Introduction
Scarlet fever is a toxin-mediated manifestation of Streptococcus pyogenes (Group A Streptococcus, GAS) infection, responsible for considerable morbidity and mortality in the 19th and early 20th centuries, when its incidence was high [Reference Lamagni1, Reference You2]. Whilst the incidence of scarlet fever subsequently declined, there has been a global resurgence in the last 10–15 years, particularly in Asia during 2005–2015 [Reference Lamagni1–Reference Park4]. It is unclear whether the incidence of scarlet fever is increasing in Australia. To our knowledge, there have only been two studies which describe the epidemiology of scarlet fever in Australia [Reference Feeney5, Reference Walker6], both limited by small sample sizes (n < 15), short study periods and non-population-based designs. Further data are required to elucidate the epidemiology of scarlet fever in Australia. Such information would be useful to help guide public health policy and resource allocation, particularly given scarlet fever’s potential morbidity and global resurgence. The aim of this study was to examine the incidence, predictors and severity of scarlet fever in Victoria, Australia’s second most populous state, from 2007 to 2017. The study period coincides with the resurgence of scarlet fever in Asia, and focuses on the period prior to the coronavirus disease 2019 (COVID-19) pandemic. Since recent data from Australia and elsewhere suggests the epidemiology of GAS may have been disrupted during COVID-19 [Reference Abo7, Reference Li8], this pre-pandemic analysis provides a baseline, against which future incidence can be compared.
Methods
We performed a retrospective population-based analysis of routinely collected, administrative health data. We analyzed emergency department (ED) presentations, hospital admissions, and deaths with scarlet fever in Victoria, Australia using three linked datasets: the Victorian Emergency Minimum Dataset; the Victorian Admitted Episodes Dataset; and the National Cause of Death Unit Record File, from 1 January 2007 to 31 December 2017. Scarlet fever cases and deaths were identified by the International Classification of Disease (ICD-10) code, A38. We calculated annual incidence rates of scarlet fever using the Australian Bureau of Statistics’ mid-year estimated resident population data, and incidence rate ratios (IRR) and their 95% confidence intervals (CI) using Poisson regression. Multivariable analysis included sex, age group and year, generating adjusted IRRs (aIRR) and 95% CIs. We formally tested for seasonal variation using Edwards’ test (Supplementary Methods). Ethics approval was obtained from The University of Melbourne Human Research Ethics Committee (reference, 2021-12232-14388-3).
Results
From 2007 to 2017, there were 1 578 ED presentations and hospital admissions with scarlet fever identified in Victoria, Australia. Of these, 1 344 (85%) occurred in children aged <10 years and 882 (56%) were male (Table 1). Approximately one in four cases (374, 24%) were admitted to hospital, however, few (177, 11%) were admitted for two or more days (Table 1, Supplementary Results). There were no deaths with scarlet fever.
Table 1. Scarlet fever emergency department presentations and hospitalisations, Victoria, Australia, 2007–2017, by sex and age group

aIRR, adjusted incidence rate ratio (adjusting for sex, age group and year); CI, confidence interval; IRR, incidence rate ratio.
The annual incidence of scarlet fever was generally stable from 2007 to 2017 (Figure 1), ranging from 1.9 cases per 100 000 in 2012 and 2014 to 3.1 cases per 100 000 in 2016 (mean, 2.5 per 100 000). There was no significant trend in annual incidence across the study period, adjusting for sex and age group (aIRR, 0.99, 95%CI 0.98–1.01) (Table 1). Most cases (918, 57%) occurred during the winter and spring months, June–November (Figure 1), with Edwards’ test suggesting a seasonal tendency, albeit the goodness-of-fit was equivocal (Χ2 = 309.8, p < 0.001).
