cushion-shaped sponges covering the substrate and occupying up to 6 cm2 (Figure 2A). Surface strongly hispid, covered with sediment, with up to several tens of cylindrical or flattened, greyish or whitish papillae which are 1–12 mm long and 1–5 mm wide. In preserved sponges exhalant and inhalant papillae do not differ in size or shape.
Anatomy
Choanosome in alcohol yellowish or greyish, dense. Main choanosomal skeleton composed of radiating tracts (88–417 µm thick) of principal spicules crossing the cortex and forming a surface hispidation reinforced with exotyles (Figure 2B). Ascending tracts also form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered small spicules, being especially abundant in the subcortical area. Cortex in alcohol light-coloured, firm, not detachable. Cortical skeleton constituted by a superficial palisade (116–232 µm thick) of small spicules, a middle layer (40–272 µm thick) of collagen fibres with low density of spicules and an internal layer (56–170 µm thick) of tangentially arranged intermediary spicules (Figure 2C). Skeleton of the papilla walls composed of two layers only, the superficial palisade and the internal tangential layer. Single small and intermediary spicules reinforce the bulkheads separating aquiferous canals and vestibules in the papillae.
Spicules
(Measurements based on seven specimens, individual variation presented in Table 2)
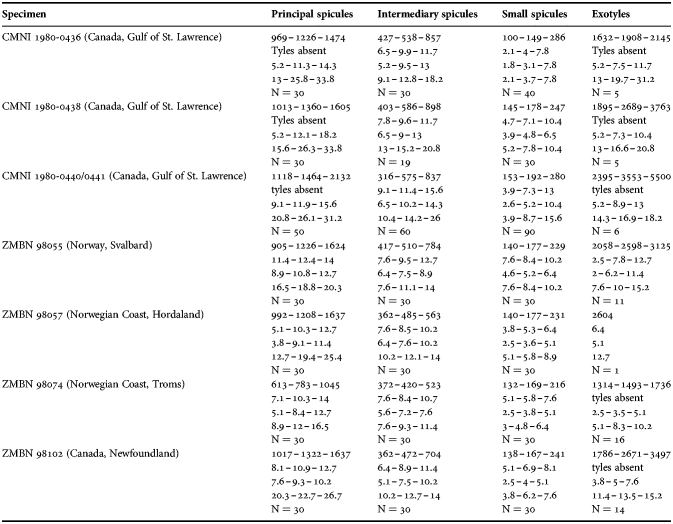
Table 2. Individual variation of spicule dimensions of Polymastia andrica (given in μm as minimum–mean–maximum). Parameters: length, diameter of tyle, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).
Principal spicules – styles (in the Canadian sponges) or subtylostyles to styles (Norwegian sponges), usually straight, fusiform, with tyles (if present) slightly displaced along the shafts, occasionally polytylote. Length 613–1248–2132 µm, diameter of tyle (if present) 5.1–11.1–14.3 µm, proximal diameter of shaft 3.8–11.1–18.2 µm, maximum diameter of shaft 8.9–23.9–33.8 µm, N = 230.
Intermediary spicules – subtylostyles to styles, straight or gently bent, fusiform, occasionally with tyles slightly displaced along the shafts. Length 316–517–898 µm, diameter of tyle (if present) 6.4–9.9–15.6 µm, proximal diameter of shaft 5.1–9.1–14.3 µm, maximum diameter of shaft 7.6–13.2–26.0 µm, N = 229.
Small spicules – tylostyles, often gently bent in the proximal part, usually slender, occasionally stout. Length 100–176–286 µm, diameter of tyle 2.1–6.5–13 µm, proximal diameter of shaft 1.8–4.5–10.4 µm, maximum diameter of shaft 2.1–7.1–15.6 µm, N = 280.
Exotyles – filiform styles (Canadian sponges and individual ZMBN 98074 from Troms) or filiform subtylostyles with weakly developed, often slightly displaced tyles (other Norwegian sponges). Length 1314–2358–5500 µm, diameter of tyle (if present) 2.5–6.7–12.7 µm, proximal diameter of shaft 2.0–5.6–13 µm, maximum diameter of shaft 5.1–12.6–31.2 µm, N = 58.
Strongyles (registered only in individual CMNI1980-0436 from the Gulf of St. Lawrence) – straight, fusiform or slender, occasionally with one or two weakly developed tyles. Length 26–47–156 µm, proximal diameter of shaft 9.1–15.3–18.2 µm, maximal diameter of shaft 9.1–21–41.6 µm, N = 30.
Genetic data
CO1 sequences obtained from five individuals of Polymastia andrica are identical, but an intragenomic polymorphism was observed in 28S rDNA of one individual (Matrix M34256 in TreeBase). Polymastia andrica is closely related to P. arctica and P. grimaldii, sharing with them five synapomorphies in CO1 (Online resource 2, p. 1) and four synapomorphies in 28S rDNA (Online resource 3, p. 1), which distinguish these three species from all other polymastiids. 28S rDNA of P. andrica, P. arctica and P. grimaldii displays a high level of intraspecific and intragenomic polymorphism with some identical gene versions found in the individuals from different species (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; Matrices M34250 and M34256 in TreeBase). On the contrary, the CO1 data are consistent. In this gene P. andrica and P. arctica share one synapomorphy distinguishing them from all other polymastiids, and, additionally P. andrica has one autapomorphy (Online resource 2, p. 1). Apart from this autapomorphy, P. andrica differs from P. arctica by eight base pairs (bps), from P. grimaldii by 12 bps and from the type species of Polymastia, P. mamillaris, by 32 bps in CO1 (Matrix M34248 in TreeBase).
OCCURRENCE
(Figure 2D)
Canadian Atlantic Coast: Gulf of St. Lawrence (218–382 m according to Lambe, Reference Lambe1896), Newfoundland (619–699 m). Norwegian Coast: Hordaland (28–300 m), Nordland (120–721 m), Troms (25–220 m), Finnmark (30–80 m). Norwegian Sea, offshore areas (626–628 m). Svalbard (215 m).
DISCUSSION
Before our study, Polymastia andrica was recorded only from the type locality, the Gulf of St. Lawrence (de Laubenfels, Reference de Laubenfels1949). We have identified as P. andrica a sponge from Newfoundland and 10 Norwegian individuals based on their morphological similarities with the material from the type locality (although the exotyles in the Norwegian specimens are shorter than those in the Canadian sponges) and the identity of CO1 from the Newfoundland specimen and the Norwegian specimens. Polymastia andrica is morphologically very similar to P. arctica and P. mamillaris, but differs from these two by the presence of exotyles. Additionally P. andrica differs from P. arctica by the absence of threads with buds at the summits of the inhalant papillae and by the absence of size difference between the inhalant and exhalant papillae. All genetic data obtained support the discrimination between P. andrica and P. mamillaris based on morphology. The morphological differences between P. andrica and P. arctica are only confirmed by the CO1 data, but not by 28S rDNA.
Polymastia arctica (Merejkowsky, Reference Merejkowsky1878)
(Figure 3)
Original description: Rinalda arctica Merejkowsky, Reference Merejkowsky1878, p. 4, pl. I figures 7–12, pl. II figures 6–8, pl. III figures 1–3, 6–10, 20–22, 30–39.
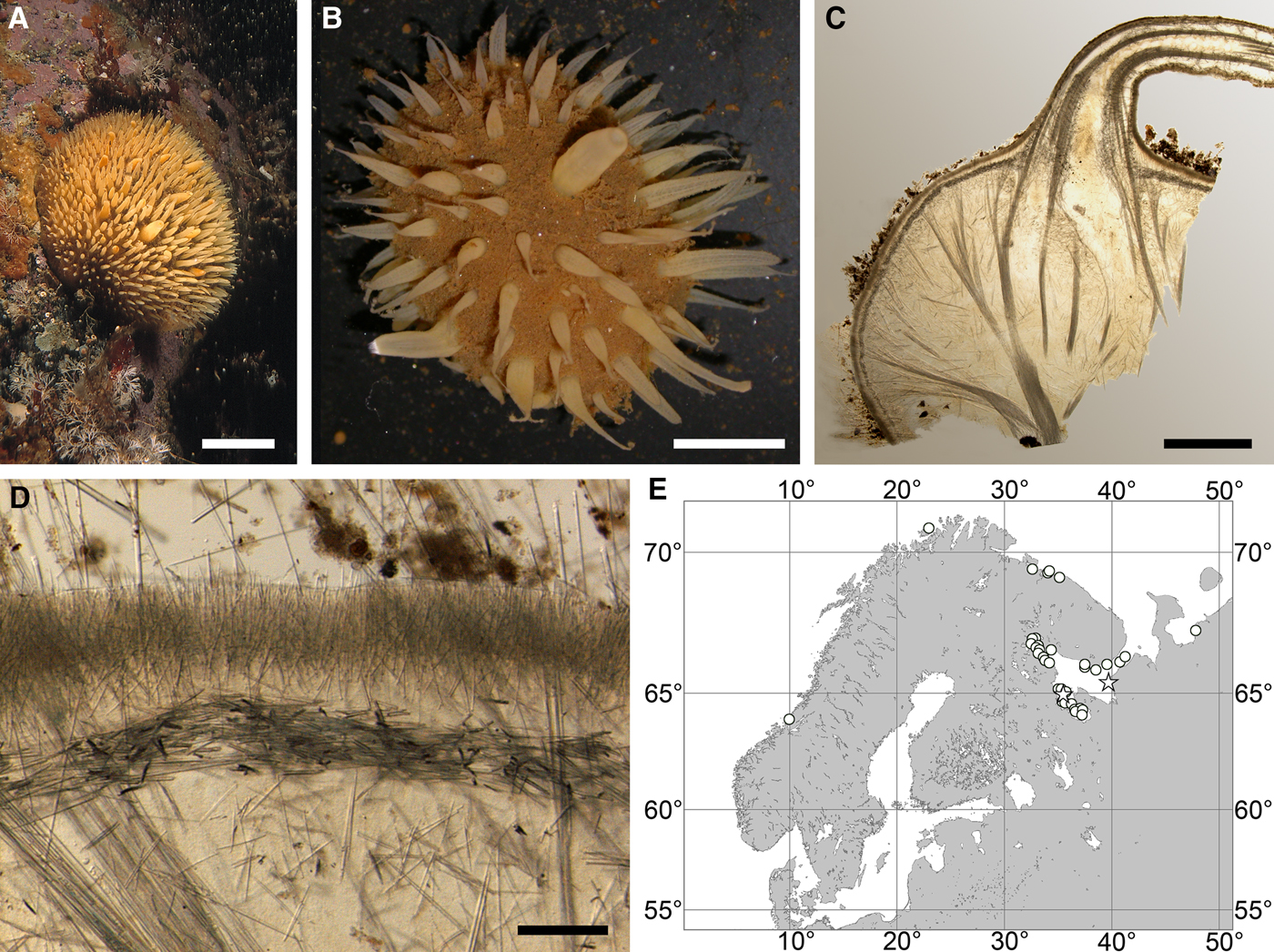
Fig. 3. Polymastia arctica: (A) an individual in situ, Kandalaksha Bay, White Sea (courtesy of M. Fedyuk, St. Petersburg State University); (B) an individual in aquarium; (C) ZMBN 098068, longitudinal section through the body, general view; (D) the same section, detail of cortex; (E) distribution: white stars, type localities; white circles, our data. Scale bars: A, 2 cm; B, 1 cm; C, 3 mm; D, 0.2 mm.
SYNONYMS AND CITATIONS
Polymastia arctica (Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 541, figures 1a & 2a; Plotkin & Boury-Esnault, Reference Plotkin and Boury-Esnault2004, p. 15, figures 1–3).
Polymastia mammillaris (Arnesen, Reference Arnesen1918, p. 8, pl. 1 figures 1–4, pl. 2 figures 1–5, pl. 3 figures 6–9, pl. 4 figures 1–2; pl. 5 figures 1–2, pl. 6 figures 1–4; Ereskovsky, Reference Ereskovsky1993a, p. 22, Reference Ereskovsky1995c, p. 724; Plotkin & Ereskovsky, Reference Plotkin and Ereskovsky1997, p. 127).
Polymastia mammillaris mammillaris (Koltun, Reference Koltun1966, p. 69, text-figure 38, pl. XX figure 6 pars.).
Polymastia penicillus (Swarczewsky, Reference Swarczewsky1906, p. 313, pl. 13 figure 1).
Rinalda arctica (Merejkowsky, Reference Merejkowsky1880, p. 421).
TYPE MATERIAL
Lectotype (designated by Plotkin & Boury-Esnault, Reference Plotkin and Boury-Esnault2004): ZIN RAS 10610 (specimen and slide 5526a), Archipelago of Solovki, Onega Bay, White Sea, 64°57.0′N 35°29.4′E – 65°10.8′N 35°51.6′E, 9–22 m, summer 1877, coll. Merejkowsky.
Paralectotypes: ZIN RAS 10611 (specimen and slide 5526b) and ZIN RAS 10612 (four specimens), from the same sample as the lectotype.
Paralectotypes: ZIN RAS 10613 (two specimens and slide 9112), Cape Kerets, Dvina Bay, White Sea, 65°25′N 39°38′E, 11 m, 22.06.1876, coll. Merejkowsky.
Detailed description of the type material was presented by Plotkin & Boury-Esnault (Reference Plotkin and Boury-Esnault2004).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Norway: Sør-Trøndelag: NTNU-VM-55865 (two specimens), Finnmark: ZMBN 098065 and ZMBN 098068 (two specimens).
Russia: Murman Coast: ZIN RAS ocpm078, ZIN RAS ocpm079, ZIN RAS ocpm131, ZIN RAS ocpm132, ZIN RAS ocpm148 (five specimens), Chyosha Bay of the Barents Sea: ZIN RAS ocpm145 (one specimen), White Sea: ZMBN 098060, ZMBN 098062, ZMBN 098063 (three specimens) and 114 specimens deposited in ZIN RAS.
DESCRIPTION
External morphology
Cushion-shaped sponges covering the substrate and occupying up to 100 cm2 (Figure 3A, B). Surface thickly or thinly hispid, usually covered with sediment, with up to several hundred papillae. In living sponges the colour of papillae and the areas of the surface free of sediment cream to yellowish. Most papillae inhalant, cylindrical in shape, 2–18 mm in length and 1–6 mm in diameter. Average density of the inhalant papillae 13 per 1 cm2 of the surface. The inhalant papillae may bear at the summits threads with up to six buds arranged in line (Figure 3B). Exhalant papillae usually conical, 3–12 mm long, 3–7 mm wide at base and 1–5 mm wide at summit, with oscula about 0.5 mm in diameter. One sponge may have up to 19 exhalant papillae.
Anatomy
Choanosome in life orange, dense. Main choanosomal skeleton composed of radial, or longitudinal tracts (170–460 µm thick) of principal spicules branching in the subcortical area, crossing the cortex and forming a surface hispidation (Figure 3C). Ascending tracts also form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered bundles, each of two to five small spicules, being especially abundant in the subcortical area. Cortex in life cream-coloured, firm, not detachable. Cortical skeleton constituted by a superficial palisade (180–310 µm thick) of small spicules, a middle layer (90–180 µm thick) of collagen fibres with low density of spicules and an internal layer (160–250 µm thick) of tangentially arranged intermediary spicules (Figure 3D). Skeleton of the papilla walls composed of two layers only, the superficial palisade and the internal tangential layer. Single intermediary spicules reinforce the bulkheads separating aquiferous canals and vestibules in the papillae.
Spicules
(measurements based on 43 specimens)
Principal spicules – subtylostyles, straight, fusiform. Length 620–868–1100 µm, maximal diameter of shaft 8.8–14.3–20.0 µm, N = 500.
Intermediary spicules – subtylostyles, straight or gently bent, slender. Length 270–414–550 µm, diameter of shaft 5.0–9.5–17.5 µm, N = 500.
Small spicules – tylostyles, gently bent, fusiform. Length 120–161–215 µm, diameter of tyle 3.8–5.5–7.5 µm, maximal diameter of shaft 3.8–4.8–6.3 µm, N = 500.
Genetic data
CO1 sequences obtained from five individuals of Polymastia arctica are identical, but these individuals differ in 28S rDNA (Matrix M34250 in TreeBase) and, moreover, three of them exhibit a polymorphism in this gene (Matrix M34256 in TreeBase). By both genes P. arctica is closely related to P. andrica and P. grimaldii (see the synapomorphies in the Genetic data section for P. andrica above). 28S rDNA of these three species displays a high level of intraspecific and intragenomic polymorphism, while the CO1 data are consistent (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). In this gene P. arctica has two autapomorphies (Online resource 2, p. 1). Apart from them, P. arctica differs from P. andrica by 7 bps, from P. grimaldii by 11 bps and from the type species of Polymastia, P. mamillaris, by 28 bps in CO1 (Matrix M34248 in TreeBase).
OCCURRENCE
(Figure 3E)
Literature data: Norwegian Coast: Troms and Finnmark (73–182 m) (as Polymastia mammilaris – Arnesen, Reference Arnesen1918). Norwegian and Barents Sea (as P. mamillaris – Koltun, Reference Koltun1966). White Sea (as Rinalda arctica – Merejkowsky, Reference Merejkowsky1878, Reference Merejkowsky1880; as P. penicillus – Swarczewsky, Reference Swarczewsky1906; as P. mamillaris – Koltun, Reference Koltun1966).
Our data: Norwegian Coast: Sør-Trøndelag (27–50 m), Finnmark (127 m). Barents Sea: Murman Coast (60–108 m), Chyosha Bay (7 m). White Sea (4–109 m).
DISCUSSION
Polymastia arctica is morphologically very similar to P. andrica and P. mamillaris. The main feature distinguishing P. arctica from the latter two is the presence of threads with buds at the summits of some inhalant papillae (Arnesen, Reference Arnesen1918; Plotkin & Ereskovsky, Reference Plotkin and Ereskovsky1997), although the budding intensity in the populations displays a considerable seasonal fluctuation with some individuals stopping bud formation in the warmest period (Plotkin & Ereskovsky, Reference Plotkin and Ereskovsky1997). Additionally P. arctica differs from P. andrica by the absence of exotyles and from P. mamillaris by the relatively thicker middle cortical layer and the presence of spicules in the bulkheads separating aquiferous canals in the papillae. Some minute differences between these three species in the shape of spicules were also reported, e.g. principal spicules usually being fusiform subtylostyles in P. arctica and strongyloxeas in P. mamillaris (Plotkin & Boury-Esnault, Reference Plotkin and Boury-Esnault2004), but our study has revealed instability of this character. All genetic data obtained support the discrimination between P. arctica and P. mamillaris based on morphology. The morphological differences between P. arctica and P. andrica are only confirmed by the CO1 data, but not by 28S rDNA.
Polymastia cf. bartletti de Laubenfels, Reference de Laubenfels1942
(Figure 4)
Original description: Polymastia bartletti de Laubenfels, Reference de Laubenfels1942, p. 265.
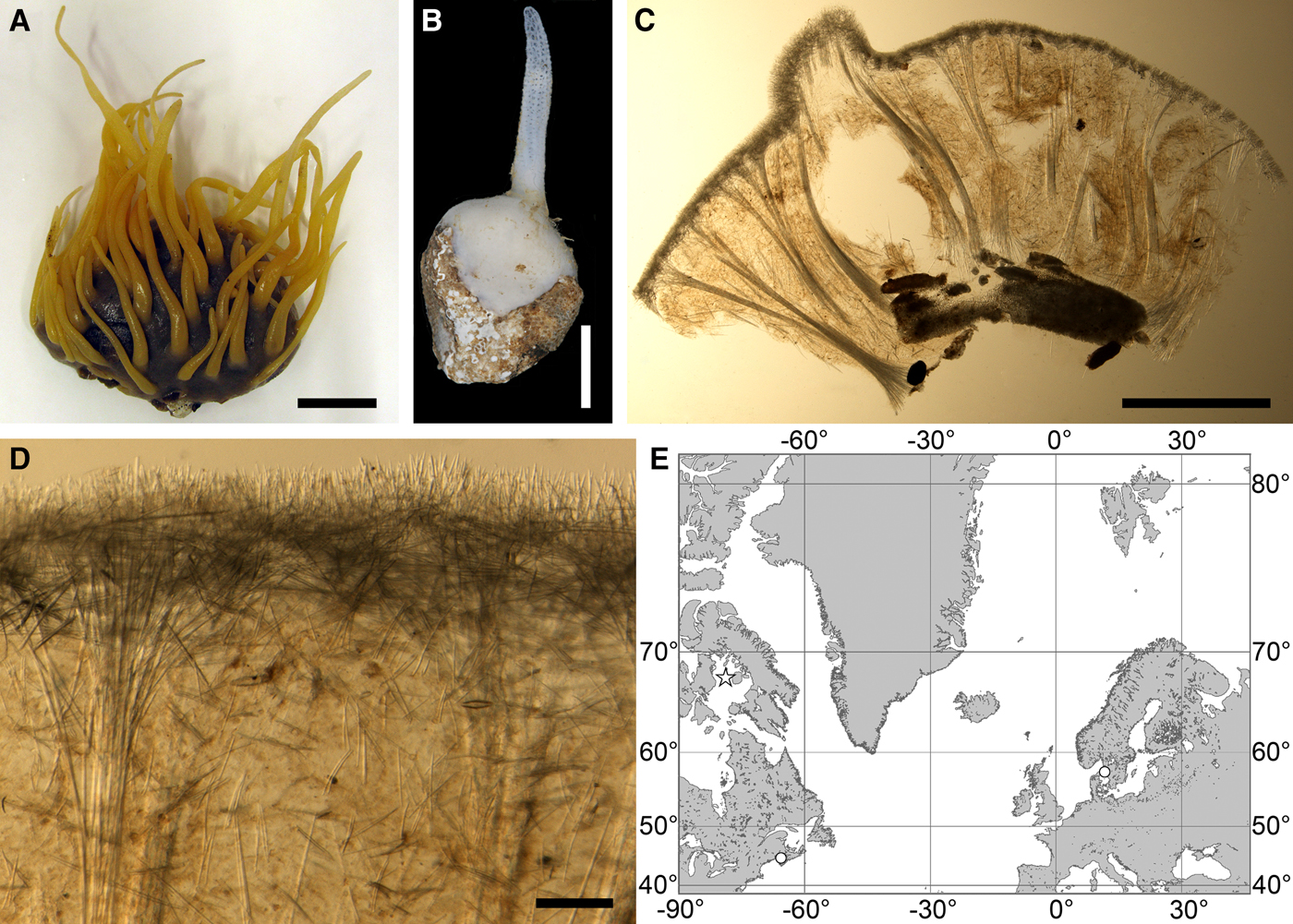
Fig. 4. Polymastia bartletti: (A) ZMBN 098111, habitus; (B) GNM 904:1, habitus; (C) ZMBN 098111, longitudinal section through the body, general view; (D) the same section, detail of cortex; (E) distribution: white stars, type localities; white circles, our data. Scale bars: A, 2 cm; B, 0.5 cm; C, 3 mm; D, 0.2 mm.
TYPE MATERIAL
Holotype (not studied): USNM 22692 (in the Smithsonian National Museum of Natural History, Washington), Foxe Basin, Nunavut, Canada, 67°45′N 79°09′W, 69 m, 24.08.1927, coll. Bartlett.
MATERIAL EXAMINED
(see Online resource 1 for details)
Canada: Nova Scotia: ZMBN 098111 (one specimen).
Sweden: Västra Götaland, Kattegat: GNM 904:1 (one specimen, identification under doubt).
DESCRIPTION
External morphology
Cushion-shaped sponges covering the substrate. Surface smooth, free of sediment, with long papillae lacking visible oscula. Canadian sponge ~51 × 42 × 4 mm in size, with 45 cylindrical papillae, which are 14–44 mm long and 1–4 mm wide. In life the surface is brown and the papillae are yellowish (Figure 4A). In alcohol both the surface and the papillae have become whitish. Swedish sponge 12 × 9 × 0.7 mm in size, with one cylindrical papilla which is 12 mm long and 1.8 mm wide (Figure 4B). Surface and papilla are whitish in alcohol.
Anatomy
Choanosome in alcohol whitish, dense. In both sponges studied main choanosomal skeleton composed of tracts of principal spicules (Figure 4C). The tracts, 71–135 µm thick in the middle of the body, radiate towards the base and the cortex. Ascending tracts also form a framework of the papilla skeleton. Examination of the auxiliary choanosomal skeleton and the cortex in the Swedish individual was not possible because of its small size. In the Canadian sponge the auxiliary choanosomal skeleton comprises small and intermediary spicules, most free-scattered, some in bundles of three to seven. Cortex dense, but friable, not detachable. Cortical skeleton constituted by a superficial palisade (106–166 µm thick) of small spicules, which is overlapped with an inner layer (203–286 µm thick) of criss-cross intermediary spicules (Figure 4D).
Spicules
GNM 904:1 (Sweden):
Principal spicules – mainly styles, occasionally subtylostyles with weakly developed tyles, usually straight, fusiform. Length 568–752–905 µm, proximal diameter of shaft 2.5–10.2–12.7 µm, maximum diameter of shaft 6.4–14.1–16.5 µm, N = 30.
Intermediary spicules – subtylostyles to styles, usually gently curved, slightly fusiform. Length 246–391–503 µm, diameter of tyle (if present) 6.4–9.2–12.7 µm, proximal diameter of shaft 5.1–8.0–10.2 µm, maximum diameter of shaft 7.6–10.1–12.7 µm, N = 30.
Small spicules – tylostyles, usually gently curved, slender. Length 94–134–165 µm, diameter of tyle 3.8–5.2–7.6 µm, proximal diameter of shaft 2.0–3.6–5.1 µm, maximum diameter of shaft 2.5–4.0–5.1 µm, N = 30.
ZMBN 098111 (Canada):
Principal spicules – styles, usually straight, fusiform. Length 930–1162–1327 µm, proximal diameter of shaft 10.2–12.1–15.2 µm, maximum diameter of shaft 14.0–17.1–20.3 µm, N = 30.
Intermediary spicules – subtylostyles to styles, usually gently curved, slightly fusiform. Length 467–565–648 µm, diameter of tyle (if present) 8.9–10.2–12.7 µm, proximal diameter of shaft 7.6–8.4–10.2 µm, maximum diameter of shaft 10.2–11.8–15.2 µm, N = 30.
Small spicules – tylostyles, usually gently curved, slender. Length 127–161–192 µm, diameter of tyle 4.6–5.3–6.4 µm, proximal diameter of shaft 2.5–3.5–5.1 µm, maximum diameter of shaft 3.8–5–6.4 µm, N = 30.
Holotype USNM 22692 (according to de Laubenfels, Reference de Laubenfels1942):
Principal choanosomal spicules – tylostyles. Length 540–600 µm, diameter of shaft 9–12 µm.
Small choanosomal spicules – length 200 µm, diameter of shaft 4 µm.
Cortical spicules (de Laubenfels did not distinguish between small and intermediary cortical spicules) – tylostyles. Length 350–400 µm, diameter of shaft 6 µm.
Genetic data
The Canadian Polymastia bartletti and the Swedish Polymastia cf. bartletti are distinguished by two bps in CO1 (Matrix M34248 in TreeBase) and four bps in 28S rDNA (Matrix M34250 in TreeBase). At the same time these sponges share nine synapomorphies in CO1 (Online resource 2, p. 3) and two synapomorphies in 28S rDNA (Online resource 3, p. 3) distinguishing them from other polymastiids. Apart from these synapomorphies, both P. bartletti and P. cf. bartletti differ from morphologically similar P. nivea by 27 bps in CO1 (Matrix M34248 in TreeBase) and 60 bps in 28S rDNA (Matrix M34250 in TreeBase) and from the type species of Polymastia, P. mamillaris, by 61 bps in CO1 (Matrix M34248 in TreeBase) and 84 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
(Figure 4E)
Literature data: Canadian Atlantic Coast: Foxe Basin (69 m) (de Laubenfels, Reference de Laubenfels1942).
Our data: Canadian Atlantic Coast: Nova Scotia (depth unknown). Swedish Western Coast: Kattegat (19–31 m).
DISCUSSION
Before our study Polymastia bartletti was known only from the type locality, the Foxe Basin (de Laubenfels, Reference de Laubenfels1942). We have identified as P. bartletti a specimen from Newfoundland based on its external and anatomical similarities with the original description, and a specimen from Sweden based on the similarities of its external features and DNA with the Newfoundland sponge. But we cannot exclude that the Swedish individual may in fact represent another species since its spicules in all categories are slightly shorter than those in the Canadian individual, and the sequences of the phylogenetic markers from these sponges are not completely identical. More careful morphological examination and genetic studies of larger material are required to check this assumption.
Polymastia bartletti is morphologically very similar to the NE Atlantic species, P. nivea. Discrimination between these two species is based mainly on their large genetic difference. In its turn P. nivea was often confused with P. robusta Bowerbank, Reference Bowerbank1862 and P. boletiformis (e.g. Koltun, Reference Koltun1966). Polymastia nivea and P. boletiformis in fact differ considerably both in morphology (Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012; present study) and genetics (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; present study), while the status of P. robusta is questionable (Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012; present study). The records of P. robusta from the Canadian Atlantic (e.g. Lambe, Reference Lambe1896; Whiteaves, Reference Whiteaves1901) may indicate P. bartletti, but the respective material should be re-examined to test this assumption.
Polymastia boletiformis (Lamarck, Reference Lamarck1815)
(Figure 5)
Original description: Alcyonium boletiforme Lamarck, Reference Lamarck1815, p. 332.

Fig. 5. Polymastia boletiformis: (A) an individual in situ, Tingelsædet, Egersund, Norway (courtesy of E. Svensen, OceanPhoto/Dalane Tidende AS, Egersund); (B) ZMBN 107563, longitudinal section through the body, general view; (C) the same section, detail of cortex and subcortical area; (D) distribution along the Scandinavian Coast: white circles, our data; for distribution in other regions see Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987; as Polymastia robusta). Scale bars: B, 2 mm; C, 0.5 mm.
SYNONYMS AND CITATIONS
Polymastia boletiformis (Burton, Reference Burton, Fridriksson and Tuxen1959a, 11 pars.; Van Soest et al., Reference Van Soest, Picton and Morrow2000, Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016; Van Soest, Reference Van Soest, Costello, Emblow and White2001, p. 74; Morrow et al., Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012, p. 177; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1i).
Polymastia robusta (Topsent, Reference Topsent1900, p. 147, pl. IV figures 3–7, 14; Arndt, Reference Arndt, Arndt, Broch, Krumbach, Pax and Lieberkind1928, p. 31, figure 29a;, Reference Topsent1933, p. 45; Burton, Reference Burton1930a, p. 496; Arndt, Reference Arndt, Grimpe and Wagler1935, p. 34, figure 51; Alander, Reference Alander1942, p. 75; Borojevic, Reference Borojevic1967, p. 1, pls I–II; Cabioch, Reference Cabioch1968, p. 215; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 44, figure 8).
TYPE MATERIAL
Lamarck (Reference Lamarck1815) reported neither the museum number, nor the type locality in the original description. Topsent (Reference Topsent1933) examined a sponge with Lamarck's original label ‘Alcyonium boletiforme’ considered as the holotype of this species and stored in MNHN. Since then nobody has re-examined this individual and it is regarded as lost by MNHN.
MATERIAL EXAMINED
(see Online resource 1 for details)
Sweden: Västra Götaland: GNM 901:1, GNM 903:1 and GNM 903:2 (three specimens).
Norway: Vest-Agder: ZMBN 098088 and ZMBN 098089 (two specimens), Rogaland: ZMBN 107584 and ZMBN 107585 (three specimens), Hordaland: ZMBN 098047, ZMBN 098048, ZMBN 098081, ZMBN 107559, ZMBN 107560, ZMBN 107562, ZMBN 107563, ZMBN 107564, ZMBN 107565, ZMBN 107566, ZMBN 107567, ZMBN 107568, ZMBN 107569, ZMBN 107571 (14 specimens), Møre and Romsdal: ZMBN 107493, ZMBN 107570 (two specimens).
DESCRIPTION
External morphology
Sponges cushion-shaped, covering the substrate or massive (Figure 5A). The largest individuals may occupy up to 100 cm2. Surface smooth, sometimes covered with sediment, with cylindrical or conical papillae. In living sponges both the surface and the papillae bright orange or yellow. Inhalant papillae 6–18 mm long and 2–5 mm wide. About 2–3 inhalant papillae per 1 cm2 of the surface. Exhalant papillae 16–36 mm long and 3–6 mm wide, with well visible oscula at the summits. A sponge may bear 1–6 exhalant papillae.
Anatomy
Choanosome in life slightly darker than cortex, crumbly. Main choanosomal skeleton composed of tracts of principal spicules forming a reticulation or meanders (Figure 5B, C). Ascending tracts form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered principal spicules. Cortex leather-like, easily detachable. Cortical skeleton constituted by a superficial palisade (80–250 µm thick) of small spicules and an internal layer (169–420 µm thick) of criss-cross principal spicules (Figure 5C). Aquiferous cavities connected with ostia in the surface and separated by bundles of intermediary spicules lie in a space (125–400 µm thick) between the cortex and the choanosome. Both cortical layers extend to the walls of papillae. Each papilla bears several inhalant canals, and in exhalant papilla there are also one to three exhalant canals located midmost. Bulkheads separating the canals are reinforced with free-scattered principal spicules.
Spicules
(Measurements based on four specimens)
Principal spicules – subtylostyles, straight or gently curved, slender or slightly fusiform. Length 261–540–735 µm, proximal diameter of shaft 5.1–6.7–9.0 µm, maximum diameter of shaft 7.5–10.4–12.7 µm, N = 120.
Small spicules – subtylostyles with weakly developed tyles, usually gently bent in the proximal part, slender. Length 91–153–232 µm, proximal diameter 2.0–3.1–3.9 µm, maximum diameter of shaft 2.0–3.2–3.9 µm, N = 124.
Genetic data
In both CO1 and 28S rDNA phylogenies Polymastia boletiformis is the sister to morphologically quite distinct Quasillina brevis (Bowerbank, Reference Bowerbank1866) (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These species share three synapomorphies in CO1 (Online resource 2, p. 4) and 24 synapomorphies in 28S rDNA (Online resource 3, p. 4). CO1 data were obtained from two specimens of P. boletiformis, of which one differs from Q. brevis just by one bp in this gene, while the other differs from Q. brevis by six bps (Matrix M34248 in TreeBase). 28S rDNA sequences obtained from six Scandinavian P. boletiformis are identical to the sequences of a British P. boletiformis (GenBank accessions HQ379232, HQ379306 and HQ379372, Morrow et al., Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012) and display six synapomorphies distinguishing them from all other polymastiids (Online resource 3, p. 4). Apart from these synapomorphies, P. boletiformis differs from Q. brevis by 17 bps in 28S rDNA (Matrix M34250 in TreeBase) and from the type species of Polymastia, P. mamillaris by 64–67 bps in CO1 (considering the intraspecific polymorphism, Matrix M34248) and 89 bps in 28S rDNA (Matrix M34250).
OCCURRENCE
Literature data: Portuguese, Spanish and French Atlantic Coast (as Polymastia robusta – Topsent, Reference Topsent1900; Borojevic, Reference Borojevic1967; Cabioch, Reference Cabioch1968; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987). Mediterranean Sea, English Channel (as Polymastia robusta – Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987). British Isles (as P. robusta – Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; as P. boletiformis – Van Soest et al., Reference Van Soest, Picton and Morrow2000, Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016). Sweden (as P. robusta – Alander, Reference Alander1942). Depths – from 10 m and deeper down the shelf (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987). Records from the continental slope, 2354 m by Burton (Reference Burton, Fridriksson and Tuxen1959a) and 1267 m by Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987), cause doubt.
Our data (Figure 5D): Skagerrak: Swedish Western Coast (22–60 m). Norwegian Coast: Vest-Agder (30–40 m), Rogaland (18–31 m), Hordaland (18–40 m), Møre and Romsdal (20–130 m).
DISCUSSION
Polymastia boletiformis has a confused taxonomic history. Since the description of Alcyonium boletiforme from an unknown locality by Lamarck (Reference Lamarck1815) this name had not been in use until Topsent (Reference Topsent1933) reported that Lamarck's type material and Polymastia robusta Bowerbank, Reference Bowerbank1862 from the British Isles were the same species. Although A. boletiforme was an older name than P. robusta Topsent (Reference Topsent1933) relegated the former to a synonym of the latter, and this was followed by most of the later authors (e.g. Arndt, Reference Arndt, Grimpe and Wagler1935; Alander, Reference Alander1942; Koltun, Reference Koltun1966; Cabioch, Reference Cabioch1968; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987) except for Burton (Reference Burton, Fridriksson and Tuxen1959a) who retained the name P. boletiformis. In the recent literature (Van Soest et al., Reference Van Soest, Picton and Morrow2000; Van Soest, Reference Van Soest, Costello, Emblow and White2001, Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016; Morrow et al., Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012) the name P. boletiformis was, however, prioritized instead of P. robusta following the principle of priority (Article 23.1 in Anonymous, 1999).
Polymastia robusta/P. boletiformis was recorded from a large geographic area from the Canadian Atlantic Coast (Lambe, Reference Lambe1896; Whiteaves, Reference Whiteaves1901) and Iceland (Burton, Reference Burton, Fridriksson and Tuxen1959a) to the European Atlantic Coast (Topsent, Reference Topsent1900; Arndt, Reference Arndt, Grimpe and Wagler1935; Borojevic, Reference Borojevic1967; Cabioch, Reference Cabioch1968; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987), British Isles (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; Van Soest et al., Reference Van Soest, Picton and Morrow2000), Swedish Coast (Alander, Reference Alander1942), Norwegian Coast (Burton, Reference Burton1930a), Barents Sea (Topsent, Reference Topsent1913) and Arctic Ocean (Koltun, Reference Koltun1966). The depth range recorded for P. robusta/P. boletiformis was also very large with the extreme deep-sea records by Burton (Reference Burton, Fridriksson and Tuxen1959a) and Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987). Furthermore, four species were relegated to synonyms of P. robusta: P. bulbosa Bowerbank, Reference Bowerbank1866 and P. ornata Bowerbank, Reference Bowerbank1866 from the British Isles by Topsent (Reference Topsent1900), Reniera nivea Hansen, Reference Hansen1885 from the Norwegian Sea by Burton (Reference Burton1930a) and P. euplectella Rezvoj, Reference Rezvoj1927 from the Barents and White Sea by Koltun (Reference Koltun1966).
However, Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) demonstrated clear morphological distinctions between P. euplectella and P. robusta, a radial choanosomal skeleton and three size categories of spicules in the former against a reticulate skeleton and two spicule categories in the latter. The present study has confirmed these differences by genetic data. Meanwhile, we have revealed the strong similarities between P. euplectella and Reniera nivea, relegating the former to a synonym of the latter (see Description of Polymastia nivea below). We can now assume that all records of P. robusta/P. boletiformis to the North and North-East from Nordmøre Coast in Norway very probably indicate P. nivea. Moreover, the present study has shown that P. bartletti, a Canadian species morphologically very similar to the Arctic-Scandinavian P. nivea, differs greatly from the latter as well as from the European P. boletiformis by genetics. We can therefore assume that the records of P. robusta/P. boletiformis from Canada may indicate P. bartletti (see the description of this species above). Finally, we have examined one of the dry syntypes of P. robusta BMNH 1930.7.3.20 and found that its choanosomal skeleton is radial as distinct from the commonly accepted definition of P. boletiformis (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; Van Soest et al., Reference Van Soest, Picton and Morrow2000; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012), but the condition of the syntypes prevents us from more detailed study. Unfortunately we have not examined P. bulbosa and P. ornata, and therefore we cannot conclude whether these two are separate species or conspecific with P. boletiformis, P. robusta or some other species.
Thus, for the moment, we gather under the name P. boletiformis South European, British and South Scandinavian Polymastia with intensive orange or yellow colour, a smooth surface with differentiated exhalant and inhalant papillae, a reticulate choanosomal skeleton and two spicule categories. These morphological similarities are confirmed by the genetic identity of the British and South Scandinavian individuals.
Polymastia grimaldii (Topsent, Reference Topsent1913)
(Figure 6)
Original description: Trichostemma grimaldii Topsent, Reference Topsent1913, p. 21, pl. I figure 4.
