Chronic kidney disease (CKD) is a progressive loss of kidney function over time, leading to irreversible kidney failure( Reference Levey and Coresh 1 ). The pathophysiological process involved in CKD is characterised by a background of low-grade chronic inflammation( Reference Miyamoto, Carrero and Stenvinkel 2 ). Together with coagulation disorders and neutrophil–endothelium interaction, inflammation is believed to play a role in the genesis of kidney injury, potentially leading to chronically impaired kidney function( Reference Leung, Tonelli and James 3 ).
Diet may play a central role in the regulation of chronic inflammation( Reference Giugliano, Ceriello and Esposito 4 ) and, possibly, in kidney health. Anti-inflammatory nutrients such as n-3 PUFA, fibre and many vitamins( Reference Dunkler, Dehghan and Teo 5 ) have been associated with better kidney function, lower risk of albuminuria and slower decline in kidney function( Reference Dunkler, Dehghan and Teo 5 – Reference Xu, Huang and Riserus 7 ). Conversely, nutrients assumed to have pro-inflammatory effects such as SFA or sugar( Reference Huang, Sjogren and Arnlov 8 ) have been linked with worsening of kidney function( Reference Lin, Judd and Le 9 ). Studies have concluded that Dietary Approaches to Stop Hypertension (DASH) and Mediterranean dietary patterns, which are rich in protective nutrients such as antioxidant vitamins including vitamin E, C, A, K, Mg, Ca, fibre, PUFA, MUFA and phytochemicals and poor in SFA, trans-fatty acids and simple carbohydrate, can affect kidney function and decrease the risk of CKD( Reference Farhadnejad, Asghari and Mirmiran 10 , Reference Huang, Jiménez-Moleón and Lindholm 11 ).
The Dietary Inflammatory Index (DII®) was designed to assess the inflammatory potential of the diet based on the pro- and anti-inflammatory properties of its various components. The DII was developed to provide a means for estimating the overall inflammatory potential of the diet( Reference Shivappa, Steck and Hurley 12 , Reference Cavicchia, Steck and Hurley 13 ). Previously, the DII was associated with a range of outcomes including markers of systemic inflammation, CVD, telomere length and overall mortality( Reference Wirth, Burch and Shivappa 14 – Reference Neufcourt, Assmann and Fezeu 18 ). However, there is very limited evidence on the association between dietary patterns and kidney health( Reference Sabatino, Regolisti and Gandolfini 19 ). The main objectives in the management of CKD are the reduction of unfavourable symptoms of uraemia, delaying the start of renal replacement therapy and quality-of-life improvement( Reference Deniz Ayli, Ayli and Ensari 20 ). Diet, specifically through a focus on foods with anti-inflammatory properties, including those with high concentrations of vitamins and minerals, has the potential to decrease oxidative stress. Dietary restrictions imposed in CKD subjects make it difficult to ensure adequate micronutrient content in the diet, while, at the same time, it has been suggested that these patients have an impaired intestinal absorption of minerals and vitamins( Reference Vaziri, Said and Hollander 21 ).
The aim of this study was to investigate the association of the inflammatory potential of diet with kidney function and prevalent CKD in adult Americans. We hypothesised that those living with prevalent CKD would have more pro-inflammatory diets than those without CKD.
Methods
Population
We used data from the Nutrition and Health Examination Surveys (NHANES), which is described in greater detail elsewhere( 22 ). In brief, these are periodic cross-sectional surveys conducted by the US National Center for Health Statistics, and during which home visits are conducted to administer questionnaires to collect data on demographics, diet and other health behaviours. NHANES applies complex multistage probability sampling procedure to ensure selection of participants from various geographical locations and adequate racial/ethnic representation( 22 ). Trained interviewers collected participants’ demographic, socio-economic, dietary and health-related information using questionnaires administered during home visits. Clinical examination and dietary assessment are conducted by skilled personnel using a mobile examination centre (MEC)( 22 ). All procedures were carried out in accordance with relevant approved guidelines and regulations( Reference Needham, Adler and Gregorich 23 – 26 ). Informed consent was obtained from all participants, and the National Centre for Health Statistics Research Ethics Review Board approved the protocol. For the present analysis, four survey cycles (i.e. 2005–2006, 2007–2008, 2009–2010 and 2011–2012) were combined to produce estimates with greater precision and smaller sampling error (n 40 790). The analytical sample was limited to adults aged ≥18 years. After excluding pregnant and lactating women (n 986), as well as participants with missing information on the variables of interest (n 1547), the final analytical sample included 21 649 respondents from NHANES 2005–2012.
