Sustained overeating and physical inactivity often leads to increased body weight. Despite numerous advances in dietary and lifestyle approaches, body weight regulation remains challenging. One major obstacle when adhering to an energy-restricted diet is to endure hunger, a predictor of success for long term weight loss and maintenance( Reference Drapeau, King and Hetherington 1 , Reference Rogers and Brunstrom 2 ).
Several promising approaches for satiety regulation have emerged, including the consumption of low energy density (ED) foods, increased intake of dietary fibre, and by manipulation of the food matrix, such as the physical form or viscosity of the meal( Reference Almiron-Roig, Palla and Guest 3 – Reference Rolls 5 ). Although several foods have been identified to possess some of these characteristics, few alternatives are available when replacing high ED foods in the diet without any major dietary modifications. An emerging novel food that appears to embody all three satiety approaches are low-energy foods made from a dietary fibre known as konjac glucomannan (KGM).
KGM is a highly viscous dietary fibre derived from the perennial plant Amorphophallus konjac and has a long history of use as a traditional food in Eastern Asia( Reference Chua, Baldwin and Hocking 6 ). At low concentrations, KGM is capable of forming a strong gel (KGM-gel) that is approximately 97 % water when in the presence of a coagulant, representing one of the lowest ED (approximately 0·25 kJ/g (0·06 kcal/g) foods available( Reference Parry 7 ). KGM-gel has a neutral taste and can be formed into various food shapes such as long, noodle-like strands popularly known as ‘shirataki noodles’. This can provide a potential method to reduce energy intake with minimal effort when replacing similar shaped foods that are high in ED without changing the volume or sensory characteristics of the meal. In addition, KGM is resistant to typical gastric digestion like most fibres due to the β-1,4-linkages within the fibre chain that cannot be hydrolysed by salivary or pancreatic amylase. Another feature of KGM-gels is its high firmness, which has been estimated to be between 0·8–1·6 N, which exceeds the strength typically exerted by the stomach during digestion( Reference Marciani, Gowland and Fillery-Travis 8 , Reference Vuksan, Rogovik and Jovanovski 9 ). This additional property results in KGM-gels being retained longer in the stomach, and may be a mechanism that independently promotes satiety responses to reduce energy intake.
Despite these favourable characteristics and increasing public interest for low-energy foods that are satiety-enhancing, no study to date has investigated the role of KGM-gel foods and its effect on energy intake and satiety in a controlled clinical setting. It is also unclear whether the energy deficit incurred from substituting high ED foods with KGM-gel foods will be compensated at a subsequent meal, abolishing its effectiveness as a potential weight management tool. Therefore, we sought to evaluate the effects of substituting KGM-gel shirataki noodles administrated at two levels in place of a high-carbohydrate preload of equal volume, but different energy contents, on subsequent food intake and subjective satiety in healthy individuals. We hypothesised that increasing KGM-gel noodles would lead to greater energy compensation in a dose-dependent manner at the subsequent meal due to the large differences in energy and macronutrient content between preloads.
Methods
Participants
Healthy participants were recruited into the study by advertisement flyers placed on the campus of the University of Toronto and in St. Michael’s Hospital, Toronto, Canada. Individuals who responded to the advertisements were interviewed by telephone to ensure they met the initial criteria for inclusion into the study: age 18–65 years, BMI between 18 and 25 kg/m2, no presence or known history of major diseases, not using prescription medication and/or natural health products during the study period, not pregnant or lactating, no known food allergies and weight stable for the past 2 months.
Potential participants meeting the initial criteria were invited to the clinic, where anthropometrics (height, weight, % body fat) were taken, and several questionnaires were completed regarding dietary and lifestyle regimens. All participants gave informed written consent before participating and conducted according to the Declaration of Helsinki. The study was approved by the St. Michael’s Hospital Research Ethics Board and was conducted at the Risk Factor Modification Centre, St. Michael’s Hospital (Toronto, Canada). The trial protocol was registered with ClinicalTrials.gov, identifier NCT01875627.