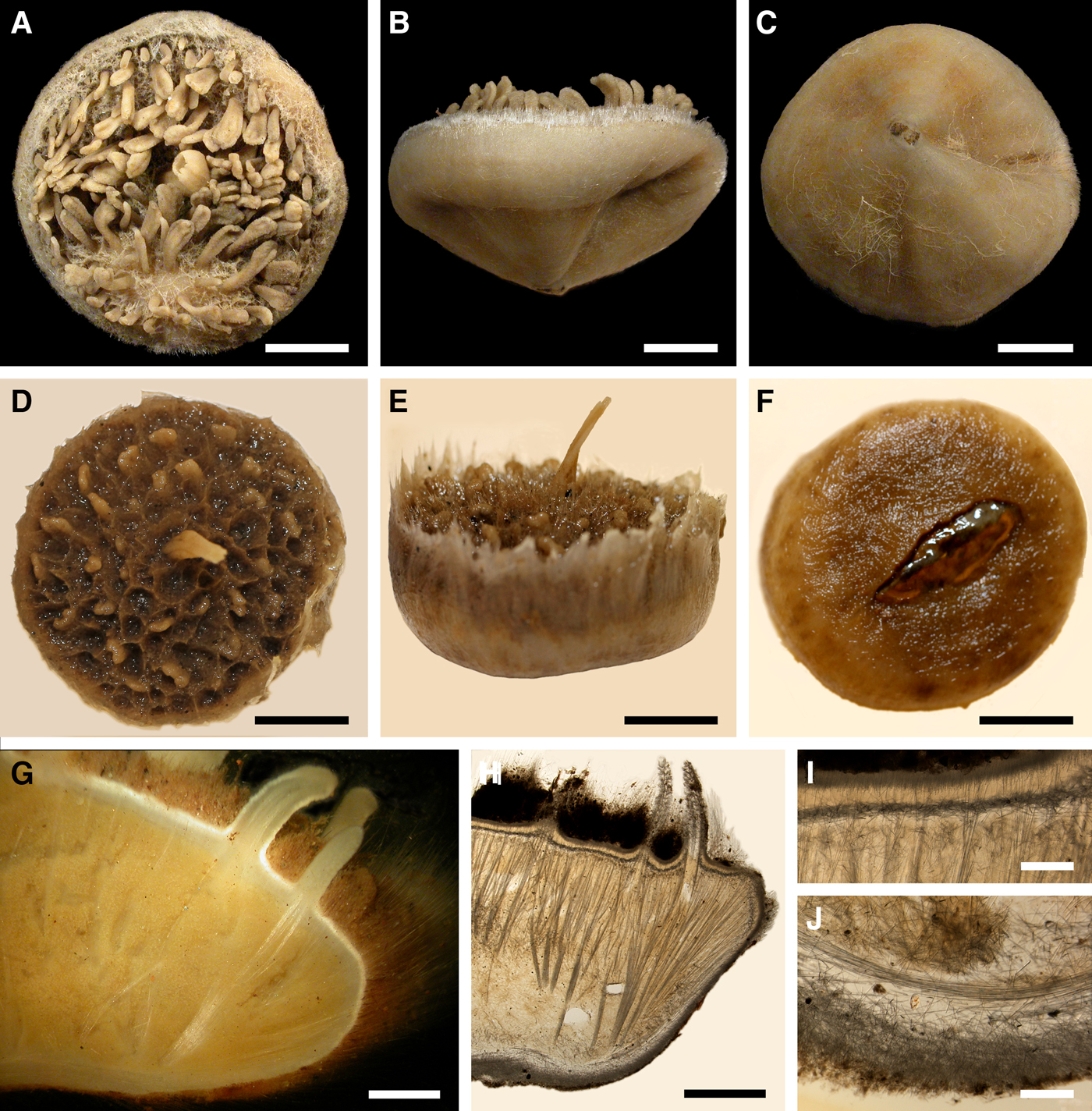
Fig. 6. Polymastia grimaldii: (A) lectotype of Trichostemma grimaldii, MOM 04-0840e, habitus, view from above; (B) the same, side view; (C) the same, bottom view; (D) holotype of Polymastia mamillaris var. hyperborea (synonym of P. grimaldii), UPSZTY 2103, habitus, view from above; (E) the same, side view; (F) the same, bottom view; (G) a fresh dissected individual from the Kandalaksha Bay, White Sea, (H) ZMBN 107576, longitudinal section through the body, general view; (I) the same section, detail of upper cortex; (J) the same section, detail of basal cortex. D–F: courtesy of P. Cárdenas, BioMedical Centre, University of Uppsala. Scale bars: A–F, 1 cm; G–H, 3 mm; I–J, 0.5 mm.
SYNONYMS AND CITATIONS
Polymastia grimaldii (Topsent, Reference Topsent1927a, p. 257; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 42, figure 7; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 542, figures 1e & 2e; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 2l).
Polymastia mamillaris (Vosmaer, Reference Vosmaer1885, p. 14, text-figure 5, pl. I figure 5, pl. III figures 10, 11–14, 21).
Polymastia mamillaris grimaldii (Koltun, Reference Koltun1966, p. 70, text-figures 39–40, pl. XX figures 1–5).
Polymastia mamillaris var. hyperborea Hentschel, Reference Hentschel, Théel and Lönnberg1916, p. 8 (Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929, pp. 868 and 923).
Polymastia penicillus (Vosmaer, Reference Vosmaer1882, p. 26, pl. I figures 12 & 13, pl. IV figures 127–132; Hansen, Reference Hansen1885, p. 9; Fristedt, Reference Fristedt1887, p. 434; Levinsen, Reference Levinsen and Lütken1887, p. 346).
Radiella grimaldii (Burton, Reference Burton, Fridriksson and Tuxen1959a: 13; Koltun, Reference Koltun1964, p. 149).
TYPE MATERIAL
Lectotype of Trichostemma grimaldii (designated herein, Figure 6A–C): MOM 04-0840e, East off Iceland, 65°21′N 10°42′W, 650 m, Campagnes scientifiques accomplies par le Prince Albert I de Monaco, RV ‘Princesse-Alice’, station 1040, 07.09.1898.
Paralectotypes of Trichostemma grimaldii: MOM 04-0840a–d, f–l (11 specimens), from the same sample as the lectotype.
Holotype of Polymastia mamillaris var. hyperborea Hentschel, Reference Hentschel, Théel and Lönnberg1916 (Figure 6D–F; herein considered as a synonym of Polymastia grimaldii): UPSZTY 2103, mouth of Nordfjorden, Svalbard, ~ 78°27.5′N 15°03.0′E, 197–190 m, Swedish expedition to Spitzbergen, station 99, 27.08.1908.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: Newfoundland: ZIN RAS ocpg059, ZIN RAS ocpg060, ZIN RAS ocpg061, ZIN RAS ocpg083, ZIN RAS ocpg096 and ZMBN 098110 (six specimens).
Greenland: Kangerdlugssuaqfjord: ZMBN 107579 (one specimen).
Denmark Strait: ZIN RAS ocpg133 (one specimen).
Norwegian Sea, offshore: ZIN RAS ocpg029, ZIN RAS ocpg030, ZIN RAS ocpg082 and ZIN RAS ocpg116 (five specimens).
Barents Sea, offshore: ZMBN 098112, ZMBN 107576 (two specimens) and 156 specimens deposited in ZIN RAS.
Norway: Troms: ZIN RAS ocpg075 (one specimen), Finnmark: ZMBN 098064 (one specimen).
Russia: Murman Coast: ZIN RAS ocpg051 (one specimen), White Sea: ZIN RAS ocpg001, ZIN RAS ocpg002, ZIN RAS ocpg003, ZIN RAS ocpg004, ZIN RAS ocpg147, ZIN RAS ocpg165, ZIN RAS ocpg166 and ZIN RAS ocpg167 (10 specimens), Kanin Peninsula: ZIN RAS ocpg088 (one specimen), Novaya Zemlya: ZIN RAS ocpg10, ZIN RAS ocpg020 and ZIN RAS ocpg097 (seven specimens), Taymyr Peninsula: ZIN RAS ocpg079, ZIN RAS ocpg126, ZIN RAS ocpg135, ZIN RAS ocpg143 and ZIN RAS ocpg158 (five specimens), Severnaya Zemlya: ZIN RAS ocpg160 (one specimen).
Kara Sea: ZIN RAS ocpg015, ZIN RAS ocpg064, ZIN RAS ocpg069, ZIN RAS ocpg095, ZIN RAS ocpg098, ZIN RAS ocpg100, ZIN RAS ocpg105, ZIN RAS ocpg113, ZIN RAS ocpg125 and ZIN RAS ocpg131 (10 specimens).
Laptev Sea: ZIN RAS ocpg146 (one specimen).
East Siberian Sea: ZIN RAS ocpg163 (seven specimens).
Arctic Ocean: ZIN RAS ocpg164 (four specimens).
DESCRIPTION
External morphology
Lectotype with almost circular, flat upper surface, 40–41 mm in diameter, and convex basal surface with a central point 22 mm distant from the upper surface (Figure 6A–C). Upper surface strongly hispid, greyish, with a single central exhalant papilla, cylindrical in shape, 9 mm long and 4 mm in diameter, and 118 inhalant papillae, most flattened, slightly widened towards the top, 1–6 mm in length and 0.7–2 mm in diameter at base (Figure 6A). Basal surface sleek, beige to grey, damaged in a central point indicating that the sponge was attached to a tiny substrate (Figure 6C). A fringe of extra-long spicules, 1.5 mm wide, developed at the sponge edge between the upper and basal surface (Figure 6B). Other sponges discoid, hemispherical with either the upper or the basal surface being convex, or sometimes lenticular. Upper surface up to 200 cm2, strongly hispid, usually covered with sediment, with up to 300 papillae, of which most are inhalant and one to six are exhalant, with well-visible oscula. The exhalant papillae cylindrical or conical, up to 16 mm in length. The inhalant papillae flattened, leaf-shaped, or sometimes cylindrical, up to 9 mm in length. Basal surface smooth, sometimes even sleek, attached to a small substrate only by a central point. Marginal fringe of extra-long spicules preventing sinking of the sponge into the sediment may be reduced in some individuals.
Anatomy
Choanosome in life pale orange or beige, firm (Figure 6G). Main choanosomal skeleton composed of tracts (65–655 µm thick) of principal spicules radiating from sponge base and dividing into two to four thinner tracts, which cross the upper cortex and form a surface hispidation (Figure 6G, H). Ascending tracts also form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered small spicules, especially concentrating below the upper cortex. Cortex in life whitish, firm, not detachable (Figure 6G). Skeleton of the upper cortex constituted by a superficial palisade (170–210 µm thick) of small spicules, a middle layer (100–180 µm thick) of collagen fibres with low density of spicules and an internal layer (100–140 µm thick) of tangentially arranged intermediary spicules (Figure 6I). Skeleton of the basal cortex (520–700 µm thick) formed by the peripheral tracts of principal spicules running parallel to the surface overlapped by a superficial palisade of small spicules and an inner confused mass of intermediary spicules (Figure 6J). Marginal fringe composed of bundles of extra-long spicules (exotyles) embedded into the cortex. Skeleton of the papilla walls composed of the superficial palisade and the internal tangential layer. Both inhalant and exhalant papillae with single central canals.
Spicules
(measurements based on 15 specimens)
Principal spicules – strongyloxeas, straight or gently curved. Length 1450–2358–3235, maximum diameter of shaft 21.0–24.8–29.2 µm, N = 450.
Intermediary spicules – tylostyles, straight or gently bent, fusiform. Length 212–445–671 µm, maximum diameter of shaft 15.8–18.4–24.0 µm, N = 450.
Small spicules – tylostyles to subtylostyles, straight or gently bent, usually slender. Length 147–228–287 µm, maximum diameter of shaft 3.8–6.4–7.8 µm, N = 450.
Exotyles (spicules of the marginal fringe) – flexuous styles, occasionally subtylostyles. Length 2020–4527–7000 µm, maximum diameter 8.0–9.4–10.6 µm, N = 150.
Genetic data
CO1 sequences obtained from three individuals of Polymastia grimaldii are identical. 28S rDNA available only from one of these individuals is polymorphic (Matrix M34256 in TreeBase). By both genes P. grimaldii is closely related to P. andrica and P. arctica (see the synapomorphies in the Genetic data section for P. andrica above). 28S rDNA of these three species displays intraspecific and intragenomic polymorphism, while the CO1 data are consistent (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). In CO1 P. grimaldii has one autapomorphy (Online resource 2, p. 1). Apart from the latter, this species differs from P. andrica by 12 bps, from P. arctica by 12 bps and from the type species of Polymastia, P. mamillaris, by 31 bps in CO1 (Matrix M34248 in TreeBase).
OCCURRENCE
(Figure 7)
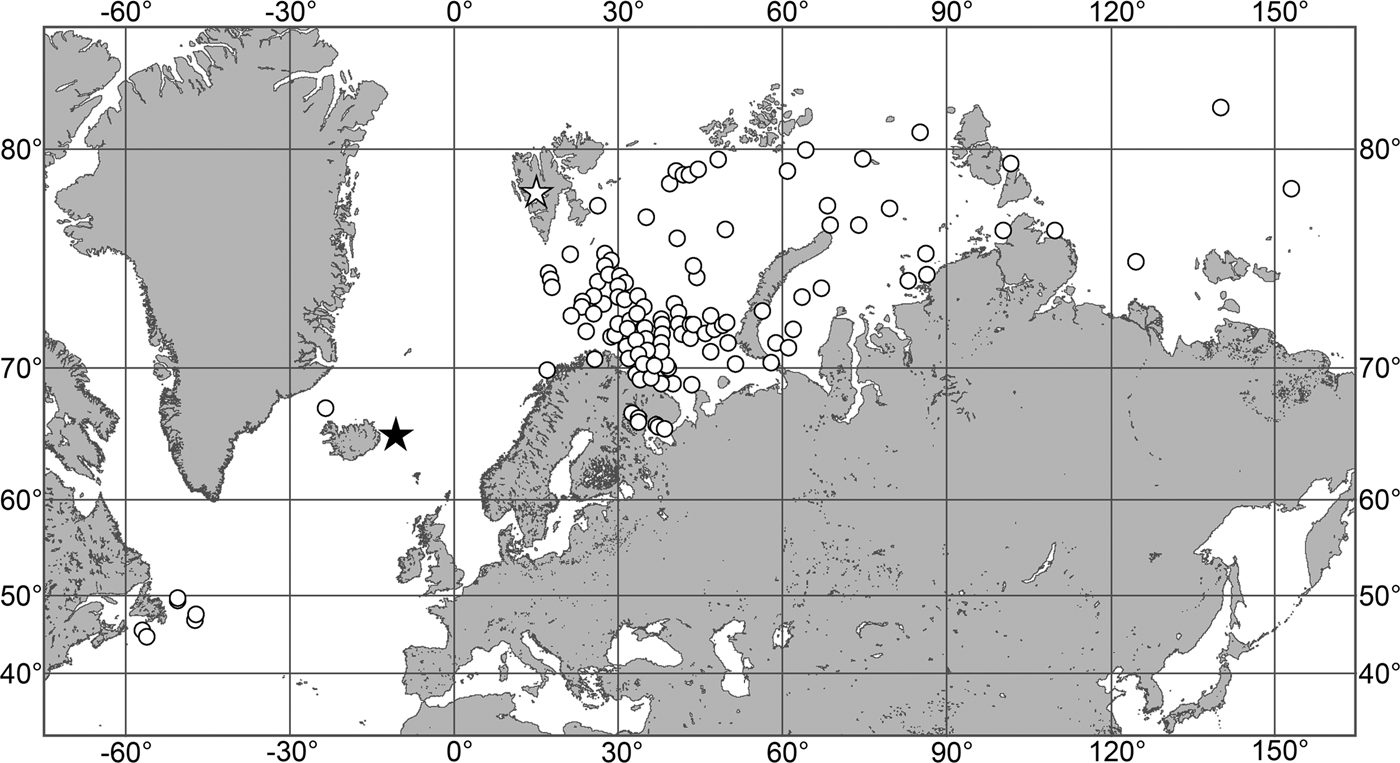
Fig. 7. Polymastia grimaldii, distribution: black star, type locality of Trichostemma grimaldii; white star, type locality of Polymastia mamillaris var. hyperborea (synonym of P. grimaldii); white circles, our data.
Our data (agree with the literature data): Canadian Atlantic Coast: Newfoundland (315–440 m). Greenland Sea: (640–680 m). Denmark Strait: (511 m). Norwegian Sea, offshore areas (120–420 m). Norwegian Coast: Troms (320 m), Finnmark (211 m). Barents Sea: Murman Coast (190 m), Kanin Peninsula (62 m), offshore areas (53–460 m). White Sea (18–100 m). Svalbard (190–197 m). Novaya Zemlya (93–459 m). Taymyr Peninsula (23–58 m). Severnaya Zemlya (237 m). Kara Sea (49–305 m). Laptev Sea (51 m). East Siberian Sea (73 m). Arctic Ocean (1900–1630 m).
DISCUSSION
Polymastia grimaldii was a key species in a long discussion on the relationships between a broadly acknowledged genus Polymastia and two genera with uncertain status, Radiella Schmidt, Reference Schmidt1870 and Trichostemma Sars, Reference Sars1872. The latter two names were since Schmidt (Reference Schmidt1880) often regarded as the synonyms for the same genus, but there were some debates about which of them should be considered as the senior name (see Discussion on Polymastia hemisphaerica (Sars, Reference Sars1872) below) until Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) relegated Trichostemma to a synonym of Radiella following the principle of priority (Article 23.1 in Anonymous, 1999).
Radiella/Trichostemma was usually distinguished from Polymastia by a radial growth pattern (a sponge attached to the substrate by a small point of the basal surface), the presence of a basal cortex distinct from the upper cortex and the presence of a fringe of extra-long monactines at the boundary between the upper and basal surface (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). All these features are displayed by Polymastia grimaldii, but at the same time this species possesses numerous papillae and a three-layered cortex including a middle layer of collagen fibres that rather resemble the type species of Polymastia, P. mamillaris, than Radiella spp. or Trichostemma spp. (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). Based on the similarities between P. grimaldii, P. mamillaris and other Polymastia spp. several early authors identified some sponges with evident distinctive features of Radiella/Trichostemma as P. penicillus (Vosmaer, Reference Vosmaer1882; Hansen, Reference Hansen1885; Fristedt, Reference Fristedt1887; Levinsen, Reference Levinsen and Lütken1887) or P. mamillaris (Vosmaer, Reference Vosmaer1885). It was Topsent (Reference Topsent1913) who established a new species, Trichostemma grimaldii, for the sponges combining the features of Radiella/Trichostemma and Polymastia. But, after a time, he re-considered the generic allocation of this species transferring it to Polymastia (Topsent, Reference Topsent1927a). In the same manner Koltun (Reference Koltun1964) initially placed grimaldii in Radiella, but two years later (Koltun, Reference Koltun1966) relegated it to a subspecies of Polymastia mamillaris. The uncertainty about the taxonomic affinities of P. grimaldii was perfectly expressed by Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 44): ‘P. grimaldii may be considered as a step on the evolutionary line which starts at Polymastia advancing to Trichostemma’.
This uncertainty was recently resolved by the phylogenies reconstructed from CO1 and 28S rDNA datasets (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b), where P. grimaldii formed a clade with Radiella hemisphaerica (formerly Trichostemma hemisphaericum, the type species of Trichostemma), P. mamillaris (the type species of Polymastia) and four other Polymastia spp. At the same time two species of Radiella grouped with the type species of Spinularia Gray, Reference Gray1867, S. spinularia (Bowerbank, Reference Bowerbank1866), outside the Polymastia-clade (see Discussion on the genus Spinularia below). Consequently, grimaldii and hemisphaerica are now affiliated with Polymastia.
Polymastia hemisphaerica (Sars, Reference Sars1872)
(Figure 8)
Original description: Trichostemma hemisphaericum Sars, Reference Sars1872, p. 62, pl. VI figures 1–15.
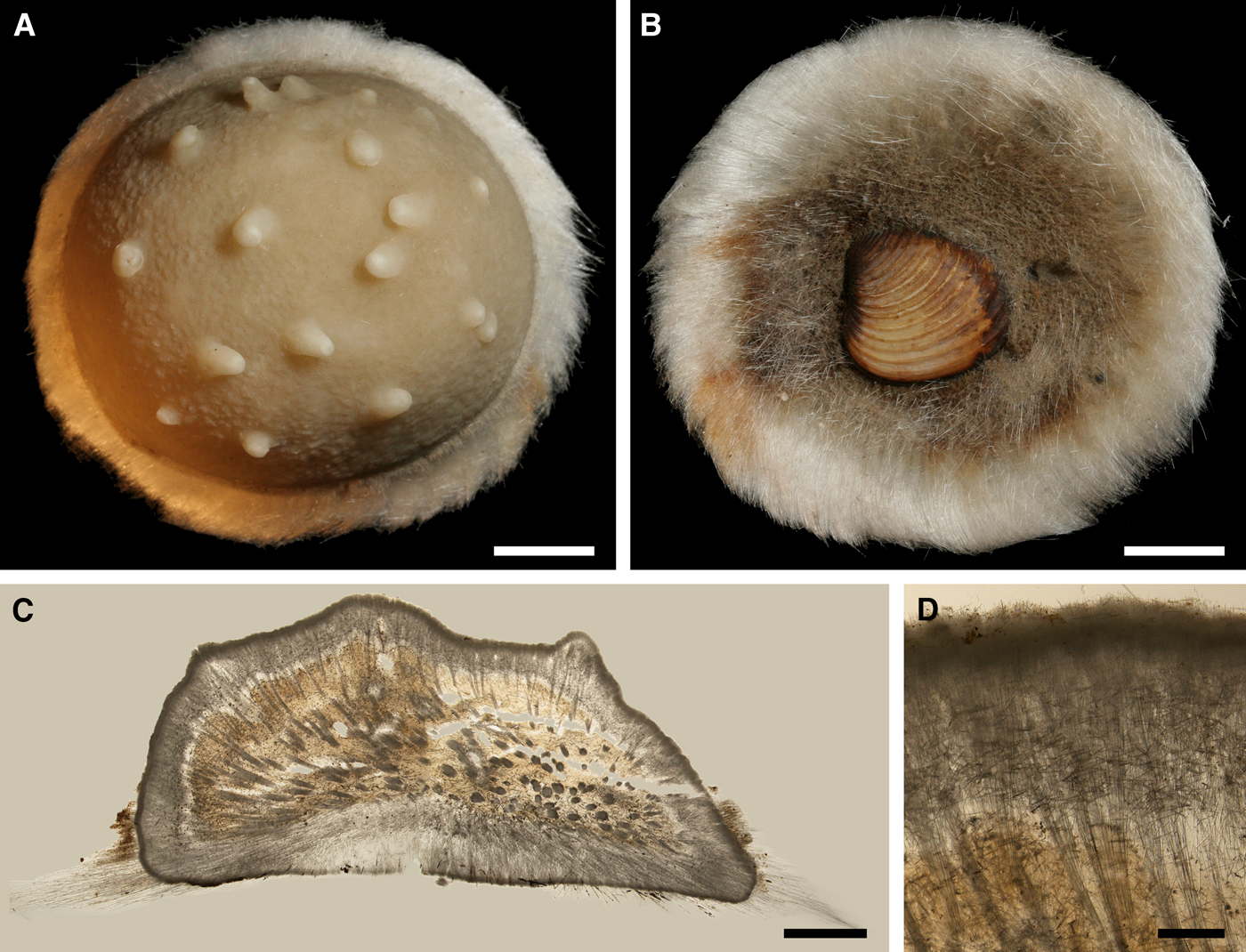
Fig. 8. Polymastia hemisphaerica: (A) holotype, NHMUO B862, habitus, view from above; (B) the same, bottom view; (C) ZMBN 098043, longitudinal section through the body, general view; (D) the same section, detail of upper cortex. Scale bars: A–B, 1 cm; C, 3 mm; D, 0.5 mm.
SYNONYMS AND CITATIONS
Halicnemia hemisphaerica (von Marenzeller, Reference von Marenzeller1878, p. 371).
Polymastia hemisphaerica (Vosmaer, Reference Vosmaer1885, p. 12; Topsent, Reference Topsent1892, p. 132; Rezvoj, Reference Rezvoj1924, p. 242).
Polymastia hemisphaericum (Koltun, Reference Koltun1966, p. 78, text-figure 51, pl. XXIX figures 1–5).
Radiella hemisphaerica (Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 542, figures 1f & 2f; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 27, figure 2k).
Radiella sol (Hansen, Reference Hansen1885, p. 7; Burton, Reference Burton1930a: 510 pars., Reference Burton, Fridriksson and Tuxen1959a: 12 pars.).
Suberites radians Hansen, Reference Hansen1885, p. 10, pl. II figure 7.
Trichostemma hemisphaericum (Sars, Reference Sars1869, p. 250, 265, 268 nomen nudum; Whiteaves, Reference Whiteaves1874, p. 184, Reference Whiteaves1901, p. 14; Lambe, Reference Lambe1896, p. 197, pl. II figures 7 & 7a–e; Lundbeck, Reference Lundbeck1909, p. 451; Topsent, Reference Topsent1913, p. 20, pl. I figure 2, pl. II figures 1 & 2).
TYPE MATERIAL
Holotype (specimen in alcohol): NHMUO B862, Lofoten, Nordland, Norway, 218–546 m.
Paralectotypes (three dry specimens): NHMUO B863, Brattesnes, Lofoten, Nordland, Norway, 182–218 m.
Paralectotype (specimen in alcohol): ZMBN 000136, Lofoten, Nordland, Norway, 218 m.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: Nova Scotia: ZIN RAS ocph002 (one specimen), Labrador and Newfoundland: ZIN RAS ocph004, ZIN RAS ocph024, ZIN RAS ocph025 and ZIN RAS ocph032 (four specimens), offshore areas of NW Atlantic: ZIN RAS ocph022, ZIN RAS ocph023, ZIN RAS ocph026, ZIN RAS ocph031 and ZIN RAS ocph038 (five specimens).
Greenland, SE Coast: ZIN RAS ocph037 and ZIN RAS ocph043 (two specimens).
Iceland: ZIN RAS ocph007, ZIN RAS ocph042 and ZMBN 098069 (three specimens).
Norwegian Sea, offshore: ZIN RAS ocph029, ZIN RAS ocph034, ZIN RAS ocph036 and ZIN RAS ocph041 (four specimens).
Barents Sea, offshore: ZIN RAS ocph008, ZIN RAS ocph011, ZIN RAS ocph012, ZIN RAS ocph013, ZIN RAS ocph014, ZIN RAS ocph015, ZIN RAS ocph016, ZIN RAS ocph017, ZIN RAS ocph018, ZIN RAS ocph019, ZIN RAS ocph020, ZIN RAS ocph027, ZIN RAS ocph028, ZIN RAS ocph030, ZIN RAS ocph035, ZIN RAS ocph040, ZIN RAS ocph044, ZMBN 098071 and ZMBN 107577 (19 specimens).
Norway: Hordaland: ZMBN 098043, ZMBN 098056, ZMBN 098058, ZMBN 098077 and ZMBN 107561 (five specimens), Møre and Romsdal: ZMBN 107486 (one specimen); Nord-Trøndelag: NTNU-VM-72542 (21 specimens), Nordland: NTNU-VM-66581 and NTNU-VM-72513 (two specimens).
Russia: Novaya Zemlya: ZIN RAS ocph001 and ZIN RAS ocph021 (two specimens).
Kara Sea: ZIN RAS ocph006 (one specimen).
DESCRIPTION
External morphology
Holotype hemispherical, 80–86 mm in diameter. Upper surface convex, knobbly, cream-coloured, with 18 papillae (Figure 8A). Papillae conical, 1–5 mm long and 1.5–3.5 mm wide at base, with considerably contracted oscula at the summits. Basal surface shaggy, pale grey in colour, attached to a bivalve shell by the central point (Figure 8B). A fringe of extra-long spicules, 4–9 mm wide, developed at the sponge edge separating the upper and basal surface. Other sponges hemispherical or discoid, up to 65 mm in diameter, with the marginal spicule fringe up to 13 mm in width. Upper surface whitish or cream-coloured in life, knobbly, with up to 30 conical papillae. In living sponges the papillae with well visible oscula. Under sampling and preservation the papillae stretch and the oscula contract. Basal surface shaggy or hispid, attached to a small substrate.
Anatomy
Choanosome in life yellowish or pale orange, firm. Main choanosomal skeleton composed of tracts (190–416 µm thick) of principal spicules radiating from the basal area and entering the cortex (Figure 8C). In the upper cortex the tracts run perpendicular to the surface and do not protrude. Some tracts ascend to the papillae. In the basal cortex the tracts run obliquely to the surface and stick out forming a thick hispidation. Auxiliary choanosomal skeleton comprises free-scattered small spicules and bundles of intermediary spicules concentrated in the subcortical area. Cortex in life whitish, firm, not detachable. Cortical skeleton constituted by a notched superficial palisade (366–522 µm thick) of small spicules and an internal layer (663–930 µm thick in the upper cortex and up to 1280 µm thick at the basal central point) of criss-cross intermediary spicules (Figure 8C, D). Marginal fringe composed of bundles of extra-long spicules (exotyles) embedded into the cortex. In the upper part of the body the cortex and the choanosome separated by an area with low concentration of spicules (127–206 µm thick). Papilla walls reinforced with the cortical palisade. Each papilla with a central exhalant canal and several peripheral inhalant canals.
Spicules
(Measurements based on 10 specimens)
Principal spicules – styles to subtylostyles, straight or gently curved, slightly fusiform. Length 1920–3125–5400, maximum diameter of shaft 10.9–20.2–32.3 µm, N = 200.
Intermediary spicules – tylostyles, straight, fusiform. Length 390–512–618 µm, maximum diameter of shaft 17.2–19.9–26.2 µm, N = 300.
Small spicules – tylostyles, usually gently bent in the distal part, slender. Length 160–229–305 µm, maximum diameter of shaft 4.2–5.8–8.4 µm, N = 300.
Exotyles (spicules of the marginal fringe) – styles, straight, slightly fusiform. Length 4990–6511–8015 µm, maximum diameter 45.8–47.5–50.1 µm, N = 100.
Genetic data
CO1 was obtained from six individuals of Polymastia hemisphaerica, while 28S rDNA were sequenced only from three of them. By both genes P. hemisphaerica is closely related to morphologically quite different P. thielei (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These species share two synapomorphies in CO1 (Online resource 2, p. 2) and two synapomorphies in 28S rDNA (Online resource 3, p. 2) and, apart from them, differ from the type species of Polymastia, P. mamillaris, by 18 bps in CO1 (Matrix M34248 in TreeBase) and six bps in 28S rDNA (Matrix M34250 in TreeBase). At the same time P. hemisphaerica demonstrates an intraspecific polymorphism. In CO1 five individuals of this species differ from P. thielei just by one bp, while one individual, ZMBN 098056, differs from P. thielei by two bps (Matrix M34248 in TreeBase). On the contrary, by 28S rDNA ZMBN 098056 is identical to two other conspecific sponges differing from P. thielei just by one insertion in this gene, while another individual of P. hemisphaerica, ZMBN 098043 possessing the same insertion, is distinguished from both the conspecific individuals and P. thielei by three bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
(Figure 9)
Fig. 9. Polymastia hemisphaerica, distribution: white star, type locality; black crosses, data from Rezvoj (Reference Rezvoj1924); black diamond, data from Topsent (Reference Topsent1892); white diamond, data from Topsent (Reference Topsent1913); white circles, our data.
Literature data: Canadian Atlantic Coast: Gulf of St. Lawrence (204 m) (Whiteaves, Reference Whiteaves1874, Reference Whiteaves1901; Lambe, Reference Lambe1896).
Our data: Canadian Atlantic Coast: Nova Scotia (355–480 m), Newfoundland (our data: 383–415 m, Topsent, Reference Topsent1892: 1267 m), Labrador (225–485 m), offshore areas (230–450 m). Greenland, SE Coast (212–405 m). Iceland (214–650 m). Norwegian Sea, offshore (250–480 m). Norwegian Coast: Hordaland (300–312 m), Møre and Romsdal (100 m), Nord-Trøndelag (200 m), Nordland (182–850 m). Barents Sea, offshore (180–369 m). Russia: Novaya Zemlya (153–270 m). Kara Sea (our data: 368 m, Rezvoj, Reference Rezvoj1924: 91–200 m).
DISCUSSION
Polymastia hemisphaerica was originally described as Trichostemma hemisphaericum Sars, Reference Sars1872 designated as the type species of Trichostemma Sars, Reference Sars1872. In fact, this genus and this species were first mentioned in a list of the Norwegian sponges three years earlier (Sars, Reference Sars1869), although without any description. Von Marenzeller (Reference von Marenzeller1878) regarded Trichostemma as a synonym of Halicnemia Bowerbank, Reference Bowerbank1864, while Schmidt (Reference Schmidt1880) put T. hemisphaericum in synonymy with Radiella sol Schmidt, Reference Schmidt1870, one of the two species for which he had earlier established Radiella Schmidt, Reference Schmidt1870 (for more details on the taxonomic history of Radiella see Discussion on the genus Spinularia below). Consequently, Trichostemma was regarded as a synonym of Radiella (Schmidt, Reference Schmidt1880). This was encouraged by Hansen (Reference Hansen1885) and Burton (Reference Burton1930a, Reference Burtonb, Reference Burton, Fridriksson and Tuxen1959a). However, all other authors recognized Trichostemma hemisphaericum and T. sol as two different species, although they agreed that Trichostemma and Radiella were synonyms. Most authors encouraged the precedence of the former over the latter, referring to its first record in Sars (Reference Sars1869) (Whiteaves, Reference Whiteaves1874, Reference Whiteaves1901; Ridley & Dendy, Reference Ridley and Dendy1886, Reference Ridley and Dendy1887; von Lendenfeld, Reference von Lendenfeld1887; Lambe, Reference Lambe1896; Topsent, Reference Topsent1904, Reference Topsent1913, Reference Topsent1928; Lundbeck, Reference Lundbeck1909; Wilson, Reference Wilson1925; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; Uriz & Rosell, Reference Uriz and Rosell1990; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994). Conversely, Vacelet (Reference Vacelet1961) and Koltun (Reference Koltun1964) considered Radiella as the senior synonym, while Vosmaer (Reference Vosmaer1885), Levinsen (Reference Levinsen and Lütken1887) and Rezvoj (Reference Rezvoj1924) relegated Trichostemma to a synonym of Polymastia and Vosmaer (Reference Vosmaer and Bronn1887) and Koltun (Reference Koltun1966) relegated both Radiella and Trichostemma to synonyms of Polymastia. Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) recognized Radiella as a valid genus and the record of Trichostemma by Sars (Reference Sars1869) as nomen nudum, and hence acknowledged the synonymization of Trichostemma with Radiella based on the principle of priority (Article 23.1 in Anonymous, 1999). This decision was further encouraged by Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) and Plotkin et al. (Reference Plotkin, Gerasimova and Rapp2012).
However, very recently, Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) re-considered the relationships between Trichostemma, Radiella and Polymastia based on genetic data. In the CO1 and 28S rDNA phylogenies Trichostemma hemisphaericum (referred to as Radiella hemisphaerica by Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b) appeared in a strongly supported clade including the type species of Polymastia, P. mamillaris, and five other Polymastia spp. At the same time two species of Radiella, R. sarsii (Ridley & Dendy, Reference Ridley and Dendy1886) and Radiella sp., fell in a remote clade (for details on the current status of Radiella see Discussion on the genus Spinularia below). Since Polynastia was an older name than Trichostemma, the latter was relegated to a synonym of the former (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). This is followed in the present study.
Polymastia mamillaris (Müller, Reference Müller1806)
(Figure 10)
Original description: Spongia mamillaris Müller, Reference Müller1806, p. 44.
Fig. 10. Polymastia mamillaris: (A) holotype, ZMUC-DEM-394, habitus; (B) ZMBN 098083 in situ, Fedafjorden, Norway (courtesy of E. Svensen, OceanPhoto/Dalane Tidende AS, Egersund); (C) holotype, ZMUC-DEM-394, longitudinal section through the body, general view; (D) the same section, detail of upper cortex; (E) distribution: white star, type locality; white diamonds, data from Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000); white circles, our data. Scale bars: A, 1 cm; C, 1 mm; D, 0.2 mm.
SYNONYMS AND CITATIONS
Pencillaria penicillus (Gray, Reference Gray1867, p. 527 pars.).
Polymastia mamillaris (Fristedt, Reference Fristedt1885, p. 15; Alander, Reference Alander1942, p. 75; Morrow & Boury-Esnault, Reference Morrow and Boury-Esnault2000, p. 329, figures 1 & 2D–F; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 203, figure 2; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 11).
Non Polymastia mamillaris (Bowerbank, Reference Bowerbank1862, p. 1104, Reference Bowerbank1864, p. 178, Reference Bowerbank1866, p. 71; Vosmaer, Reference Vosmaer1885, p. 14, text-figure 5, pl. I figure 5, pl. III figures 10, 11–14, 21; Verrill, Reference Verrill1874, p. 505; Whiteaves, Reference Whiteaves1874, p. 184; Lambe, Reference Lambe1896, p. 196, pl. III figure 1; Whiteaves, Reference Whiteaves1901, p. 13; Arnesen, Reference Arnesen1918, p. 8, pl. 1 figures 1–4, pl. 2 figures 1–5, pl. 3 figures 6–9, pl. 4 figures 1–2; pl. 5 figures 1–2, pl. 6 figures 1–4; Koltun, Reference Koltun1966, p. 69; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 32, figure 1; Ereskovsky, Reference Ereskovsky1993a, p. 22; Plotkin & Ereskovsky, Reference Plotkin and Ereskovsky1997, p. 127).
TYPE MATERIAL
Holotype (Figure 10A): ZMUC-DEM-394, East of Aspholmen in Byfjorden, Orust, Västra Götaland, Sweden, 58°15′N 11°50′E, depth unknown.
Detailed description of the holotype was presented by Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Norway: Vest-Agder: ZMBN 098083 (one specimen), Hordaland: ZMBN 098078 (one specimen).
DESCRIPTION
External morphology
Cushion-shaped sponges covering the substrate and occupying up to 9 cm2. Surface hispid, usually covered with sediment, with up to 30 papillae, 5–12 mm long and 2–5 mm wide at base (Figure 10A, B). In living sponges the papillae tapering towards the summits, some with visible oscula (Figure 10B). Colour of papillae whitish in life and cream-coloured in alcohol.
Anatomy
Choanosome in life cream-coloured, firm. Main choanosomal skeleton of radiating tracts (240–370 µm thick) of principal spicules dividing into two to three thinner tracts, which cross the cortex and form a surface hispidation (Figure 10C). Ascending tracts also form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered bundles, each of two to five small spicules, being especially abundant in the subcortical area. Cortex in life whitish, firm, not detachable. Cortical skeleton constituted by a superficial palisade (120–150 µm thick) of small spicules, a middle layer (65–105 µm thick) of collagen fibres with low density of spicules and an internal layer (85–155 µm thick) of tangentially arranged intermediary spicules (Figure 10D). Skeleton of the papilla walls composed of two layers only, the superficial palisade and the internal tangential layer.
Spicules
(Measurements based on three specimens, individual variation presented in Table 3)
Table 3. Individual variation of spicule dimensions of Polymastia mamillaris (given in μm as minimum–mean–maximum). Parameters: length, diameter of tyle, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).
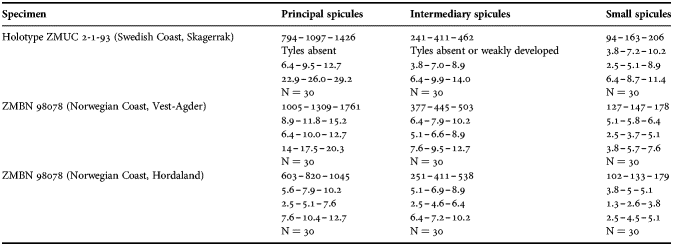
Principal spicules – straight strongyloxeas or straight, fusiform styles to subtylostyles. Length 603–1075–1761 µm, diameter of tyle (if present) 5.6–9.8–15.2 µm, proximal diameter of shaft 2.5–8.3–12.7 µm, maximum diameter of shaft 7.6–18.0–29.2 µm, N = 90.
Intermediary spicules – styles, occasionally subtylostyles, usually straight, slightly fusiform. Length 241–422–538 µm, diameter of tyle (if present) 5.1–7.4–10.2 µm, proximal diameter of shaft 2.5–6.1–8.9 µm, maximum diameter of shaft 6.4–8.9–14.0 µm, N = 90.
Small spicules – tylostyles, gently curved or straight, stout. Length 94–148–206 µm, diameter of tyle 3.8–6.0–10.2 µm, proximal diameter of shaft 1.3–3.8–8.9 µm, maximum diameter of shaft 2.5–6.3–11.4 µm, N = 90.
Genetic data
CO1 sequences obtained from two individuals of Polymastia mamillaris are identical (Matrix M34248 in TreeBase), but these sponges are distinguished by one bp in 28S rDNA (Matrix M34250 in TreeBase). Polymastia mamillaris differs from all other polymastiids by five autapomorphies in CO1 (Online resource 2, p. 1) and one autapomorphy in 28S rDNA (Online resource 3, p. 1). More details on the differences between P. mamillaris and other Polymastia spp. are presented in the Genetic data sections for these species.
OCCURRENCE
(Figure 10E)
Literature data: Swedish Western Coast: Skagerrak (76–225 m) (Morrow & Boury-Esnault, Reference Morrow and Boury-Esnault2000).
Our data: Norwegian Coast: Vest-Agder (40–45 m), Hordaland (300–310 m).