Biochemical analysis
Methods for Biochemical analyses are described in the NHANES Laboratory/Medical Technologists Procedures Manual( Reference Needham, Adler and Gregorich 23 – 26 ). A blood specimen was drawn from the participant’s antecubital vein by a trained phlebotomist according to a standardised protocol. Fasting glucose was measured in plasma by a hexokinase method using a Roche/Hitachi 911 Analyzer and Roche Modular P Chemistry Analyzer. The concentration of creatinine in serum was determined using the modular chemistry side of a Beckman Coulter DxC800 using the Jaffe reaction method (kinetic alkaline picrate). The creatinine calibration is traceable to an isotope dilution MS reference method( Reference Selvin, Manzi and Stevens 27 ). Urinary creatinine by the Jaffe rate reaction, and urinary albumin by solid-phase fluorescent immunoassay, from a random urine sample( Reference Chavers, Simonson and Michael 28 ) were used to calculate the urinary albumin:creatinine ratio (ACR). The CKD Epidemiology Collaboration equation( Reference Levey, Stevens and Schmid 29 ) was used to estimate glomerular filtration rate (eGFR, in ml/min per 1·73 m2), and eGFR lower than 60 ml/min per 1·73 m2 was used to define low eGFR. ACR >30 mg/g was used to define albuminuria, and the presence of either low eGFR or albuminuria was used to define CKD in line with Kidney Disease: Improving Global Outcomes 2012 recommendations( 30 ).
Diet and Dietary Inflammatory Index
Dietary data in NHANES were collected using a single 24-h dietary recall interview at the MEC( 22 ). The development and validation of the DII has been discussed in detail elsewhere( Reference Shivappa, Steck and Hurley 12 ). The 24-hour-derived dietary information was used to calculate energy-adjusted DII (E-DIITM) scores for all participants( Reference Shivappa, Steck and Hurley 12 ). The DII food parameters available in NHANES database included carbohydrates; protein; fat; grams of alcohol; fibre; cholesterol; SFA, MUFA and PUFA; n-3 and n-6 PUFA; niacin; vitamins A, B1, B2, B6, B12, C, D and E; Fe; Mg; Zn; Se; folic acid; β-carotene; and caffeine. Higher (i.e. more positive) scores tend to indicate more pro-inflammatory diets and more negative values are more anti-inflammatory( Reference Shivappa, Steck and Hurley 12 ). To control for the effect of total energy intake, the E-DII was calculated per 4184 kJ (1000 kcal) of food consumed. We used energy-adjusted food parameters wherein we calculated all the food parameters per 4184 kJ (1000 kcal) of consumption. This required using an energy-adjusted world database.
Statistical analysis
Data analyses followed the CDC guidelines for complex NHANES data analysis, accounting for the masked variance and using the recommended weighting methodology( 31 ), implemented with the use of SPSS® complex sample module version 22.0 (IBM Corp). We used means with their standard errors for continuous variables (with groups compared via ANOVA) and percentages for categorical variables (with groups compared using the χ 2 test). The Kolmogorov–Smirnov test was used to evaluate the normal distribution of continuous variables. The natural logarithm of ACR and urinary albumin were taken to approximate a normal distribution. Adjusted mean of kidney function markers across E-DII quartiles was calculated using ANCOVA. These models were adjusted for age, sex, race, fasting blood glucose, systolic and diastolic blood pressure, BMI (kg/m2), diabetes (self-reported history of diabetes or fasting plasma glucose ≥7·0mmol/l(126mg/dl)) and hypertension. Logistic regressions models, using a similar adjustment strategy, were then used to derive the OR and 95 % CI for the association of E-DII (by quartile) with prevalent CKD, always using the lowest quartile as reference. A P value <0·05 was used as the nominal cut-off point to indicate statistically significant results.