Design
In a randomised, single-blinded, controlled, dose–response, cross-over design, participants were administered one of three intervention preloads in a randomised sequence determined by a random number table generated by a statistician. Investigators, study personnel and statisticians were blinded to intervention preloads. Participants visited the clinic between 08.00 and 10.00 hours after a 10–12 h overnight fast on three separate occasions separated by a washout period of at least 2 d. Upon arrival at the clinic, anthropometric data were collected and participants were seated in a quiet, isolated area for the remainder of the visit. Participants were told to maintain their usual dietary and lifestyle regimens during the study period and this was assessed by a questionnaire. Baseline (0 min) satiety and symptoms were recorded and the preloads were served subsequently. Participants rated the palatability of the preload immediately after consumption and satiety and symptoms questionnaires were completed at 15, 30, 45, 60, 75 and 90 min after the first bite of the preload. Participants were given 15 min to consume the preload and water served. Participants were only allowed to consume the foods given to them at the clinic. Immediately after completing the satiety and symptoms questionnaires at 90 min, participants were offered an ad libitum dessert. Dessert was served in 150 g portions and participants were told to consume as much of the dessert as they desired until they felt comfortably full. Preload and dessert meals were served with ad libitum water in portions of 240 ml and the same amount of water was served with the respective meal during subsequent visits for each participant. All food and drinks served were weighed using a digital scale to the nearest 0·1 g before and after serving to determine the intake of food.
Study materials
Macronutrient composition and characteristics of the preloads are described in Table 1. The intervention preloads were the following: (1) cooked pasta noodles with no KGM-gel noodles (control); (2) substitution of half cooked pasta noodles with KGM-gel noodles (50-KGM); (3) complete substitution of cooked pasta noodles with KGM-gel noodles (100-KGM). Preloads were matched for volume and appearance, as they were mixed with the same quantity of pasta sauce before serving. All preloads were cooked and prepared on the same day as the clinical visit by a trained research personnel independent of the study to maintain blinding. The ad libitum dessert consisted of bite-sized (approximately 2·5 cm) pieces of hazelnut-flavoured wafer cookies (Quadratini Wafer Cookies; Loaker©) that contains 21·63 kJ/g (5·17 kcal/g) (48 % carbohydrate, 47 % fat, 5 % protein). All study materials were commercially available food products purchased from a local supermarket and were prepared in accordance with the instructions found on the manufacturer’s label.
Table 1 Composition and characteristics of the preloads
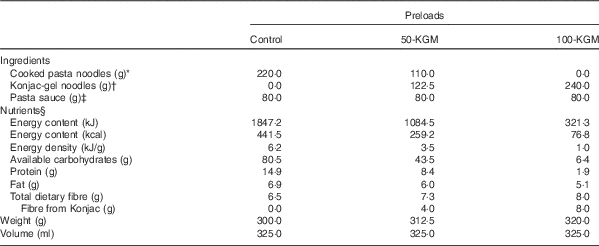
* Capellini; Barilla America Inc.
† Konjac Noodles; Wellbond Import Export Inc.
‡ Rosée Pasta Sauce; Irresistibles©.
§ Adapted from the nutrition facts table presented on the manufacturer’s packaging.
Measurements
Participants were asked to remove any heavy clothing before collecting anthropometrics. Body weight was collected using a digital scale (TANITA) to ensure no major changes in anthropometrics occurred between visits. If major changes occurred, the participant was excluded from the study. Height was measured using a wall mounted stadiometer.
Participants completed satiety questionnaires in the form of 100 mm visual analogue scales (VAS) assessing four subjective satiety measurements: desire to eat, hunger, fullness and prospective consumption. Each VAS were anchored on both sides with descriptors adapted from Blundell et al.( Reference Blundell, de Graaf and Hulshof 10 ). The satiety recorded before serving the preload were used as baseline (0 min) measurements and satiety after the first bite of the preload were recorded every 15 min thereafter until 90 min. Response of satiety to the preloads were presented as mean satiety and evaluated by calculating the total AUC after 90 min using the trapezoid method( Reference Ledikwe, Rolls and Smiciklas-Wright 11 ).