DISCUSSION
Polymastia mamillaris has a very confused taxonomic history. This species was originally described from the Swedish Western Coast as Spongia mamillaris (Müller, Reference Müller1806). Johnston, Reference Johnston1842 transferred it to Halichondria Fleming, Reference Fleming1828 and simultaneously relegated a British species Spongia penicillus Montagu, Reference Montagu1814 to a synonym of H. mamillaris. Bowerbank (Reference Bowerbank1862) erected a new genus, Polymastia, for H. mamillaris. For unclear reason Gray (Reference Gray1867) erected another new genus, Pencillaria, for Polymastia mamillaris, but this was not acknowledged by the later authors. Vosmaer (Reference Vosmaer1882) admitted that Spongia mamillaris and S. penicillus were the same species of Polymastia, but considered penicillus as the senior synonym for this species despite it having been described earlier than mamillaris. Moreover, in the same paper Vosmaer synonymized a White Sea species Rinalda arctica Merejkowsky, Reference Merejkowsky1878 with P. penicillus and also placed in this species some Barents Sea sponges which were in fact distinguished from the original descriptions of Spongia mamillaris, S. penicillus and R. arctica by a radial growth type and a marginal spicule fringe (i.e. the evident features of the species now recognized as P. grimaldii, see the respective Description and Discussion above). Three years later (Vosmaer, Reference Vosmaer1885) he re-considered the synonymy by declaring Polymastia mamillaris the senior synonym. Fristedt (Reference Fristedt1885) recorded P. mamillaris along the Swedish Western Coast. Later (Fristedt, Reference Fristedt1887) he identified as P. penicillus some sponges with the radial growth type and the marginal spicule fringe from Greenland, Svalbard and the Siberian Seas referring to Vosmaer (Reference Vosmaer1882) and noted that P. penicillus (Montagu, Reference Montagu1814) sensu Vosmaer (Reference Vosmaer1882) from the Arctic region and Polymastia mamillaris (Müller, Reference Müller1806) from Sweden were different species. Levinsen (Reference Levinsen and Lütken1887) recorded P. penicillus from the Kara Sea and agreed with Vosmaer (Reference Vosmaer1882), although with some doubt, that P. mamillaris and Rinalda arctica were the synonyms of P. penicillus. Whiteaves (Reference Whiteaves1874, Reference Whiteaves1901) and Lambe (Reference Lambe1896) recorded P. mamillaris in the Canadian Atlantic. But de Laubenfels (Reference de Laubenfels1949) established a new species P. andrica for these Canadian sponges, although without good argumentation. Topsent (Reference Topsent1913) erected a new species, Trichostemma grimaldii, for the Nordic Polymastia-looking sponges with the radial growth type and the marginal spicule fringe. But later (Topsent, Reference Topsent1927a) he transferred grimaldii to Polymastia. Hentschel (Reference Hentschel, Théel and Lönnberg1916) described a new variety, P. mamillaris var. hyperborea from Svalbard, which in fact resembled P. grimaldii. Koltun (Reference Koltun1966) relegated grimaldii to a subspecies of P. mamillaris. According to his extended definition P. mamillaris included three subspecies, P. mamillaris mamillaris comprising all sponges similar to Spongia mamillaris Müller, Reference Müller1806 and Rinalda arctica Merejkowsky, Reference Merejkowsky1878 from the North Atlantic, Nordic Seas and Arctic, P. mamillaris grimaldii comprising all sponges from the same regions, resembling P. mamillaris mamillaris by numerous papillae and architecture of the upper cortex, but differing by the radial growth type and the marginal spicule fringe, and P. mamillaris rara Koltun, Reference Koltun1966, a NW Pacific subspecies differing from P. mamillaris mamillaris by longer principal spicules.
Since Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987) P. mamillaris and P. grimaldii are, however, recognized as two separate species. Later, based on a careful comparison between the holotypes of Spongia mamillaris and S. penicillus and additional material Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000) demonstrated that P. mamillaris and P. penicillus were different species too. According to this study most of the previous records of P. mamillaris from the British Isles, French, Spanish and Portuguese Coasts in fact represent P. penicillus characterized by a two-layered cortex and spicules in all size categories being tylostyles. Polymastia mamillaris distributed only along the Swedish Coast is distinguished by a three-layered cortex (with a middle layer of collagen fibres), principal spicules being strongyloxeas and intermediary spicules being styles. Furthermore, Plotkin & Boury-Esnault (Reference Plotkin and Boury-Esnault2004) proved that Polymastia arctica (originally placed in Rinalda) commonly synonymized either with P. mamillaris or with P. penicillus was actually a valid species distributed in the White and Barents Sea and distinguished by the bud formation on the papillae, relatively thick middle and intermediate layers in the cortex and the presence of spicules in the bulkheads separating canals in the papillae. Now we can finally confirm the morphological differences between P. arctica, P. grimaldii, P. mamillaris and P. penicillus by genetic evidence (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; present study). Moreover, we have revealed that P. andrica erected by de Laubenfels (Reference de Laubenfels1949) for the Canadian records of P. mamillaris is a valid species distributed from Canada to the Norwegian Coast and Svalbard, differing from the similar Polymastia spp. by genetics and morphologically distinguished by the presence of extra-long cortical spicules (exotyles) (see the respective description above).
Polymastia nivea (Hansen, Reference Hansen1885)
(Figure 11)
Original description: Reniera nivea Hansen, Reference Hansen1885, p. 5, pl. I figure 6.
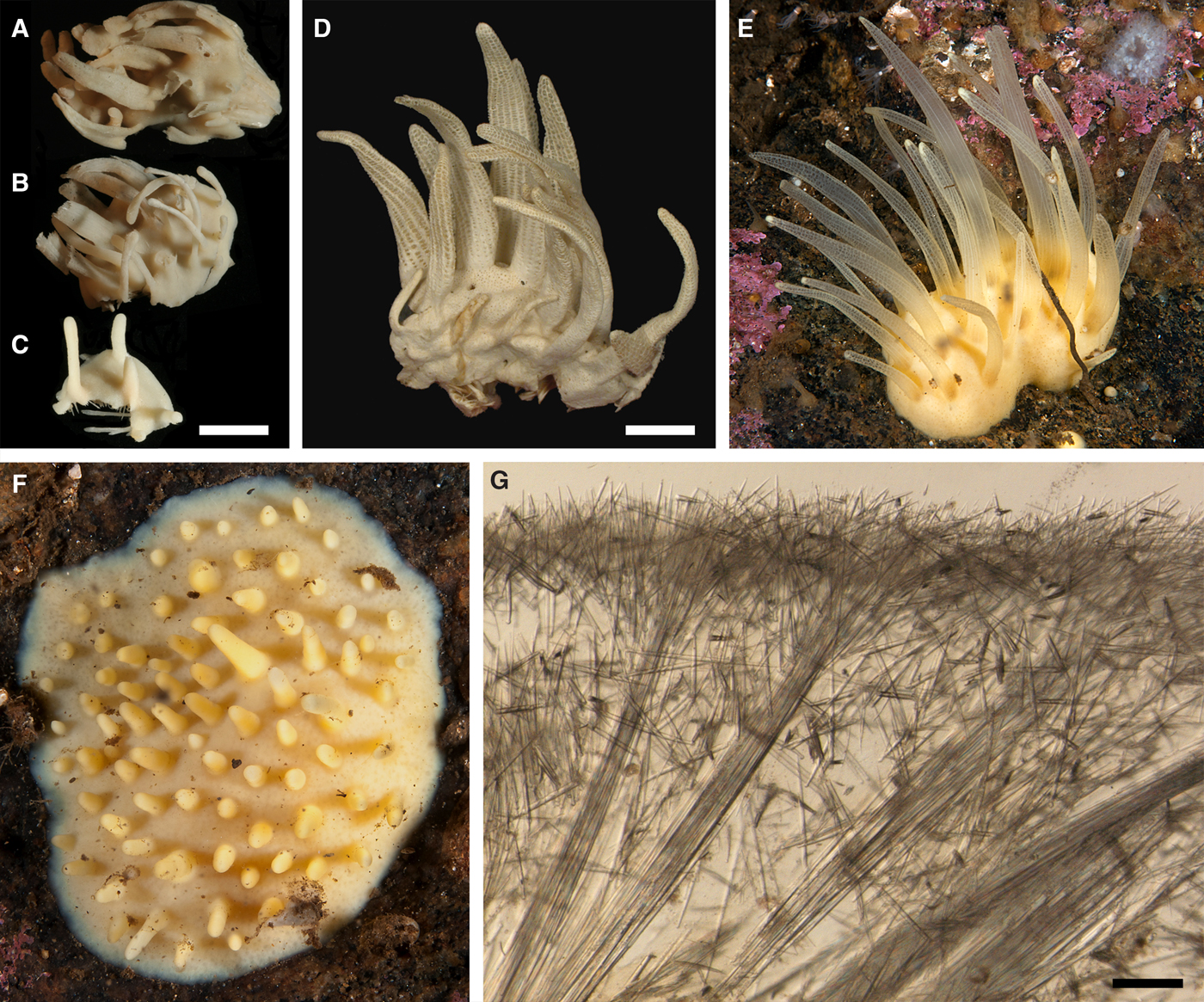
Fig. 11. Polymastia nivea: (A) lectotype of Reniera nivea, ZMBN 000055, habitus; (B) and (C) paralectotypes of Reniera nivea, ZMBN 000055, habitus; (D) holotype of Polymastia euplectella (synonym of P. nivea), ZIN RAS 7102/7103, habitus; (E) an individual in situ, Sør-Trøndelag, Norway (courtesy of E. Svensen, OceanPhoto/Dalane Tidende AS, Egersund); (F) an individual in situ, Fedafjorden, Norway (courtesy of E. Svensen); (G) lectotype of Reniera nivea, ZMBN 000055, longitudinal section through the body. Scale bars: A–D, 1 cm; G, 0.2 mm.
SYNONYMS AND CITATIONS
Polymastia euplectella Rezvoj, Reference Rezvoj1927, p. 301, figure a, b (Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 541, figures 1b & 2b; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1k).
Polymastia robusta robusta (Koltun, Reference Koltun1966, p. 74, text-figure 44, pl. XXI figures 1–4).
TYPE MATERIAL
Lectotype (designated herein, Figure 11A) and two paralectotypes of Reniera nivea Hansen, Reference Hansen1885: ZMBN 000055, Norwegian Sea, precise locality and depth unknown, Norwegian North Atlantic Expedition, 1876–1878.
Holotype of Polymastia euplectella Rezvoj, Reference Rezvoj1927: ZIN RAS 7102/7103 (slide 125), Three Sisters Rocks, Kildin Strait, Murman Coast of the Barents Sea, Russia, 69°18.31′N 34°18.82′E, depth unknown, 31.07.1924. Herein P. euplectella is relegated to a synonym of P. nivea.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Norway: Vest-Agder: ZMBN 098085, ZMBN 098086 and ZMBN 098087 (three specimens), Hordaland: ZMBN 009667 (one specimen), Møre and Romsdal: ZMBN 107484 (one specimen), Sør-Trøndelag: NTNU-VM-54701 (one specimen), Nordland: NTNU-VM-55006, NTNU-VM-58254, NTNU-VM-72508, NTNU-VM-72514 (18 specimens), Finnmark: NTNU-VM-54880 and ZMBN 098044 (three specimens).
Barents Sea, offshore: ZIN RAS ocpe014 (one specimen).
Russia: Murman coast: ZIN RAS ocpe001, ZIN RAS ocpe009, ZIN RAS ocpe010, ZIN RAS ocpe011, ZIN RAS ocpe012, ZIN RAS ocpe12 and ZIN RAS ocpe15 (seven specimens), White Sea: ZIN RAS ocpe002, ZIN RAS ocpe004, ZIN RAS ocpe005, ZIN RAS ocpe006 and ZIN RAS ocpe008 (12 specimens).
DESCRIPTION
External morphology
All type specimens of Reniera nivea cream-coloured in alcohol, with smooth surface bearing damaged cylindrical papillae (Figure 11A–C). Lectotype ~30 × 17 × 6 mm, with 19 papillae (Figure 11A). Larger paralectotype 24 × 18 × 5 mm, with 20 papillae (Figure 11B). Smaller lectotype is a small sponge fragment with five papillae (Figure 11C). Holotype of Polymastia euplectella ~30 × 12 × 5 mm (Figure 11D). Surface smooth, with 18 papillae, 10–40 mm long and 3–7 mm wide at base, gently pointed at the summits. Colour of both the surface and papillae whitish in alcohol. Papilla walls display a conspicuous tracery network of spicules.
Other sponges cushion-shaped, covering the substrate and occupying up to 20 cm2. Surface smooth, free of sediment, with up to 30 papillae (Figure 11E, F). Colour of the surface in life pale orange or pale yellow, sometimes whitish, in alcohol always becoming whitish. Papillae of most living individuals cylindrical, 8–60 mm in length and 2–8 mm in diameter, semitransparent with well-visible spicule network, oscula not visible (Figure 11E). Some sponges with much smaller (2–6 mm in length and 1–4 mm in diameter) papillae of the same colouration as the surface (Figure 11F).
Anatomy
Choanosome dense. Main choanosomal skeleton composed of longitudinal or radial tracts (110–310 µm thick) of principal spicules (Figure 11G). Auxiliary choanosomal skeleton comprises small and intermediary spicules, most free-scattered, some in bundles of three to seven. Cortex 275–460 µm thick, dense, but friable, not detachable, with small aquiferous cavities connected with ostia in the surface. Cortical skeleton constituted by a superficial palisade of small spicules, which is overlapped with an inner layer of criss-cross intermediary spicules (Figure 11G). Papilla with a single central canal enveloped by a network made of the tracts of principal spicules ascending from the choanosome crossed by bundles of intermediary spicules from the outside. Superficial layer of small spicules covers this network.
Spicules
(Measurements based on four specimens, individual variation presented in Table 4)
Table 4. Individual variation of spicule dimensions of Polymastia nivea (given in μm as minimum–mean–maximum). Parameters: length, diameter of tyle, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).
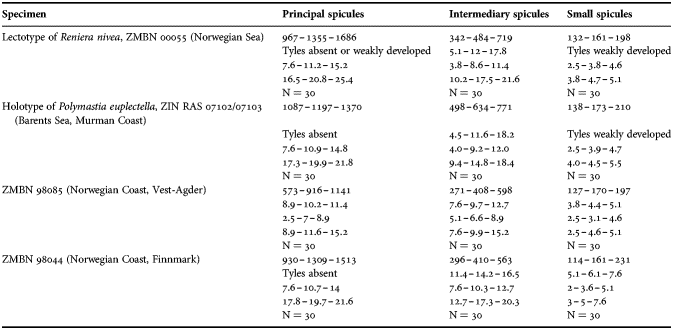
Principal spicules – strongyloxeas (ZMBN 000055), slender styles to subtylostyles (ZIN RAS 07102/07103, ZMBN 098044), or fusiform subtylostyles with displaced tyles (ZMBN 098085), usually straight, occasionally gently curved. Length 573–1194–1686 µm, diameter of tyle (if present) 8.9–10.2–11.4 µm, proximal diameter of shaft 2.5–9.6–15.2 µm, maximum diameter of shaft 8.9–17.4–25.4 µm, N = 120.
Intermediary spicules – gently curved tylostyles, usually stout or slightly fusiform (most sponges), occasionally slenderer (ZMBN 098085). Length 271–434–771 µm, diameter of tyle (if present) 5.1–11.9–18.2 µm, proximal diameter of shaft 3.8–8.4–12.7 µm, maximum diameter of shaft 7.6–14.7–21.6 µm, N = 120.
Small spicules – subtylostyles with weakly developed tyles, usually curved and slender. Length 114–164–231 µm, diameter of tyle 3.8–5.2–7.6 µm, proximal diameter of shaft 2.0–3.5–5.1 µm, maximum diameter of shaft 2.5–4.8–7.6 µm, N = 120.
Genetic data
CO1 was obtained from four individuals of Polymastia nivea, 28S rDNA was sequenced from three of them and no intraspecific polymorphism was revealed. In the phylogenies based on these genes P. nivea is closely related to P. penicillus (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b), although these species share just two synapomorphies in 28S rDNA distinguishing them from other polymastiids (Online resource 3, p. 3) and no such synapomorphies in CO1. At the same time P. nivea possesses one autapomorphy in CO1 (Online resource 2, p. 3) and four autapomorphies in 28S rDNA (Online resource 3, p. 3). Apart from these autapomorphies, P. nivea differs from P. penicillus by 6 bps in CO1 (Matrix M34248 in TreeBase) and 19 bps in 28S rDNA (Matrix M34250 in TreeBase) and from the type species of Polymastia, P. mamillaris, by 56 bps in CO1 (Matrix M34248 in TreeBase) and 85 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
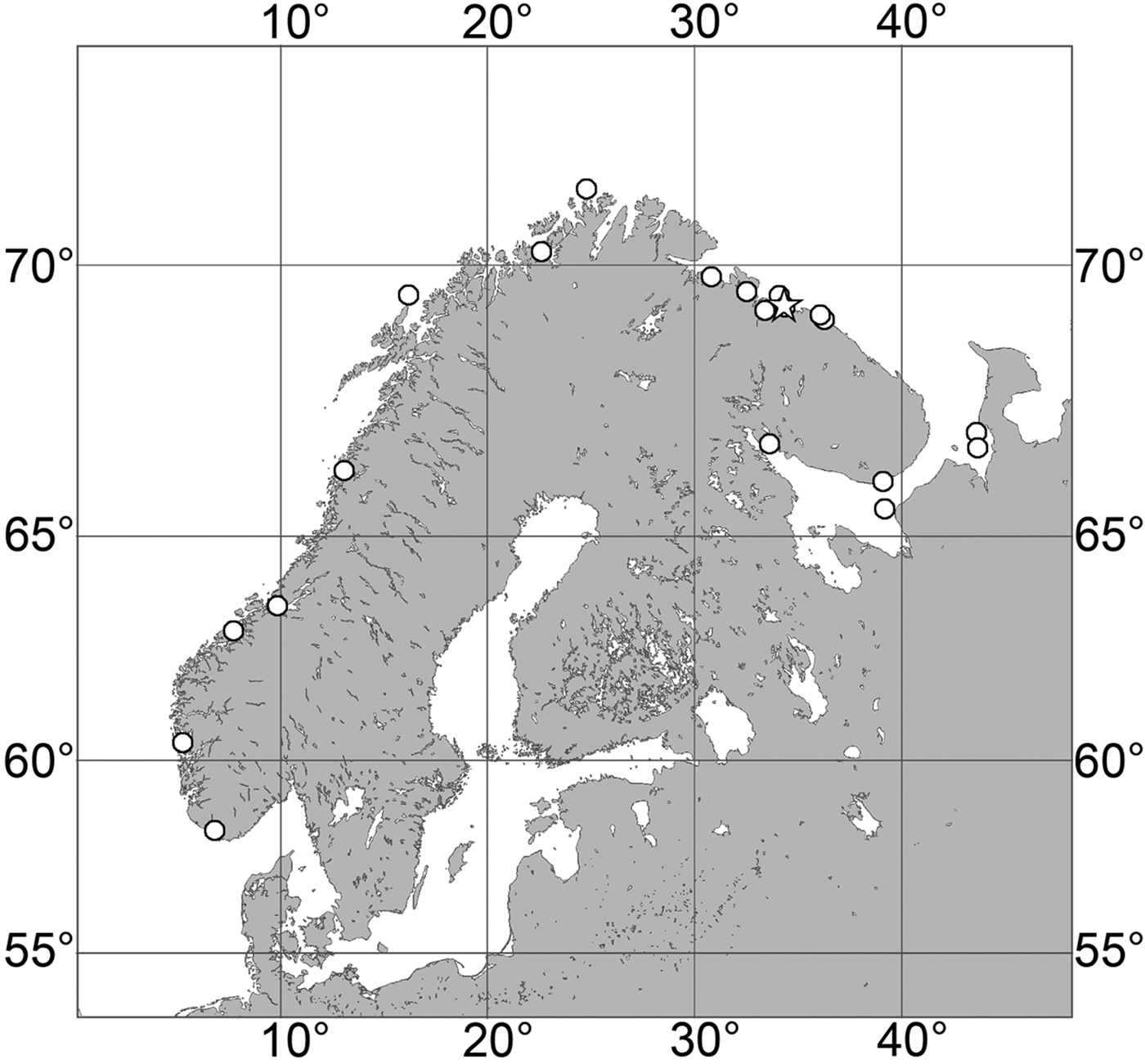
Fig. 12. Polymastia nivea, distribution: white star, type locality of Polymastia euplectella (synonym of P. nivea); white circles, our data. Precise type locality of Reniera nivea is unknown.
Our data: Norwegian Coast: Vest-Agder (40–45 m), Hordaland (75 m), Møre and Romsdal (70–130 m), Sør-Trøndelag (100–300 m), Nordland (80–360 m), Finnmark (250–284 m). Barents Sea: Murman Coast (9–127 m), offshore areas (122 m). White Sea (10–78 m).
DISCUSSION
Describing his new species, Reniera nivea from the Norwegian Sea, Hansen (Reference Hansen1885, p. 5) apparently confused individuals with quite different morphology, pyriform sponges (‘isolated individuals’ in his interpretation, depicted in pl. I figure 6a in his paper) and encrusting sponges with papillae (‘collections of individuals on plates’ depicted in pl. I figure 6b). Burton (Reference Burton1930a) synonymized Hansen's sponges bearing papillae with Polymastia robusta and pyriform sponges with Quasillina brevis. Later P. robusta was synonymized with P. boletiformis (Topsent, Reference Topsent1933; see also Discussion on the latter species above in the present paper). Meanwhile, a new species P. euplectella strongly resembling Hansen's sponges with papillae was described from the Barents Sea by Rezvoj (Reference Rezvoj1927). Koltun (Reference Koltun1966) relegated P. euplectella to a synonym of P. robusta, but Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) resurrected the status of P. euplectella. Based on the re-examination of the type material of both P. euplectella and R. nivea (except for Hansen's pyriform sponges which are considered as lost) we can conclude that they belong to the same species, which gets the name Polymastia nivea in accordance with the principle of priority (Anonymous, 1999). This species is distinguished from P. boletiformis by its pale colouration, longer papillae, all without visible oscula, longitudinal choanosomal skeleton, overlapping spicule layers in the cortex and the presence of three spicule categories. Meanwhile, P. nivea is morphologically very similar to P. bartletti (see the description of the latter above). Genetic data clearly indicate that P. bartletti, P. boletiformis and P. nivea are three separate species (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b).
Polymastia penicillus (Montagu, Reference Montagu1814)
(Figure 13)
Original description: Spongia penicillus Montagu, Reference Montagu1814, p. 93, pl. XIII figure 7.

Fig. 13. Polymastia penicillus: (A) BELUM MC6505 in situ, North Channel, Irish Coast (courtesy of B.E. Picton, Ulster Museum, Belfast); (B) the same individual in preserved state, (C), the same individual, longitudinal section through the body; (D) distribution of the individuals studied; for other documented localities see Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987; as P. mamillaris). Scale bars: B, 1 cm; C, 1 mm.
SYNONYMS AND CITATIONS
Halichondria mamillaris (Jonhston, Reference Johnston1942, p. 142, pl. XVI figure 2).
Pencillaria mamillaris (Gray, Reference Gray1867, p. 527).
Polymastia mamillaris (Bowerbank, Reference Bowerbank1862, p. 1104, Reference Bowerbank1864, p. 178, Reference Bowerbank1866, p. 71; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 32, figure 1).
Polymastia penicillus (Morrow & Boury-Esnault, Reference Morrow and Boury-Esnault2000, p. 330, figures 2A–C & 3).
Non Polymastia penicillus (Vosmaer, Reference Vosmaer1882, p. 26, pl. I figures 12 & 13, pl. IV figure 127–132; Hansen, Reference Hansen1885, p. 9; Fristedt, Reference Fristedt1887, p. 434; Levinsen, Reference Levinsen and Lütken1887, p. 346; Swarczewsky, Reference Swarczewsky1906, p. 313, pl. 13 figure 1).
TYPE MATERIAL
Holotype: BMNH 1930.7.3.26, Devon Coast, England, precise locality and depth unknown.
Detailed description of the holotype was presented by Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Northern Ireland, Co Antrim, North Channel: BELUM MC6505 (one specimen).
Sweden: Västra Götaland, Skagerrak: GNM 460:1 and GNM 460:2 (two specimens).
DESCRIPTION
External morphology
Cushion-shaped sponges up to 10 mm thick, covering the substrate and occupying up to 300 cm2 (Figure 13A, B). Surface velvety rather than hispid, with up to 200 papillae, of which several (one to five depending on sponge size) with visible oscula at the summits. In life the surface dirty grey to yellow or pale orange. The papillae cream-coloured to pale yellow, up to 12 mm in length and 5 mm in diameter, cylindrical or gently tapering towards the summits (Figure 13A). Under sampling the papillae contract and the oscula close (Figure 13B). Sponges usually buried in sediment with only the papillae protruding outwards.
Anatomy
Choanosome in alcohol firm, cream-coloured. Main choanosomal skeleton composed of longitudinal or radial tracts (125–360 µm thick) of principal spicules fanning under the cortex, crossing the cortex and forming a fine surface hispidation (Figure 13C). Auxiliary choanosomal skeleton comprises free-scattered intermediary spicules. Cortex in alcohol whitish, firm, not detachable. Cortical skeleton constituted by a superficial palisade (110–140 µm thick) of small spicules and an internal layer (350–500 µm thick) of tangentially arranged intermediary spicules (Figure 13C). Skeleton of the papilla walls is a framework of the tracts ascending from the choanosome and covered with the cortical layers.
Spicules
(Measurements based on four specimens)
Principal spicules – tylostyles (holotype and the Irish individual) or styles to subtylostyles with weakly developed, displaced tyles (Swedish sponges), usually straight and fusiform. Length 603–959–1490 µm, proximal diameter of shaft 2.5–8.3–12.7 µm, maximum diameter of shaft 7.6–12.3–20.3 µm, N = 120.
Intermediary spicules – tylostyles, occasionally subtylostyles with weakly developed tyles, gently curved or straight, usually slender, occasionally fusiform. Length 300–478–710 µm, diameter of tyle 5.1–8.0–10.2 µm, proximal diameter of shaft 2.5–6.4–8.9 µm, maximum diameter of shaft 5.1–10.0–15.2 µm, N = 120.
Small spicules – tylostyles, gently curved, slender or slightly fusiform. Length 79–155–201 µm, diameter of tyle 3.8–4.7–6.4 µm, proximal diameter of shaft 1.5–3.0–5.1 µm, maximum diameter of shaft 1.5–3.3–6.4 µm, N = 120.
Genetic data
28S rDNA sequences obtained from three individuals of Polymastia penicillus, two from Sweden and one from Northern Ireland, are identical. CO1 was obtained only from the Irish sponge, and this sequence is identical to the CO1 sequence from a Portuguese P. penicillus (GenBank accessions KF225486 and KF225487, Alex et al., Reference Alex, Silva, Vasconcelos and Antunes2013). In both 28S rDNA and CO1 phylogenies P. penicillus is related to P. nivea (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b), although these species share just two synapomorphies and display many differences (for details see the Genetic data section for P. nivea above). Polymastia penicillus is distinguished from all other polymastiids by two autapomorphies in 28S rDNA (Online resource 3, p. 3). Apart from them, P. penicillus differs from the type species of Polymastia, P. mamillaris, by 51 bps in CO1 (Matrix M34248 in TreeBase) and 82 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
Literature data: Portuguese, Spanish and French Atlantic Coast, Mediterranean Sea, English Channel, British Isles (as Polymastia mamillaris – Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987; as P. penicillus – Van Soest et al., Reference Van Soest, Picton and Morrow2000, Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016). Depth – 0–600 m (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987). Records of Polymastia penicillus from the deep-sea, 2500 m near Jan Mayen and 1267 m near Newfoundland (Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987) most likely represent other species.
Our data (Figure 13D): North Channel between Ireland and Great Britain: Irish Coast (depth unknown). Skagerrak: Swedish Western Coast (23–44 m).
DISCUSSION
Polymastia penicillus was for a long time confused with the sympatric species P. mamillaris (Johnston, Reference Johnston1842; Bowerbank, Reference Bowerbank1862; Gray, Reference Gray1867; Vosmaer, Reference Vosmaer1885; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987) and P. grimaldii (Vosmaer, Reference Vosmaer1882; Fristedt, Reference Fristedt1887; Levinsen, Reference Levinsen and Lütken1887) until Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000) demonstrated that these three were separate species (see the detailed taxonomic history in Discussion on P. mamillaris above), that is now confirmed by genetic data (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; present study). Morphologically P. penicillus is distinguished by a surface smoother than in P. mamillaris and P. grimaldii and by a two-layered cortex against the three-layered cortex in the latter two species. At the same time P. penicillus and P. mamillaris share the encrusting growth pattern and the presence of just three spicule categories that differentiate them from P. grimaldii. Minor differences between P. penicillus and P. mamillaris in spicule shape emphasized by Morrow & Boury-Esnault (Reference Morrow and Boury-Esnault2000) appear to be unstable.
Polymastia svenseni sp. nov.
(Figure 14)
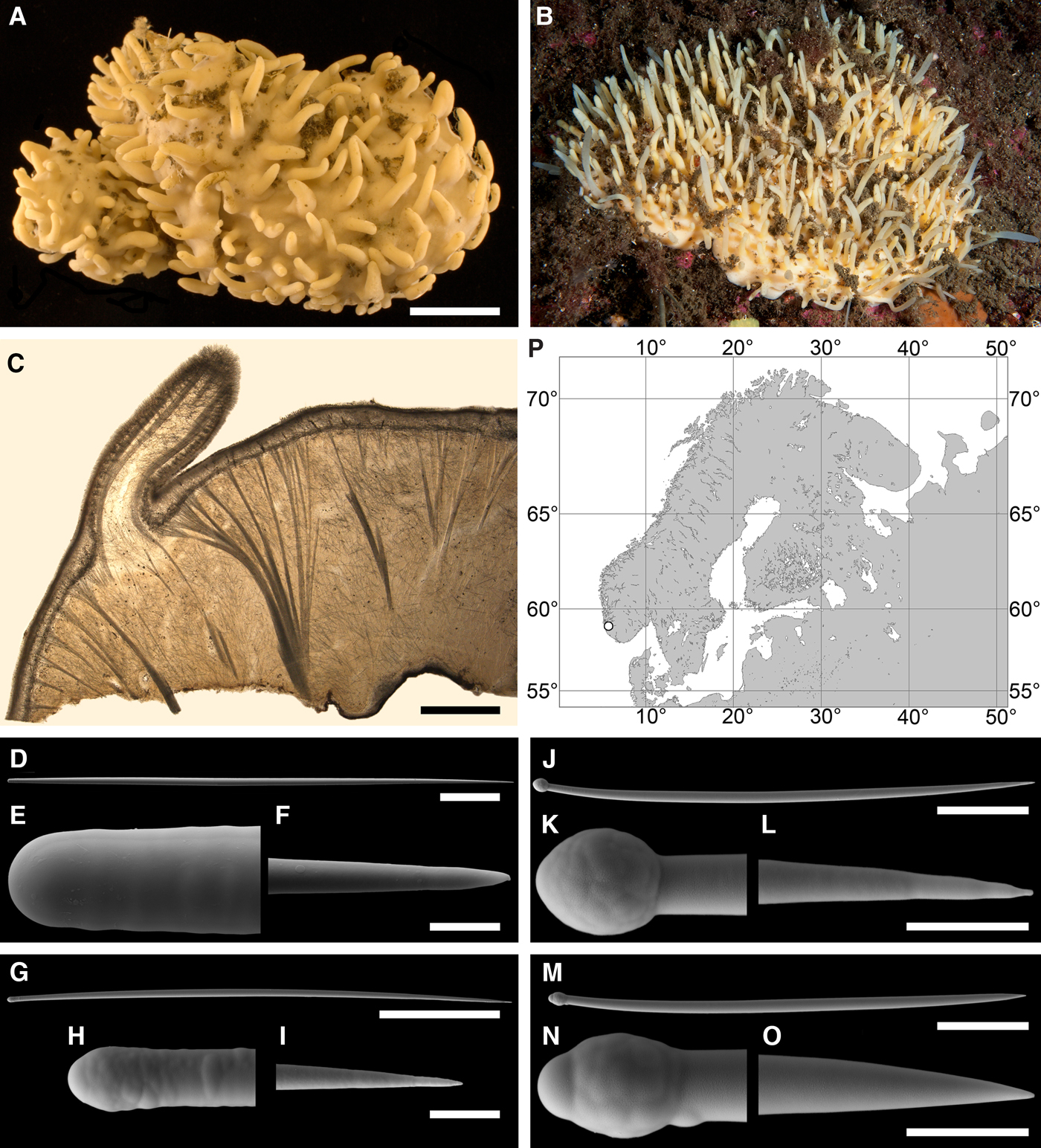
Fig. 14. Polymastia svenseni: (A) holotype ZMBN 098092, habitus; (B) an individual in situ, Ramsvik, Stavanger, Norway (courtesy of E. Svensen, OceanPhoto/Dalane Tidende AS, Egersund); (C) holotype ZMBN 098092, longitudinal section through the body; (D) – (O) spicules of the holotype: (D) large style, general view; (E) the same style, detailed view of the proximal tip; (F) the same style, detailed view of the distal tip; (G) large subtylostyle, general view; (H) the same subtylostyle, detailed view of the proximal tip; (I) the same subtylostyle, detailed view of the distal tip; (J) small tylostyle with terminal tyle, general view; (K) the same tylostyle, detailed view of the tyle; (L) the same tylostyle, detailed view of the distal tip; (M) small tylostyle with displaced tyle, general view; (N) the same tylostyle, detailed view of the tyle; (O) the same tylostyle, detailed view of the distal tip; (P), type locality (white circle). Scale bars: A, 2 cm; C, 2 mm; D, 0.1 mm; E and F, 0.005 mm; G, 0.1 mm; H and I, 0.005 mm; J, 0.03 mm; K and L, 0.005 mm; M, 0.03 mm; N and O, 0.005 mm.
TYPE MATERIAL
Holotype: ZMBN 098092, Ramsvik in Stavanger, Rogaland, Norway, 58°57.404′N 05°45.796′E, 25–18 m, coll. Plotkin and Svensen, 02.09.2012.
Paratype: ZMBN 098091, from the same sample as the holotype.
COMPARATIVE MATERIAL
ZMBN 107558, from the same sample as the holotype.
ETYMOLOGY
Named after Erling Svensen, a Norwegian underwater photographer, who has discovered a large population of this species in Stavanger.
DESCRIPTION
External morphology
Both holotype and paratype cushion-shaped, removed from a rock cliff. Surface, for the most part, smooth, cream-coloured in alcohol, with sparse rests of sediment and papillae, which may be conical tapering towards the summits, cylindrical or leaf-shaped expanding towards the summits. Holotype 105 × 52 × 13 mm, with 157 papillae 2–13 mm long and 1.5–5.5 mm (Figure 14A). Paratype 67 × 44 × 12 mm, with 76 papillae 2–15 mm long and 1.4–6.7 mm wide. Other sponges cushion-shaped, occupying up to 250 cm2 of the substrate (Figure 14B). Surface cream-coloured or whitish in life, smooth, partly covered with sediment, with up to 400 papillae tapering towards the summits. Few papillae with visible oscula at the summits.
Anatomy
Choanosome in life pale orange or yellowish, firm. Main choanosomal skeleton composed of tracts (112–321 µm thick) of large spicules radiating from the base and ending in the cortex (Figure 14C). Ascending tracts also form a framework of the papilla skeleton. Auxiliary choanosomal skeleton comprises free-scattered large and small spicules. Cortex in life whitish, firm, not detachable. Cortical skeleton constituted by a superficial palisade (220–240 µm thick) of small spicules, a middle layer (130–150 µm thick) and an internal layer (100–125 µm thick), both composed of criss-cross large spicules lying loosely in the middle layer and much more condensed in the internal layer (Figure 14C). Skeleton of the papilla walls composed of two cortical layers, the superficial palisade and the internal layer.
Spicules
(Measurements based on three specimens, individual variation presented in Table 5)
Table 5. Individual variation of spicule dimensions of Polymastia svenseni (given in μm as minimum–mean–maximum). Parameters: length, diameter of tyle, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).
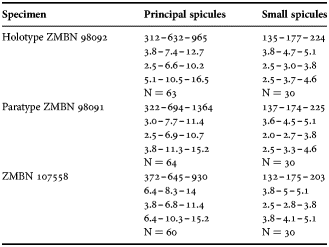
Large spicules – mainly styles (Figure 14D–F), occasionally subtylostyles with weakly developed and displaced tyles (Figure 14G–I), usually straight, sometimes gently curved, fusiform to a greater or lesser extent. Length 312–658–1364 µm, diameter of tyle (if present) 3.0–7.8–14.0 µm, proximal diameter of shaft 2.5–6.8–11.4 µm, maximum diameter of shaft 3.8–10.9–16.5 µm, N = 187. The large spicules vary greatly in size, both in the cortex and choanosome, but the frequency distribution of their dimensions exhibits just one peak and hence they cannot be divided into two size categories.
Small spicules – tylostyles, usually gently curved, slender (Figure 14J–O). Length 132–175–225 µm, diameter of tyle 3.6–4.7–5.1 µm, proximal diameter of shaft 2.0–2.8–3.8 µm, maximum diameter of shaft 2.5–3.7–5.1 µm, N = 90.
Genetic data
CO1 and 28S rDNA obtained from two individuals of Polymastia svenseni display no intraspecific polymorphism. B10–C1 region of 28S rDNA sequenced by Morrow et al. (Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012) from an unidentified Irish Polymastia sp. (GenBank accession KF017187) is identical to the corresponding gene fragment of P. svenseni (about 1/3 length of our sequences). By both CO1 and 28S rDNA P. svenseni is closely related to a Norwegian Polymastia sp. described below, and by CO1 alone these two are closely related to an unidentified Canadian Polymastia (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b), for which no 28S rDNA is available and which is not covered by the present study. These sponges share nine synapomorphies in CO1 (Online resource 2, p. 3, Matrix M34248 in TreeBase). Polymastia svenseni and the Norwegian Polymastia sp. also share two synapomorphies in 28S rDNA distinguishing these taxa from all other polymastiids (Online resource 3, p. 3). At the same time P. svenseni is distinguished by two autapomorphies in 28S rDNA (Online resource 3, p. 3). Apart from these autapomorphies, it differs from the Norwegian Polymastia sp. by 21 bps in CO1 (Matrix M34248 in TreeBase), from the Canadian Polymastia sp. by 17 bps in CO1 (Matrix M34248 in TreeBase), and from the type species of Polymastia, P. mamillaris, by 65 bps in CO1 (Matrix M34248 in TreeBase) and 21 bps in 28S rDNA (Matrix M34250 in TreeBase). In the CO1 phylogeny the trio Polymastia svenseni + Norwegian Polymastia sp. + Canadian Polymastia sp. is the sister to the clade of seven other Polymastia spp. including P. mamillaris, although with a weak Bayesian support (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). In the 28S rDNA phylogeny the pair Polymastia svenseni + Norwegian Polymastia sp. is the sister to the same clade with a high support (the same study).
OCCURRENCE
Literature data: Ireland (Morrow et al., Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012: the record based only on the genetic data).
Our data (Figure 14P): Norwegian Coast: Rogaland (18–25 m).
DISCUSSION
Polymastia svenseni is established as a new species of Polymastia primarily based on the CO1 and 28S rDNA phylogenies and on its autapomorphies in the latter gene. However, no morphological autapomorphies distinguishing this species from other Polymastia spp. are revealed. Polymastia svenseni resembles P. boletiformis by the smooth surface and the presence of only two categories of spicules. On the contrary, by its radial main choanosomal skeleton and three-layered cortex P. svenseni resembles P. andrica, P. arctica, P. grimaldii and P. mamillaris.
Polymastia thielei Koltun, Reference Koltun1964
(Figure 15)
Original description: Polymastia thielei Koltun, Reference Koltun1964, p. 149, figure 4.
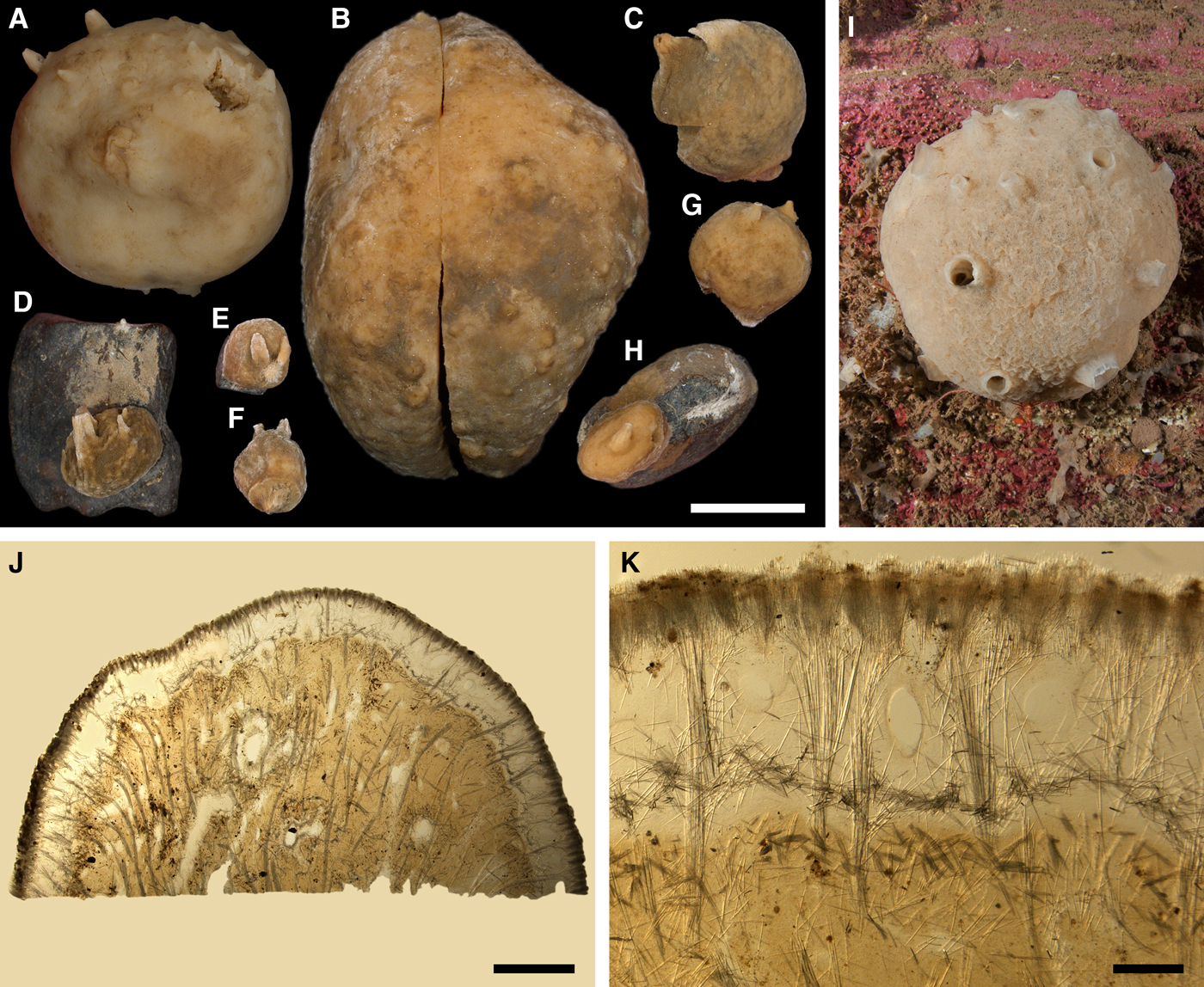
Fig. 15. Polymastia thielei: (A) lectotype ZIN RAS 10640, habitus; (B) paralectotype ZIN RAS10638, habitus; (C)–(H) paralectotypes ZIN RAS10639, habitus; (I) an individual in situ, Hinlopenstretet, Svalbard, Norway (courtesy P. Leopold, University of Tromsø); (J) ZMBN 98070, longitudinal section through the body, general view; (K), the same section, detail of cortex and subcortical area. Scale bars: A–H, 2 cm; J, 3 mm, K, 0.5 mm.