Results
Of the 21 649 participants included in the analyses, 1634 (6·8 %) had prevalent CKD. The characteristics of participants overall and across E-DII quartiles are summarised in Table 1. The E-DII score ranged from −5·66 to+4·33, with a median of 0·44 (25th–75th percentiles (i.e. interquartile range −1·04 to 1·62). Mean age decreased from 53·6 to 42·1 years (P<0·001), whereas the proportion of women decreased from 58·3 % to 47·1 % (P<0·001) across increasing quartiles of E-DII. Across increasing E-DII quartiles, the proportion of non-Hispanic White (the largest ethnic group) followed a U-shape; the proportions of Mexican–American and other Hispanics followed a reversed U-shape; the proportions of non-Hispanic Blacks increased; and the proportion of the remaining racial groups decreased (P<0·001). The proportion of participants with more than high school education (the larger group) followed a reversed U-shape, those with high school education (the second larger group) followed a U-shape pattern and participants with less than high school education decreased from 16·8 % in the lowest quartile to 8 % in the top quartile of the E-DII distribution (P<0·001). The cardio-metabolic risk profile systematically deteriorated across increasing quartiles of the E-DII (all P<0·001). For instance, values (highest v. lowest E-DII quartiles) were 5·66 v. 5·48 mmol/l (102 v. 98·7 mg/dl) for mean fasting blood glucose, 124·2 v. 121·6 mmHg for systolic blood pressure, 29·2 v. 28·2 kg/m2 for BMI, 11·2 v. 8·0 % for prevalent diabetes mellitus and 34·1 v. 28·1 % for prevalent hypertension, as shown in Table 1.
Table 1 Descriptive characteristics of participants across quartiles of energy-adjusted Dietary Inflammatory Index (E-DII) (Mean values with their standard errors)
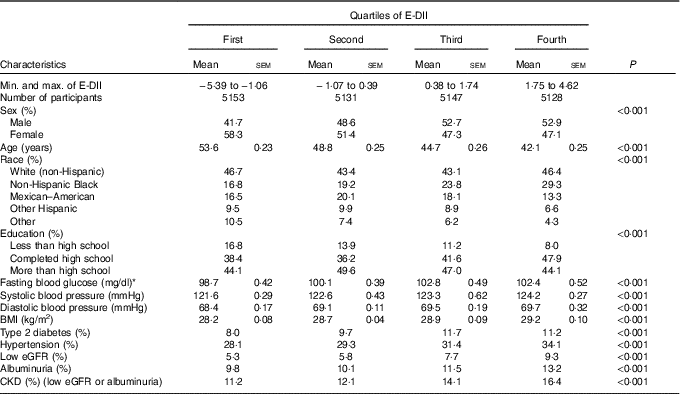
eGFR, estimate glomerular filtration rate; CKD, chronic kidney disease.