To investigate the efficiency and capacity of the preload to influence satiety, the satiety quotient (SQ) was calculated for each satiety measurement, except for sensations of fullness, at each postprandial time measured following the equation adapted from Green et al.( Reference Green, Delargy and Joanes 12 ): SQ (mm/kJ)=(baseline satiety (mm)−postprandial satiety (mm))/(energy content of preload (kJ)). Fullness used a reversed SQ where (postprandial satiety (mm)−baseline satiety (mm)) was used instead to facilitate the comparison between the other satiety measurements. A higher SQ for each satiety measurement would represent a greater satiety response and a lower SQ would represent a weaker satiety response because the SQ accounts for baseline satiety and the energy content of the preload.
Gastrointestinal symptoms of bloating, belching, diarrhoea, flatulence and nausea were reported by presence of absence of the symptom at each time interval satiety were measured. If present, severity was recorded using a 100mm VAS. VAS were anchored with the descriptors ‘low’ and ‘high’ on the left and right ends of the VAS, respectively.
Preload palatability was measured using a 100 mm VAS immediately after consumption of the preload anchored with the phrases ‘very unpalatable’ to ‘very palatable’ on the left and right ends of the VAS, respectively.
Statistical analysis
The primary outcome measured was energy compensation defined as the energy intake 90 min after preload administration. Total food intake (g and kJ), satiety (baseline, 90 min AUC and SQ), palatability ratings and gastrointestinal symptoms were evaluated as secondary outcomes. A composite score (CS) for satiety adapted from a previous study( Reference Vuksan, Panahi and Lyon 13 ) was calculated to reflect the overall effect of the preload on satiety using each satiety measurement: CS (mm)=(hunger+desire to eat+(100−fullness)+prospective consumption)/4.
Data are presented as means with their standard errors unless otherwise specified. All statistical procedures were performed using the Statistical Analysis System software package, University Edition (SAS Institute Inc.). Results were considered significant at P<0·05. Data normality was assessed visually and using the Shapiro-Wilk procedure. The effect of the interventions on satiety was assessed using a mixed model ANOVA (proc mixed; SAS) with preload, time, and time×preload interaction as fixed factors and participants as the repeated factor. Pairwise analyses were conducted to assess the differences between interventions and adjusted for multiple comparisons using the Tukey–Kramer method for multiple comparisons. Mean satiety, AUC for satiety, mean SQ, time to eat, palatability, subsequent energy intake and cumulative energy intake were assessed with a similar procedure without time and its interactive factor. For all satiety measures, baseline fasting (0 min) values were included as a covariate to adjust for the variability between intervention days and individuals. For symptoms, data were grouped by presence during the clinical visit and assessed using a χ 2 test.
The power calculation was based on a 628 kJ (150 kcal) difference between groups in a two-tailed test with a significance level of 0·05 and a power of 80 %. Based on a previous study of fibres and food intake, sixteen individuals were to be recruited into the study( Reference Samra and Anderson 14 ).
Results
Participants
In all, sixteen participants (twelve females: four males) with mean age of 26·0 (sd 11·8) years (range: 18–62 years) and BMI of 23·1 (sd 3·2) kg/m2 were enrolled in the study. All participants completed the study and were compliant with the study protocols. No significant changes in body weight or body fat occurred over the duration of the study (data not shown). Participants did not report any significant changes to diet or physical activity levels during the study period.