SYNONYMS AND CITATIONS
Polymastia thielei (Koltun, Reference Koltun1966, p. 76, text-figures 47–48, pl. XXVII figures 1–5; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 541, figures 1c & 2c).
Polymastia uberrima (Lundbeck, Reference Lundbeck1909, p. 450, pl. XIV figure 4; Topsent, Reference Topsent1913, p. 18, pl. II figure 5; Burton, Reference Burton, Fridriksson and Tuxen1959a: 12 pars.; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 48, figure 10).
Rinalda uberrima (Hansen, Reference Hansen1885, p. 8, pl. VI figure 18; Thiele, Reference Thiele1903, p. 376, figure 2).
TYPE MATERIAL
Lectotype (designated herein, see figure 1c in Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004 and Figure 15A in the present study): ZIN RAS 10640 (slide 6153), Greenland Sea, 80°31′N 06°59′W, 259 m, RV ‘Ob’’, station 33 28.08.1956, coll. Koltun.
Paralectotype: ZIN RAS 10638 (slide 6024), North-East off Franz Josef Land, Arctic Ocean, 81°52.5′N 60°32′E, 230–246 m, RV ‘Litke’, station 14, 15.09.1955, coll. Koltun.
Six paralectotypes: ZIN RAS 10639 (slide 6085), North-West off Franz Josef Land, Arctic Ocean, 82°00′N 42°00′E, 418–415 m, RV ‘Litke’, station 26, 18.09.1955, coll. Koltun.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada, Newfoundland: ZIN RAS ocpt017 (one specimen).
Denmark Strait: ZIN RAS ocpt030 and ZMBN 098107 (two specimens).
Iceland: ZIN RAS ocpt004 and ZMBN 098070 (two specimens).
Iceland Sea: ZIN RAS ocpt026 (one specimen).
Faroes: ZIN RAS ocpt013 (one specimen).
Norwegian Sea, offshore: MOM 04-0851 (identified as Polymastia uberrima by Topsent, Reference Topsent1913), NTNU-VM-54993, NTNU-VM-72524, ZMBN 098109, ZMBN 107580 and ZMBN 107581 (six specimens).
Barents Sea, offshore: ZIN RAS ocpt007, ZIN RAS ocpt009, ZIN RAS ocpt011, ZIN RAS ocpt014, ZIN RAS ocpt027 and ZIN RAS ocpt029 (six specimens).
Norway: Møre and Romsdal: |NTNU-VM-54960, ZMBN 107495 and ZMBN 107496 (three specimens), Nord-Trøndelag: NTNU-VM-54884, NTNU-VM-54887, NTNU-VM-54888 and NTNU-VM-72537 (five specimens), Nordland: NTNU-VM-72519 and NTNU-VM-72540 (two specimens), Troms: ZIN RAS ocpt005 (one specimen), Finnmark: ZIN RAS ocpt001 (one specimen), Svalbard: ZIN RAS ocpt002, ZIN RAS ocpt028, ZMBN 098052 and ZMBN 098053 (four specimens).
Russia: Murman Coast: ZIN RAS ocpt006 (one specimen), Franz Josef Land: ZIN RAS ocpt022 (one specimen), Novaya Zemlya: ZIN RAS ocpt016 (one specimen), Taymyr Peninsula: ZIN RAS ocpt003, ZIN RAS ocpt021 and ZIN RAS ocpt025 (three specimens), Nordenskjold Archipelago: ZIN RAS ocpt020 (one specimen).
Kara Sea: ZIN RAS ocpt008, ZIN RAS ocpt012, ZIN RAS ocpt015, ZIN RAS ocpt018, ZIN RAS ocpt019, ZIN RAS ocpt023 and ZIN RAS ocpt024 (seven specimens).
DESCRIPTION
External morphology
Lectotype globular, about 50 mm in diameter, attached to small stones (Figure 15A). Surface velvety, pale beige with sparsely scattered small brown stains, bearing 18 papillae. The papillae crater-shaped (very short and wide) or conical, 1.5–5.5 mm long and 1.7–9.2 mm wide at base, most with well-visible oscula at the summits. Paratype ZIN RAS 10638 irregularly ovoid, 81 × 60 mm in diameter, split across, removed from the substrate (Figure 15B). Surface velvety, pale brown with darker stains, bearing 23 wart-like small papillae, the largest with oscula at the summits. Paratypes ZIN RAS 10639 are small, massive sponges on stones or removed from the substrates (Figure 15C–H). Surface, for the most part, velvety or knobbly, porous in some individuals, brownish, with one or few conical or crater-shaped papillae, all with oscula at the summits. Some paratypes with minute hispidation around the base. Other sponges massive, fist-shaped, globular or ovoid, up to 250 mm in diameter (Figure 15I). Surface knobbly, velvety or smooth, with well-visible ostia. Colour in life whitish, cream-coloured or beige, occasionally with large dark brown spots. In alcohol the colouration darkens and the ostia contract. Up to 30 papillae, all with oscula at the summits, crater-shaped in life and often stretching under sampling.
Anatomy
Choanosome pale brown to dark brown, both in life and in alcohol, somewhat loose, porous, with prominent meandering and anastomosing exhalant canals (up to 8 mm in diameter) running to the papillae. Main choanosomal skeleton composed of tracts (90–360 µm thick) of principal spicules radiating from the sponge base (Figure 15J). In the central part of the choanosome the tracts often meander and ramify, sometimes anastomose and form a network. Auxiliary choanosomal skeleton comprises small spicules, free-scattered or grouped in little bundles, especially abundant in a subcortical area (200–350 µm thick). Within the choanosome the walls of the exhalant canals paved with criss-cross small and intermediary spicules. Cortex in life cream-coloured, dense, but detachable. Cortical skeleton includes a superficial layer (340–650 µm thick) formed by bouquets of small spicules and an internal layer (150–250 µm thick) of criss-cross intermediary spicules (Figure 15K). A space (650–880 µm thick) between the spicule layers is occupied by oval aquiferous cavities (190–480 × 70–240 µm in diameter) connected with ostia which are located between the superficial spicule bouquets. Bulkheads between the aquiferous cavities reinforced with the ascending choanosomal tracts and criss-cross intermediary spicules. The papillae walls reinforced with the ascending choanosomal tracts and covered with the spicule cortical layers.
Spicules
(Measurements based on 10 specimens)
Principal spicules – styles to subtylostyles, usually straight and fusiform. Length 693–1277–1705 µm, proximal diameter of shaft 5.0–9.8–14.2 µm, maximum diameter of shaft 12.5–18.4–25.1 µm, N = 300.
Intermediary spicules – subtylostyles to tylostyles, straight or gently curved, fusiform. Length 445–539–648 µm, diameter of tyle 4.2–7.0–8.2 µm, proximal diameter of shaft 3.2–5.8–7.1 µm, maximum diameter of shaft 10.5–11.2–12.3 µm, N = 300.
Small spicules – tylostyles, gently curved, slender or slightly fusiform. Length 219–297–363 µm, diameter of tyle 3.8–5.5–7.4 µm, proximal diameter of shaft 1.7–3.8–5.7 µm, maximum diameter of shaft 4.1–6.8–9.3 µm, N = 300.
Genetic data
CO1 sequences obtained from five individuals of Polymastia thielei are identical. 28S rDNA was obtained only from one of these sponges. By both genes P. thielei is closely related to morphologically quite different P. hemisphaerica (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). The synapomorphies and differences between these species are described above in the Genetic data section for P. hemisphaerica.
OCCURRENCE
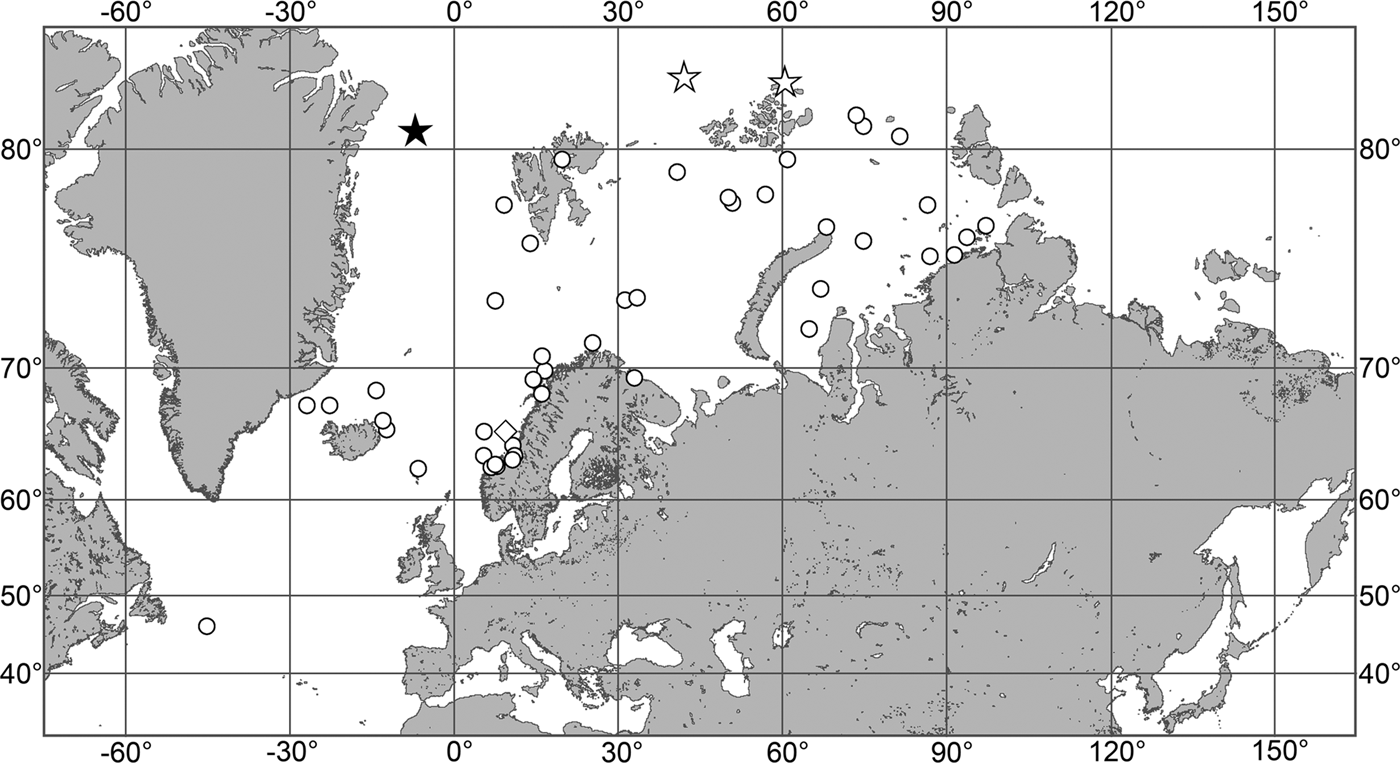
Fig. 16. Polymastia thielei, distribution: black star, locality of lectotype; white stars, localities of paralectotypes; white diamond, locality of MOM 04-0851 identified as P. uberrima by Topsent (Reference Topsent1913) and re-identified as P. thielei herein; white circles, our data.
Our data (agree with the literature data): Canadian Atlantic Coast: Newfoundland (340–375 m). Denmark Strait (317–650 m). Iceland (165–318 m). Iceland Sea (1280 m). Faroes (350 m). Greenland Sea (259 m). Norwegian Coast: Møre and Romsdal: (120–200 m), Nord-Trøndelag: (28–400 m), Nordland: (200–800 m), Troms (280 m), Finnmark (275 m). Norwegian Sea, offshore areas (440–1580 m). Barents Sea: Murman Coast: (272 m), offshore areas: (165–365 m). Svalbard (40–820 m). Franz Josef Land: (122–246 m). Novaya Zemlya (293 m). Taymyr Peninsula (49–60 m). Nordenskjold Archipelago (75 m). Kara Sea (85–446 m). Arctic Ocean (415–418 m).
DISCUSSION
Before Koltun (Reference Koltun1964) established Polymastia thielei the sponges with the characteristic features of this species had usually been identified as P. uberrima because of some external similarity between these species (e.g. Hansen, Reference Hansen1885; Thiele, Reference Thiele1903; Lundbeck, Reference Lundbeck1909; Topsent, Reference Topsent1913). Polymastia thielei also displays some similarities in body shape and architecture of the cortex with Weberella bursa (Müller, Reference Müller1806). The main distinctive features of P. thielei are much smaller number of papillae and their conspicuous crater-like shape. Weberella bursa differs from both P. thielei and P. uberrima by the pale colouration of both the cortex and the choanosome, much thinner cortex, the reticulate choanosomal skeleton and the presence of just two size categories of spicules. Polymastia uberrima is distinguished by the presence of a marginal hispid collar absent in both P. thielei and W. bursa and the regular radial choanosomal skeleton, while in P. thielei the choanosomal skeleton is less regular, with a tendency to meandering and anastomosing. The morphological differences between P. thielei, P. uberrima and W. bursa are clearly confirmed by the genetic data.
Polymastia uberrima (Schmidt, Reference Schmidt1870)
(Figure 17)
Original description: Rinalda uberrima Schmidt, Reference Schmidt1870, p. 51, pl. VI figure 3.
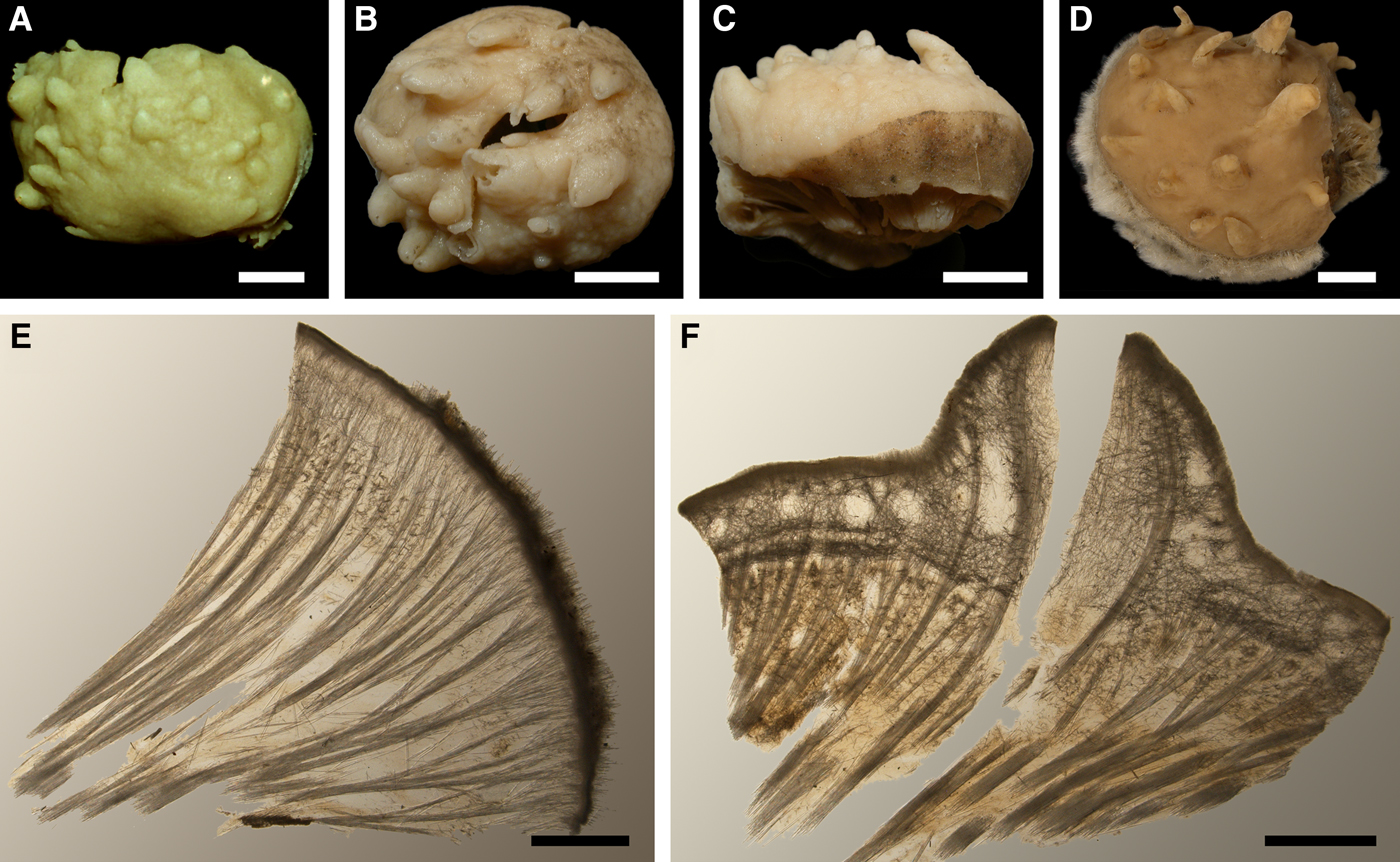
Fig. 17. Polymastia uberrima: (A) holotype of Rinalda uberrima, ZMUC-DEM-395, habitus; (B) lectotype of Polymastia infrapilosa (a synonym of P. uberrima), MOM 04-1449, habitus, view from above; (C) the same, side view; (D) ZIN RAS ocpu005, habitus; (E) holotype of Rinalda uberrima, ZMUC-DEM-395, longitudinal section through the body, peripheral area; (F) the same individual, longitudinal section through a papilla and adjacent area. Scale bars: A–D, 1 cm; E, 3 mm; F, 2 mm.
SYNONYMS AND CITATIONS
Polymastia infrapilosa Topsent, Reference Topsent1927b, p. 4 (Topsent, Reference Topsent1928, p. 147, pl. II figures 25–26, pl. VI figure 3; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 40, figure 6).
Polymastia uberrima (Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929, p. 868 and 923; Arndt, Reference Arndt, Grimpe and Wagler1935, p. 35 pars.; Gorbunov, Reference Gorbunov1946, p. 37; Burton, Reference Burton, Fridriksson and Tuxen1959a, p. 12 pars.; Koltun, Reference Koltun1966, p. 75, text-figure 46, pl. XXV figures 1–3; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 542, figures 1d & 2d; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1f).
Rinalda uberrima (? von Marenzeller, Reference von Marenzeller1878, p. 369, pl. II figure 2).
Non Polymastia uberrima (Lundbeck, Reference Lundbeck1909, p. 450, pl. XIV figure 4; Topsent, Reference Topsent1913, p. 18, pl. II figure 5; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987, p. 48, figure 10).
Non Rinalda uberrima (Hansen, Reference Hansen1885, p. 8, pl. VI figure 18; Thiele, Reference Thiele1903, p. 376, figure 2).
TYPE MATERIAL
Holotype of Polymastia uberrima (Figure 17A): ZMUC-DEM-395, Iceland, precise locality unknown.
Lectotype (designated herein, Figure 17B, C) and two paralectotypes of Polymastia infrapilosa Topsent, 1927: MOM 04-1449, SE of Halifax, Nova Scotia, Canada, 44°10′N 62°27.5′W, 75 m, Scientific campaigns by Albert the 1st of Monaco, station 3425, 13.08.1913. Herein P. infrapilosa is relegated to a synonym of P. uberrima (see Discussion on this species below).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: Nova Scotia: ZIN RAS ocpu004, ZIN RAS ocpu005 and ZIN RAS ocpu094 (three specimens), Newfoundland: ZIN RAS ocpu002, ZIN RAS ocpu015, ZIN RAS ocpu020, ZIN RAS ocpu022, ZIN RAS ocpu023, ZIN RAS ocpu024, ZIN RAS ocpu026, ZIN RAS ocpu027, ZIN RAS ocpu031, ZIN RAS ocpu033, ZIN RAS ocpu034, ZIN RAS ocpu043, ZIN RAS ocpu044 and ZIN RAS ocpu045 (17 specimens), Labrador: ZIN RAS ocpu003, ZIN RAS ocpu016, ZIN RAS ocpu017, ZIN RAS ocpu021, ZIN RAS ocpu025 and ZIN RAS ocpu028 (six specimens), offshore areas of the NW Atlantic: ZIN RAS ocpu046 (one specimen).
Greenland: SW Coast/Davis Strait: ZIN RAS ocpu018, ZIN RAS ocpu019, ZIN RAS ocpu029 and ZIN RAS ocpu032 (four specimens), SE Coast: ZIN RAS ocpu042, ZIN RAS ocpu049, ZIN RAS ocpu097 and ZIN RAS ocpu102 (four specimens).
Iceland: ZIN RAS ocpu035, ZIN RAS ocpu039, ZIN RAS ocpu041, ZIN RAS ocpu048 and ZIN RAS ocpu050 (11 specimens).
North-East Atlantic, offshore: ZIN RAS ocpu036, ZIN RAS ocpu040, ZIN RAS ocpu038 and ZIN RAS ocpu096 (four specimens).
Norwegian Sea, offshore: ZIN RAS ocpu006, ZIN RAS ocpu007, ZIN RAS ocpu008, ZIN RAS ocpu009, ZIN RAS ocpu010, ZIN RAS ocpu013, ZIN RAS ocpu099 and ZMBN 098066 (10 specimens).
Barents Sea, offshore: ZIN RAS ocpu011, ZIN RAS ocpu053, ZIN RAS ocpu057, ZIN RAS ocpu061, ZIN RAS ocpu063, ZIN RAS ocpu066, ZIN RAS ocpu067, ZIN RAS ocpu070, ZIN RAS ocpu073, ZIN RAS ocpu080, ZIN RAS ocpu081, ZIN RAS ocpu082, ZIN RAS ocpu085, ZIN RAS ocpu100, ZIN RAS ocpu101 and ZIN RAS ocpu104 (21 specimens).
Norway: Nordland: NTNU-VM-55002, NTNU-VM-72520 and NTNU-VM-72534 (three specimens), Troms: NTNU-VM-72530, ZMBN 098073, ZMBN 107574 and ZMBN 107575 (four specimens), Finnmark: NTNU-VM-72503, NTNU-VM-72528, ZIN RAS ocpu095 and ZMBN 107578 (five specimens), Svalbard: ZIN RAS ocpu012 and ZIN RAS ocpu014 (three specimens).
Russia: Murman Coast: ZIN RAS ocpu051, ZIN RAS ocpu056, ZIN RAS ocpu062, ZIN RAS ocpu064, ZIN RAS ocpu071, ZIN RAS ocpu074, ZIN RAS ocpu075, ZIN RAS ocpu076, ZIN RAS ocpu077, ZIN RAS ocpu083, ZIN RAS ocpu084, ZIN RAS ocpu088, ZIN RAS ocpu089, ZIN RAS ocpu090, ZIN RAS ocpu091, ZIN RAS ocpu092 and ZIN RAS ocpu103 (21 specimens), White Sea: ZIN RAS ocpu072 (one specimen), Franz Josef Land: ZIN RAS ocpu0107 (one specimen), Severnaya Zemlya: ZIN RAS ocpu105 and ZIN RAS ocpu106 (two specimens).
Arctic Ocean, offshore: ZIN RAS ocpu60 and ZIN RAS ocpu98 (three specimens).
DESCRIPTION
External morphology
Holotype of Rinalda uberrima massive, ~46 × 27 × 29 mm, removed from the substrate (Figure 17A). Surface, for the most part, smooth, cream-coloured, with about 20 papillae and a minutely hispid greyish edging, 5–6 mm wide, on the undamaged side. Most papillae wart-like, weakly developed, but some conical, up to 5 mm long and 7 mm wide at base, with visible oscula at the summits. Lectotype and paralectotypes of Polymastia infrapilosa massive, removed from the substrates. Surface, for the most part, smooth, cream-coloured, with a minute hispidation, 6–7 mm wide, along the undamaged edge and conical, occasionally cylindrical, papillae bearing oscula at the summits. Lectotype ~42 × 38 × 21 mm, split across, with 49 papillae, which are 2–12 mm long, 2–8 mm wide at base and 1–2 mm wide at summit (Figure 17B, C). Paralectotypes ~36 × 30 × 16 mm, with 23 papillae, and 37 × 29.5 × 10 mm, with 30 papillae. The latter with several surface protuberances, each bearing one to three buds, 2–3 mm in diameter. Other sponges massive or globular, occupying up to 100 mm2 of the substrate. Except for a marginal collar, the surface smooth or velvety, whitish in life, but often darkening in alcohol, with up to 150 conical or cylindrical papillae, in life all with visible oscula. The marginal collar is a rough, hispid or even shaggy area of various widths (Figure 17D).
Anatomy
Choanosome yellowish in life, but may become dark brown in alcohol, dense. Main choanosomal skeleton composed of tracts (207–571 µm thick) of principal spicules radiating from the sponge base and occasionally ramifying under the cortex (Figure 17E, F). Most tracts end in the cortex except for the marginal area where they protrude above the surface. Some tracts ascend to the papillae (Figure 17F). Auxiliary choanosomal skeleton comprises small bundles of intermediary spicules, especially abundant in a subcortical area (423–1484 µm thick). Cortex in life cream-coloured, firm, not detachable. Cortical skeleton includes a superficial palisade (201–257 µm thick) of small spicules, a middle layer of loosely and confusedly lying bundles of intermediary spicules (672–1437 µm thick) and an internal layer (181–326 µm thick) with a high concentration of criss-cross intermediary spicules. In the areas under the papillae the middle layer contains aquiferous cavities connected with the surface ostia (Figure 17F). The papillae walls reinforced with the ascending choanosomal tracts and covered with the superficial palisade and internal cortical layer. Several exhalant and inhalant canals running into the papillae are separated by bulkheads reinforced with the ascending tracts and free-scattered intermediary spicules.
Spicules
(Measurements based on nine specimens, individual variation presented in Table 6)
Table 6. Individual variation of spicule dimensions of Polymastia uberrima (given in μm as minimum–mean–maximum). Parameters: length, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).
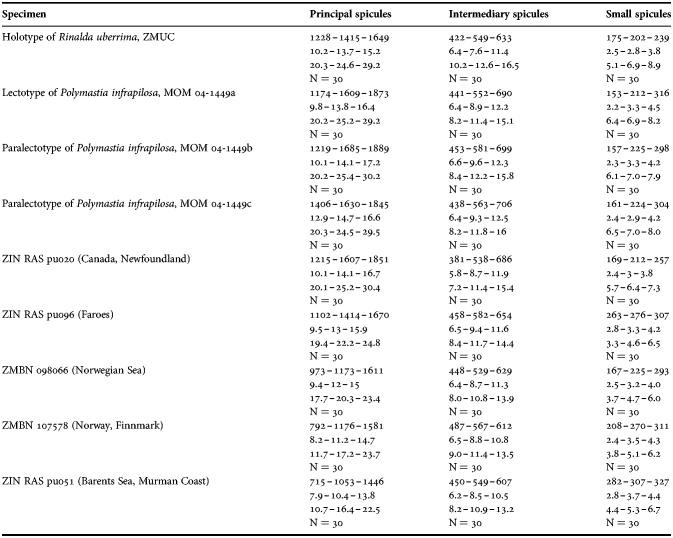
Principal spicules – styles to subtylostyles, straight, fusiform. Length 715–1409–1889 µm, proximal diameter of shaft 7.9–13.1–17.2 µm, maximum diameter of shaft 10.7–23.1–30.4 µm, N = 270.
Intermediary spicules – styles to subtylostyles, occasionally tylostyles, straight or occasionally gently curved, fusiform. Length 381–550–706 µm, proximal diameter of shaft 5.8–8.5–12.5 µm, maximum diameter of shaft 7.2–11.8–16.5 µm, N = 270.
Small spicules – subtylostyles to tylostyles, gently curved or straight, fusiform. Length 153–218–327 µm, proximal diameter of shaft 2.2–3.1–4.5 µm, maximum diameter of shaft 3.3–6.4–8.9 µm, N = 270.
Genetic data
CO1 sequences obtained from two individuals of Polymastia uberrima are identical, but these sponges differ by one bp in 28S rDNA (Matrix M34250 in TreeBase). In both CO1 and 28S rDNA phylogenies P. uberrima falls in the clade with six other Polymastia spp. including the type species of this genus, P. mamillaris (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). However, P. uberrima does not have sister relation to any of these species. It is distinguished by two autapomorphies in CO1 (Online resource 2, p. 2) and three autapomorphies in 28S rDNA (Online resource 3, p. 2) from all other polymastiids. Apart from these autapomorphies, P. uberrima differs from P. mamillaris by 31 bps in CO1 (Matrix M34248 in TreeBase) and 15 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
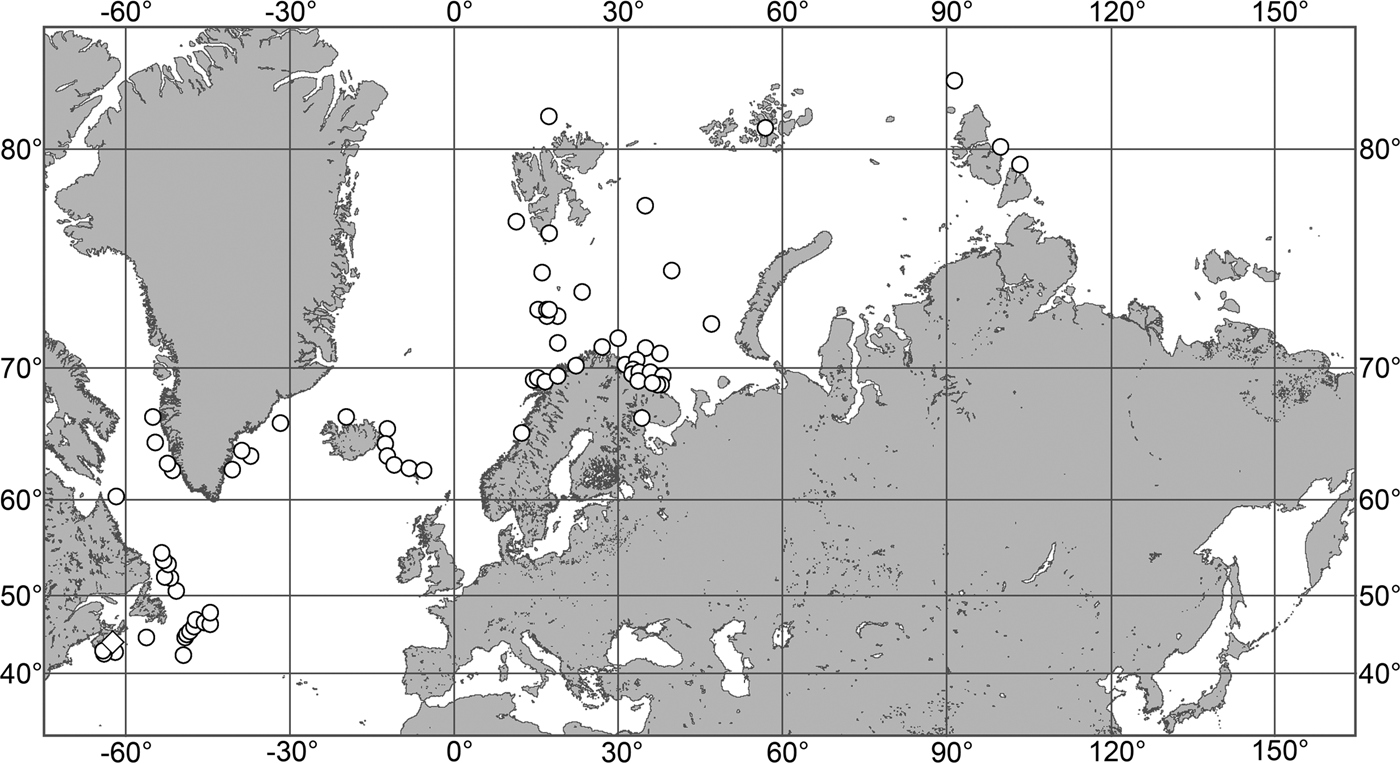
Fig. 18. Polymastia uberrima, distribution: white diamond, type locality of Polymastia infrapilosa (a synonym of P. uberrima); white circles, our data. Precise type locality of Rinalda uberrima is unknown.
Our data (agree with the literature data): Canadian Atlantic Coast: Nova Scotia (75–480 m), Newfoundland and Labrador (135–700 m), offshore areas (2070 m). Davis Strait (110–350 m). South-East Greenland (245–450 m). Denmark Strait (132–345 m). Iceland (165–475 m). North-East Atlantic, offshore areas (430–485 m). Norwegian Sea, offshore areas (220–500 m). Norwegian Coast: Nordland (120–1100 m), Troms (35–220 m), Finnmark (130–286 m). Barents Sea: Murman Coast (31–270 m), offshore areas (80–350 m). Svalbard (100–230 m). Franz Josef Land (317 m). White Sea (depth unknown). Laptev Sea/Severnaya Zemlya (108–139 m). Arctic Ocean (360–477 m).
DISCUSSION
Polymastia uberrima exhibits some similarity with P. thielei in external morphology, but is well-distinguished by the presence of a marginal more or less hispid collar. This collar may be destroyed during sampling, and that might be the reason why some early authors did not distinguish between P. uberrima and P. thielei (von Marenzeller, Reference von Marenzeller1878; Hansen, Reference Hansen1885; Thiele, Reference Thiele1903; Lundbeck, Reference Lundbeck1909; Topsent, Reference Topsent1913; Boury-Esnault, Reference Boury-Esnault, Vacelet and Boury-Esnault1987). Furthermore, P. uberrima differs greatly from P. thielei by the architecture of the cortex and choanosomal skeleton (see Discussion on P. thielei above) and these morphological distinctions between the two species are confirmed by genetic data. At the same time P. infrapilosa established by Topsent (Reference Topsent1927b) for Polymastia possessing the marginal collar in fact has no differences from P. uberrima and hence is regarded as a junior synonym of P. uberrima.
Polymastia sp.
(Figure 19)
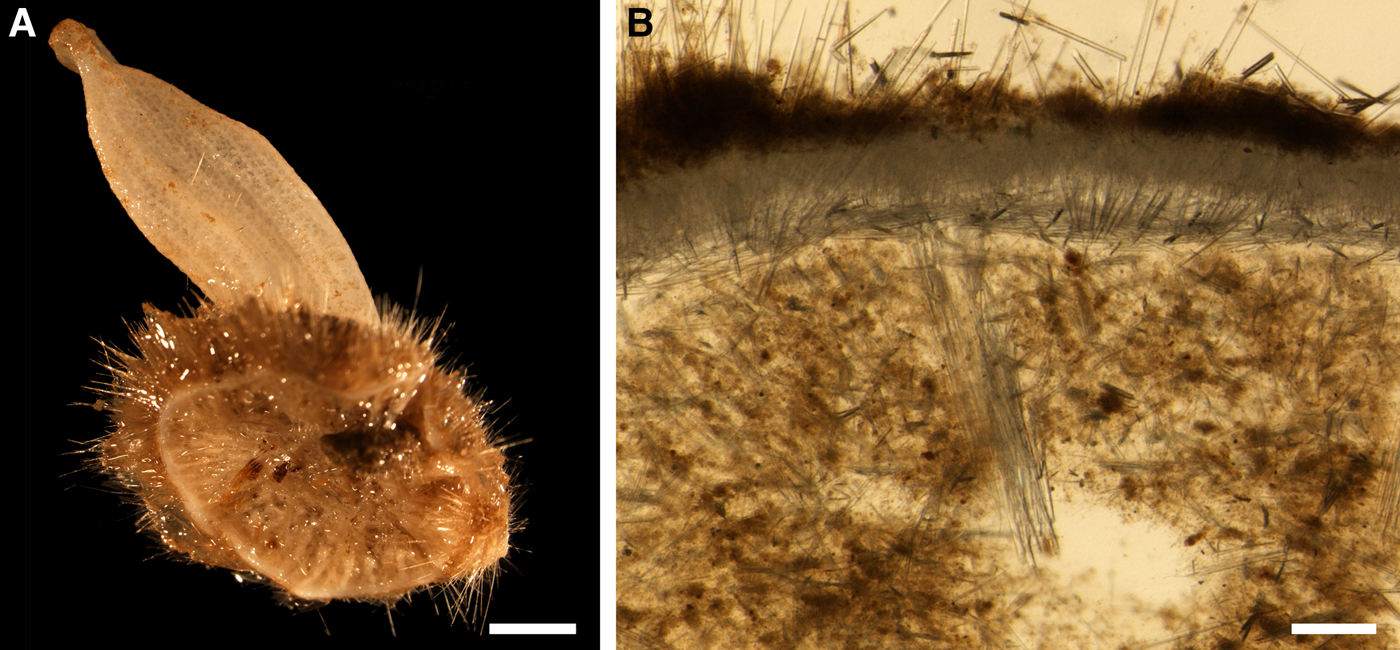
Fig. 19. Polymastia sp., ZMBN 098080: (A) habitus; (B) longitudinal section through the body. Scale bars: A, 3 mm; B, 0.2 mm.
MATERIAL EXAMINED
ZMBN 098080 (one specimen), West off Marstein, Hordaland, Norway, 60°07.9′–60°07.8′N 04°50.5′–04°51.4′E, 312 m, RV ‘Hans Brattstrøm’, station 2, coll. Plotkin, 03.07.2012.
DESCRIPTION
External morphology
Encrusting sponge, about 12 mm in diameter and 5 mm thick, removed from the substrate (Figure 19A). Surface strongly hispid, covered with sediment, bearing a conical papilla, 16.5 mm long and 4.6 mm wide at base, with a small osculum at the summit.
Anatomy
Choanosome in alcohol pale orange, firm. Main choanosomal skeleton composed of radiating tracts (92–106 µm thick) of principal spicules crossing the cortex and forming a surface hispidation reinforced with exotyles (Figure 19B). Auxiliary choanosomal skeleton comprises bundles of free-scattered small spicules. Cortex in alcohol light-coloured, firm, not detachable. Cortical skeleton constituted by a superficial palisade (170–200 µm thick) of small spicules and an internal layer (139–184 µm thick) of criss-cross intermediary spicules (Figure 19B).
Spicules
Principal spicules – subtylostyles with slightly displaced tyles, occasionally polytylote, straight, fusiform. Length 880–1257–2034 µm, diameter of tyle 8.1–12.2–17.8 µm, proximal diameter of shaft 6.4–9.7–11.4 µm, maximum diameter 12.7–16.8–20.3 µm, N = 30.
Intermediary spicules – subtylostyles, straight, slender. Length 442–557–693 µm, diameter of tyle 7.6–8.7–10.2 µm, proximal diameter of shaft 5.1–6.7–8.9 µm, maximum diameter 7.6–10.0–11.4 µm, N = 30.
Small spicules – tylostyles, often bent in the proximal part, stout. Length 132–162–183 µm, diameter of tyle 3.8–5.6–7.6 µm, proximal diameter of shaft 2.5–3.7–5.1 µm, maximum diameter of shaft 2.5–5.0–6.4 µm, N = 30.
Exotyles – filiform subtylostyles. Length 1860–2590–3770 µm, diameter of tyle 12.7–14.1–16.5 µm, proximal diameter of shaft 7.6–10.9–14.0 µm, maximum diameter of shaft 10.2–13.8–17.8 µm, N = 10.
Genetic data
The Norwegian Polymastia sp. described above is genetically very close to P. svenseni and an unidentified Canadian Polymastia (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). The relationships of this trio with other polymastiids and the synapomorphies between the Norwegian Polymastia sp. and P. svenseni are described in the Genetic data section for the latter species above. Three synapomorphies in CO1 distinguish the Norwegian Polymastia sp. and the Canadian Polymastia sp. from all other polymastiids (Online resource 2, p. 3, Matrix M34248 in TreeBase). At the same time the Norwegian Polymastia sp. is distinguished by two autapomorphies in this gene (Online resource 2, p. 3). Apart from them, it differs from the Canadian Polymastia sp. by four bps in CO1 (Matrix M34248 in TreeBase), from P. svenseni by 19 bps in CO1 (Matrix M34248 in TreeBase) and two bps in 28S rDNA (Matrix M34250 in TreeBase) and from the type species of Polymastia, P. mamillaris, by 68 bps in CO1 (Matrix M34248 in TreeBase) and 21 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
Fig. 20. Polymastia sp., ZMBN 098080: locality (white circle).
