To the Editor—Owing to the widespread prevalence of carbapenemase-producing Enterobacteriaceae resistant to last-resource therapeutic options, including extended-spectrum β-lactams, fluoroquinolones, and aminoglycosides, an interest in old antimicrobial agents, such as polymyxins and fosfomycin, has reignited.Reference Giske 1 The latter is an agent that acts inhibiting the formation of a precursor of peptidoglycan (ie, a cell wall–acting agent); it was first used in the treatment of uncomplicated urinary tract infections but, nowadays, is being used (still on a small scale in our institution) as an adjunct to other active agents for the treatment of Klebsiella pneumoniae carbapenemase (KPC)–2-producing K. pneumoniae (KPC-2-Kp) infections.Reference Michalopoulos, Virtzili and Rafailidis 2
Although high in vitro frequency of fosfomycin resistance mutations has been reported,Reference Karageorgopoulos, Wang, Yu and Falagas 3 resistance rates to this agent have remained relatively low since its introduction in clinical practice. On the other hand, a substantially higher resistance rate has been noted when carbapenemase producers are considered.Reference Karageorgopoulos, Wang, Yu and Falagas 3 , Reference Jiang, Shen and Wei 4 Furthermore, a report by Karageorgopoulos et alReference Karageorgopoulos, Miriagou, Tzouvelekis, Spyridopoulou and Daikos 5 identified patients who were treated with fosfomycin for an initially fosfomycin-susceptible KPC-2-Kp bacteremia but from whom a fosfomycin-resistant isolate was subsequently collected. However, the potential of in vivo emergence of fosfomycin-resistance among KPC-2-Kp isolates has not been systematically investigated so far.
Thus, this study aimed to perform a survey on the subsequent emergence of fosfomycin resistance among intensive care unit patients from whom a fosfomycin-susceptible KPC-2-Kp isolate was previously collected at a tertiary hospital in southern Brazil from April 1, 2013, through May 31, 2015. KPC-2-Kp was defined according to carbapenem resistance patterns, with phenotypic testing results determined as proposed by Clinical and Laboratory Standards Institute guidelines 6 and through bla KPC-2 gene detection by polymerase chain reaction as previously reported.Reference Perez, Rodrigues and Dias 7 Cases were defined as patients from whom a fosfomycin-resistant isolate was recovered from urine and/or blood cultures more than 48 hours but less than 90 days after the day a urinary fosfomycin-susceptible isolate was collected. Data on antibiotic exposures between the first KPC-2-Kp isolation and the day on which a positive culture for a fosfomycin-resistant isolate was obtained were recorded.
Eighty-five patients had a urinary KPC-2-Kp isolate collected during the period of this study and 35 of them (41.2%; 95% CI, 31.3%–51.8%) had a subsequent isolation of this same pathogen: 20 patients with a recurrent bacteriuria, 10 patients presenting a bloodstream infection, and 5 patients with both. Each patient was considered only once as a case and therefore for those patients in whom a KPC-2-Kp was recovered from a recurrent bacteriuria and blood, only the latter was considered. Among these 35 patients, in 32 (91.4%) a fosfomycin-susceptible KPC-2-Kp isolate had been previously recovered. For the 3 patients presenting a fosfomycin-resistant KPC-2-Kp isolate, the subsequent fosfomycin susceptibility remained unaltered. On the other hand, for those 32 patients with a prior fosfomycin-susceptible isolate, in 8 patients (25%) the subsequent KPC-2-Kp isolate was resistant to fosfomycin. When evaluating the previous use of antibiotics, 5 of these 8 patients (62.5%; odds ratio, 9.6 [95% CI, 1.6–56.9], P=.013) had already received fosfomycin to treat the first urinary KPC-2-Kp isolate (Table 1).
TABLE 1 Microbiologic Features and Patients’ Data in Study of Emergence of Fosfomycin Resistance
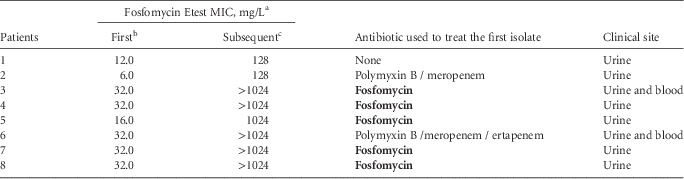
NOTE. All isolates were Klebsiella pneumoniae with carbapenem resistance via bla KPC-2. MIC, minimum inhibitory concentration.
a Considering ≤64 mg/L and >64 mg/L as susceptible and resistant, respectively.
b First urinary K. pneumoniae carbapenemase (KPC)–2-producing K. pneumoniae (KPC-2-Kp) isolate.
c Subsequent KPC-2-Kp isolate, considering a hospitalization period of >48 hours and <90 days following first isolation.
Although fosfomycin used to be primarily designated for urinary tract infection treatments, the lack of available antibiotics to treat carbapenemase producers has given fosfomycin an important adjuvant role, mainly in more severe infection cases. Despite that, according to results reported by Karageorgopoulos et alReference Karageorgopoulos, Miriagou, Tzouvelekis, Spyridopoulou and Daikos 5 as well as this present study where the emergence of fosfomycin resistance was reported just shortly after its introduction in clinical practices (mid-2014), fosfomycin resistance has become a concern because the endemic level reached by the KPC-2-Kp is due to its great ability to adapt and survive,Reference Perez 8 , Reference Perez and Dias 9 characteristics that came as an advantage mainly through antimicrobial selective pressure, strongly driven by the previous use, showing the need to establish a rigorous protocol for antimicrobial consumption.
The limitation of this study is due to the unknown genetic background information on which mechanism is involved to confer resistance to fosfomycin. So, further studies should be performed in order to detect possible genetic targets, such as fosA3 gene, that encode for a specific enzyme and which have recently resulted in a high resistance level to fosfomycin among European KPC-producers.Reference Mendes, Rodrigues and Pires 10
In conclusion, this study reports a significant emergence of fosfomycin resistance among KPC-2-Kp isolates in a relatively short period after the introduction of this antibiotic as an effective agent to treat KPC infections. Strict control practices are urgently required in order to avoid the resistance rate increase, regardless of the mechanism by which it occurs.
ACKNOWLEDGMENTS
Financial support. Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Potential conflicts of interest. The author reports no conflicts of interest relevant to this article.