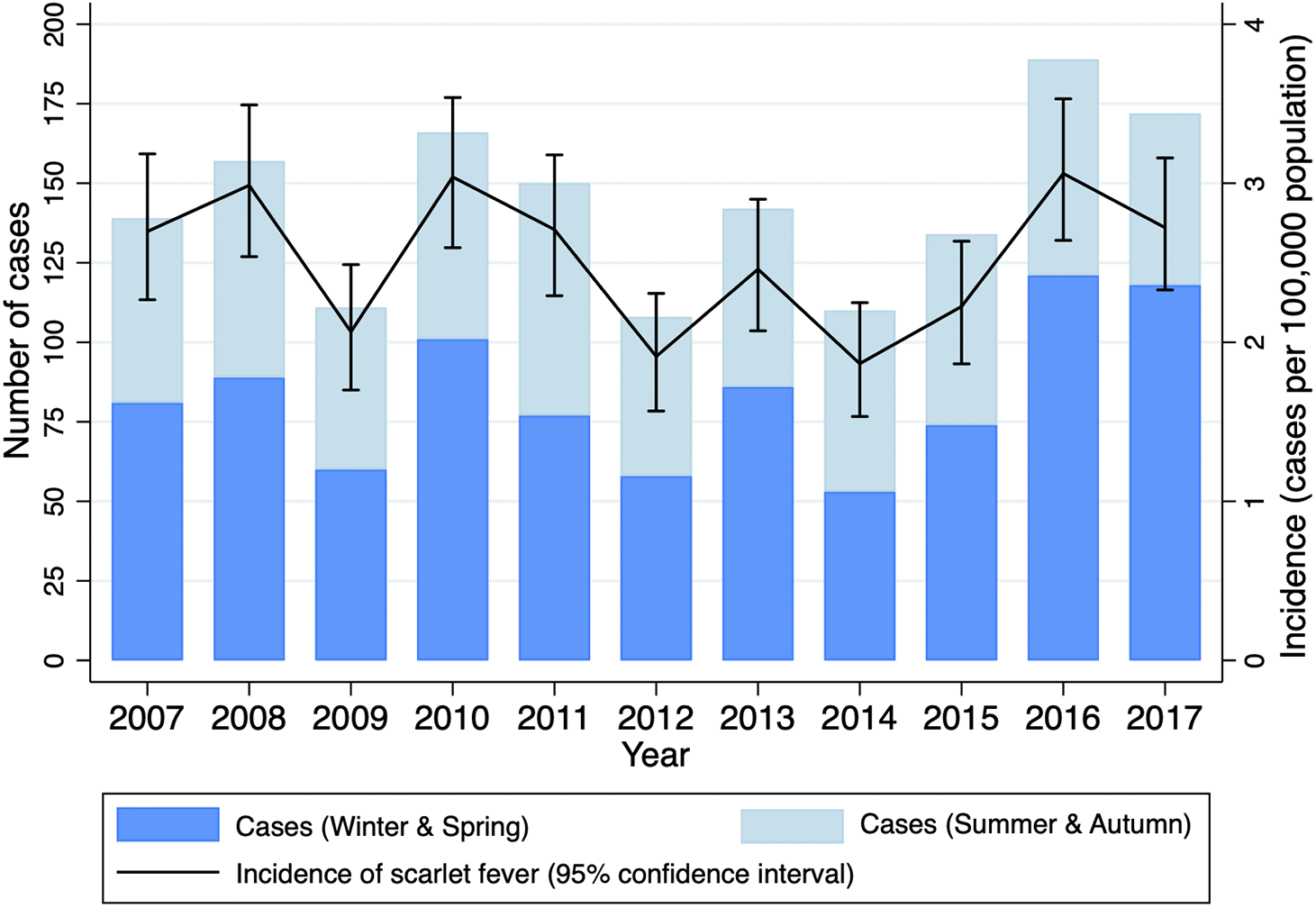
Figure 1. Annual scarlet fever hospital presentations (emergency department and hospitalization admissions), Victoria, Australia, 2007–2017.