Norwegian Coast: Hordaland (312 m).
DISCUSSION
The sponge described above is placed in Polymastia based on the CO1 and 28S rDNA phylogenies. Meanwhile, it cannot be identified as any known Polymastia spp. It strongly resembles Polymastia andrica by the presence of four categories of spicules and a surface hispidation formed by the tracts of principal spicules crossing the cortex and reinforced with exotyles. However, in the molecular phylogenies Polymastia sp. is closely related to the morphologically quite distinct P. svenseni, while P. andrica falls in another subclade. We assume that Polymastia sp. may be a species new to science, but postpone the formal erection of this species until more material in addition to the single tiny individual becomes available.
Genus Quasillina Norman, Reference Norman1869
Original description: Quasillina Norman, Reference Norman1869, p. 329.
SYNONYMS
Bursalina Schmidt, Reference Schmidt1875, p. 116.
TYPE SPECIES
Polymastia brevis Bowerbank, Reference Bowerbank1866 (by original designation).
DIAGNOSIS
Polymastiidae of pedunculate or columnar body shape with a smooth surface and a single osculum located either directly at the summit of the main body or at the summit of a short papilla. Choanosomal skeleton is a mass of small monactines. Cortex comprises a superficial palisade of small monactines, a middle layer of criss-cross large or intermediary monactines and an inner layer of longitudinal tracts of large monactines lying parallel to the surface.
DISCUSSION
Quasillina Norman, Reference Norman1869 is very similar to Ridleia Dendy, Reference Dendy1888. Both genera lack one of the main morphological synapomorphies usually defined for Polymastiidae (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002), the presence of main choanosomal skeleton composed of tracts of principal spicules. In Ridleia the choanosomal skeleton is restricted to a subcortical layer of criss-cross small spicules, whereas in Quasillina it is an unordered mass of small spicules scattered over the choanosome that corresponds to the auxiliary choanosomal skeleton in other polymastiids. At the same time in both Quasillina and Ridleia longitudinal tracts of principal spicules are located in the inner part of the cortex making it very similar to the papilla wall in other polymastiids. Of the alternative phylogenies of Polymastiidae based on 25 morphological characters, one, suggesting the absence of papillae in Quasillina and Ridleia, displayed the position of these genera outside the clade of other polymastiids, whereas the other, interpreting their body as a single hyper-developed papilla with reduced choanosome, testified for the monophyly of Polymastiidae including Quasillina and Ridleia (Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). Phylogenies based on CO1 and 28S rDNA confirmed that Quasillina is a polymastiid (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; Figure 1 in this study), but no molecular data on Ridleia are available so far.
Quasillina brevis (Bowerbank, Reference Bowerbank1866)
(Figure 21)
Original description: Polymastia brevis Bowerbank, Reference Bowerbank1866, p. 64.
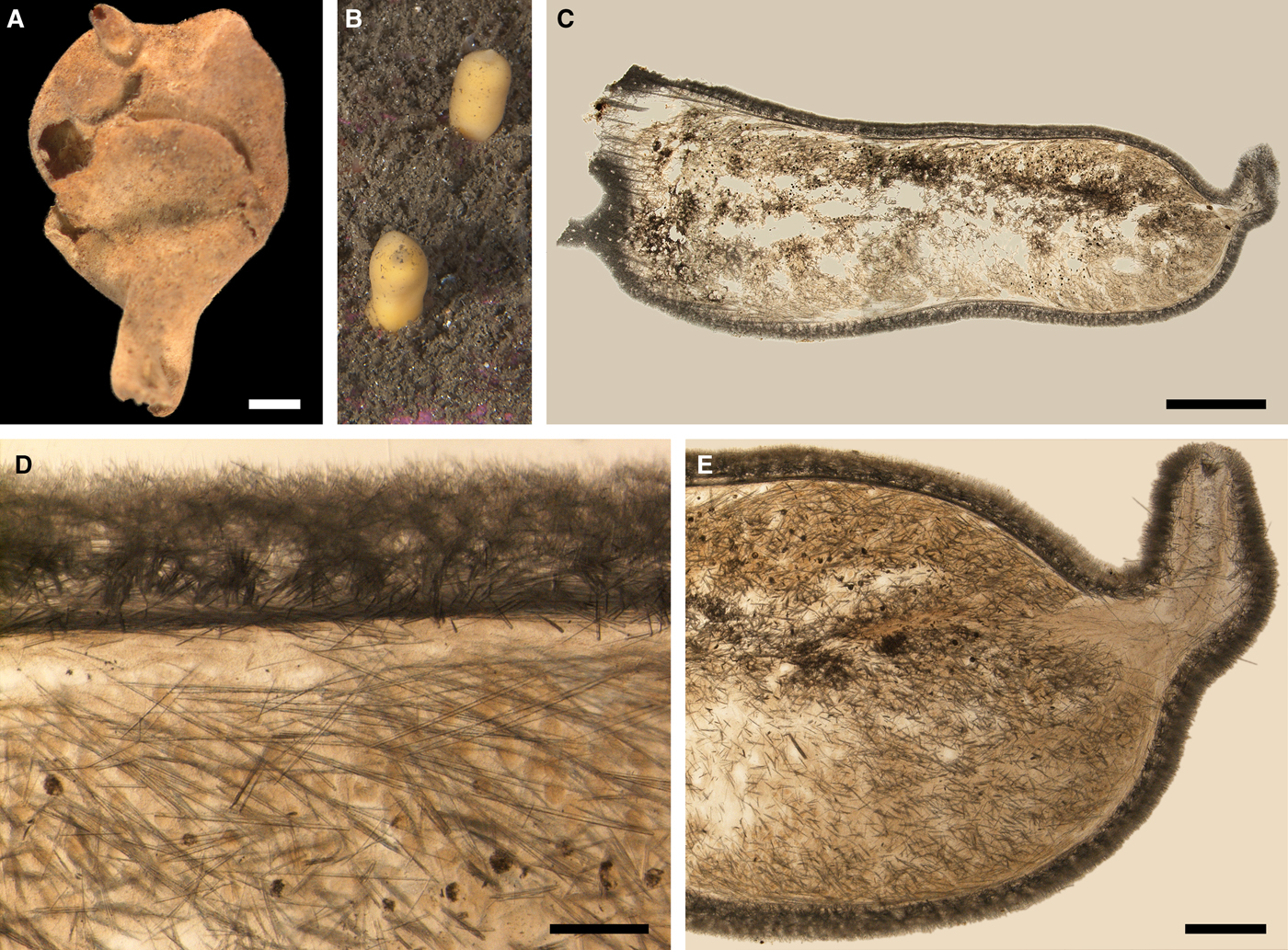
Fig. 21. Quasillina brevis: (A) lectotype BMNH 1910.1.1.5-6, habitus; (B) individuals in situ, Tingelsædet, Egersund, Norway (courtesy of E. Svensen, OceanPhoto/Dalane Tidende AS, Egersund); (C) ZMBN 098084, longitudinal section through the body, general view; (D) the same section, detail of cortex; (E) ZMBN 098084, longitudinal section through a papilla and adjacent area. Scale bars: A, 0.5 cm; C, 3 mm; D, 0.3 mm; E, 1 mm.
SYNONYMS AND CITATIONS
Bursalina muta (Schmidt, Reference Schmidt1875, p. 116, pl. I figures 3 & 4).
Euplectella brevis (Bowerbank, Reference Bowerbank and McAndrew1861, p. 236 nomen nudum; 1861, p. 71 nomen nudum).
Polymastia brevis (Bowerbank & Norman, Reference Bowerbank and Norman1882, p. 16 and 31; Fristedt, Reference Fristedt1887, p. 433; Topsent, Reference Topsent1888, p. 140).
Quasillina brevis (Norman, Reference Norman1869, p. 329; Vosmaer, Reference Vosmaer1885, p. 20, Reference Vosmaer and Bronn1887, p. 330, pl. XXVI, figure 12; Ridley & Dendy, Reference Ridley and Dendy1886, p. 490, Reference Ridley and Dendy1887, p. 226, figure 10; Dendy, Reference Dendy1888, p. 520, figures 8–12; Topsent, Reference Topsent1892, p. 132, Reference Topsent1900, p. 158; Hanitsch, Reference Hanitsch1894, p. 175 and 203; Whiteaves, Reference Whiteaves1901, p. 14; Lundbeck, Reference Lundbeck1909, p. 452; Brøndsted, Reference Brøndsted1914, p. 522;, Reference Brøndsted, Jensen, Lundbeck and Ragnar Spärck1932, p. 6, Reference Brøndsted1933, p. 14; Stephens, Reference Stephens1915, p. 30; Rezvoj, Reference Rezvoj1928, p. 81; Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929, p. 869; Burton, Reference Burton1930a, p. 496;, Reference Burton, Fridriksson and Tuxen1959a, p. 13; Alander, Reference Alander1942, p. 76; Lévi, Reference Lévi1950, p. 9; Koltun, Reference Koltun1966, p. 89, text-figure 89, pl. XIX figures 9 & 10, pl. XXVI figures 3–5); Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994, p. 71, figure 46; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 211, figure 8; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 545, figures 1l & 2l; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1h).
Quasillina richardi Topsent, Reference Topsent1913, p. 19, pl. III figure 7 and pl. V figure 14 (Alander, Reference Alander1942, p. 76; Gorbunov, Reference Gorbunov1946, p. 37; Burton, Reference Burton, Fridriksson and Tuxen1959a, p. 13).
TYPE MATERIAL
Lectotype of Polymastia brevis (designated herein, Figure 21A): BMNH 1910.1.1.5–6 (dry specimen), Shetland, UK, precise locality unknown, 109–164 m.
Paralectotypes of Polymastia brevis: BMNH 1910.1.1.5–6 (six intact specimens and several fragments, all dry), BMNH 1898.5.7.59 (two specimens in alcohol) and BMNH 1910.1.1.597A (two specimens in alcohol), from the same sample as the lectotype.
Syntypes of Quasillina richardi Topsent, Reference Topsent1913: MOM 04-0817 (two specimens in alcohol), between Bear Island and Norwegian Coast, 72°37′N 20°00′E, 394 m, Campagnes scientifiques accomplies par le Prince Albert I de Monaco, RV ‘Princesse-Alice’, station 960, 29.07.1898. Herein Q. richardi is regarded as a synonym of Q. brevis.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: offshore areas of NW Atlantic: ZIN RAS ocqb007 (one specimen).
Iceland/Denmark Strait: ZMBN 098067 (one specimen).
North-East Atlantic, offshore: ZIN RAS ocqb006 (one specimen).
Norwegian Sea, offshore: ZIN RAS ocqb010 (one specimen).
Barents Sea, offshore: ZIN RAS ocqb001, ZIN RAS ocqb002, ZIN RAS ocqb004, ZIN RAS ocqb009, ZIN RAS ocqb012, ZIN RAS ocqb013, ZIN RAS ocqb014, ZIN RAS ocqb015, ZIN RAS ocqb016, ZIN RAS ocqb017, ZIN RAS ocqb018, ZIN RAS ocqb019 and ZIN RAS ocqb020 (13 specimens).
Norway: Vest-Agder: ZMBN 098084, ZMBN 098090 and ZMBN 107573 (three specimens), Rogaland: ZMBN 098082 (one specimen), Sogn and Fjordane: ZMBN 098049 (one specimen), Møre and Romsdal: ZMBN 107489 and ZMBN 107491 (two specimens), Nord-Trøndelag: NTNU-VM-54600, NTNU-VM-54747, NTNU-VM-54751, NTNU-VM-54754, NTNU-VM-54755 and NTNU-VM-72545 (15 specimens), Nordland: NTNU-VM-72516 (two specimens), Finnmark: NTNU-VM-72501 (two specimens). Svalbard: ZIN RAS ocqb008 (one specimen).
Russia: Murman Coast: ZIN RAS ocqb011 (one specimen), Novaya Zemlya: ZIN RAS ocqb005 (one specimen).
Arctic Ocean, offshore: ZIN RAS ocqb003 (one specimen).
DESCRIPTION
External morphology
Lectotype of Polymastia brevis has an irregularly ovoid main body, ~25 mm in diameter and 28 mm high, sitting on a stalk, ~11 mm high and 7 mm in diameter and bearing a well developed cylindrical papilla, ~5 mm long and 3 mm in diameter, with an osculum at the summit (Figure 21A). Paralectotypes smaller, some with weakly developed papillae, others lacking papillae and bearing oscula directly on the body summits. Most other sponges columnar, up to 35 mm high and up to 8 mm in diameter, with no segregation between main body and stalk (Figure 21B). Few sponges with ovoid or pyriform main bodies sitting on stalks. Papillae, if present, are weakly developed. Colour in life pale orange or yellowish. Syntypes of Quasillina richardi are small fragments, one on a stalk lacking the major part of the main body, the other being a residual of the main body.
Anatomy
In life choanosome is an intensive yellow or orange, unstructured, semi-fluid mass without any spicule tracts. This mass is often washed away under preservation. Choanosomal skeleton comprises free-scattered bundles of small spicules (Figure 21C). Cortex in life pale orange or pale yellow, leather-like. Cortical skeleton comprises a superficial palisade (150–200 µm thick) of bouquets of small spicules and two inner overlapping layers, a layer of criss-cross large spicules (270–300 µm thick) located midst in the cortex and an internal layer (35–80 µm thick) of longitudinal tracts of large spicules (Figure 21D). Skeleton of the papilla wall is the same as the cortical skeleton (Figure 21E).
Spicules
(Measurements based on 15 specimens)
Principal spicules – subtylostyles to styles, straight, or occasionally gently curved, fusiform. Length 570–862–1098 µm, maximum diameter 11.2–17.8–25.5 µm, N = 450.
Small spicules – styles to subtylostyles, often bent in the distal part, slender. Length 140–227–306 µm, maximum diameter of shaft 1.9–4.0–5.8 µm, N = 450.
Genetic data
Data obtained from five individuals of Quasillina brevis are identical in both genes studied. Quasillina brevis is closely related, especially in the CO1 phylogeny, to morphologically distinct Polymastia boletiformis (see the synapomorphies in the Genetic data section for the latter species above). At the same time Q. brevis possesses six autapomorphies in 28S rDNA distinguishing it from all other polymastiids (Online resource 3, p. 4) and, apart from them, differs from P. boletiformis by 18 bps in this gene (Matrix M34250 in TreeBase).
OCCURRENCE
Fig. 22. Quasillina brevis, distribution: black star, type locality of Polymastia brevis; white star, type locality of Quasillina richardi (synonym of Q. brevis); black squares, data from Boury-Esnault et al. (Reference Boury-Esnault, Pansini and Uriz1994); white square, data from Brøndsted (Reference Brøndsted1933); black trefoils, data from Burton (Reference Burton, Fridriksson and Tuxen1959a); black diamond, data from Hentschel (Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929); white diamond, data from Fristedt (Reference Fristedt1887); black heart, data from Lundbeck (Reference Lundbeck1909); white heart, data from Ridley & Dendy (Reference Ridley and Dendy1886); black triangle, data from Stephens (Reference Stephens1915), white triangle, data from Topsent (Reference Topsent1892); white circles, our data.
Literature data:
Canadian Atlantic Coast: Nova Scotia (155 m) (Ridley & Dendy, Reference Ridley and Dendy1886, Reference Ridley and Dendy1887). Azores (318 m) (Topsent, Reference Topsent1892). West of Gibraltar (218–524 m) (Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994). Mediterranean Sea: Moroccan Coast (144–145 m) (Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994). Northern French Coast (Lévi, Reference Lévi1950). British Isles: Ireland (706 m) (Stephens, Reference Stephens1915), Shetland (73–309 m) (Bowerbank, Reference Bowerbank1866; Norman, Reference Norman1869). Iceland (83–557 m) (as Q. brevis and Q. richardi – Burton, Reference Burton, Fridriksson and Tuxen1959a). South Greenland (120 m) (Brøndsted, Reference Brøndsted1933). East Greenland (231 m) (Lundbeck, Reference Lundbeck1909). Faroes (160 m) (Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Spärck1932). Swedish Western Coast (85–360 m) (as Q. brevis and Q. richardi – Alander, Reference Alander1842). Norwegian Coast: Rogaland (193 m) (as Bursalina muta – Schmidt, Reference Schmidt1875; as Q. brevis – Burton, Reference Burton1930a), Hordaland (Burton, Reference Burton1930a), Sør-Trøndelag (Burton, Reference Burton1930a), Troms (Burton, Reference Burton1930a), Finnmark (273 m) (Fristedt, Reference Fristedt1887). Norwegian Sea (67–500 m) (as Q. brevis – Vosmaer, Reference Vosmaer1885; Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929; Koltun, Reference Koltun1966; as Q. richardi – Topsent, Reference Topsent1913). Barents Sea (67–500 m) (Vosmaer, Reference Vosmaer1885; Rezvoj, Reference Rezvoj1928; Koltun, Reference Koltun1966). Kara and Laptev Sea (67–500 m) (Gorbunov, Reference Gorbunov1946; Koltun, Reference Koltun1966).
Our data: Canadian Atlantic Coast: offshore areas (380–415 m). Iceland/Denmark Strait (118 m). North-East Atlantic (400 m). Norwegian Sea, offshore (394–500 m). Norwegian Coast: Vest-Agder (30–45 m), Rogaland (25–31), Sogn and Fjordane (241–254 m), Møre and Romsdal (100–120 m), Nord-Trøndelag (15–110 m), Nordland (360 m), Finnmark (250 m). Barents Sea: Murman Coast (110–146 m), offshore areas (90–442 m). Svalbard (759 m). Novaya Zemlya (8 m). Arctic Ocean, offshore (360 m).
DISCUSSION
Quasillina brevis is recorded in a wide geographic area, but there is some doubt whether all these records indicate the same species. Topsent (Reference Topsent1913) established a new species of Quasillina, Q. richardi, for the sponges from the Northern Norwegian Sea based on their difference from Q. brevis from more southern areas (British Isles, French Coast, Azores). In the former species small spicules were bent in the distal part, while in the latter they were straight. However, the correlation of the spicule shape in Quasillina to geography was questioned by several records of both Q. brevis and Q. richardi from the same localities, e.g. from Iceland (Burton, Reference Burton, Fridriksson and Tuxen1959a) and the Swedish Western Coast (Alander, Reference Alander1942). In our material from the Nordic Seas we have found both individuals with bent and straight small spicules, without any correlation to geography. Therefore, until molecular data on the British, South European and Azorean Quasillina become available and comparable with our data on the Nordic sponges, we follow Koltun (Reference Koltun1966) and Plotkin (Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) and conclude that Q. richardi should be regarded as a junior synonym of Q. brevis.
Genus Sphaerotylus Topsent, Reference Topsent1898
Original description: Sphaerotylus Topsent, Reference Topsent1898, p. 244.
TYPE SPECIES
Polymastia capitata Vosmaer, Reference Vosmaer1885 (by original designation).
DIAGNOSIS
Polymastiidae of hemispherical, dome, cushion-like or button-like body shape, always with papillae. Spicule assortment comprises smooth monactines in at least two size categories and exotyles with ornamented distal extremities, which may be rough, spined, granulated, tuberculated or wrinkled, often with knobs varying from spherical to hemispherical, fungiform, umbrelliform or lobate. Main choanosomal skeleton composed of radial or longitudinal tracts of principal monactines. Auxiliary choanosomal skeleton comprises free-scattered, small and/or intermediary monactines, and, occasionally exotyles. A superficial cortical palisade composed of either exotyles with sparse small monactines or small monactines reinforced with sparse exotyles. An inner layer of criss-cross intermediary monactines may also be present.
DISCUSSION
According to the CO1 and 28S rDNA phylogenies Sphaerotylus Topsent, Reference Topsent1898 is not monophyletic (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; Figure 1 in this study). The two species described below, the type species, S. capitatus (Vosmaer, Reference Vosmaer1885), and S. borealis (Swarczewsky, Reference Swarczewsky1906) fall in remote clades. However, here we follow the traditional taxonomy of Sphaerotylus spp. (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Plotkin et al., Reference Plotkin, Morrow, Gerasimova and Rapp2016a), since no alternative classification is proposed for Polymastiidae so far (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b).
Sphaerotylus borealis (Swarczewsky, Reference Swarczewsky1906)
(Figure 23)
Original description: Proteleia borealis Swarczewsky, Reference Swarczewsky1906, p. 315, pl. X figure 1, pl. XIII figure 2.
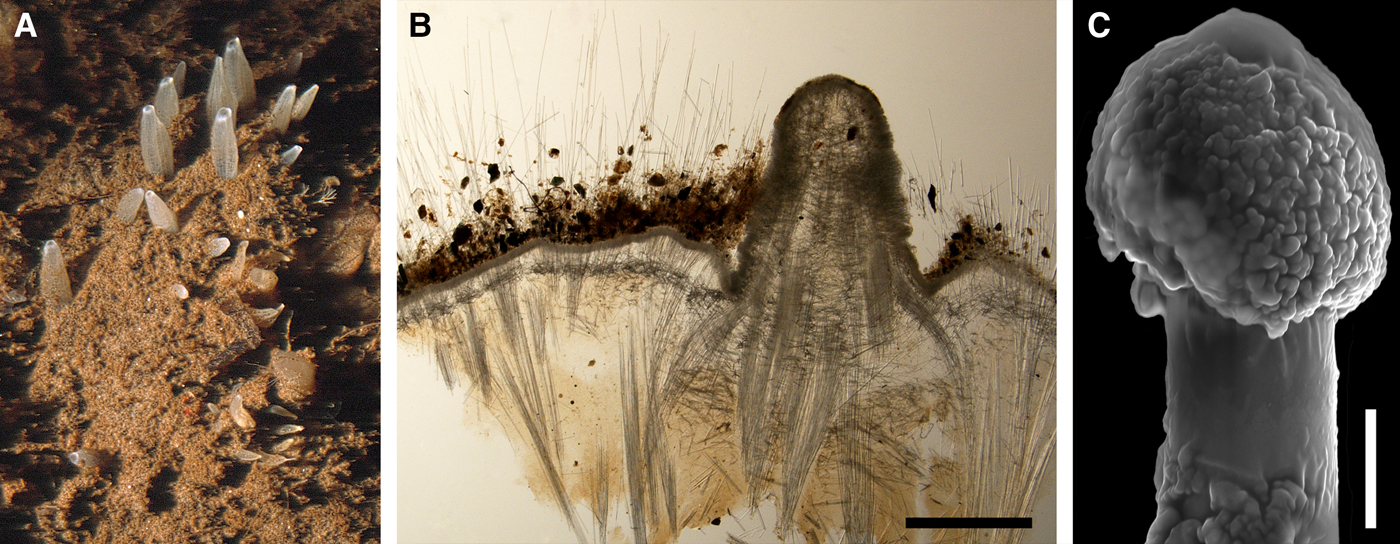
Fig. 23. Sphaerotylus borealis: (A) an individual in situ, Kandalaksha Bay, White Sea (courtesy of M. Fedyuk, St. Petersburg State University); (B) ZMBN 098059, longitudinal section through the body; (C) ZMBN 098036, proximal tip of exotyle. Scale bars: B, 2 mm; C, 0.005 mm.
SYNONYMS AND CITATIONS
Proteleia borealis (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 204).
Sphaerotylus borealis (Rezvoj, Reference Rezvoj1928, p. 78, figures 4 & 5; Koltun, Reference Koltun1966, p. 83, pl. XXX figures 1–5, text-figure 55; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 543, figures 1i, 2i & 4b).
Sphaerotylus schoenus var. borealis (Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929, p. 925).
TYPE MATERIAL
Neotype: ZIN RAS 11194, Sredny Island, Keret’ Inlet, Kandalaksha Bay, White Sea, 66°17.391′N 33°38.025′E, 10–13 m, 12.07.2000, coll. Plotkin.
Designation and detailed description of the neotype was done by Plotkin et al. (Reference Plotkin, Morrow, Gerasimova and Rapp2016a).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Iceland: ZIN RAS 11169 and ZIN RAS 11184 (two specimens).
Norwegian Sea, offshore: ZIN RAS 11145 (one specimen).
Barents Sea, offshore: ZIN RAS 11146, ZIN RAS 11156, ZIN RAS 11157, ZIN RAS 11158, ZIN RAS 11159, ZIN RAS 11160, ZIN RAS 11163, ZIN RAS 11165, ZIN RAS 11166, ZIN RAS 11167, ZIN RAS 11170, ZIN RAS 11171, ZIN RAS 11176, ZIN RAS 11177 and ZIN RAS 11181 (15 specimens).
Russia: Murman Coast: ZIN RAS 11164, ZIN RAS 11168, ZIN RAS 11174 and ZIN RAS 11183 (four specimens), White Sea: ZIN RAS 11144, ZIN RAS 11147, ZIN RAS 11148, ZIN RAS 11149, ZIN RAS 11150, ZIN RAS 11151, ZIN RAS 11155, ZIN RAS 11161, ZIN RAS 11162, ZIN RAS 11175, ZIN RAS 11180, ZIN RAS 11182, ZIN RAS 11195, ZMBN 098036, ZMBN 098059 and ZMBN 098061(31 specimens), Severnaya Zemlya: ZIN RAS 11179 (one specimen).
Arctic Ocean, offshore: ZIN RAS 11178 (one specimen).
DESCRIPTION
(according to Plotkin et al., Reference Plotkin, Morrow, Gerasimova and Rapp2016a)
External morphology
Sponges thickly encrusting or cushion-shaped, the largest up to 100 cm2. Surface shaggy, silted with sediment making it dirty greyish or brownish in colour. Up to 50 cylindrical or conical papillae, whitish in life, but usually becoming pale yellow, brownish or pinkish in alcohol. On soft bottoms living sponges are often completely covered by sediment with only erect papillae protruding above (Figure 23A). On hard bottoms the sponges may contract the papillae. After sampling and fixation the papillae always considerably contract and invaginate into the surface hispidation. Oscula not visible in preserved sponges.
Skeleton
Choanosome in life orange, dense. Main choanosomal skeleton composed of longitudinal tracts of principal spicules which cross the cortex and make up a dense and thick surface hispidation (Figure 23B). Auxiliary choanosomal skeleton comprises small, occasionally intermediary, spicules often arranged in bundles, 3–7 spicules each. Cortex in life whitish, dense, not detachable. Cortical skeleton composed of a 115–120 µm thick superficial palisade of small spicules and an internal layer (~210 µm thick) of tangentially arranged intermediary spicules (Figure 23B). In areas around papillae these layers are separated by an intermediate, aspicular zone (about 100 µm thick). Exotyles cross the cortex and join the surface hispidation. Walls of papillae lack the tangential cortical layer. Single intermediary spicules scattered both in the walls and in the bulkheads between canals.
Spicules
(Measurements based on 10 specimens)
Principal spicules – styles to subtylostyles, often polytylote straight, slender. Length 1100–2423–5000 µm, diameter of shaft 12.0–16.2–19.0 µm, N = 100.
Intermediary spicules – tylostyles, usually straight, occasionally curved, slightly fusiform. Length 200–502–796 µm, diameter of tyle 6.9–9.2–11.1 µm, proximal diameter of shaft 5.0–7.1–9.0 µm, maximum diameter of shaft 6.9–10.8–14.3 µm, N = 100.
Small spicules – tylostyles, straight or curved, usually slender. Length 94–125–160 µm, diameter of tyle 3.9–4.6–5.1 µm, diameter of shaft 3.0–3.5–4.0 µm, N = 100.
Exotyles slender, 5100–6117–7520 µm long, usually with weakly developed or completely reduced proximal tyles. Shafts 13.8–17.2–20 µm in maximum diameter. Distal knobs (14.1–19.9–27.0 µm in diameter) usually irregularly fungiform or umbrelliform (Figure 23C), more rarely hemispherical or spherical, occasionally with short protuberances on the edges, sometimes slightly displaced along the shaft or comprising several swellings. Surface of the knobs and the adjacent portions of the shafts rough, wrinkled, granulated or tuberculated.
Genetic data
Of the three individuals of Sphaerotylus borealis, from which genetic data were obtained, two sponges differ neither by CO1, nor by 28S rDNA. The third individual is distinguished by two bps in 28S rDNA (Matrix M34250 in TreeBase), but no CO1 was obtained from it. In both CO1 and 28S rDNA phylogenies S. borealis does not group with any of the congeners (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). The difference between S. borealis and the type species of Sphaerotylus, S. capitatus, is 26 bps in CO1 (Matrix M34248 in TreeBase) and 86 bps in 28S rDNA, excluding the intraspecific polymorphism in both species (Matrix M34250 in TreeBase). At the same time S. borealis possessing just one autapomorphy in 28S rDNA (Online resource 3, p. 3) forms a clade with two morphologically quite distinct species, Polymastia cf. conigera Bowerbank, Reference Bowerbank1874 (a British species not covered by the present study) and Weberella bursa (the type species of Weberella Vosmaer, Reference Vosmaer1885 described below) in both CO1 and 28S rDNA phylogenies (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). However, the Bayesian support for this clade is weak (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These three species share just two synapomorphies in 28S rDNA distinguishing them from all other polymastiids (Online resource 3, p. 3, Matrix M34250 in TreeBase), while the differences between them are large. Sphaerotylus borealis differs from P. cf. conigera by 17 bps in CO1 (Matrix M34248 in TreeBase) and 35 bps in 28S rDNA (Matrix M34250 in TreeBase) and from W. bursa by 7 bps in CO1 (Matrix M34248 in TreeBase) and 33 bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
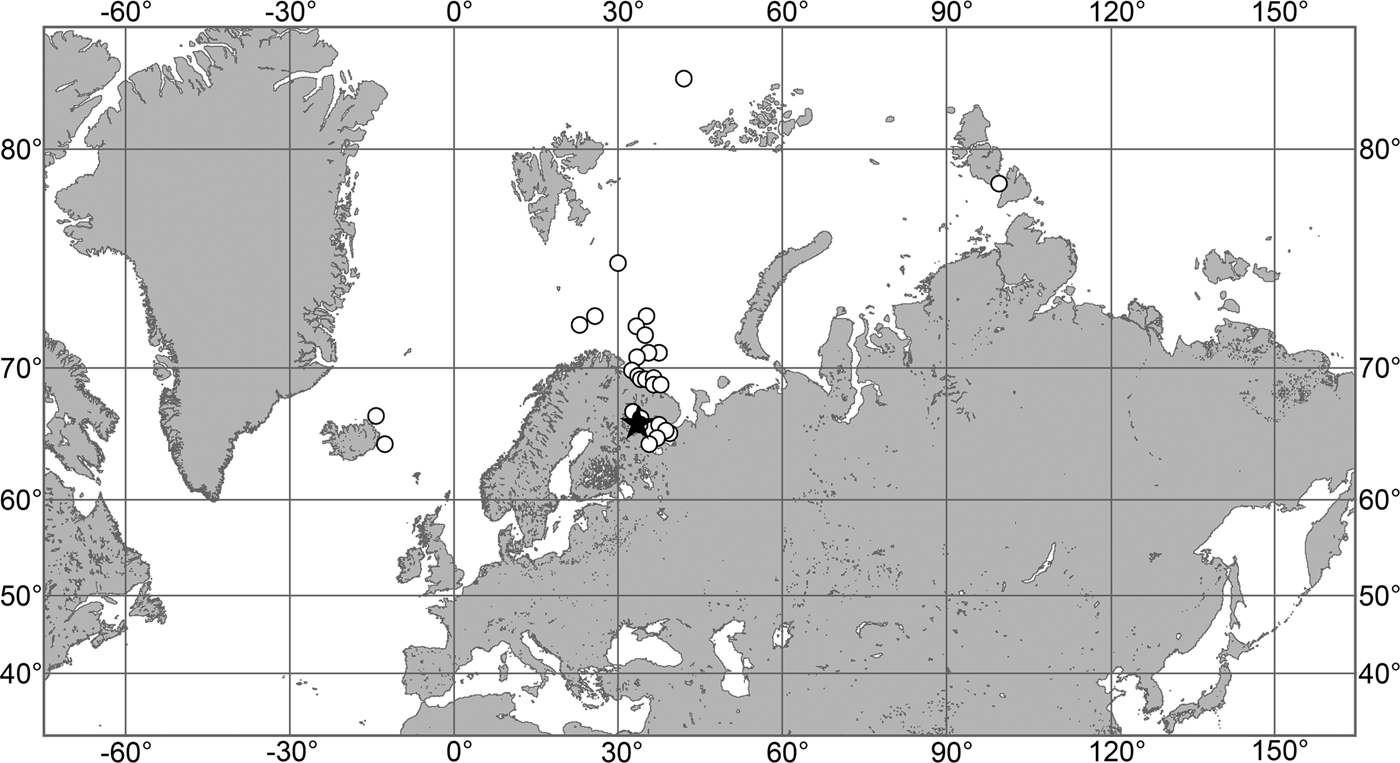
Fig. 24. Sphaerotylus borealis, distribution: black star, type locality; white circles, our data.
Our data (agree with the literature data): Iceland (157–240 m). Norwegian Sea, offshore areas (342 m). Barents Sea: Murman Coast (91–130 m), offshore areas (124–420 m). White Sea (10–57 m). Severnaya Zemlya (43 m). Arctic Ocean, offshore areas (415 m).
DISCUSSION
Sphaerotylus borealis (Swarczewsky, Reference Swarczewsky1906) was originally assigned to Proteleia Dendy & Ridley, Reference Dendy and Ridley1886, but later (Koltun, Reference Koltun1966) transferred to Sphaerotylus. The large morphological differences between S. borealis and the type species of Proteleia, P. sollasi Dendy & Ridley, Reference Dendy and Ridley1886, were described by Plotkin et al. (Reference Plotkin, Morrow, Gerasimova and Rapp2016a). At the same time S. borealis shares many features, e.g. the shaggy surface and the giant exotyles with umbrelliform or fungiform distal ornaments, with S. antarcticus Kirkpatrick, Reference Kirkpatrick1907. By these features both S. borealis and S. antarcticus are distinguished from the type species of Sphaerotylus, S. capitatus. Meanwhile, in CO1 and 28S rDNA phylogenies S. borealis and S. capitatus fall in remote clades, while the relationships between S. antarcticus and S. capitatus are ambiguous (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). Grouping of S. borealis with Polymastia cf. conigera and Weberella bursa in the molecular phylogenies has a weak Bayesian support, and no morphological synapomorphies can, for the moment, be defined for this grouping (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). Thus, the taxonomic position of S. borealis remains unclear.
Sphaerotylus capitatus (Vosmaer, Reference Vosmaer1885)
(Figure 25)
Original description: Polymastia capitata Vosmaer, Reference Vosmaer1885, p. 16, pl. IV figures 25–29.
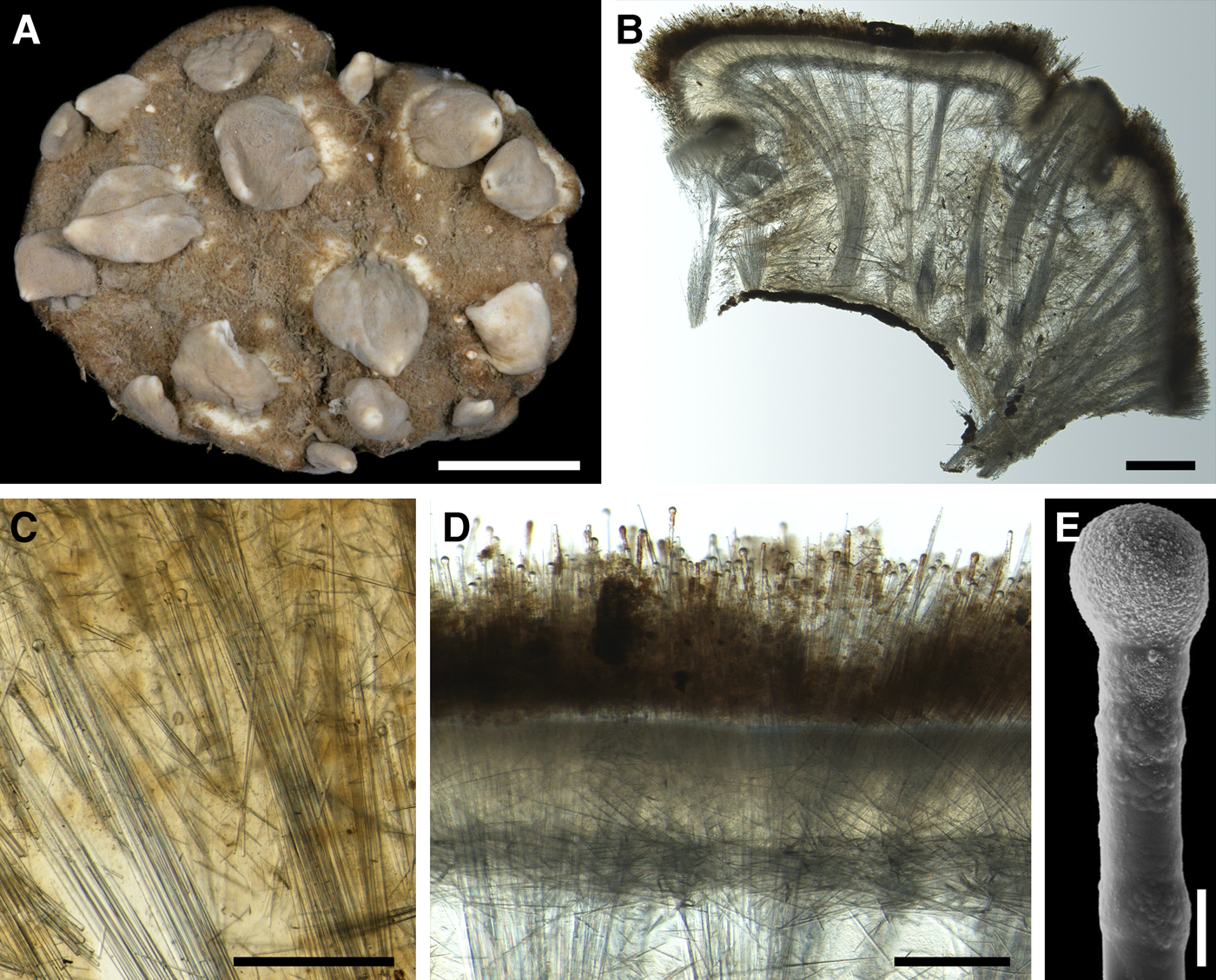
Fig. 25. Sphaerotylus capitatus: (A) GNM 899, habitus; (B) ZMBN 107485, longitudinal section through the body, general view; (C) paralectotype BMNH 1910.1.1.1199, detail of choanosome with exotyles; (D) ZMBN 107485, longitudinal section through the body, detail of cortex; (E) ZMB 10855, proximal tip of exotyle. Scale bars: A, 1 cm; B, 1 mm; C–D, 0.3 mm; E, 0.02 mm.
SYNONYMS AND CITATIONS
Polymastia capitata (Breitfuss, Reference Breitfuss1911, p. 218).
Polymastia schoenus (Dendy & Ridley, Reference Dendy and Ridley1886, p. 155, text-figure).
Radiella schoenus (Sollas, Reference Sollas1882, p. 162, considered as nomen nudum by Kirkpatrick, Reference Kirkpatrick1908, p. 18).
Sphaerotylus capitatus (Topsent, Reference Topsent1898, p. 244; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 206, figure 4; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 543, figures 1H, 2H & 4A; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 27, figure 2d).
Sphaerotylus schoenus (Topsent, Reference Topsent1913, p. 23, pl. II figure 6, Reference Topsent1928, p. 154; Koltun, Reference Koltun1966, p. 85, pl. XXX figures 6 & 7, text-figure 56; Desqueyroux-Faúndez & Van Soest, Reference Desqueyroux-Faúndez and Van Soest1997, p. 421).
TYPE MATERIAL
Lectotype and one paralectotype: RMNH 704, Norwegian Sea, 72°14.8′N 22°30.9′E, about 300 m, ‘Willem Barentz’ Expedition, station 28, 30.06.1881.
Paralectotype: BMNH 1910.1.1.612 (specimen in alcohol) and BMNH 1910.1.1.1196-1200 (slides), from the same sample as the lectotype.
Paralectotype: ZMA 1841 (specimen, not studied), from the same sample as the lectotype.
Detailed description of the type material was presented by Plotkin et al. (Reference Plotkin, Morrow, Gerasimova and Rapp2016a).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Greenland: ZIN RAS 1193 (one specimen).
Sweden: Västra Götaland: GNM 899, GNM 900:1-2 and GNM 902 (four specimens).
Norway: Hordaland: ZMB 10855 and ZMBN 098042 (two specimens), Møre and Romsdal: |NTNU-VM-69133, ZMBN 107485, ZMBN 107487 and ZMBN 107490 (four specimens), Sør-Trøndelag: NTNU-VM-54686 and NTNU-VM-57267 (two specimens), Nord-Trøndelag: NTNU-VM-54629 and NTNU-VM-66578 (two specimens), Nordland: NTNU-VM-55862 and NTNU-VM-72521 (three specimens), Troms: ZMBN 098075 (one specimen), Finnmark: ZIN RAS 1190 (one specimen), Svalbard: ZIN RAS 1185 and ZIN RAS 1192 (two specimens).
Russia: Murman Coast: ZIN RAS 1186, ZIN RAS 1187 and ZIN RAS 1188 (three specimens).
Barents Sea, offshore: ZIN RAS 1189 and ZIN RAS 1191 (two specimens).