* To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
Adjusted mean levels of kidney function markers by quartile of E-DII score are shown in Table 2. Across increasing E-DII quartiles, mean serum uric acid increased from 316 to 329 μmol/l (5·31 to 5·53 mg/dl) (P<0·001), urine albumin increased from 2·01 to 2·25 mg/l) (P<0·001) and eGFR decreased from 96·3 to 90·7 ml/min/1·73 m2 (P<0·001). Log ACR also increased from 2·10 to 2·19 (P=0·023). The proportion of participants with prevalent low eGFR, albuminuria or CKD systematically increased across increasing quartiles of E-DII (all P<0·001 for linear trend). Proportions of participants with CKD, by E-DII quartile, were 11·2 % in the first (lowest) quartile, 12·1 % in the second quartile, 14·1 % in the third quartile and 16·4 % in the top quartile (Table 1). In age-, sex- and race-adjusted logistic regressions, compared with the lowest quartile of the E-DII, the OR was 1·14 (95 % CI 0·95, 1·37) for the second quartile, 1·24 (95 % CI 1·02, 1·50) for the third quartile and 1·35 (95 % CI 1·08, 1·69) for the top quartile (P for trend<0·001, CKD diagnosed by eGFR). In logistic regression models adjusted for age, sex, race, blood glucose, blood pressure, BMI, diabetes and hypertension status, compared with the lowest quartile of E-DII, the OR of low eGFR was 1·09 (95 % CI 0·90, 1·32) for the second quartile, 1·18 (95 % CI 0·97, 1·44) for the third quartile and 1·29 (95 % CI 1·03, 1·62) for the top quartile (P for trend<0·001). Equivalent figures were 1·11 (95 % CI 0·78, 1·59), 1·13 (95 % CI 1·06, 1·22) and 1·19 (95 % CI 1·10, 1·28) for albuminuria (P for trend<0·001), and 1·08 (95 % CI 1·02, 1·13), 1·15 (95 % CI 1·08, 1·23) and 1·23 (95 % CI 1·10, 1·35) for CKD (P for trend<0·001).
Table 2 Adjusted (age, sex, race, fasting blood glucose, systolic and diastolic blood pressure, BMI, diabetes and hypertension) mean levels of markers of chronic kidney disease (CKD) across quartiles of energy-adjusted Dietary Inflammatory Index (E-DII) (Mean values with their standard errors)
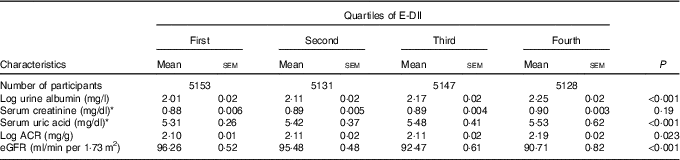
eGFR, estimated glomerular filtration rate, ACR, urine albumin:creatinine ratio.
* To convert creatinine in mg/dl to μmol/l, multiply by 88·4; to convert uric acid in mg/dl to μmol/l, multiply by 59·48.
Discussion
This study examined the association between the E-DII and prevalent CKD in a large population of adult Americans. A pro-inflammatory diet was associated with adverse profiles of kidney function markers, translating into higher rates of prevalent CKD, even after adjustment for a range of extraneous factors.
In line with our findings, recent investigations have reported that a pro-inflammatory diet was associated with systemic inflammation, as well as with reduced kidney function, in elderly individuals( Reference Xu, Sjögren and Ärnlöv 32 ). In the prospective observational Nurses’ Health Study, it was reported that, compared with the lowest quartile, the highest quartile of Western pattern score ‘pro-inflammatory’ was associated with a high risk of microalbuminuria (OR 2·17; 95 % CI 1·18, 3·66) and rapid eGFR decline ≥3 ml/min per 1·73 m2 per year (OR 1·77; 95 % CI 1·03, 3·03)( Reference Lin, Fung and Hu 33 ). On the other hand, women in the top quartile of the DASH score (highly loaded with anti-inflammatory materials such as fruit and vegetables) had decreased risk of rapid eGFR decline (OR 0·55; 95 % CI 0·38, 0·80)( Reference Lin, Fung and Hu 33 ).