The annual incidence of scarlet fever was 21% higher in males than females, adjusting for age group and year (aIRR, 1.21, 95%CI 1.09–1.34) (Table 1). Among males and females, there was no significant difference in the proportion of scarlet fever cases by age (Table 2, Supplementary Results). The proportion of males and females admitted to hospital, managed in a metropolitan or rural location, and their length of hospital stay were also similar. Annual incidence of scarlet fever was highest in those aged <5 years and 5–9 years (19.3 and 14.8 cases per 100 000, respectively) (Table 1).
Discussion
To our knowledge, this is the first longitudinal population-based study of scarlet fever in Australia. We found ED presentations and hospitalizations for scarlet fever in Victoria were stable from 2007 to 2017, ranging from 1.9 to 3.1 cases per 100 000. Whilst annual incidence rates did not increase, incidence in Victoria was similar to China, which experienced a scarlet fever outbreak during this time. The incidence of scarlet fever in China was 4.14 per 100 000 from 2011 to 2016, which was 2.6 times higher than during 2003–2010 prior to the outbreak when the incidence was 1.58 per 100 000 [Reference You2]. In contrast, scarlet fever notification rates in England and Wales were higher during the scarlet fever resurgence from 2013 to 2016, increasing from 8 to 33 per 100 000 [Reference Lamagni1].
Whilst our findings demonstrate incidence of ED presentations and hospitalizations for scarlet fever were stable in Victoria over a > 10-year period, additional information would be useful. Future studies could analyze scarlet fever presentations within Australian primary care registries, given most cases present to general practice alongside the ED [Reference Basetti9]. This could give a more accurate estimate of disease incidence in Victoria, as our analysis included only cases who presented to ED or were hospitalized. Furthermore, this study did not include microbiological analysis of scarlet fever cases. We were unable to retrieve data on the GAS serotype for scarlet fever cases (e.g., from throat swabs taken and analyzed) to investigate a potential association between particular GAS strains and scarlet fever.
In China, emm12 has been a common emm-type causing scarlet fever [Reference You2]. The finding of specific exotoxin prophage genes (such as ssa and speC) and antibiotic-resistant elements (such as macrolides and tetracyclines) amongst emm12 isolates could explain the potential selection and expansion of this lineage [Reference You2]. Alongside emm12, in the United Kingdom, other emm-types frequently associated with scarlet fever are emm1, emm3 and emm4 [Reference Lamagni1]. A new emm1 clonal lineage – M1UK – has been particularly linked with seasonal surges of scarlet fever and outbreaks of invasive GAS disease in the United Kingdom [Reference Lynskey10].
Interestingly, this same M1UK variant has recently become established in Australia [Reference Davies11], which may suggest Australia is at risk of a scarlet fever epidemic, as experienced in England, Wales, China and Hong Kong [Reference Lamagni1–Reference Lee, Cowling and Lau3], where scarlet fever is notifiable. Thus, clinicians and public health officials should remain vigilant for scarlet fever. Despite the clear epidemic risk, scarlet fever is not notifiable in Australia. To aid early detection and management of potential future outbreaks in Australia, scarlet fever could be made notifiable. However, since scarlet fever is more often a clinical, rather than laboratory diagnosis, alternatives such as adding scarlet fever to general practice-based or ED sentinel surveillance networks could also be considered.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268824001298.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
We thank Kathleen Bapaume and Tom Liu at the Centre for Victorian Data Linkage, Victorian Department of Health and Human Services, for providing the linked data.
Author contribution
Conceptualisation and design: S.P., P.T.C., K.B.G.; Data collection and analysis: S.P., P.T.C., K.B.G.; Data interpretation: S.P., P.T.C., K.B.G.; Writing – original draft: S.P.; Writing – editing and critical review: S.P., P.T.C., K.B.G.
Funding statement
K.B.G. receives salary support from a Medical Research Future Fund Investigator Grant (MRF1193727).
Competing interest
The authors declare no conflicts of interest.