DESCRIPTION
External morphology
Sponges thickly encrusting, cushion-shaped or massive, fist- or dome-shaped, occupying up to 50 cm2 of the substrate. Surface velvety, knobbly, dark brown in colour, with up to 30 papillae (Figure 25A). Papillae of living sponges whitish or pale yellow in colour, conical, with small, scarcely visible oscula at the summits. In alcohol-preserved specimens the papillae may be considerably contracted looking like tubercles, while their colour does not change much.
Skeleton
Choanosome in life yellowish or pale orange, dense. Main choanosomal skeleton composed of radial or longitudinal tracts of principal spicules which enter the cortex (Figure 25B). Auxiliary choanosomal skeleton comprises small and intermediary spicules usually scattered singly or sometimes arranged in small groups. Besides these spicules some individuals possess exotyles between the choanosomal tracts (Figure 25C). Cortex in life whitish, firm, not detachable. Cortical skeleton composed of a superficial palisade (~110 µm thick) of small spicules, an internal layer (about 170 µm thick) of tangentially arranged intermediary spicules and a middle layer (180–190 µm thick) with a low concentration of spicules (Figure 25D). Exotyles cross the cortex forming a dense superficial layer with their distal knobs rising above the palisade. Papillae walls without internal cortical layer. Single intermediary spicules scattered both in the papillae walls and in the bulkheads between the canals.
Spicules
(Measurements based on five specimens)
Principal spicules – subtylostyles to styles, often polytylote, straight, slightly fusiform or slender. Length 650–998–1505 µm, diameter of tyle, if present, 10.0–12.8–16.0 µm, proximal diameter of shaft 8.9–11.5–15.1 µm, maximum diameter of shaft 14.0–19.5–26.0 µm, N = 50.
Intermediary spicules – tylostyles, straight or gently curved, slender or slightly fusiform. Length 314–484–650 µm, diameter of tyle 9.1–11.4–14.0 µm, proximal diameter of shaft 6.9–8.8–11.0 µm, maximum diameter of shaft 9.0–13.0–16.5 µm, N = 50.
Small spicules – tylostyles, straight or curved, usually slender. Length 96–155–221 µm, diameter of tyle 2.9–4.6–6.1 µm, proximal diameter of shaft 1.1–2.3–3.2 µm, maximum diameter of shaft 2.0–5.0–7.0 µm, N = 50.
Exotyles straight or gently curved, slender, 650–974–1250 µm long. Proximal tyles varying from well-developed (6.8–11.0–14.0 µm in diameter) to reduced. Distal knobs usually regularly spherical (Figure 25E), occasionally hemispherical or elongated, 18.0–22.8–30.0 µm in diameter. Surface of the knobs and the adjacent portions of the shafts usually rough, spined or granulated. Shafts gradually expanding towards the distal knobs, N = 50.
Genetic data
CO1 sequences obtained from three individuals of Sphaerotylus capitatus are identical (Matrix M34248 in TreeBase). 28S rDNA sequences obtained only from two of these sponges differ by two bps (Matrix M34250 in TreeBase) and exhibit one synapomorphy distinguishing S. capitatus from other polymastiids (Online resource 3, p. 4). In the CO1 and 28S rDNA phylogenies S. capitatus forms a clade with two British unidentified species, Sphaerotylus sp. 1 and Sphaerotylus sp. 2, not covered by the present study (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These three species share three synapomorphies in CO1 (Online resource 2, p. 4, Matrix M34248 in TreeBase) and three synapomorphies in 28S rDNA (Online resource 3, p. 4, Matrix M34250 in TreeBase) distinguishing them from all other polymastiids. One more synapomorphy in 28S rDNA is shared only by S. capitatus and Sphaerotylus sp. 1 (Online resource 3, p. 4, Matrix M34250 in TreeBase). Meanwhile, S. borealis, a Nordic species described above, does not fall in this clade (see the differences between S. borealis and S. capitatus in the Genetic data section for the former species).
OCCURRENCE
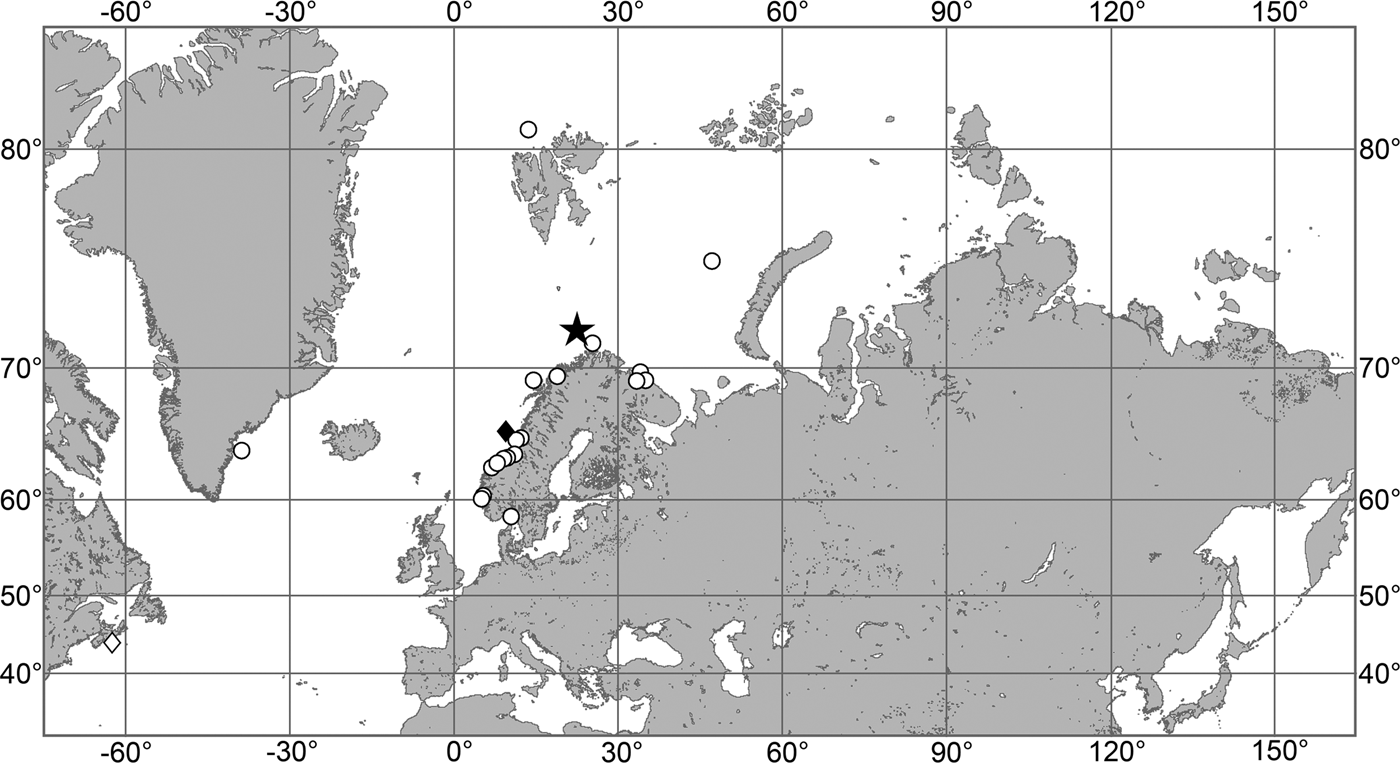
Fig. 26. Sphaerotylus capitatus, distribution: black star, type locality; black diamond, data from Topsent (Reference Topsent1913); white diamond, data from Topsent (Reference Topsent1928); white circles, our data.
Literature data (only the locality distinct from our data is given): Canadian Atlantic Coast: Nova Scotia (75 m) (as Sphaerotylus schoenus – Topsent, Reference Topsent1928).
Our data (agree with most literature data): Greenland (420–450 m). Norwegian Sea, offshore areas (300 m), Skagerrak: Swedish Western Coast (239–317 m). Norwegian Coast: Hordaland (200–500 m), Møre and Romsdal (130–250 m), Sør-Trøndelag (45–100 m), Nord-Trøndelag (20–130 m), Nordland (150–800 m), Troms (25 m), Finnmark (275 m). Barents Sea: Murman Coast (31–235 m), offshore areas (142–309 m). Svalbard (819 m).
DISCUSSION
Sphaerotylus capitatus is a well-defined species and identification of it usually causes no difficulties. Meanwhile, molecular data on a larger set of species are required for reconstruction of the relationships between S. capitatus and its congeners.
Genus Spinularia Gray, Reference Gray1867
Original description: Spinularia Gray, Reference Gray1867, p. 524.
SYNONYMS
Radiella Schmidt, Reference Schmidt1870, p. 48 pars.
Rhaphidorus Topsent, Reference Topsent1898, p. 244.
TYPE SPECIES
Tethea spinularia Bowerbank, Reference Bowerbank1866 (by original designation).
DIAGNOSIS
Polymastiidae of discoid, hemispherical, lenticular or cushion-like body shape with a shaggy or minutely hispid surface and one to 15 weakly developed exhalant papillae. Main choanosomal skeleton composed of longitudinal or radial tracts of principal monactines crossing the cortex. Auxiliary choanosomal skeleton comprises free-scattered small and/or intermediary (sub)tylostyles and may also include raphides in trichodragmata. Cortical skeleton may, in addition to the superficial palisade of small tylostyles, include extra spicule layers. Basal cortex, if present, reinforced with the peripheral tracts of principal spicules lying parallel to the surface. A spicule fringe is always present at the edge of the body.
GENETIC SYNAPOMORPHIES
Spinularia spp. are distinguished from other polymastiids by two synapomorphies in CO1 (Online resource 2, p. 5) and one synapomorphy in 28S rDNA (Online resource 3, p. 5).
DISCUSSION
Taxonomic history of Spinularia is closely related to Radiella and Trichostemma. Spinularia was established by Gray (Reference Gray1867) for a new species name Spinularia tetheoides Gray, Reference Gray1867 for unknown reasons proposed as a replacement for Tethea spinularia Bowerbank, Reference Bowerbank1866. This replacement was, however, not acknowledged by the subsequent authors. Radiella was established by Schmidt (Reference Schmidt1870) for two species, his new species Radiella sol Schmidt, Reference Schmidt1870 and Tethea spinularia, without designation of the type species. In that way Spinularia was consequently regarded as a synonym of Radiella, although the former had precedence over the latter. But three years later Schmidt (Reference Schmidt1880) reconsidered the status of Radiella spinularia (ex Tethea spinularia) acknowledging that it was conspecific with Halicnemia patera Bowerbank, Reference Bowerbank1864. Moreover, he admitted that Radiella might be a synonym of Halicnemia Bowerbank, Reference Bowerbank1864. However, Fristedt (Reference Fristedt1885, Reference Fristedt1887) preferred to retain Tethea spinularia in Radiella as R. spinularia, while Hanitsch (Reference Hanitsch1894) transferred it to Polymastia Bowerbank, Reference Bowerbank1862, although with some doubt. Spinularia was resurrected by Stephens (Reference Stephens1915) who defined the main feature distinguishing this genus from Polymastia, Halicnemia and Radiella, the presence of raphides (filiform oxea-like spicules) packed in trichodragmata. In the same paper Stephens stated that the type species of Rhaphidorus Topsent, Reference Topsent1898, R. setosus Topsent, Reference Topsent1898, which also possessed raphides in trichodramata, is synonymous with S. spinularia, and, consequently, Rhaphidorus was synonymized with Spinularia. The actions performed by Stephens were acknowledged by most subsequent authors (Topsent, Reference Topsent1928; Burton, Reference Burton1930a; Alander, Reference Alander1942; Lévi, Reference Lévi and Crosnier1993; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). Since Tethea spinularia was the type species of Spinularia, Radiella sol was designated as the type species of Radiella (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002).
Trichostemma was established by Sars (Reference Sars1872) for his new species T. hemisphaericum Sars, Reference Sars1872, although these names first appeared in a faunistic list three years earlier (Sars, Reference Sars1869). Schmidt (Reference Schmidt1880) considered T. hemisphaericum to be a synonym of Radiella sol and, consequently, Trichostemma to be a synonym of Radiella. However, most subsequent authors recognized T. hemisphaericum and R. sol as different species, although they agreed that Trichostemma and Radiella were synonymous, and there were debates on which of these names preceded until Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) acknowledged the synonymization of Trichostemma with Radiella based on the principle of priority (for references and other details see Discussion on Polymastia hemisphaerica above).
The relationships between Polymastia, Spinularia, Radiella and Trichostemma were re-considered by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b). Based on the CO1 and 28S rDNA phylogenies Radiella sarsii and Radiella sp. (here described as Spinularia njordi sp. nov., see below) forming a strongly supported clade with Spinularia spinularia (Figure 1) were transferred to Spinularia. Conversely, Trichostemma hemisphaericum, falling into a clade with the type species of Polymastia (P. mamillaris), was transferred to Polymastia. Consequently, Trichostemma Sars, Reference Sars1872 was regarded as a junior synonym of Polymastia Bowerbank, Reference Bowerbank1862 (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These actions are followed in the present study, and the definition of Spinularia is extended to cover the species lacking raphides in trichodragmata (see Diagnosis above). Spinularia australis Lévi, Reference Lévi and Crosnier1993, a species from New Caledonia originally placed in Spinularia, perfectly fits with this diagnosis. It possesses raphides like S. spinularia and, at the same time, resembles S. sarsii by body shape and the presence of basal cortex. We also agree that Rhaphidorus is a synonym of Spinularia, although we question the synonymization of R. setosus with S. spinularia (for details see Discussion on S. spinularia below).
Meanwhile, the status of the type species of Radiella, R. sol, remains uncertain. Its type material is lost (Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012) and no fresh material is available. The age-old non-type specimen identified as R. sol by Schmidt (Reference Schmidt1880) and re-described by Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) resembles Polymastia hemisphaerica by its relatively narrow marginal spicule fringe (Plotkin & Janussen, Reference Plotkin, Janussen, Martínez Arbizu and Brix2008; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). But it does not match the drawing in the original description of R. sol (Schmidt, Reference Schmidt1870: pl. 4, figure 6), which displays a wider marginal fringe rather resembling Spinularia sarsii (Plotkin & Janussen, Reference Plotkin, Janussen, Martínez Arbizu and Brix2008; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). However, regardless of the relationships of R. sol with P. hemisphaerica and S. sarsii, Radiella is abandoned since both Polymastia and Spinularia are older names (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b).
Spinularia njordi sp. nov.
(Figure 27)
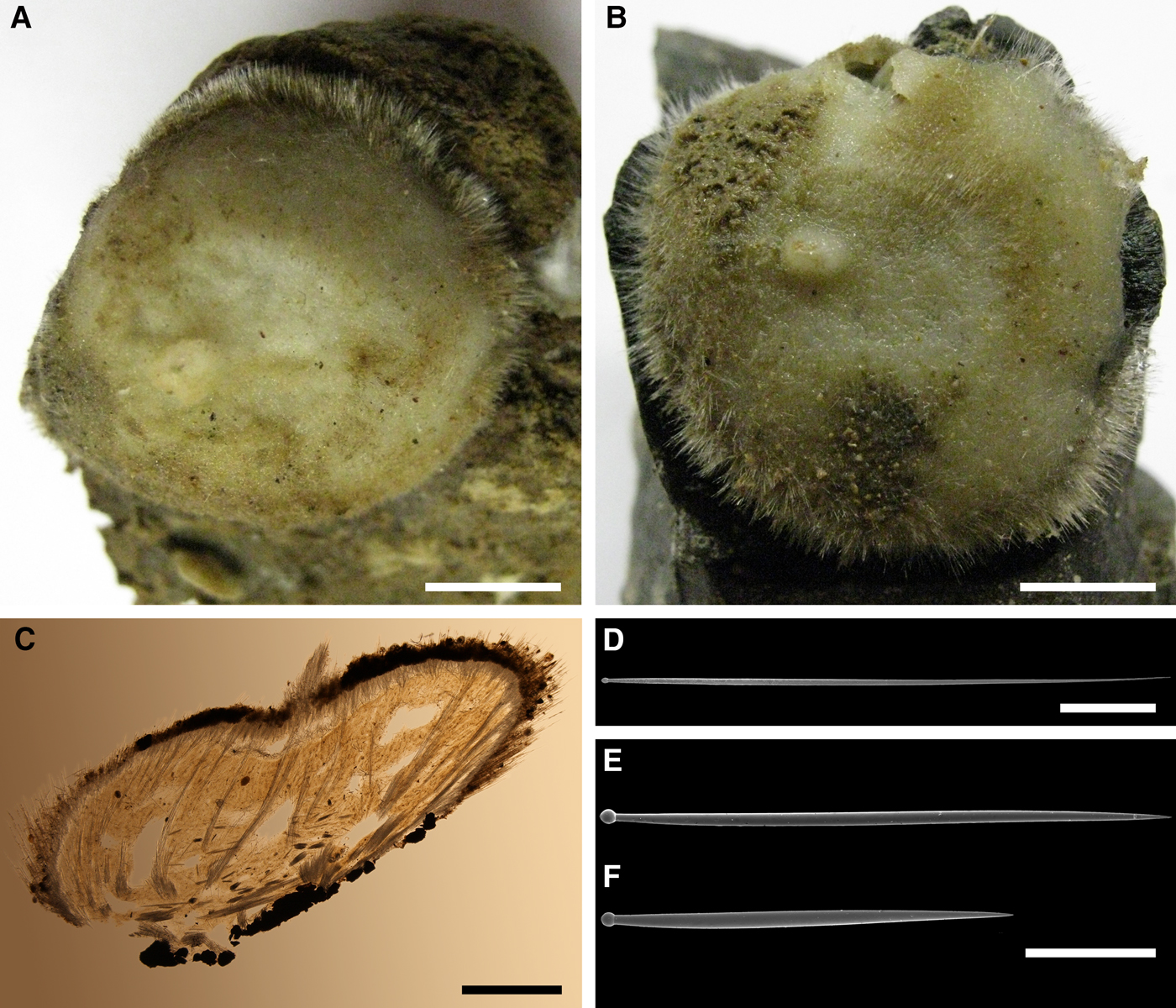
Fig. 27. Spinularia njordi: (A) holotype ZMBN 098040, habitus; (B) paratype ZMBN 098041, habitus; (C) paratype ZMBN 098038, longitudinal section through the body; (D) the same individual, principal subtylostyle; (E) the same individual, intermediary tylostyle; (F) the same individual, small tylostyle. Scale bars: A–B, 0.5 cm; C, 2 mm; D, 0.2 mm; E–F, 0.1 mm.
TYPE MATERIAL
Holotype: ZMBN 098040 (specimen), Low-angle fault to the west of Loki's Castle, North Norwegian Sea, 73°34.738′–73°35.005′N 08°03.034′–08°01.085′E, 2457–1997 m, RV ‘G.O. Sars’, expedition H2DEEP, station 09-40-DR 03, 09.08.2009.
Paratype: ZMBN 098041 (specimen), from the same sample as the holotype.
Paratype: ZMBN 098038 (sections and spicule slides; the specimen was completely used for the preparations), Schultz Massive, North Norwegian Sea, 73°47.385′N 07°40.413′E, 1997 m, RV ‘G.O. Sars’, expedition BIODEEP, 07-90-ROV 10 rock 2, 14.07.2007, coll. Augustin.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Norwegian Sea: Southern part, Storegga: ZMBN 107497, ZMBN 107498 and ZMBN 107499 (three specimens), Eastern part, ~160 km North from Andenes: NTNU-VM-72525 (10 specimens), Northern part, Schultz Massive – Loki's Castle: 58 specimens deposited in ZMBN.
ETYMOLOGY
Named after Njord, the god of ocean, seafaring and fishing in Norse mythology. Referring to the occurrence of this species in the offshore areas of the Norwegian Sea.
DESCRIPTION
External morphology
Holotype and both paratypes hemispherical, with a minutely and unevenly hispid surface edged with a narrow spicule fringe and bearing a single weakly developed papilla with an osculum at the summit. Holotype 16–17 mm in diameter and ~9 mm in height, attached to a volcanic porous concretion (Figure 27A). Surface dirty grey, with a whitish papilla. Paralectotype ZMBN 098041 17–18 mm in diameter and ~6 mm in height, attached to a basalt piece (Figure 27B). Surface dirty grey, with a whitish papilla. Paratype ZMBN 098038 was, before dissection, 11 mm in diameter and 4 mm in height, attached to small gravels. Surface was brownish, with a yellowish papilla located in a small depression. Other sponges hemispherical, lenticular or irregular, up to 24 mm in diameter. Surface, for the most part, hispid, occasionally shaggy, dark brown or dark grey because of the covering sediment. A single papilla and the surrounding area on the body top, which may be gently depressed, are smooth and pale in colour. Marginal spicule fringe up to 3 mm wide in the largest individuals.
Anatomy
Choanosome in alcohol light or dark brown, dense. Main choanosomal skeleton composed of tracts (96–310 µm thick) of principal spicules radiating from the basal area, where the sponge is attached to the substrate, occasionally ramifying, crossing the cortex and protruding above the surface (Figure 27C). At the body edge the protruding tracts form a prominent fringe. Some tracts ascend to the papilla walls. Auxiliary choanosomal skeleton comprises free-scattered small and intermediary spicules, the latter occasionally gathered in small bundles. Cortex in alcohol whitish or pale brown, firm, not detachable. Cortical skeleton comprises a superficial palisade (217–300 µm thick) of small spicules. In the central area of the upper cortex there is also an internal layer (116–494 µm thick) of criss-cross intermediary spicules. Under the papilla the palisade and the internal layer are separated by an aquiferous cavity (~268–810 µm in diameter).
Spicules
(Measurements based on seven specimens, individual variation presented in Table 7)
Table 7. Individual variation of spicule dimensions of Spinularia njordi (given in μm as minimum–mean–maximum). Parameters: length, diameter of tyle, proximal diameter of shaft, maximum diameter of shaft, number of spicules measured (N).

Principal spicules (Figure 27D) – subtylostyles to tylostyles, usually straight and slender. Length 1116–1492–2579 µm, diameter of tyle (if present) 8.9–13.3–16.5 µm, proximal diameter of shaft 6.4–9.1–14.0 µm, maximum diameter of shaft 8.9–14.9–21.6 µm, N = 284.
Intermediary spicules (Figure 27E) – tylostyles to subtylostyles, usually straight, occasionally gently curved, slender. Length 422–685–1128 µm, diameter of tyle (if present) 8.9–13.0–16.5 µm, proximal diameter of shaft 5.1–7.9–11.4 µm, maximum diameter of shaft 8.9–13.3–17.8 µm, N = 254.
Small spicules (Figure 27F) – tylostyles, often gently bent at the proximal part, occasionally straight, usually slightly fusiform. Length 201–328–422 µm, diameter of tyle 8.9–12.6–16.5 µm, proximal diameter of shaft 3.8–7.8–12.7 µm, maximum diameter of shaft 8.9–13.3–20.3 µm, N = 253.
Genetic data
Data obtained from three individuals of Spinularia njordi are identical in both genes studied. Moreover, the CO1 sequences from S. njordi are identical to those from the type species of Spinularia, S. spinularia, and the Norwegian individuals of S. sarsii, but not the Mozambican S. cf. sarsii (Online resource 2, p. 5). At the same time S. njordi displays seven autapomorphies in 28S rDNA distinguishing it from the congeners (Matrix M34250 in TreeBase). Of these autapomorphies one is unique among all polymastiid species from which 28S rDNA has ever been obtained (Online resource 3, p. 5).
OCCURRENCE
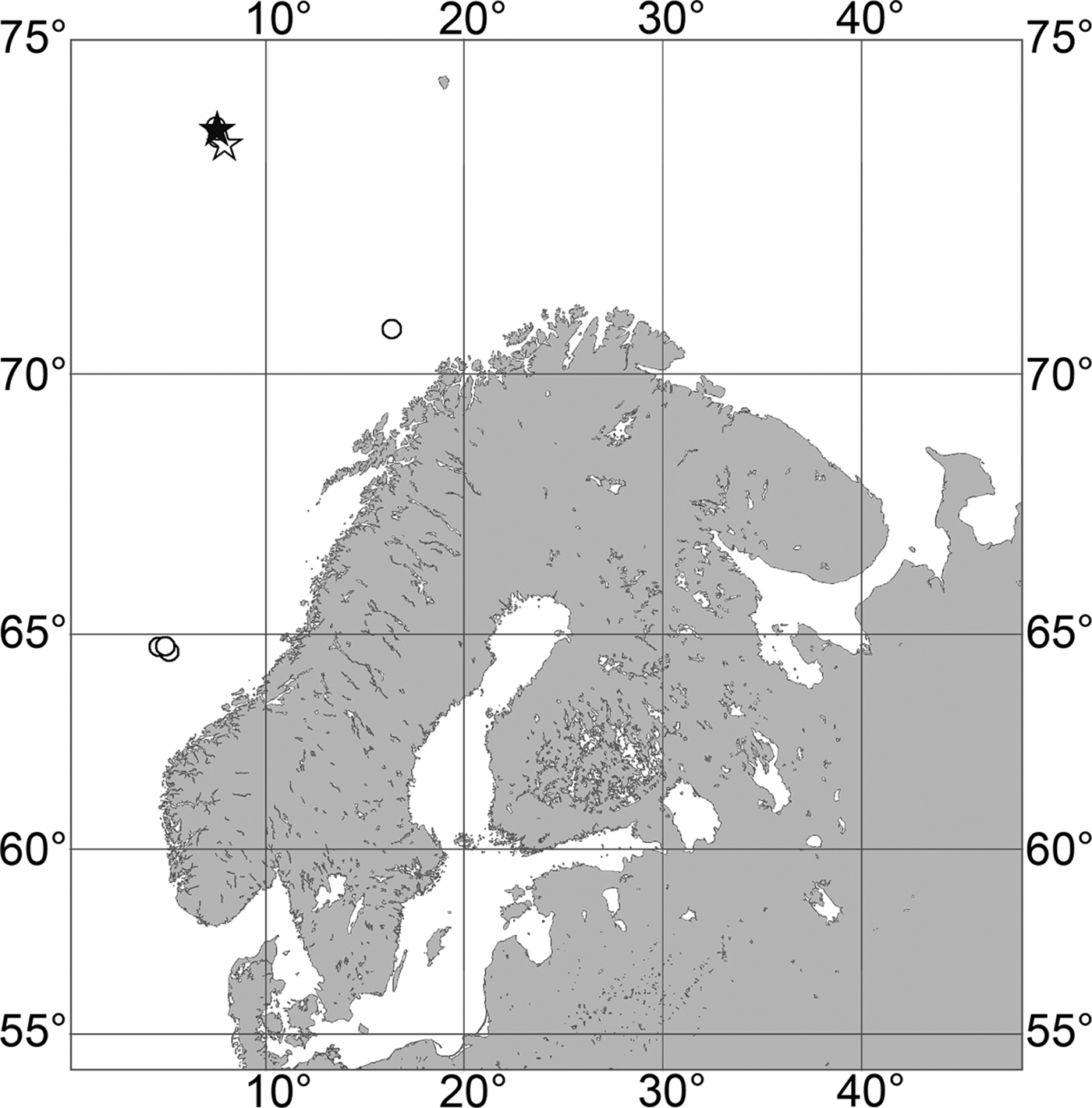
Fig. 28. Spinularia njordi, distribution: black star, locality of holotype and paratype ZMBN 098041; white star, locality of paratype ZMBN 098038; white circles, other data.
Norwegian Sea, offshore: southern areas (733–790 m), eastern areas (1580 m), northern areas (1262–2457 m).
DISCUSSION
Spinularia njordi resembles the type species of Spinularia, S. spinularia, by the encrusting growth pattern, the consequent absence of the basal cortex and the relatively small marginal fringe composed of the spicules of the same category as those forming the main choanosomal tracts. These features distinguish S. njordi and S. spinularia from other Spinularia spp. At the same time S. njordi differs from S. spinularia by the shaggy surface, the presence of an additional cortical layer made of intermediary monactines and the absence of raphides in trichodragmata in the choanosome. These features rather resemble S. sarsii. Based on the absence of raphides S. njordi and S. sarsii were provisionally allocated to Radiella, as Radiella sp. and R. sarsii respectively (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). However, based on the 28S rDNA phylogeny and the identity of the CO1 5′-end barcodes from these species and S. spinularia, they were transferred to Spinularia (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). Spinularia njordi is established as a new species primarily based on its autapomorphies in 28S rDNA (see the Genetic data above).
Spinularia sarsii (Ridley & Dendy, Reference Ridley and Dendy1886)
(Figure 29)
Original description: Trichostemma sarsii Ridley & Dendy, Reference Ridley and Dendy1886, p. 488.
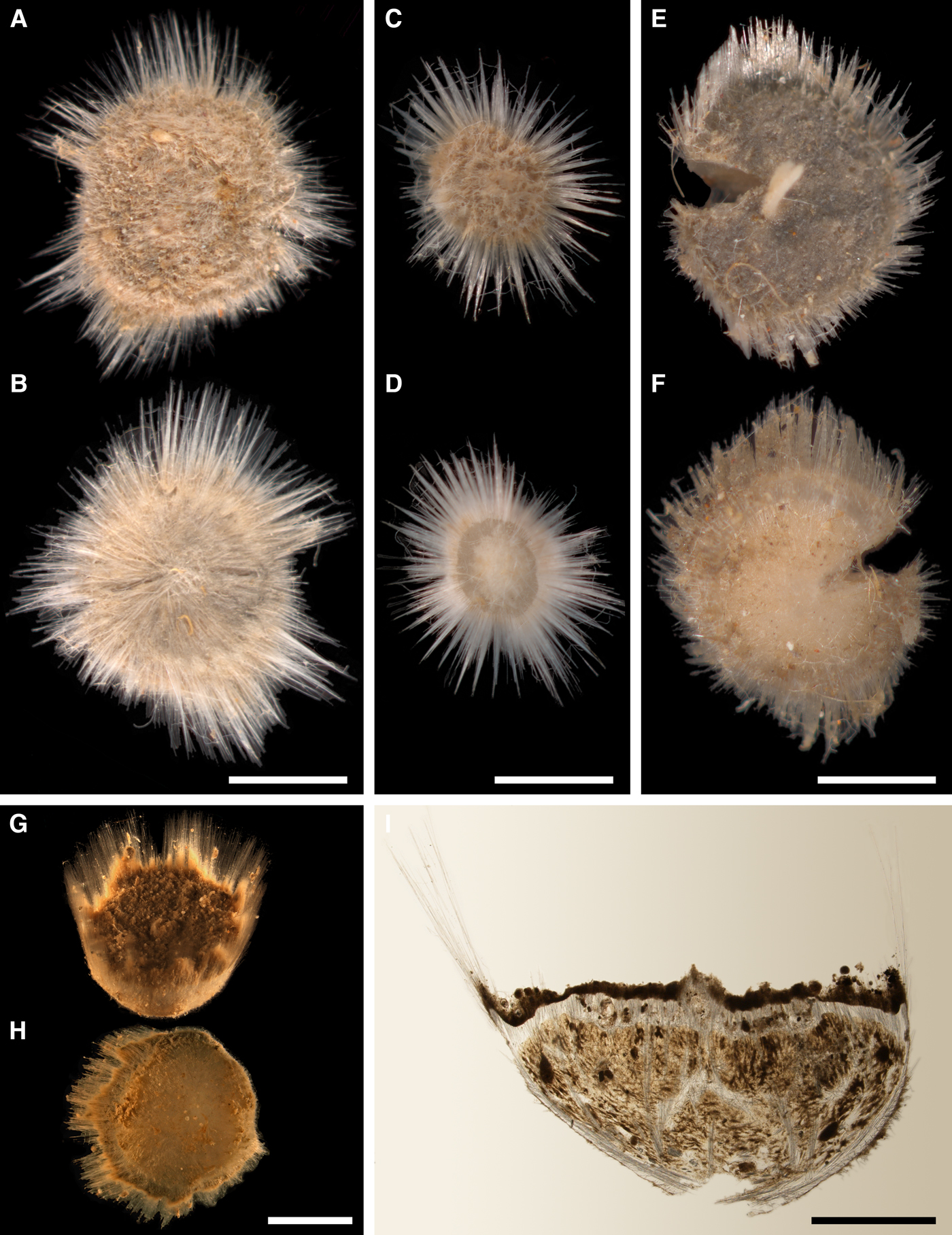
Fig. 29. Spinularia sarsii, type series, habitus: (A) lectotype BMNH 1887.5.2.38.1, view from above; (B) the same, bottom view; (C) paralectotype BMNH 1887.5.2.66, view from above; (D) the same, bottom view; (E) paralectotype BMNH 1887.5.2.40, view from above; (F) the same, bottom view; (G) ZMBN 107582, habitus, side view; (H) the same, bottom view; (I) the same individual, longitudinal section through the body. Scale bars: A–H, 5 mm; I, 3 mm.
SYNONYMS AND CITATIONS
Radiella sarsii (Gorbunov, Reference Gorbunov1946, p. 37; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 543, figures 1g & 2g; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 27, figure 2j).
Radiella sol (Koltun, Reference Koltun1964, p. 149, figure 3).
Polymastia sol sol (Koltun, Reference Koltun1966, p. 81, text-figure 52, pl. XXX figures 8 & 9, pl. XXXI figures 10 & 11).
Trichostemma sarsii (Ridley & Dendy, Reference Ridley and Dendy1887, p. 218, pl. XLIII figures 1, la, 2, 2a, 3 & 3a; Topsent, Reference Topsent1892, p. 132, Reference Topsent1904, p. 120, Reference Topsent1928, p. 154; Uriz & Rosell, Reference Uriz and Rosell1990, p. 380, figures 3, 5e & 6; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994, p. 73, figure 49).
Trichostemma sol (Barthel & Tendal, Reference Barthel and Tendal1993, p. 88, figure 5).
Non Radiella sarsii (Burton, Reference Burton1959b, p. 208).
Non Trichostemma sarsii (Dendy, Reference Dendy1922, p. 151).
TYPE MATERIAL
Lectotype (designated herein, Figure 29A, B): BMNH 1887.5.2.38.1, off Azores, 38°30′N 31°14′W, RV ‘Challenger’, 1820 m, station 73, 30.06.1873.
Paralectotypes: BMNH 1887.5.2.38.2 (small fragment), BMNH 1887.5.2.61 (two specimens) and BMNH 1887.5.2.66 (one specimen, Figure 29C, D), from the same sample as the lectotype.
Paralectotype: BMNH 1887.5.2.40 (one specimen, Figure 29E, F), SE off Cape York, Australia, 12°08′S 145°10′E, 2548 m, RV ‘Challenger’, station 184, 29.08.1874.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Greenland: East Coast: ZIN RAS ocrs002 (one specimen), NE Coast: ZIN RAS ocrs003 (one specimen).
Greenland Sea: ZIN RAS ocrs001 and ZIN RAS ocrs004 (two specimens).
Norwegian Sea, offshore: NTNU-VM-72506, ZMBN 098039, ZMBN 098098, ZMBN 107582 and ZMBN 107583 (five specimens).
Indian Ocean: Mozambican Coast: ZMBN 098103 (one specimen identified as Radiella cf. sarsii by Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b).
DESCRIPTION
External morphology
Lectotype discoid, flattened, with hispid, grey upper and basal surfaces and a marginal spicule fringe (Figure 29A, B). Body ~9 mm in diameter (excluding the fringe) and ~6 mm thick. Upper surface bears four tiny papillae with oscula at the summits (Figure 29A). Basal surface without any trace of the substrate (Figure 29B). Fringe 1.5–4 mm wide. All paralectotypes externally resemble the lectotype. Paralectotype BMNH 1887.5.2.38.2 is a body sector with a 3.0–3.5 mm wide fringe and without papillae. Paralectotype BMNH 1887.5.2.40 discoid, ~9 mm in diameter, with a convex, smooth basal surface and a flattened, hispid upper surface bearing a single papilla damaged at the summit (Figure 29E, F). Fringe 1.5–2.5 mm wide. One of the paralectotypes BMNH 1887.5.2.61 flattened, irregular, ~6 × 8 mm, with hispid upper and basal surfaces and without visible papillae. Fringe 1–2 mm wide. The other paralectotype BMNH 1887.5.2.61 discoid, flattened, ~4 mm in diameter, with an almost smooth basal surface and a hispid upper surface bearing a single papilla. Fringe 0.7–3.0 mm wide. Paralectotype BMNH 1887.5.2.66 discoid, ~5.0–5.5 mm in diameter, with a convex, almost smooth basal surface and a flattened, strongly hispid upper surface lacking visible papillae (Figure 29C, D). Fringe 1.3–2.6 mm wide. Other sponges discoid, lenticular, hemispherical, occasionally irregular, up to 13 mm in diameter (Figure 29G, H). Upper surface flattened, hispid or shaggy, covered with sediment, bearing a single papilla, more rarely few, small papillae with oscula at the summits (Figure 29G). Basal surface often convex, smooth, attached to the substrate with a central point (Figure 29H). A small substrate may be plunged into the basal cortex. Some individuals are free of any substrates. Width of the marginal fringe may reach half of body diameter.
Anatomy
Choanosome in alcohol light or dark brown, dense. Main choanosomal skeleton composed of tracts (119–283 µm thick) of principal spicules radiating from the central basal point, crossing the upper cortex and protruding above the surface (Figure 29I). Some tracts ascend to the papilla walls. Auxiliary choanosomal skeleton comprises free-scattered bundles of intermediary spicules. Cortex in alcohol whitish, firm, not detachable. Skeleton of the upper cortex comprises a superficial palisade (about 300 µm thick) of small spicules, a middle space (340–405 µm thick) with low concentration of spicules except for ascending choanosomal tracts and an internal layer (100–110 µm thick) of criss-cross intermediary spicules. Skeleton of the basal cortex formed of peripheral tracts of principal spicules running parallel to the surface and free-scattered single small spicules. Extra-long spicules (exotyles) composing the marginal fringe are embedded in the tracts.
Spicules
(Measurements based on 13 specimens)
Principal spicules – styles, occasionally subtylostyles, usually straight and fusiform. Length 871–1787–2900 µm, maximum diameter of shaft 13.0–19.3–26.6 µm, N = 130.
Intermediary spicules – subtylostyles to tylostyles, usually straight, slightly fusiform. Length 250–471–632 µm, maximum diameter of shaft 6.1– 7.6–8.7 µm, N = 390.
Small spicules – tylostyles, often gently bent at the proximal part, fusiform. Length 152–232–298 µm, maximum diameter of shaft 8.1–9.8–10.4, N = 390.
Exotyles (spicules of the marginal fringe) – styles, occasionally subtylostyles, straight or slightly curved, fusiform. Length 3890–5010–6030, μm, maximum diameter of shaft 24.5–27.3–29.8 µm, N = 70.
Genetic data
Certain genetic differences are revealed between the morphologically very similar Spinularia sarsii from Norway and Spinularia cf. sarsii from Mozambique. Data obtained from two Norwegian individuals are identical in both genes studied. Moreover, the CO1 sequences from these individuals are identical to those from the type species of Spinularia, S. spinularia, and S. njordi and display one synapomorphy distinguishing them from other polymastiids including the Mozambican S. cf. sarsii (Online resource 2, p. 5). In 28S rDNA the Norwegian S. sarsii differs from S. spinularia by 6 bps and from S. njordi by 12 bps (Matrix M34250 in TreeBase) and shares with them two synapomorphies (Online resource 3, p. 5). At the same time the Mozambican S. cf. sarsii is distinguished from all polymastiids by three autapomorphies in CO1 (Online resource 2, p. 5). Apart from these autapomorphies, it differs from the congeners by five bps in CO1 (Matrix M34248 in TreeBase). In 28S rDNA the Mozambican S. cf. sarsii differs from the Norwegian S. sarsii by 13 bps, from S. njordi by 18 bps and from S. spinularia by 14 bps (Matrix M34250 in TreeBase).