An investigation in the Multi-ethnic Study of Atherosclerosis that included almost 5000 ethnically diverse men and women reported that a dietary pattern rich in whole grains and fruit (anti-inflammatory) was associated with lower urinary ACR (20 % lower ACR across quintiles, P=0·004), whereas animal-based food (pro-inflammatory) intake was directly associated 11 % higher ACR across quintiles (P=0·03)( Reference Nettleton, Steffen and Palmas 34 ). We previously reported that higher dietary intake of animal fat was associated with the presence of microalbuminuria, whereas higher sodium intake was directly associated and higher β-carotene intake was inversely associated with faster eGFR decline over 11 years( Reference Lin, Hu and Curhan 35 ). In the same line, the ‘anti-inflammatory’ Healthy Eating Index( Reference Nettleton, Steffen and Mayer-Davis 36 ) was associated with a lower risk of albuminuria and eGFR decline in diabetic people( Reference Dunkler, Dehghan and Teo 5 , Reference Xu, Sjögren and Ärnlöv 32 ). In another observational study conducted among 1942 elderly community-dwelling participants aged 70–71 years, diet with higher pro-inflammatory load was associated with reduced kidney function( Reference Xu, Sjögren and Ärnlöv 32 ).
It has been suggested that poor dietary quality could lead to several tissue-specific and systemic metabolic dysfunctions that could promote renal dysfunction( Reference Gopinath, Harris and Flood 37 , Reference Odermatt 38 ). For example, it has been reported that diets high in whole grains, fruit or vegetables and fish (anti-inflammatory) are inversely associated with markers of inflammation including CRP and soluble ICAM-1, whereas a diet pattern rich in fats and processed meats (pro-inflammatory) is directly associated with markers of inflammation including CRP( Reference Nettleton, Steffen and Mayer-Davis 36 ). Furthermore, cytokine-mediated inflammation has been suggested to be involved in the early stages of impaired kidney function in the elderly, whereas cyclooxygenase-mediated inflammation does not appear to play a role at this stage( Reference Nerpin, Helmersson-Karlqvist and Risérus 39 ). Thus, inflammation seems to be a reasonable target for potential preventive and therapeutic interventions in patients with CKD.
Strengths and limitations
This is among the largest studies on the association of kidney disease with E-DII. Participants were a random sample of the general population, and therefore the results can be extrapolated to the general US population. Because data collection was performed on all days of the week in NHANES, the potential for day-specific information bias is very low( Reference Tooze, Midthune and Dodd 40 , Reference Guenther, Ding and Rimm 41 ). Our findings also have to be considered in the context of some study limitations. First, the cross-sectional nature of the data does not allow for direct inference about causality. Second, it is well known that a single 24-h diet recall interview is not ideal for characterising an individual’s long-term habitual intake( Reference Hebert, Hurley and Steck 42 , Reference Ma, Olendzki and Pagoto 43 ). Third, calculation of E-DII scores was based on twenty-seven out of forty-five food parameters, which may affect our findings. Despite these shortcomings, which would have increased overall error, we were able to detect a relationship between E-DII scores and CKD.
Conclusion
The potentially deleterious effect of pro-inflammatory diet on kidney health, supported by our findings and those of other investigators, suggests the potential utility of the modulation of inflammatory properties of diet, in strategies to prevent kidney disease. If confirmed in clinical trial, this knowledge may have application for both population-wide and high-risk approach to CKD prevention and control in various settings.
Acknowledgements
M. M. was supported by The world academy of science and Chinese Academy of Sciences. Drs N. S., M. D. W. and J. R. H. were supported by grant no. R44DK103377 to Connecting Health Innovations LLC (CHI) from the US National Institute of Diabetes and Digestive and Kidney Diseases.
M. M. was involved in study conception, data analysis and interpretation and drafting of the manuscript; N. S. contributed to data analysis and interpretation and critical revision of the manuscript; M. D. W. performed data analysis and interpretation and critical revision of the manuscript; J. R. H. performed data analysis and interpretation and critical revision of the manuscript; A. P. K. contributed to data interpretation and critical revision of the manuscript.
Dr J. R. H. owns controlling interest in CHI, a company planning to license the right to his invention of the Dietary Inflammatory Index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Drs N. S. and M. D. W. are employees of CHI.
The authors declare that there are no conflicts of interest.