OCCURRENCE
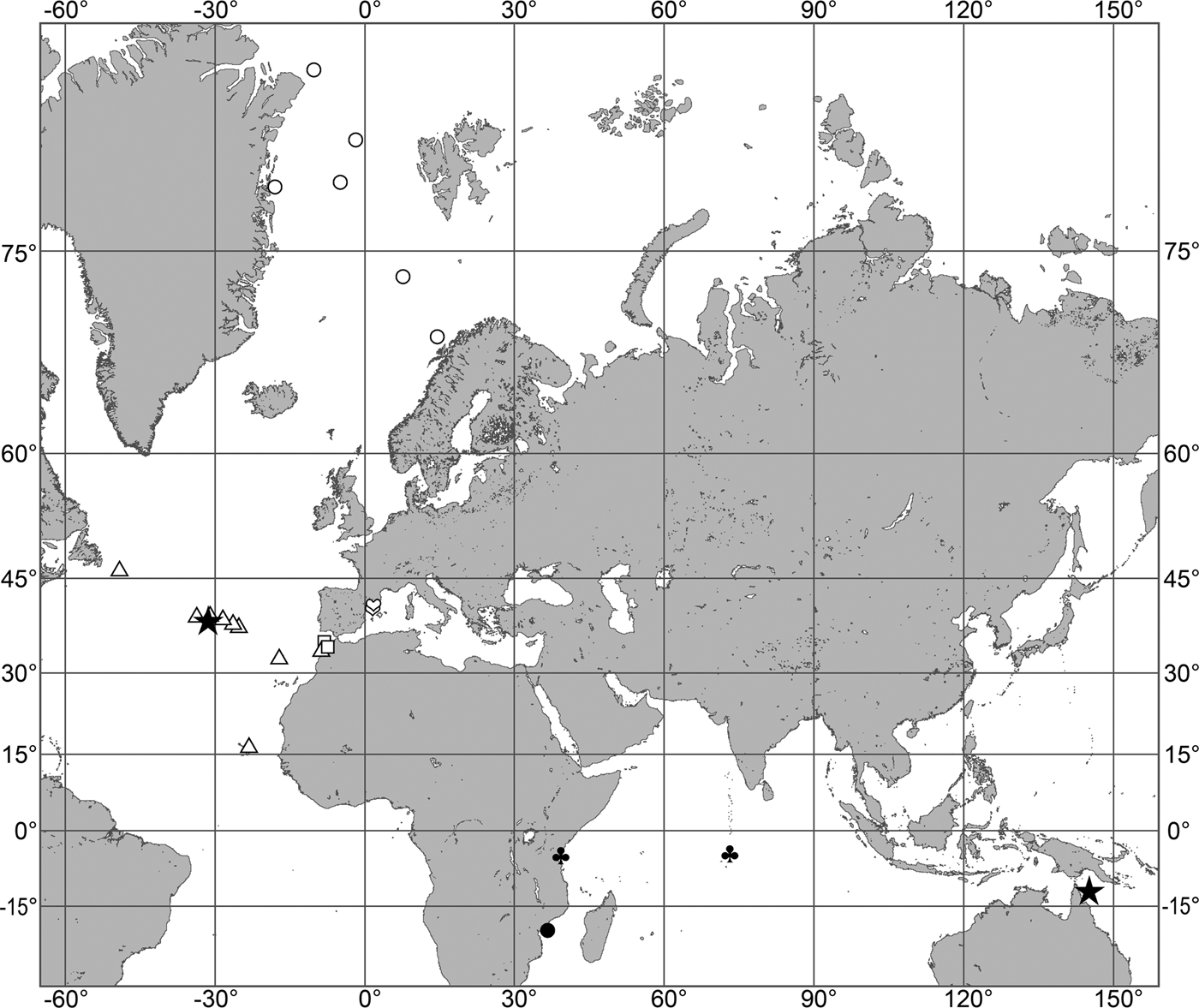
Fig. 30. Spinularia sarsii, distribution: black stars, type localities; white squares, data from Boury-Esnault et al. (Reference Boury-Esnault, Pansini and Uriz1994); black trefoils, data from Burton (Reference Burton1959b); white triangles, data from Topsent (Reference Topsent1892, Reference Topsent1904, Reference Topsent1928); white hearts, data from Uriz & Rosell (Reference Uriz and Rosell1990); white circles, our data from the Nordic Seas; black circle, our data from Mozambique.
Type locality: North-East Atlantic: Azores (1820 m). The other type locality (Coral Sea near Australia, 2548 m) probably represents another species (see Discussion below).
Other literature data: Canadian Atlantic Coast: off Newfoundland (1267 m) (Topsent, Reference Topsent1892). North-East Atlantic: Azores (861–2102 m) (Topsent, Reference Topsent1892, Reference Topsent1904), Cape Verde (1209–1417 m) (Topsent, Reference Topsent1928), Madeira (2380–3118) (Topsent, Reference Topsent1928), Moroccan Coast/Saharan Upwelling (851–2142 m) (Topsent, Reference Topsent1928; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994). Mediterranean Sea: Iberian Sea (1020–1580 m) (Uriz & Rosell, Reference Uriz and Rosell1990). Arctic Ocean, Greenland Sea and Norwegian Sea (800–2892 m) (Gorbunov, Reference Gorbunov1946; Koltun, Reference Koltun1964, Reference Koltun1966; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004). Barents Sea, Kara Sea and Laptev Sea (from 145 m and deeper) (Koltun, Reference Koltun1966).
Our data: Greenland, East and NE Coast (368–2581 m). Greenland Sea, offshore (1447–2754 m). Norwegian Sea, offshore (2387–2463 m).
DISCUSSION
Spinularia sarsii was originally described as Trichostemma sarsii Ridley & Dendy, Reference Ridley and Dendy1886, despite Trichostemma having been synonymized earlier with Radiella (Schmidt, Reference Schmidt1880). After long debates on which of the names, Trichostemma or Radiella, preceded, Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) finally proved the action by Schmidt (Reference Schmidt1880) (for details see Discussion on the genus Spinularia above). Radiella was defined as sponges with the upper and basal cortex of different architecture and a spicule fringe developed at the boundary between the upper and basal surface (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002). Radiella sarsii perfectly fitted with this definition (Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). The marginal spicule fringe was also typical of another genus, Spinularia, but the latter was distinguished from Radiella by the presence of raphides in trichodragmata (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002). However, based on the CO1 and 28S rDNA phylogenies, Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) transferred R. sarsii to Spinularia (for details see Discussion on the genus Spinularia and S. njordi above).
The other problem with Spinularia sarsii is the allegedly cosmopolitan distribution reported for this species. Its type localities are such remote regions as Azores and Australia (Ridley & Dendy, Reference Ridley and Dendy1886). Besides these S. sarsii is recorded from quite remote localities in the North Atlantic (Newfoundland (Topsent, Reference Topsent1892), Azores (Topsent, Reference Topsent1892, Reference Topsent1904), Cape Verdi (Topsent, Reference Topsent1928), Madeira (Topsent, Reference Topsent1928), Moroccan Coast (Topsent, Reference Topsent1928; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994), West Mediterranean Sea (Uriz & Rosell, Reference Uriz and Rosell1990)), Arctic Ocean, Greenland and Norwegian Sea (Gorbunov, Reference Gorbunov1946; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004) and Indian Ocean (Mozambique (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b), Zanzibar (Burton, Reference Burton1959b), Saya de Malha (Dendy, Reference Dendy1922), South off Maldives (Burton, Reference Burton1959b)). This list may also be appended with the records of Radiella sol from the Barents, Kara and Laptev Sea by Koltun (Reference Koltun1964, Reference Koltun1966) and Trichostemma sol from the Norwegian–Greenland Sea by Barthel & Tendal (Reference Barthel and Tendal1993), who at that time regarded Radiella sarsii to be a synonym of Radiella sol, which was in fact originally described from the Mexican Gulf (Schmidt, Reference Schmidt1870). Furthermore, Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016) assumed that Suberites alveus Hansen, Reference Hansen1885 and Suberites conica Hansen, Reference Hansen1885 from the Norwegian Sea were very probably conspecific with Spinularia sarsii, just displaying variation of the body shape resembling a hive-like cone in S. alveus and a flattened cone in S. conica.
The cosmopolitan distribution of Spinularia sarsii is questioned by Plotkin et al. (Reference Plotkin, Voigt, Willassen and Rapp2016b) and the present study based on genetic data. In the 28S rDNA phylogeny morphologically very similar S. sarsii (former Radiella sarsii) from the Norwegian Sea and S. cf. sarsii from the Mozambican Coast (former Radiella cf. sarsii) do not group together, although they fall in the same clade (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). Furthermore, the Mozambican sponge is distinguished from the congeners in CO1, whereas the sequences of this phylogenetic marker obtained from the Norwegian S. sarsii, S. spinularia and S. njordi are identical. Based on these data the records of S. sarsii from the northern and southern hemisphere are assumed to represent two different species. However, herein we do not formally establish a new species for the Mozambican individual because the locality is outside our study area and more material is required for a careful morphological description. At the same time there are no genetic data on S. sarsii from North Atlantic regions other than the Norwegian Sea, while the comparison of the Norwegian S. sarsii with the type material from Azores reveals no morphological differences. Neither can we check whether Suberites alveus and Suberites conica from the Norwegian Sea are indeed conspecific with Spinularia sarsii because the type material of the first two species is unfortunately lost. Therefore, for the moment we consider all records of Spinularia sarsii from the northern hemisphere as one species.
Spinularia spinularia (Bowerbank, Reference Bowerbank1866)
(Figures 31 & 32)
Original description: Tethea spinularia Bowerbank, Reference Bowerbank1866, p. 94.

Fig. 31. Spinularia spinularia: (A) lectotype BMNH 1910.1.1.34A, habitus; (B) an individual from Hardangerfjorden, Norway, habitus; (C) the same individual, longitudinal section through the body, general view; (D) the same section, detail of subcortical area with trichodragmata. Scale bars: A–B, 0.5 cm; C, 2 mm; D, 0.2 mm.
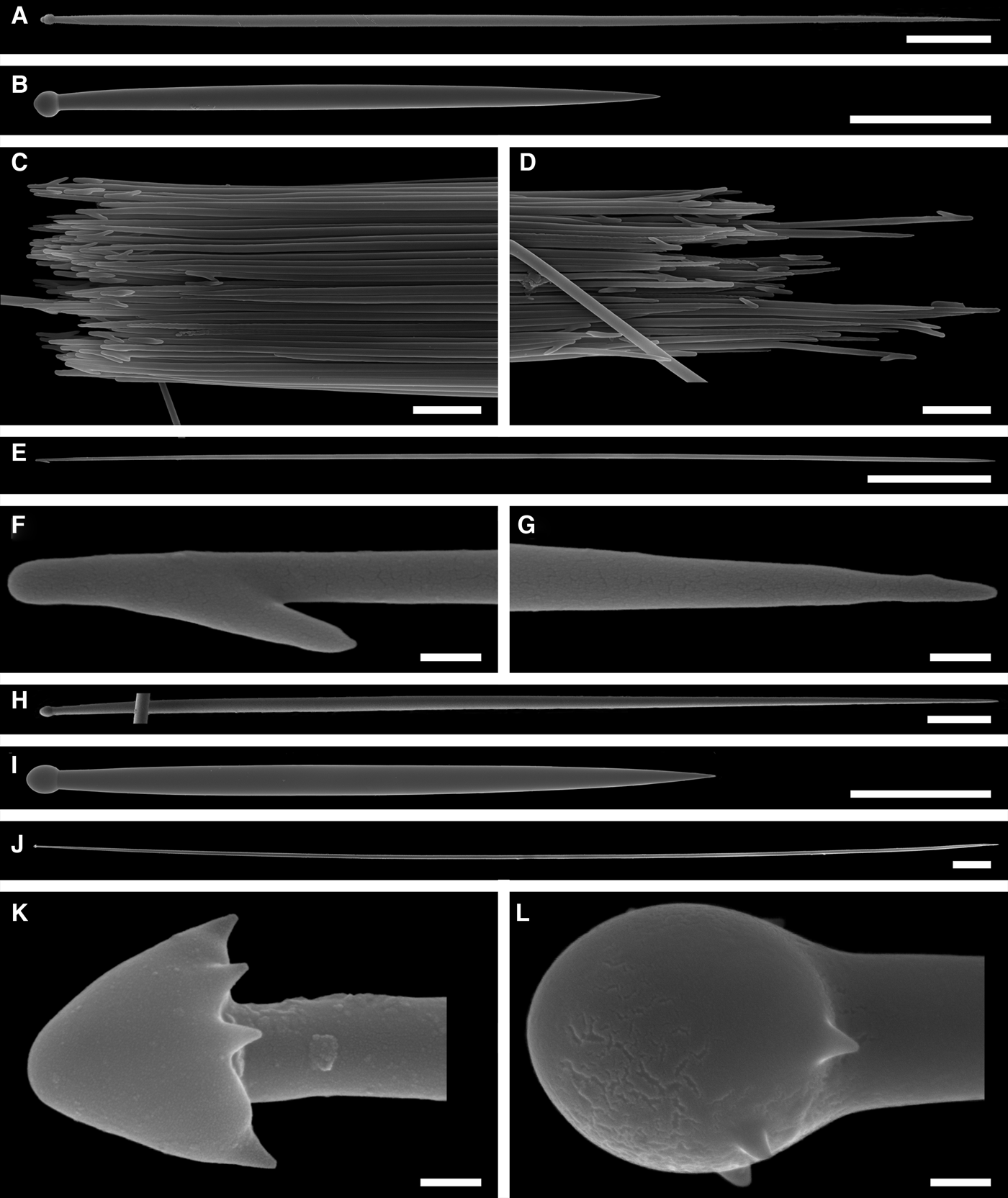
Fig. 32. Spinularia spinularia and S. setosa, spicules: (A) S. spinularia, principal tylostyle; (B) S. spinularia, small tylostyle; (C) and (D) S. spinularia, details of a trichodragma of raphides, (E) S. spinularia, raphide, general view; (F) the same spicule, detail of harpoon-shaped tip; (G) the same spicule, detail of the opposite tip; (H) S. setosa, principal tylostyle; (I) S. setosa, small tylostyle; (J) S. setosa, raphide, general view; (K) the same spicule, detail of umbrelliform tip; (L) another raphide of the same sponge, detail of subspherical tip. Scale bars: A, 0.1 mm; B, 0.05 mm; C and D, 0.002 mm; E, 0.01 mm; F and G, 0.2 µm; H, 0.1 mm; I, 0.05 mm; J, 0.01 mm; K and L, 0.2 µm.
SYNONYMS AND CITATIONS
Polymastia spinularia (Hanitsch, Reference Hanitsch1894, p. 202).
Radiella spinularia (Schmidt, Reference Schmidt1870, p. 48, pl. IV figures 7 & 8; Fristedt, Reference Fristedt1885, p. 16, Reference Fristedt1887, p. 435).
Spinularia spinularia (Stephens, Reference Stephens1915, p. 31, pl. III figure 5, pl. V figure 3; Burton, Reference Burton1930a, p. 496; Alander, Reference Alander1942, p. 76; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 216, figure 13; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 27, figure 2i).
Spinularia tetheoides (Gray, Reference Gray1867, p. 524).
Tethea spinularia (Bowerbank, Reference Bowerbank1874: pl. XV figures 23–30).
Non Spinularia spinularia (Topsent, Reference Topsent1928, p. 150).
TYPE MATERIAL
Lectotype of Tethea spinularia (designated herein, dry specimen (Figure 31A) and seven slides labelled BR 393): BMNH 1910.1.1.34A, Shetland, UK, precise locality unknown, coll. Alfred M. Norman.
Paralectotypes of Tethea spinularia: BMNH 1910.1.1.34B (one dry specimen and three fragments): from the same sample as the lectotype.
Slides from the paralectotypes of Tethea spinularia: BMNH 10.1.1.1820, BMNH 10.1.1.1821 and 10.1.1.1822.
Holotype of Rhaphidorus setosus Topsent, Reference Topsent1898 (in alcohol): MOM 04-0303, East off Sao Miguel, Azores, 38°09′N 23°15.75′W – 38°08′N 23°18.75′W, 4020 m, Campagnes scientifiques accomplies par le Prince Albert I de Monaco, RV ‘Princesse-Alice’, station 527, 25.06.1895. Rhaphidorus setosus was relegated to a synonym of Spinularia spinularia by Stephens (Reference Stephens1915), but herein it is assumed to be a separate species Spinularia setosa (see Discussion below).
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Sweden: Västra Götaland: GNM 792:1 (one specimen).
Norway: Aust-Agder: ZMBN 107587 (one specimen), Hordaland: ZMBN 098037, ZMBN 098076 and ZMBN 098079 (three specimens), Sogn and Fjordane: ZMBN 098050 (one specimen), Møre and Romsdal: ZMBN 107488 and ZMBN 107494 (two specimens), Sør-Trøndelag: NTNU-VM-54615 (one specimen), Nord-Trøndelag: NTNU-VM-54669, NTNU-VM-56952, NTNU-VM-72538 and NTNU-VM-72546 (seven specimens).
Norwegian Sea, offshore: ZMBN 107586 (one specimen).
DESCRIPTION
External morphology
Lectotype of Tethea spinularia cushion-shaped, ~23 × 15 × 12 mm, removed from the substrate (Figure 31A). Surface rough, beige in the central area and grey in the periphery, bearing 11 wart-like papillae, most with oscula at the summits. The most intact paralectotype cushion-shaped, ~9 × 8 × 5 mm, removed from the substrate. Surface minutely hispid, covered with grey sediment in the periphery and clean, pale orange in the central area around a single wart-like papilla. Two paralectotypes are fragments of cushion-shaped sponges with minutely hispid surface, without papillae, removed from the substrates. The smallest paralectotype is a tiny encrust on a stone. Holotype of Rhaphidorus setosus cushion-shaped, about 12 mm in diameter, considerably damaged, attached to a stone. Scandinavian sponges cushion-shaped, occupying up to 9 cm2 of the substrate (Figure 31B). Surface minutely hispid, occasionally smooth and free of sediment in the central area and more hispid and covered with sediment in the periphery, bearing up to 15 papillae. Many individuals with tiny spicule fringes at the body edges. Papillae small, usually wart-like, with oscula at the summits.
Anatomy
Description is based on the lectotype of Tethea spinularia and Scandinavian individuals. Anatomy of the holotype of Rhaphidorus setosus is not studied because of its considerable damage. Choanosome in life pale orange, dense. Main choanosomal skeleton composed of tracts (72–380 µm thick) of principal spicules radiating from the base, crossing the cortex and protruding above the surface (Figure 31C). Some tracts ascend to the papilla walls. Auxiliary choanosomal skeleton comprises free-scattered bundles of small spicules spread all over the body, bundles of principal spicules concentrated in the basal area and lying perpendicular to the main tracts, and trichodragmata (dense packs, 69–391 long and 56–453 µm wide) of raphides concentrated in the subcortical area (Figures 31D & 32C–D). Cortex in life whitish, cream-coloured or pale brown, firm, not detachable. Cortical skeleton comprises a superficial palisade (368–643 µm thick) of small spicules and an inner space (256–1128 µm thick) with low concentration of spicules, both reinforced with the ascending choanosomal tracts. Papilla wall covered with a superficial palisade.
Spicules
Lectotype of Tethea spinularia, two Swedish individuals and seven Norwegian individuals:
Principal spicules (Figure 32A) – tylostyles with roundish, occasionally oval, tyles, usually straight and slender. Length 404–758–1355 µm, diameter of tyle 6.8–9.3–14.4 µm, proximal diameter of shaft 3.2–6.0–10.0 µm, maximum diameter of shaft 5.8–10.0–14.6 µm, N = 300.
Small spicules (Figure 32B) – tylostyles, straight or occasionally gently bent, slightly fusiform. Length 161–290–373 µm, diameter of tyle 6.6–8.8–11.5 µm, proximal diameter of shaft 2.8–5.6–7.8 µm, maximum diameter of shaft 5.4–8.8–13.4 µm, N = 300.
Raphides (Figure 32C–G) – thin monactines with one tip acerated (Figure 32G) and the other harpoon-shaped, with a blunt point and a lateral barb (Figure 32F). Length 65–79–85 µm, maximum diameter of shaft 0.3–0.4–0.4 µm, N = 200.
Holotype of Rhaphidorus setosus:
Principal spicules (Figure 32H) – tylostyles with roundish, occasionally oval, tyles, usually straight and slightly fusiform. Length 548–1458–2185 µm, diameter of tyle 10.5–16.2–20.2 µm, proximal diameter of shaft 7.4–14.1–19.5 µm, maximum diameter of shaft 11.9–17.5–22.8 µm, N = 30.
Small spicules (Figure 32I) – tylostyles, usually straight and slender. Length 216–252–330 µm, diameter of tyle 11.1–11.7–12.5 µm, proximal diameter of shaft 7.2–7.7–8.4 µm, maximum diameter of shaft 10.9–12.0–12.7 µm, N = 30.
Raphides (Figure 32J) – thin monactines with one tip acerated and the other bearing an umbrelliform (Figure 32K) or subspherical ornamentation (Figure 32L) with tiny jags along the edge. Length 233–245–260 µm, maximum diameter of shaft 1.0–1.2–1.3 µm, N = 20.
Genetic data
28S rDNA was obtained from five individuals of Spinularia spinularia, while CO1 was sequenced only from four of them. The CO1 sequences are identical, but a polymorphism is revealed in 28S rDNA. Two subspecies groups, one comprising three individuals and the other two individuals, differ by two bps in this gene (Matrix M34250 in TreeBase). The synapomorphies and distinctions of S. spinularia from the congeners are described in the Genetic data sections for S. njordi and S. sarsii.
OCCURRENCE
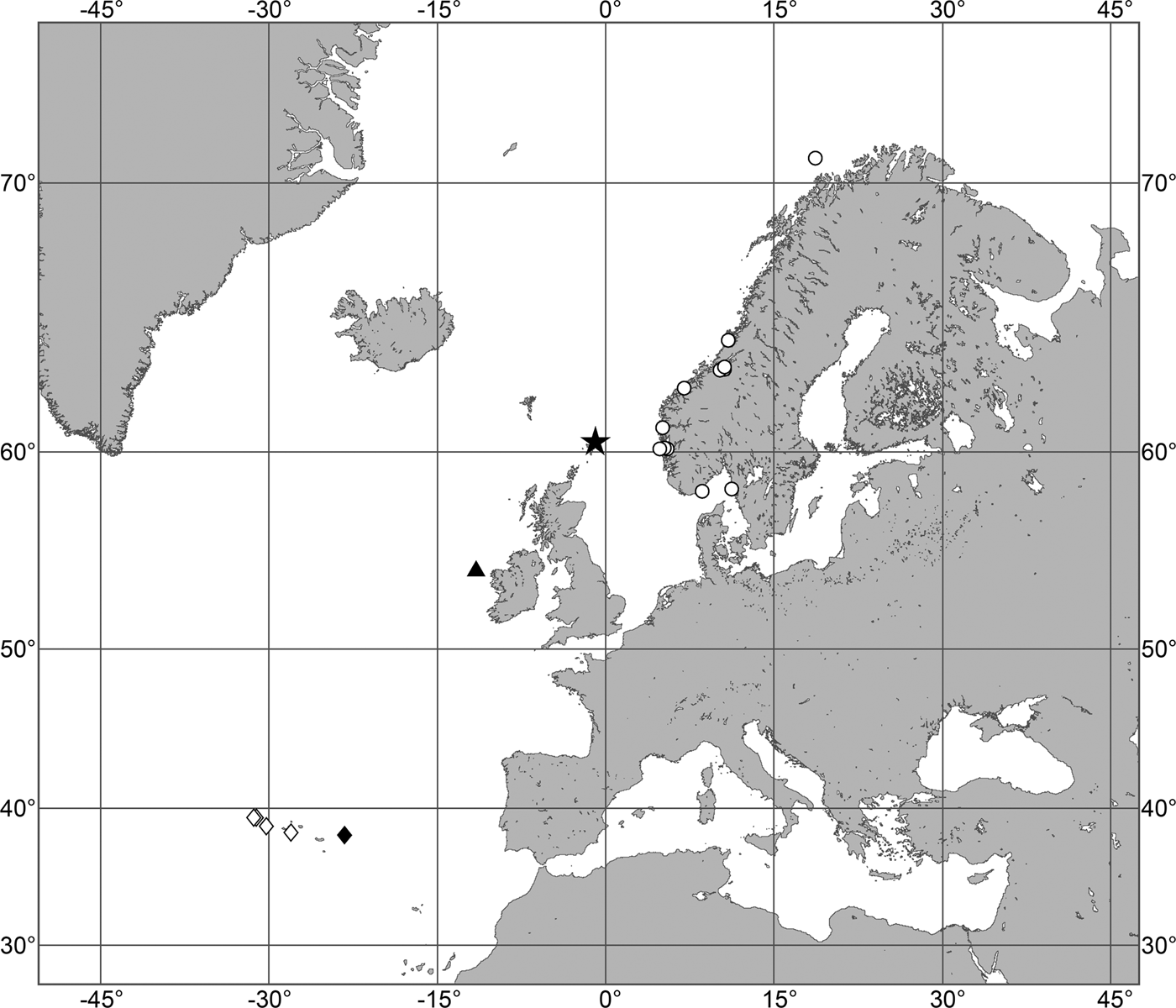
Fig. 33. Spinularia spinularia and S. setosa, distribution: black star, type locality of S. spinularia; black triangle, data on S. spinularia from Stephens (Reference Stephens1915); white circles, our data on S. spinularia; black diamond, type locality of S. setosa, white diamonds, other records of S. setosa (Topsent, Reference Topsent1904, Reference Topsent1928).
Literature data: East Greenland (237 m) (Fristedt, Reference Fristedt1887), Ireland (1001 m) (Stephens, Reference Stephens1915). Shetland (Bowerbank, Reference Bowerbank1866, Reference Bowerbank1874). Swedish Western Coast (50 m) (Fristedt, Reference Fristedt1885; Alander, Reference Alander1942). Norwegian Coast: Rogaland, Hordaland (70 m and deeper) (Burton, Reference Burton1930a; Alander, Reference Alander1942).
Our data: Skagerrak: Swedish Western Coast (45–60 m), Southern Norwegian Coast (Aust-Agder) (137–149 m). Norwegian Coast: Hordaland (30–310 m), Sogn and Fjordane (240–243 m), Møre and Romsdal (depth unknown), Sør-Trøndelag (14–35 m), Nord-Trøndelag (25–400 m). Norwegian Sea, offshore areas (272–311 m).
DISCUSSION
Since Bowerbank (Reference Bowerbank1866) established Tethea spinularia for several sponges from Shetland the validity of this species was not disputed except for the proposal by Schmidt (Reference Schmidt1880) to synonymize it with Halicnemia patera not supported by the subsequent authors. Meanwhile, there was some disagreement on what genus it should be placed in. Gray (Reference Gray1867) established for T. spinularia a new genus Spinularia. However, Schmidt (Reference Schmidt1870) and Fristedt (Reference Fristedt1885, Reference Fristedt1887) proposed to place T. spinularia in Radiella, while Hanitsch (Reference Hanitsch1894) allocated it to Polymastia. Spinularia was resurrected by Stephens (Reference Stephens1915) defining the main distinguishing feature of its type species, the presence of raphides in trichodragmata. Based on this feature she also synonymized an Azorean species Rhaphidorus setosus Topsent, Reference Topsent1898 with S. spinularia. Both actions were encouraged by most subsequent authors (Topsent, Reference Topsent1928; Burton, Reference Burton1930a; Alander, Reference Alander1942; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012). However, examination of the type and comparative material of S. spinularia and the holotype of R. setosus has revealed that in the latter both the principal spicules and the raphides are longer than in S. spinularia. Moreover, the raphides in R. setosus bear umbrelliform or subspherical distal ornaments, whereas in S. spinularia they are harpoon-shaped. Based on these differences we assume that R. setosus may be accepted as a separate species of Spinularia, S. setosa, although this should preferably be tested further with molecular data.
Genus Tentorium Vosmaer, Reference Vosmaer and Bronn1887
Original description: Tentorium Vosmaer, Reference Vosmaer and Bronn1887, p. 329, pl. II figure 4, pl. 21 figure 19.
SYNONYMS
Thecophora Schmidt, Reference Schmidt1870, p. 50 (preoccupied by Thecophora Róndani, Reference Róndani1845, a genus of flies).
TYPE SPECIES
Thecophora semisuberites Schmidt, Reference Schmidt1870 (by original designation).
DIAGNOSIS
Polymastiidae of columnar or globular body shape, always with papillae. Main choanosomal skeleton constituted by longitudinal or radial tracts of principal monactines. Skeleton of the upper cortex comprises a palisade of small monactines. Skeleton of the lateral cortex may be either the same palisade or a dense layer of criss-cross principal or intermediary spicules.
DISCUSSION
In the CO1 and 28S rDNA phylogenies Tentorium Vosmaer, Reference Vosmaer and Bronn1887 is not monophyletic (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b; Figure 1 in this study). The type species, T. semisuberites and T. papillatum Kirkpatrick, Reference Kirkpatrick1908 do not group together and, moreover, in the 28S rDNA tree the Arctic T. semisuberites and the Antarctic T. cf. semisuberites are not sisters. At the same time these phylogenies are unable to reconstruct the relationships between Tentorium spp. and other polymastiids, and therefore no alternative classification is proposed. Until more molecular data on a larger set of species become available, we recognize Tentorium as a valid genus, but emend its diagnosis proposed by Boury-Esnault (Reference Boury-Esnault, Hooper and Van Soest2002) so that all species currently allocated to Tentorium (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016) fit with it.
Tentorium semisuberites (Schmidt, Reference Schmidt1870)
(Figure 34)
Original description: Thecophora semisuberites Schmidt, Reference Schmidt1870, p. 50, pl. VI figure 2.
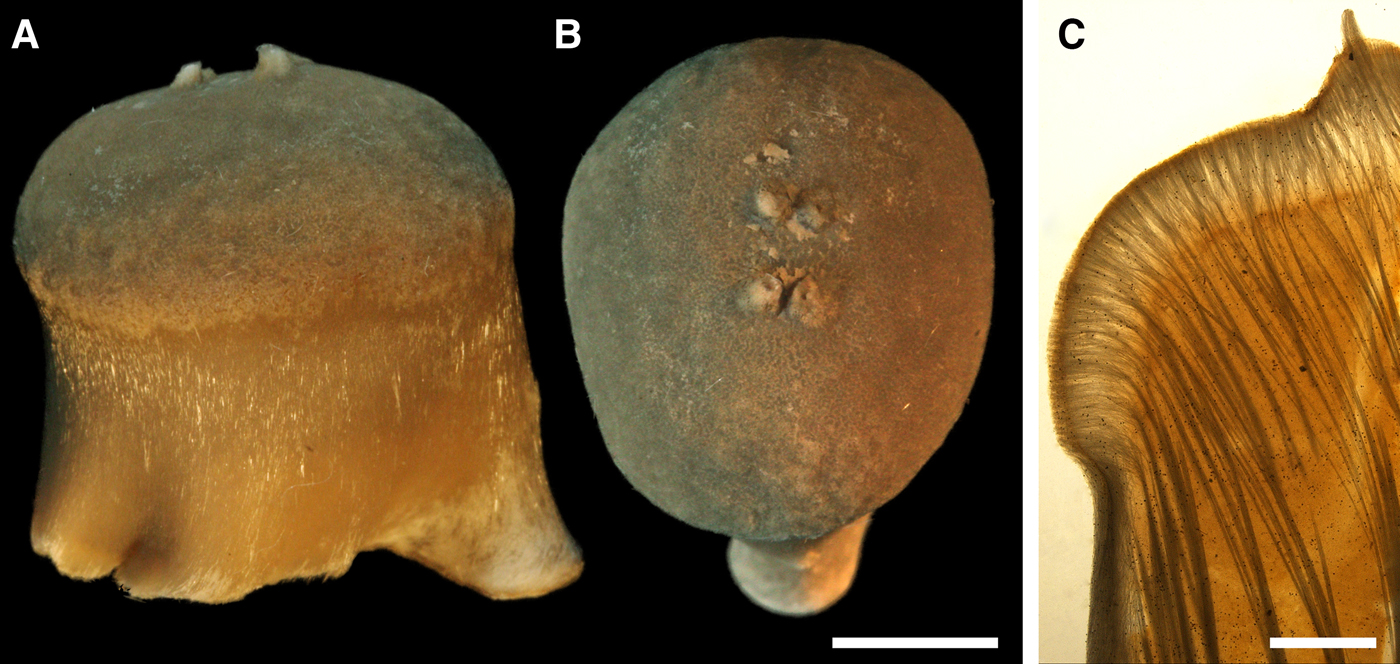
Fig. 34. Tentorium semisuberites: (A) holotype ZMUC-DEM-396, habitus, side view; (B) the same, view from above; (C) ZIN RAS octs059, longitudinal section through the body. Scale bars: A–B, 0.5 cm; C, 2 mm.
SYNONYMS AND CITATIONS
Tecophora semisuberites (Fristedt, Reference Fristedt1885, p. 17, Reference Fristedt1887, p. 433).
Thecophora elongata von Marenzeller, Reference von Marenzeller1878, p. 368, pl. II figure 4.
Thecophora ibla Thompson, Reference Thomson1873, p. 147, figure 24 (Verrill, Reference Verrill1874, p. 500, 505, pl. VIII figure 8; Whiteaves, Reference Whiteaves1874, p. 184).
Thecophora semisuberites (Thompson, Reference Thomson1873, p. 147, figure 23; Whiteaves, Reference Whiteaves1874, p. 184; von Marenzeller, Reference von Marenzeller1878, p. 368; Vosmaer, Reference Vosmaer1882, p. 30, Reference Vosmaer1885, p. 18, text-figure 9, pl. I figures 23 & 24 and pl. III figures 22–26; Hansen, Reference Hansen1885, p. 8).
Tentorium semisuberites (Vosmaer, Reference Vosmaer and Bronn1887, p. 329, pl. II figure 4 and pl. XXI figure 19; Ridley & Dendy, Reference Ridley and Dendy1887, p. 221; Lambe, Reference Lambe1896, p. 198, pl. III figures 2, 2a–c; Topsent, Reference Topsent1892, p. 132; Lambe, Reference Lambe1900, p. 25; Whiteaves, Reference Whiteaves1901, p. 14; Topsent, Reference Topsent1904, p. 124, 252–253;, Reference Topsent1913, p. 25, Reference Topsent1928, p. 151, pl. VI, figure 10; Lundbeck, Reference Lundbeck1909, p. 452; Gorbunov, Reference Gorbunov1946, p. 37; Koltun, Reference Koltun1966, p. 85, text-figure 57, pl. XIX figures 4–8, pl. XXXI figure 12; Barthel & Tendal, Reference Barthel and Tendal1993, p. 88, figure 8; Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002, p. 215, figure 12; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 544, figures 1j & 2j; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1g).
TYPE MATERIAL
Holotype: ZMUC-DEM-396, Greenland, precise locality unknown.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: Newfoundland and Labrador: ZIN RAS octs140, ZIN RAS octs142, ZIN RAS octs143 and ZIN RAS octs150 (four specimens).
Greenland: South-East Coast: ZIN RAS octs101, ZIN RAS octs138, ZIN RAS octs197, ZIN RAS octs199 and ZIN RAS octs201 (six specimens), East Coast: ZIN RAS octs092, ZIN RAS octs173 and ZIN RAS octs176 (six specimens), North-East Coast: ZIN RAS octs174 (one specimen).
Icelandic Coast and Iceland Sea: ZIN RAS octs088, ZIN RAS octs132, ZIN RAS octs141, ZIN RAS octs151 and ZIN RAS octs193 (27 specimens).
Denmark Strait: ZIN RAS octs139 (four specimens).
North-East Atlantic, offshore: ZIN RAS octs146, ZIN RAS octs147, ZIN RAS octs148 and ZIN RAS octs200 (four specimens).
Greenland Sea, offshore: ZIN RAS octs090, ZIN RAS octs091, ZIN RAS octs093, ZIN RAS octs094, ZIN RAS octs095, ZIN RAS octs096, ZIN RAS octs175, ZIN RAS octs178, ZIN RAS octs179, ZIN RAS octs180, ZIN RAS octs181, ZIN RAS octs182, ZIN RAS octs204, ZIN RAS octs209 and ZIN RAS octs216 (26 specimens).
Norwegian Sea, offshore: NTNU-VM-72502, NTNU-VM-72512, NTNU-VM-72526, ZIN RAS octs023, ZIN RAS octs034, ZIN RAS octs067, ZIN RAS octs068, ZIN RAS octs087, ZIN RAS octs104, ZIN RAS octs105, ZIN RAS octs126, ZIN RAS octs152, ZIN RAS octs161 and ZIN RAS octs203 (69 specimens).
Barents Sea, offshore: 141 specimens deposited in ZIN RAS.
Norway: Hordaland: ZMBN 098099 (one specimen), Møre and Romsdal: ZMBN 107492 (one specimen), Nord-Trøndelag: NTNU-VM-72539 and NTNU-VM-72547 (two specimens), Nordland: NTNU-VM-72517 (one specimen), Troms: NTNU-VM-72531 (one specimen), Svalbard: ZIN RAS octs084, ZIN RAS octs099, ZIN RAS octs106 and ZMBN 098054 (17 specimens).
Russia: Murman Coast: ZIN RAS octs054, ZIN RAS octs058, ZIN RAS octs100, ZIN RAS octs130, ZIN RAS octs135, ZIN RAS octs156, ZIN RAS octs157, ZIN RAS octs158, ZIN RAS octs159, ZIN RAS octs191, ZIN RAS octs194 and ZIN RAS octs211 (51 specimens), Kanin Peninsula: ZIN RAS octs053 (six specimens), Pechora Sea: ZIN RAS octs028 (five specimens), Franz Josef Land: ZIN RAS octs014, ZIN RAS octs113, ZIN RAS octs122, ZIN RAS octs145, ZIN RAS octs153, ZIN RAS octs188 and ZIN RAS octs189 (21 specimens), Novaya Zemlya: ZIN RAS octs001, ZIN RAS octs006 and ZIN RAS octs206 (four specimens), Taymyr Peninsula: ZIN RAS octs079 and ZIN RAS octs215 (four specimens), Nordenskjold Archipelago: ZIN RAS octs089, ZIN RAS octs123, ZIN RAS octs124 and ZIN RAS octs214 (92 specimens), Severnaya Zemlya: ZIN RAS octs011, ZIN RAS octs013, ZIN RAS octs015, ZIN RAS octs016, ZIN RAS octs082, ZIN RAS octs108, ZIN RAS octs109, ZIN RAS octs115, ZIN RAS octs116 and ZIN RAS octs117 (144 specimens).
Kara Sea: ZIN RAS octs002, ZIN RAS octs003, ZIN RAS octs004, ZIN RAS octs008, ZIN RAS octs009, ZIN RAS octs085, ZIN RAS octs102, ZIN RAS octs103, ZIN RAS octs154, ZIN RAS octs198, ZIN RAS octs205, ZIN RAS octs207, ZIN RAS octs208, ZIN RAS octs210, ZIN RAS octs213 and ZIN RAS octs219 (43 specimens).
Laptev Sea: ZIN RAS octs007 and ZIN RAS octs110 (three specimens).
East Siberian Sea: ZIN RAS octs086, ZIN RAS octs098, ZIN RAS octs171 and ZIN RAS octs172 (27 specimens).
Arctic Ocean, offshore: ZIN RAS octs081, ZIN RAS octs097, ZIN RAS octs107, ZIN RAS octs111, ZIN RAS octs112, ZIN RAS octs128, ZIN RAS octs149, ZIN RAS octs177, ZIN RAS octs184, ZIN RAS octs185, ZIN RAS octs186, ZIN RAS octs187 and ZIN RAS octs190 (69 specimens).
DESCRIPTION
External morphology
Holotype fungiform, ~16 mm high and 13–15 mm in diameter, removed from the substrate (Figure 34A, B). Lateral surface smooth, pale beige (Figure 34A). Upper surface minutely hispid, beige, with four small exhalant papillae at the summit (Figure 34B). Other sponges columnar, fungiform, occasionally drum-shaped, up to 35 mm high and 30 mm in diameter, with distinct difference between the smooth, palely coloured lateral surface and the more or less hispid, phaeochrous upper surface bearing one to 20 exhalant papillae. In some individuals the papillae considerably stretched, thread like, with the length exceeding the height of the main body by several times. When growing on soft sediments, the sponges often form long root-like structures made of spicule bundles.
Anatomy
Choanosome in life pale orange, pale yellow or light brown, dense. Main choanosomal skeleton composed of longitudinal tracts (430–1000 µm thick) of principal spicules running from the base, branching and entering the cortex (Figure 34C). Some tracts ascend to the papilla walls. No auxiliary choanosomal skeleton observed. Cortex in life whitish or light beige, firm, not detachable. Skeleton of the lateral cortex (500–780 µm thick) is a dense layer of criss-crossed intermediary spicules. Skeleton of the upper cortex (1000–1600 µm thick) comprises radiating tracts of principal spicules ascending from the choanosome perpendicular to the surface and a superficial palisade (450–650 µm thick) of small spicules.
Spicules
(Measurements based on 15 individuals)
Principal spicules – subtylostyles to tylostyles, usually straight and slender. Length 956–1642–2400 µm, maximum diameter of shaft 13.2–20.8–25.2 µm, N = 450.
Intermediary spicules – tylostyles, straight, fusiform. Length 810–955–1138 µm, maximum diameter of shaft 20.5–24.7–31.2 µm, N = 450.
Small spicules – tylostyles, straight, fusiform. Length 274–467–670 µm, maximum diameter of shaft 12.8–16.8–19.8 µm, N = 450.
Genetic data
In the CO1 phylogeny Tentorium semisuberites is the sister to the clade of Spinularia spp., but in the 28S rDNA it has no close relations (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). At the same time T. semisuberites possesses just one autapomorphy in CO1 distinguishing it from all other polymastiids (Online resource 2, p. 5). Moreover, an intraspecific polymorphism in both genes is observed in this species. The two individuals, from which the data were obtained, differ by two bps in CO1 (Matrix M34248 in TreeBase) and four bps in 28S rDNA (Matrix M34250 in TreeBase).
OCCURRENCE
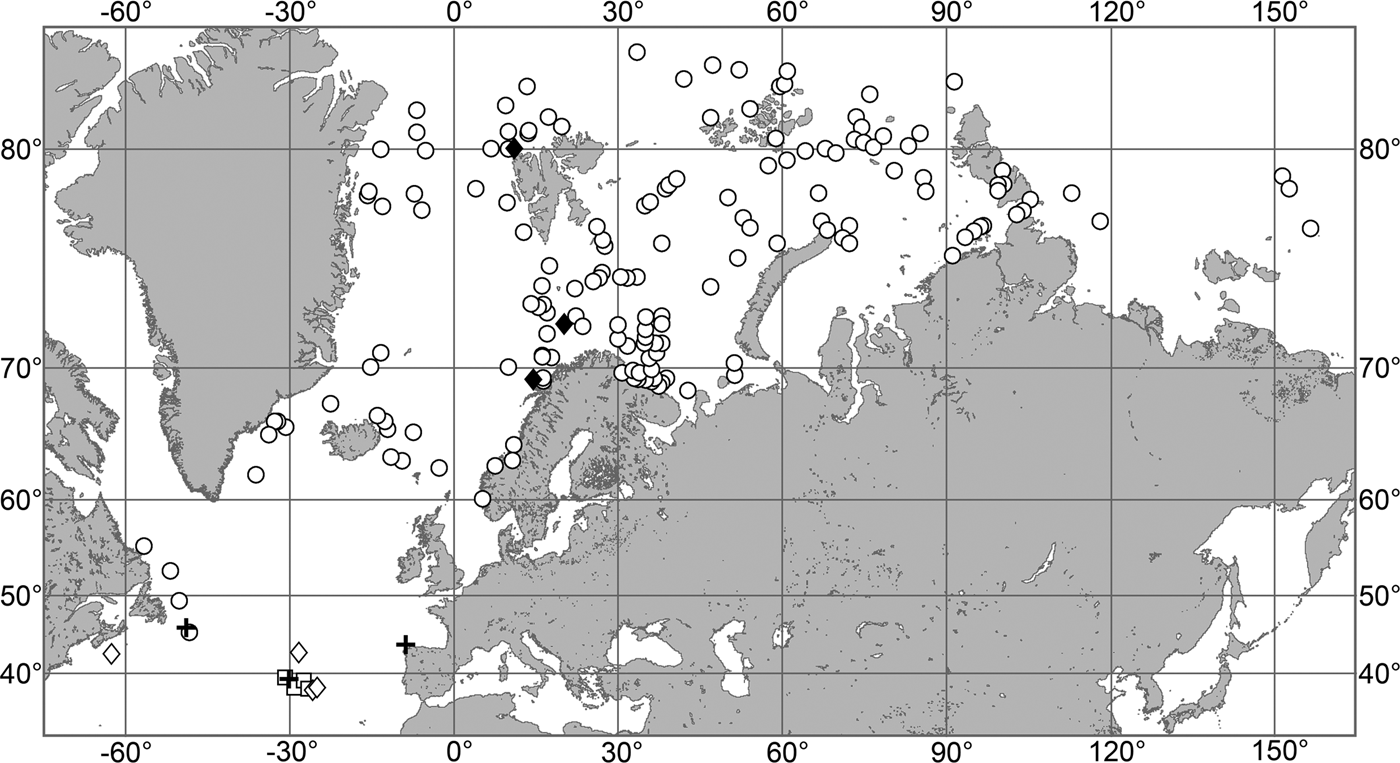
Fig. 35. Tentorium semisuberites, distribution: black crosses, data from Topsent (Reference Topsent1892); white squares, data from Topsent (Reference Topsent1904); black diamonds, data from Topsent (Reference Topsent1913); white diamonds, data from Topsent (Reference Topsent1928); white circles, our data. Precise type locality is unknown.
Literature data (only the localities distinct from our data are given): US Atlantic Coast: Gulf of Maine (Verrill, Reference Verrill1874). Canadian Atlantic Coast: Nova Scotia (1458 m) (Topsent, Reference Topsent1928), Gulf of St. Lawrence (91–175 m) (as Thecophora semisuberites and T. ibla – Whiteaves, Reference Whiteaves1874; as Tentorium semisuberites – Lambe, Reference Lambe1896; Whiteaves, Reference Whiteaves1901). Davis Strait (273 m) (Lambe, Reference Lambe1900; Whiteaves, Reference Whiteaves1901). West Greenland: Uummannaq Fjord (211–746 m) (Fristedt, Reference Fristedt1887). North Atlantic: Azores (200–3018 m) (Topsent, Reference Topsent1892, Reference Topsent1904, Reference Topsent1928), Bay of Biscay (248 m) (Topsent, Reference Topsent1892).
Our data (agree with most literature data): Canadian Atlantic Coast: Newfoundland and Labrador (208–375 m). Greenland: South-East Coast (180–400 m), East Coast (56–165 m), North-East Coast (83 m). Icelandic Coast and Iceland Sea (165–1075 m). Denmark Strait (650 m). North-East Atlantic, offshore areas (385–485 m). Greenland Sea, offshore areas (136–2800 m). Norwegian Sea, offshore areas (180–1820 m). Norwegian Coast: Hordaland (100–500 m), Møre and Romsdal (108–109 m), Nord-Trøndelag (40–400 m), Nordland (360 m), Troms (200–220 m). Barents Sea: Murman Coast (75–271 m), Kanin Peninsula (228 m), Pechora Sea (64 m), offshore areas (66–395 m). Svalbard (81–550 m). Franz Josef Land (124–285 m), Novaya Zemlya (307–368 m), Taymyr Peninsula (53 m), Nordenskjold Archipelago (53–82 m), Severnaya Zemlya (43–257 m). Kara Sea (41–580 m). Laptev Sea (86–459 m). East Siberian Sea (65–81 m). Arctic Ocean, offshore areas (165–2899 m).
DISCUSSION
Taxonomic identification of Tentorium semisuberites usually causes no difficulties. Thecophora ibla Thomson, Reference Thomson1873 and Thecophora elongata von Marenzeller, Reference von Marenzeller1878 represent in fact just varieties of body shapes in T. semisuberites. Meanwhile, the phylogenetic relationships of T. semisuberites are unresolved (see Genetic data for this species and Discussion on the genus Tentorium above).
Genus Weberella Vosmaer, Reference Vosmaer1885
Original description: Weberella Vosmaer, Reference Vosmaer1885, p. 16, text-figures 6 & 8, pl. III figures 16–19.
TYPE SPECIES
Alcyonium bursa Müller, Reference Müller1806 (by original designation).
DIAGNOSIS
Polymastiidae of massive or globular body shape, with a smooth surface always bearing papillae. Spicule assortment restricted to two size categories of smooth monactines. Main choanosomal skeleton is a reticulation formed by tracts of principal monactines. Auxiliary choanosomal skeleton comprises free-scattered small monactines. Cortical skeleton composed of a palisade of small tylostyles or subtylostyles and an internal layer of criss-cross principal monactines separated by a middle layer with aquiferous cavities.
DISCUSSION
Weberella is morphologically well-defined, but the relationships between Weberella spp. are unclear because for the moment the genetic data are only available from the type species, W. bursa.
Weberella bursa (Müller, Reference Müller1806)
(Figure 36)
Original description: Alcyonium bursa Müller, Reference Müller1806, p. 43, pl. CLVIII figures 1 & 2.
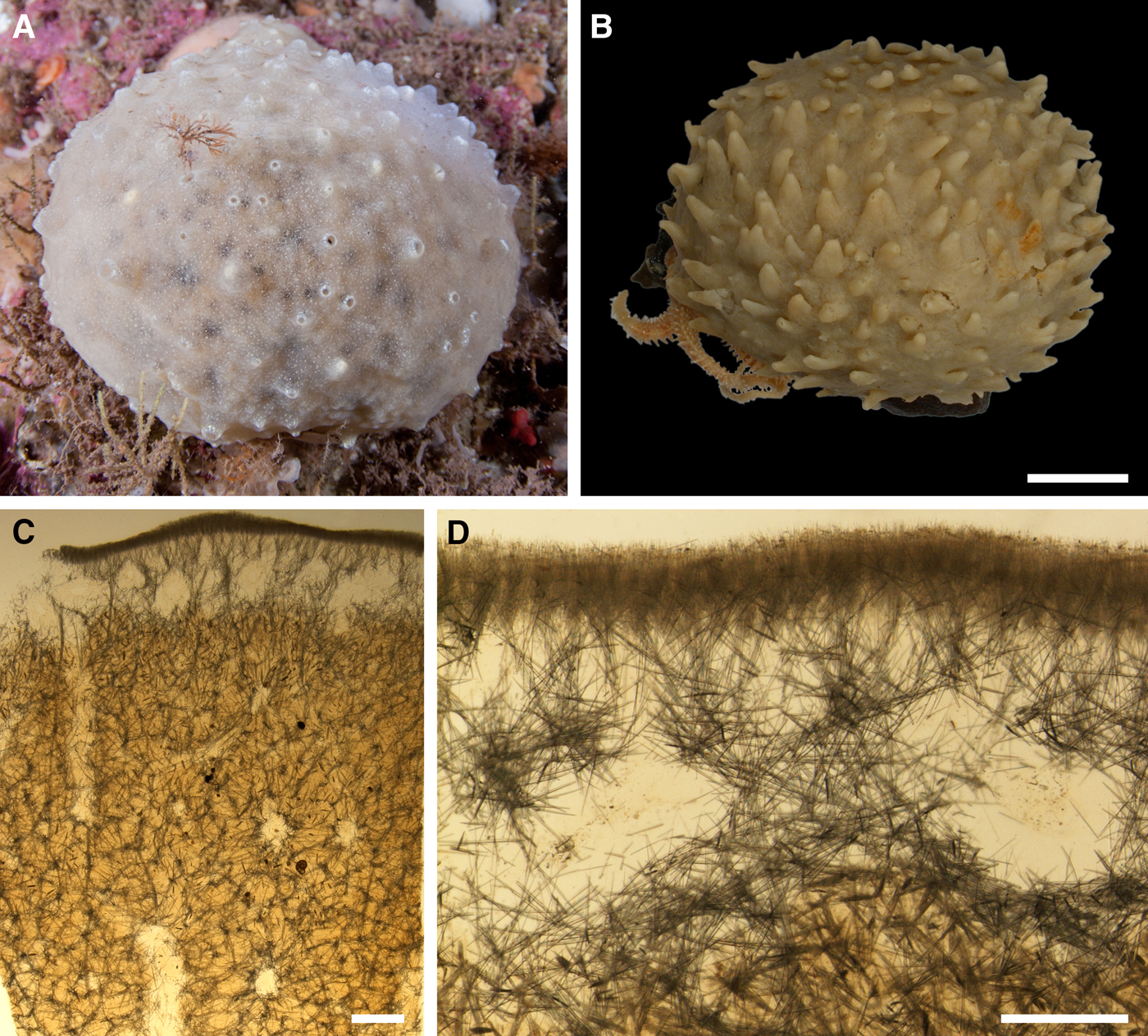
Fig. 36. Weberella bursa: (A) ZMBN 098051 in situ, Hinlopenstretet, Svalbard, Norway (courtesy P. Leopold, University of Tromsø); (B) ZIN RAS ocwb016, habitus; (C) the same, longitudinal section through the body, general view; (D) the same section, detail of cortex. Scale bars: B, 2 cm; C, 1 mm; D, 0.5 mm.
SYNONYMS AND CITATIONS
Polymastia bursa (Koltun, Reference Koltun1964, p. 149, Reference Koltun1966, p. 76, text-figure 49, pl. IX figure 2; pl. XXIII figure 1–2, pl. XXIV figures 1 & 2).
Polymastia uberrima (Burton, Reference Burton, Fridriksson and Tuxen1959a: 12 pars.).
Weberella bursa (Vosmaer, Reference Vosmaer1885, p. 16, text-figures 6 & 8, pl. III figures 16–19, Reference Vosmaer and Bronn1887, p. 329 pl. XXVI figure 10; Topsent, Reference Topsent1928, p. 149, pl. II, figure 19; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994, p. 76, figure 51, Reference Boury-Esnault, Hooper and Van Soest2002, p. 214, figure 11; Plotkin, Reference Plotkin, Pansini, Pronzato, Bavestrello and Manconi2004, p. 544, figures 1k & 2k; Plotkin et al., Reference Plotkin, Gerasimova and Rapp2012, p. 25, figure 1j).
Non Polymastia bursa (von Lendenfeld, Reference von Lendenfeld1898, p. 117, pl. VI figure 79).
TYPE MATERIAL
Unknown.
COMPARATIVE MATERIAL
(see Online resource 1 for details)
Canada: Newfoundland: ZIN RAS ocwb004 (one specimen), offshore areas of the NW Atlantic: ZIN RAS ocwb010 (one specimen).
Greenland: SW Coast/Davis Strait: ZIN RAS ocwb033 and ZIN RAS ocwb035 (two specimens), SE Coast: ZIN RAS ocwb003 and ZIN RAS ocwb031 (two specimens).
Denmark Strait: ZIN RAS ocwb025 (one specimen).
Iceland: ZIN RAS ocwb005, ZIN RAS ocwb015, ZIN RAS ocwb018 and ZIN RAS ocwb036 (four specimens).
Faroes: ZIN RAS ocwb011 (one specimen).
Norwegian Sea, offshore: ZIN RAS ocwb012 and ZIN RAS ocwb020 (two specimens).
Barents Sea, offshore: ZIN RAS ocwb001, ZIN RAS ocwb002, ZIN RAS ocwb006, ZIN RAS ocwb007, ZIN RAS ocwb008, ZIN RAS ocwb009, ZIN RAS ocwb013, ZIN RAS ocwb016, ZIN RAS ocwb019, ZIN RAS ocwb021, ZIN RAS ocwb023, ZIN RAS ocwb026, ZIN RAS ocwb027, ZIN RAS ocwb028, ZIN RAS ocwb029, ZIN RAS ocwb030 and ZIN RAS ocwb032 (17 specimens).
Norway: Sør-Trøndelag: NTNU-VM-56951 (one specimen), Nord-Trøndelag: NTNU-VM-54951 (one specimen), Nordland: NTNU-VM-72522 (one specimen), Troms: NTNU-VM-72532 and ZMBN 098072 (two specimens), Finnmark: NTNU-VM-72504 and ZIN RAS ocwb024 (two specimens), Svalbard: ZIN RAS ocwb022 and ZMBN 098051 (two specimens).
Russia: Franz Josef Land: ZIN RAS ocwb017 (one specimen), Novaya Zemlya: ZIN RAS ocwb014 and ZIN RAS ocwb034 (two specimens).
DESCRIPTION
External morphology
Massive, fist-shape, or occasionally globular sponges occupying up to 100 cm2 of the substrate (Figure 36A, B). Surface smooth, white or pale cream both in life and alcohol, with up to 200 papillae, all with oscula at the summits. The papillae conical, 2–8 mm long, up to 8 mm wide at base and 2 mm wide at summit.
Anatomy
Choanosome crumbly, pale yellow in life, but becoming slightly darker in alcohol. Main choanosomal skeleton composed of reticulating tracts (47–254 µm thick) of principal spicules (Figure 36C). Some tracts ascend to the cortex and the papillae. Auxiliary choanosomal skeleton comprises free-scattered small spicules. Cortex both in life and alcohol whitish, firm, but detachable. Cortical skeleton includes a superficial palisade (246–337 µm thick) composed of tufts of small spicules, a middle layer (513–1011 µm thick) with aquiferous cavities separated by the ascending and radiating choanosomal tracts and an internal layer (100–233 µm thick) of obliquely lying small spicules (Figure 36D). The cortical palisade and the internal layer stretch to the papillae walls. Each papilla has a large central exhalant canal and numerous small inhalant canals in the periphery. Bulkheads separating the canals reinforced with the ascending tracts of principal spicules and free-scattered small spicules.
Spicules
(Measurements based on 12 individuals)
Principal spicules – subtylostyles, occasionally styles, usually straight and slender. Length 407–538–700 µm, diameter of tyle 8.8–11.0–13.0 µm, proximal diameter of shaft 7.4–9.6–12.5 µm, maximum diameter of shaft 8.8–11.0–12.6, N = 360.
Small spicules – tylostyles, occasionally subtylostyles, usually straight and slender. Length 90–201–273 µm, diameter of tyle 2.5–4.8–7.7 µm, proximal diameter of shaft 1.9–3.3–5.0 µm, maximum diameter of shaft 1.9–3.6–5.3, N = 360.
Genetic data
Two individuals of Weberella bursa, from which the genetic data were obtained, differ neither by CO1, nor by 28S rDNA. In the phylogenies based on these genes W. bursa is the sister to morphologically quite distinct Polymastia cf. conigera (a British species not covered by the present study), although the Bayesian support for this relationship is weak (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). These species share two unique synapomorphies in CO1 (Online resource 2, p. 3, Matrix M34248 in TreeBase) and three synapomorphies in 28S rDNA, of which one is unique and two are also shared by Sphaerotylus borealis (Online resource 3, p. 3, Matrix M34250 in TreeBase). The difference between W. bursa and P. cf. conigera is 13 bps in CO1 (Matrix M34248 in TreeBase) and five bps in 28S rDNA (Matrix M34250 in TreeBase). The distinctions between W. bursa and S. borealis are described in the Genetic data section for the latter species.
OCCURRENCE
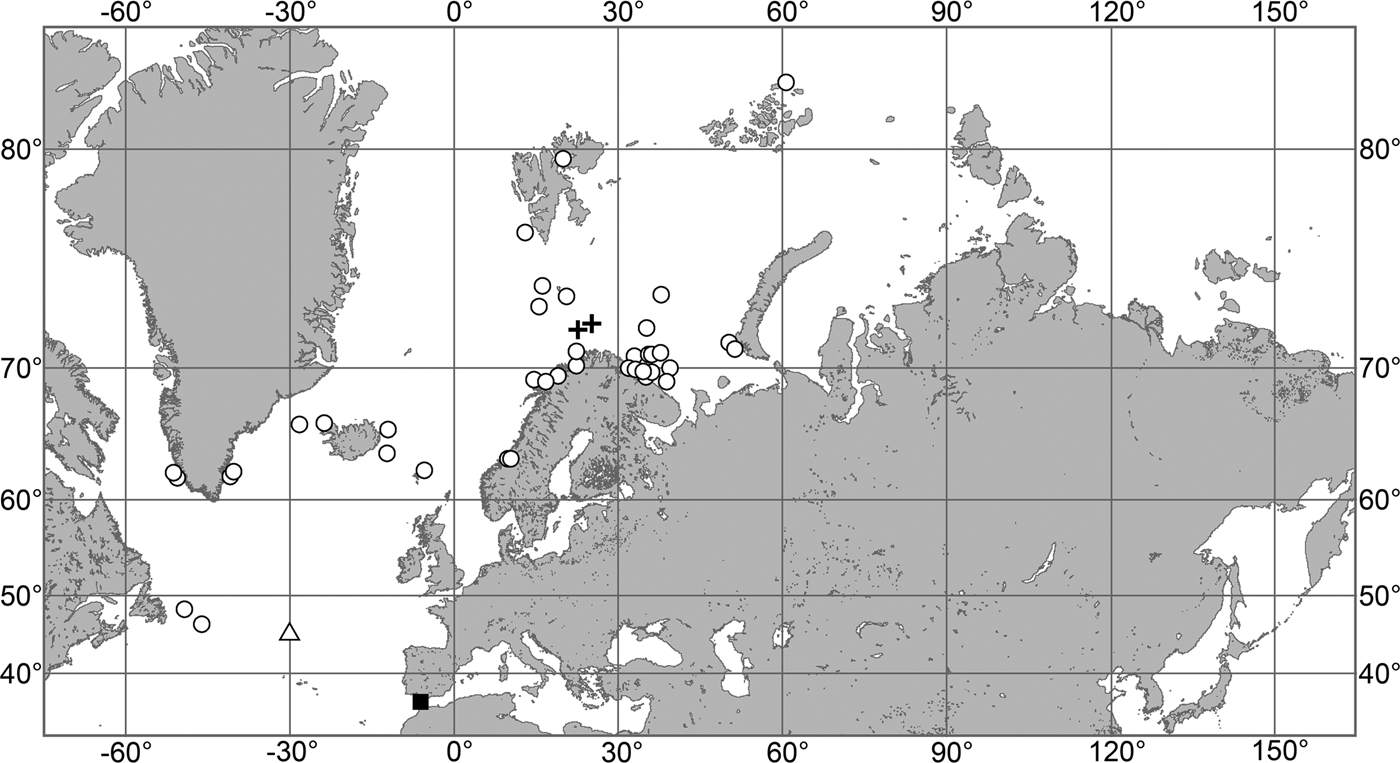
Fig. 37. Weberella bursa, distribution: black square, data from Boury-Esnault et al. (Reference Boury-Esnault, Pansini and Uriz1994); white triangle, data from Topsent (Reference Topsent1928); black crosses, data from Vosmaer (Reference Vosmaer1885); white circles, our data. Type locality is unknown.
Literature data: North-East Atlantic: North off Azores (150–932 m) (Topsent, Reference Topsent1928), West off Strait of Gibraltar (133–137 m) (Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994). Norwegian and Barents Sea: off Finnmark Coast in Norway (255–282 m) (Vosmaer, Reference Vosmaer1885). Norwegian and Barents Sea (elsewhere), Svalbard (62–485 m) (Koltun, Reference Koltun1964, Reference Koltun1966).
Our data: Canadian Atlantic Coast: Newfoundland (415 m), offshore areas (410–430 m). Davis Strait (75–195 m). South Greenland (184–300 m). Denmark Strait (348 m). Iceland (125–490 m). Faroes (525 m). Norwegian Sea, offshore areas (500–525 m). Barents Sea, offshore areas (130–250 m). Norwegian Coast: Sør-Trøndelag (50–200 m), Nord-Trøndelag (30–100 m), Nordland (750–800 m), Troms: (16–220 m), Finnmark: (200–260 m). Svalbard (40–215 m). Franz Josef Land (246 m). Novaya Zemlya (110–133 m).
DISCUSSION
Weberella bursa exhibits some external similarities with Polymastia thielei and P. uberrima, but is distinguished from these two by the reticulate choanosomal skeleton, the presence of only two size categories of spicules and some other features (see more in Discussions on P. thielei and P. uberrima). These morphological differences are confirmed by the molecular phylogenies. At the same time the sister relationships between W. bursa and Polymastia cf. conigera as well as the sister relationships between this pair and S. borealis revealed in the molecular phylogenies, but insufficiently supported, need more studies on a larger set of species.
Koltun (Reference Koltun1964, Reference Koltun1966) put Weberella in synonymy with Polymastia, that was not accepted further (Boury-Esnault, Reference Boury-Esnault, Hooper and Van Soest2002). Polymastia bursa (Müller, Reference Müller1806) sensu Koltun should not be mixed with Polymastia bursa (Schmidt, Reference Schmidt1862) sensu von Lendenfeld (Reference von Lendenfeld1898). The latter is taxon inquirendum originally described as Suberites bursa Schmidt, Reference Schmidt1862 from the Adriatic Sea (see Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schoenberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2016).
CONCLUDING REMARKS
Diversity of species
Altogether 20 species from six polymastiid genera were recorded in the Nordic and Siberian Seas (Table 8). Of these two species, Polymastia svenseni and Spinularia njordi, are new to science, one species, Polymastia andrica, is new to the Nordic Seas and two species, P. cf. bartletti and P. penicillus, are new to the Scandinavian Coast. The sponge from Western Norway herein identified as Polymastia sp. may potentially be another new species, but more material is required to check this. The new findings listed above were mainly done based on molecular data. Polymastia svenseni and S. njordi are distinguished by genetic autapomorphies, but exhibit no clear morphological autapomorphies. Polymastia cf. bartletti is morphologically very similar to P. nivea and therefore these species can be separated only based on their considerable genetic distinctions. Polymastia andrica exhibits just one clear morphological distinction, the presence of exotyles, and several distinctions in CO1 from the sibling species P. arctica, although the relationship between these two needs more studies taking into account the intragenomic polymorphism of their 28S rDNA, which probably may indicate a gene flow through hybridization between these species (Plotkin et al., Reference Plotkin, Voigt, Willassen and Rapp2016b). Polymastia penicillus was identified based on its both morphological and genetic distinctions from the congeners. Polymastia sp. is morphologically very similar to P. andrica, but considerably differs from the latter by both CO1 and 28S rDNA. Moreover, based on the 28S rDNA phylogeny Polymastia sp. is a sibling of morphologically distinct P. svenseni.
Table 8. Geographic distribution of Polymastiidae in the Nordic and Siberian Seas. + our and literature data, x only literature data. Numeration of species: 1 – Polymastia andrica, 2 – Polymastia arctica, 3 – Polymastia cf. bartletti, 4 – Polymastia boletiformis, 5 – Polymastia grimaldii, 6 – Polymastia hemisphaerica, 7 – Polymastia mamillaris, 8 – Polymastia nivea, 9 – Polymastia penicillus, 10 – Polymastia svenseni, 11 – Polymastia thielei, 12 – Polymastia uberrima, 13 – Polymastia sp., 14 – Quasillina brevis, 15 – Sphaerotylus borealis, 16 – Sphaerotylus capitatus, 17 – Spinularia njordi, 18 – Spinularia sarsii, 19 – Spinularia spinularia, 20 – Tentorium semisuberites, 21 – Weberella bursa.
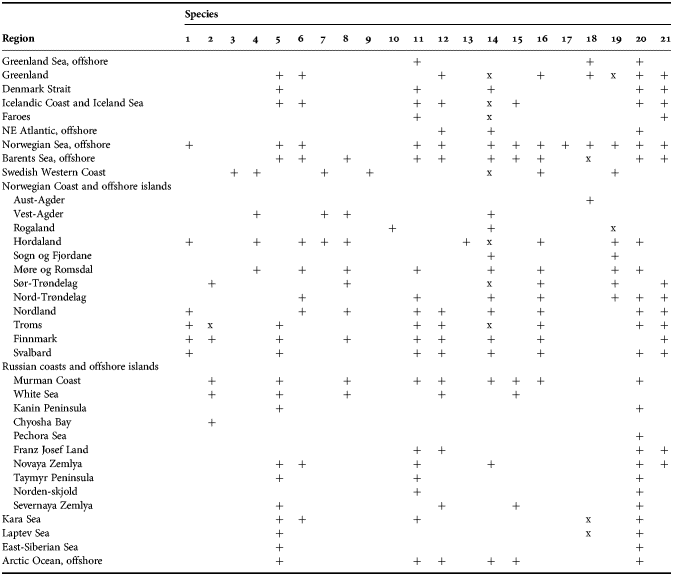
In addition to the species described in our study, one more polymastiid was recorded in the Nordic Seas – Polymastia paupera Fristedt, Reference Fristedt1887 found east off South Greenland. However, Boury-Esnault (Reference Boury-Esnault, Vacelet and Boury-Esnault1987) suggested that this was a suberitid. We agree with her opinion after examining the holotype of this species (Swedish Museum of Natural History, Stockholm, Type-1207). It is a sponge piece without papillae. The skeleton is made of tylostyles with lobate tyles (one size category) located obliquely to the surface. Cortex is not differentiated. None of these traits are found in the polymastiids.
PATTERNS OF DISTRIBUTION
Of all the species studied, 10 species, Polymastia andrica, P. grimaldii, P. hemisphaerica, P. thielei, P. uberrima, Quasillina brevis, Sphaerotylus capitatus, Spinularia sarsii, Tentorium semisuberites and W. bursa, have an amphi-Atlantic boreoarctic distribution ranging from Nova Scotia, Newfoundland and Labrador at the Canadian Atlantic Coast and north-eastwards over the Nordic Seas and along the coasts of Greenland, Iceland, Scandinavia and Russia up to the Arctic Ocean and the Siberian Seas (Table 8). In the south-western parts of this area the occurrence of these species is usually limited to the depths below 100–200 m, while in the north-east most of them spread to shallow waters (Figure 38). The distribution of two species, P. hemisphaerica and S. sarsii, is limited to the deep-sea (to 150 m for the former species and to 300 m for the latter) in all regions. The prevalence of the amphi-Atlantic boreoarctic species in the Nordic and Arctic faunas was earlier demonstrated on several demosponge genera, e.g. Geodia Lamarck, Reference Lamarck1815 from the family Geodiidae Gray, Reference Gray1867 (Cárdenas et al., Reference Cárdenas, Rapp, Klitgaard, Best, Thollesson and Tendal2013) and Thenea Gray, Reference Gray1867 (Cárdenas & Rapp, Reference Cárdenas and Rapp2012) from Theneidae Carter, Reference Carter1883, and on hexactinellids, e.g. Asconema Kent, Reference Kent1870 from Rossellidae Schulze, Reference Schulze, Tizard, Moseley, Buchanan and Murray1885 (Tabachnick & Menshenina, Reference Tabachnick and Menshenina2007).
Fig. 38. Vertical distribution of Polymastiidae in the Nordic and Siberian Seas (our and literature data): (A) NW Atlantic (offshore areas, Canadian Coast and Davis Strait); (B) NE Atlantic (offshore areas), Southern Greenland Coast, Denmark Strait, Iceland Sea, Icelandic Coast and Faroes; (C) Greenland Sea (offshore areas and Eastern Greenland Coast); (D) Norwegian Sea (offshore areas) and Scandinavian Coast from Western Sweden to Northern Norway; (E) Barents Sea (offshore areas, archipelagos and Russian Coast); (F) White Sea; (G) Siberian Seas (offshore areas, archipelagos and Russian Coast); (H) Arctic Ocean. Labelling species: P. and., Polymastia andrica, P. arc., Polymastia arctica, P. bar., Polymastia bartletti and P. cf. bartletti, P. bol., Polymastia boletiformis, P. gri., Polymastia grimaldii, P. hem., Polymastia hemisphaerica, P. mam., Polymastia mamillaris, P. niv., Polymastia nivea, P. pen., Polymastia penicillus, P. sve., Polymastia svenseni, P. thi., Polymastia thielei, P. ube., Polymastia uberrima, Pol. sp., Polymastia sp., Q. bre., Quasillina brevis, S. bor., Sphaerotylus borealis, S. cap., Sphaerotylus capitatus, S. njo., Spinularia njordi, S. sar., Spinularia sarsii, S. spi., Spinularia spinularia, T. sem., Tentorium semisuberites, W. bur., Weberella bursa.
Meanwhile, it is still unclear how far southwards in the central North Atlantic and along the European and African coasts the amphi-Atlantic boreoarctic polymastiids may be found. The records of Q. brevis and W. bursa from Azores (Topsent, Reference Topsent1892, Reference Topsent1928), the entrance to the Gibraltar Strait and the Mediterranean Sea (Uriz & Rosell, Reference Uriz and Rosell1990; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994) as well as the records of T. semisuberites from Azores (Topsent, Reference Topsent1892, Reference Topsent1904, Reference Topsent1928) and the Bay of Biscay (Topsent, Reference Topsent1892) need verification with molecular data. The allegedly cosmopolitan distribution of S. sarsii is already questioned based on the observed genetic differences between the Norwegian and Mozambican sponges, but a thorough comparison between the Norwegian, Azorean and NW African individuals is still required.
The distribution of other polymastiid species studied is narrower (Table 8). Four species, Polymastia arctica, P. nivea, Sphaerotylus borealis and Spinularia spinularia may be regarded as NE Atlantic high-boreoarctic. The known occurrence of Sphaerotylus borealis is limited to Iceland in the south-west and the eastern Kara Sea in the north-east. The distribution of Polymastia arctica and P. nivea is limited to the Norwegian Coast and Russian Coast of the Barents and White Sea. The former species has never been recorded to the south-west from Central Norway (Sør-Trøndelag), while the latter has been found up to Southern Norway (Vest-Agder). Spinularia spinularia is widely distributed along the Scandinavian Coast, East Greenland Coast and around the British Isles, while its records from Azores (Topsent, Reference Topsent1898, Reference Topsent1904, Reference Topsent1928) are considered as a separate species, S. setosa, based on distinct differences in morphology. Atlantic high-boreoarctic species were earlier recorded among other demosponge families, e.g. Geodia hentscheli Cárdenas et al., Reference Cárdenas, Rapp, Schander and Tendal2010 and G. parva Hansen, Reference Hansen1885 from Geodiidae (Cárdenas et al., Reference Cárdenas, Rapp, Klitgaard, Best, Thollesson and Tendal2013), Tetilla sibirica (Fristedt, Reference Fristedt1887) from Tetillidae Sollas, Reference Sollas1886 (Koltun, Reference Koltun1966), and Thenea abyssorum Koltun, Reference Koltun1964 from Theneidae (Cárdenas & Rapp, Reference Cárdenas and Rapp2012). A few records of the sponge species distribution limited to the Arctic Ocean and Siberian Seas, e.g. demosponges Hemimycale rhodus (Hentschel, Reference Hentschel, Römer, Schaudinn, Brauer and Arndt1929), Cladorhiza arctica Koltun, Reference Koltun1959 and Pseudosuberites sadko Koltun, Reference Koltun1966, should be verified on the additional material. It seems that the Arctic sponge fauna is predominantly composed of the species dispersed from the Atlantic.
Two species studied, Polymastia boletiformis and P. penicillus, represent the southern boreal component in the Scandinavian sponge fauna. They are quite common in the shallow depths along the European coasts (Figure 38), with their northernmost records from Møre and Romsdal in Norway for P. boletiformis and the British Isles and the Swedish Western Coast for P. penicillus. The occurrence of the southern boreal species along the Scandinavian Coast was earlier recorded for other sponge families, e.g. a calcarean Clathrina coriacea (Montagu, Reference Montagu1814) from Clathrinidae Minchin, Reference Minchin and Lankester1900 (Rapp, Reference Rapp2006), demosponges Characella pachastrelloides (Carter, Reference Carter1876) and Poecillastra compressa (Bowerbank, Reference Bowerbank1866) from Pachastrellidae Carter, Reference Carter1875 and Thenea muricata (Bowerbank, Reference Bowerbank1858) from Theneidae (Cárdenas & Rapp, Reference Cárdenas and Rapp2012).
Even though we have got numerous and well-documented records for most species studied, our knowledge of the distribution of other species is still poor. Particularly, more data on the rare species Polymastia bartletti and two new species, P. svenseni and Spinularia njordi, are required. The observed distribution patterns of the polymastiids in the area of study should therefore be considered as preliminary until more comprehensive material from adjacent waters can be included. It is recommended that further research on the biogeography of polymastiids should be based on an integrative approach including detailed morphological studies and analyses of a larger set of phylogenetic markers.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315417000285
ACKNOWLEDGEMENTS
We would like to express our gratitude to all colleagues, who kindly provided the access to the sponge collections of their institutions: Jean-Marc Gagnon (Canadian Museum of Nature, Ottawa), Erica Mejlon (Museum of Evolution, University of Uppsala), Kennet Lundin and Carola Azurduy Högström (Gothenburg Natural History Museum), Michèle Bruni (Musée Océanographique de Monaco), Carsten Lüter (Museum für Naturkunde, Berlin), Isabelle Domart-Coulon (Muséum National d'Histoire Naturelle, Paris), Clare Valentine, Andrew Cabrinovic and Emma Sherlock (Natural History Museum, London), Nicole de Voogd and J. Koos van Egmond (Naturalis Biodiversity center, Leiden), Lutz Bachmann and Åse Ingvild Wilhelmsen (Natural History Museum, University of Oslo), Ole Secher Tendal (Natural History Museum of Denmark, University of Copenhagen), Bernard E. Picton (Ulster Museum, National Museums of Northern Ireland, Belfast), Jon Anders Kongsrud (University Museum of Bergen, Natural History Collections), Torkild Bakken (University Museum of the Norwegian University of Science and Technology, Trondheim), Olga Bozhenova (Zoological Institute of Russian Academy of Sciences, Saint-Petersburg). We are also thankful to Bjørn Gulliksen (University of Tromsø), Bjørn Tore Dragnes (OMNIMAR Dragnes, Tromsø), Peter Leopold (University Centre in Svalbard) and Erling Svensen (OceanPhoto/Dalane Tidende AS, Egersund), who arranged the sampling of fresh material along the Norwegian Coast and on Svalbard and provided us with high-quality underwater images. The colleagues, who granted us fresh samples from other regions, are also greatly acknowledged: Christine Morrow (Department of Zoology, Ryan Institute, National University of Ireland, Galway) and Bernard E. Picton (Ulster Museum, National Museums of Northern Ireland, Belfast) for the samples and photos of sponges from the British Isles, Javier Cristobo and Pilar Rios (Spanish Institute of Oceanography, Madrid) for the samples from Mozambique, and Natalia Chervyakova (Moscow State University) for the material from the White Sea. Paco Cárdenas (BioMedical Centre, Uppsala) is acknowledged for the photos of sponges from Museum of Evolution, University of Uppsala. Thanks also come to Egil Severin Erichsen and Irene Heggstad (University of Bergen, Laboratory for Electron Microscopy) for their careful assistance at SEM, to Kenneth Meland, Solveig Thorkildsen and Louise Lindblom (University of Bergen, BioDiversity Laboratories at the Department of Biology) for their great assistance under the obtaining and treatment of molecular data and to Endre Willassen (University Museum of Bergen, Natural History Collections) and Oliver Voigt (Ludwig-Maximilians-Universität München, Palaeontology and Geobiology) for their inestimable help with the phylogenetic computing. Last but not least, we would like to thank the two reviewers for the great work they have done when reading our manuscript.
FINANCIAL SUPPORT
This study was supported by the Norwegian Biodiversity Information Centre, Artsdatabanken (project No. 70184219), the Norwegian Academy of Science and Letters (grant to HTR), the Research Council of Norway (through contract No. 179560) and the European Union's Horizon 2020 research and innovation programme under grant agreement No. 679849 (the SponGES project). The visit of AP to the Natural History Museum (London) in 2010 received funding from the SYNTHESYS Project (http://www.synthesys.info/) financed by European Community Research Infrastructure Action under the FP7 Integrating Activities Programme.
APPENDIX 1
KEY FOR THE NORDIC AND ARCTIC SPECIES OF THE FAMILY POLYMASTIIDAE
1. Pedunculate, columnar, fungiform or drum-shaped body.………2
– Other body shape………3
2. Body pedunculate or columnar, but never fungiform or drum-shape. Choanosome is a semi-fluid, amorphous mass with free-scattered bundles of small spicules. Tracts of principal spicules stretch in the cortex parallel to the surface.………Quasillina brevis
– Body columnar, pedunculate, fungiform or drum-shaped. Choanosome dense, with longitudinal tracts of principal spicules crossing the upper surface………Tentorium semisuberites
3. Raphids in trichodragmata present (in the subcortical area)………Spinularia spinularia
– Raphids in trichodragmata absent………4
4. Exotyles with ornamented distal knobs present.………5
– Exotyles absent or, if present, without any distal ornamentations.………6
5. Exotyles 5–7.5 mm long, with distal knobs of irregular shape varying from fungiform and umbrelliform to hemispherical or subspherical………Sphaerotylus borealis
– Exotyles not exceeding 1.5 mm in length, with regular, spherical distal knobs………Sphaerotylus capitatus
6. Body discoid, hemispherical or lenticular, attached to the substrate only by the central basal point. A prominent spicule fringe present at the boundary between the upper and basal surface………7
– Body globular, massive, cushion-shaped or encrusting. Basal surface completely attached to the substrate. Marginal spicule fringe tiny or absent.………9
7. Upper surface smooth………Polymastia hemisphaerica
– Upper surface hispid………8
8. Papillae well-developed. Body may reach 80 mm in diameter and possess up to 300 papillae, of which most are inhalant (blind) and few are exhalant (with oscula at the summits). Exhalant papillae larger than the inhalant ones.………Polymastia grimaldii
– One to five weakly developed papillae, of which all are exhalant. Body not larger than 13 mm in diameter.………Spinularia sarsii
9. Surface completely, or at least for the most part smooth or velvety………10
– Surface completely hispid or shaggy.………16
10. Main choanosomal skeleton is a reticulation of spicule tracts………11
– Main choanosomal skeleton composed of radial or longitudinal spicule tracts, occasionally meandering or anastomosing.………12
11. Colour in life intensively orange or yellow. Exhalant papillae larger than the inhalant ones.………Polymastia boletiformis
– Colour in life pale cream or whitish. All papillae are exhalant and do not differ in size or shape………Weberella bursa
12. Body massive or globular.………13
– Body cushion-shaped or encrusting………14
13. Surface uniformly smooth or velvety, with not more than 30 papillae, crater-shaped (very short and wide) in life. Aquiferous cavities all over the cortex. Choanosomal spicule tracts may meander or anastomose.………Polymastia thielei
– Surface smooth or velvety, but with a marginal hispid collar. Up to 150 conical or cylindrical papillae. Aquiferous cavities concentrated in the cortical areas around the papillae. Choanosomal skeleton spicule tracts never meander or anastomose………Polymastia uberrima
14. Although the spicules considerably vary in length, only two size categories can be distinguished. Large spicules present both in cortex and choanosome. Cortex comprises three prominent layers, a superficial palisade of small spicules, a middle layer of loosely lying criss-cross large spicules and an internal layer of densely packed criss-cross large spicules.………Polymastia svenseni
– Three size categories of spicules clearly distinguished. Large spicules comprise choanosomal tracts. Cortex of two layers, a superficial palisade of small spicules and an internal layer of densely packed criss-cross intermediary spicules………15
15. Oscula at the summits of papillae not visible. In the cortex superficial spicule palisade and internal layer of criss-cross spicules intermingle………Polymastia bartletti or Polymastia nivea (discrimination possible only based on molecular data)
– In life some papillae with visible oscula at the summits. In the cortex superficial spicule palisade and internal layer of criss-cross spicules clearly segregated………Polymastia penicillus
16. Body not exceeding 24 mm in diameter, with a single weakly developed papilla and a tiny marginal spicule fringe. Cortex comprises a superficial palisade of small spicules and an internal layer of criss-cross spicules in the central area and only the palisade in the periphery.………Spinularia njordi
– Body with numerous well-developed papillae, usually exceeds 30 mm in diameter, often reaching up to 100 mm. Marginal spicule fringe absent. Cortex uniform, comprising a superficial palisade of small spicules, a middle layer of collagen fibres with low density of spicules and an internal layer of criss-cross spicules………17
17. Four size categories of spicules including filiform monactines averaging 2.5 mm in length and reinforcing the surface hispidation………Polymastia andrica or Polymastia sp. (discrimination possible only based on molecular data)
– Three size categories of spicules with the largest not exceeding 1.8 mm in length. Filiform monactines absent.………18
18. Inhalant papillae often bear at the summits threads with buds. Middle cortical layer up to 180 µm thick………Polymastia arctica
– Inhalant papillae without buds. Middle cortical layer thinner than 110 µm………Polymastia mamillaris