Energy intake
Despite significant energy differences of the preloads, energy intakes during dessert 90 min later were similar between interventions (Fig. 1, effect of treatment: P=0·71). Subsequent energy intake was 1534·7 (sem 142) kJ (366·8 (sem 34) kcal) for control, 1631·8 (sem 142) kJ (390·1 (sem 34) kcal) for 50-KGM and 1652·3 (sem 142) kJ (394·9 (sem 34) kcal) for 100-KGM and subsequent food intake was 70·3 (sem 7) g for control, 74·7 (sem 7) g for 50-KGM and 75·7 (sem 7) g for 100-KGM. No differences in subsequent energy intake (100-KGM v. control: 117·9 (150·2) kJ (28·2 (sem 35·9) kcal), P=0·72; 50-KGM v. control: 97·9 (150·2) kJ (23·4 (sem 35·9) kcal), P=0·79; 100-KGM v. 50-KGM: 20·0 (150·2) kJ (4·8 (sem 35·9) kcal), P=0·99) or food intake (100-KGM v. control: 5·4 (sem 6·9) g, P=0·72; 50-KGM v. control: 4·5 (sem 6·9) g, P=0·79; 100-KGM v. 50-KGM: 0·9 (sem 6·9) g, P=0·99) at 90 min was observed. Cumulative energy intake across the two meals (preload and dessert) remained significantly different for 50-KGM (−841 (sem 151) kJ (−201 (sem 36) kcal), P<0·01) and 100-KGM (−1761 (sem 151) kJ (−421 (sem 36) kcal), P<0·01) relative to control and when comparing 100-KGM to 50-KGM (−946·3 (sem 150·2) kJ (−219·8 (sem 35·9) kcal), P<0·01).
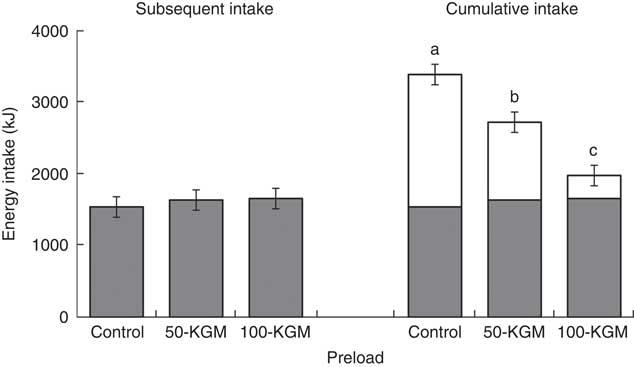
Fig. 1 Subsequent energy intake at 90 min after preload administration. Cumulative intake was defined as the total energy intake from the preload and the subsequent meal. Values are means with their standard errors represented by vertical bars. ![]() , Preload;
, Preload; ![]() , dessert; KGM, konjac glucomannan. a,b,c Preloads with unlike letters were significantly different (P<0·05, Tukey adjusted).
, dessert; KGM, konjac glucomannan. a,b,c Preloads with unlike letters were significantly different (P<0·05, Tukey adjusted).
Satiety
Mean satiety outcomes are presented in Table 2. A significant effect of time (P<0·001) and preload (P<0·001) was observed for all satiety measures, but no interaction effect was observed. Mean ratings for desire to eat were not significantly different across comparisons (100-KGM v. control: 8·5 (sem 4·0) mm, P=0·10; 50-KGM v. control: 0·1 (sem 4·0) mm, P=0·99; 100-KGM v. 50-KGM: 8·5 (sem 3·9), P=0·09). Mean ratings for hunger was significantly higher when comparing 100-KGM to control (10·0 (sem 3·9) mm, P=0·04) and not significantly different for other comparisons (100-KGM to 50-KGM: 9·0 (sem 3·7) mm, P=0·05; 50-KGM to control: 1·0 (sem 3·7) mm, P=0·96). Mean ratings for fullness were not significantly different across comparisons (100-KGM v. control: −8·7 (sem 4·1) mm, P=0·10; 50-KGM v. control: 1·4 (sem 4·1) mm, P=0·94; 100-KGM v. 50-KGM: −10·2 (sem 4·2), P=0·06). Mean ratings for prospective food consumption was significantly lower when comparing 50-KGM to 100-KGM (−9·1 (sem 3·4) mm, P=0·03) and not for other comparisons (100-KGM v. control: 7·4 (sem 3·4) mm, P=0·10; 50-KGM v. control: 1·7 (sem 3·5) mm, P=0·87). Mean CS were not significantly different across comparisons (100-KGM v. control: 8·2 (sem 3·7) mm, P=0·09; 50-KGM v. control: −0·4 (sem 3·7) mm, P=0·99; 100-KGM v. 50-KGM: 8·6 (sem 3·6), P=0·06).
Table 2 Mean ratings of satiety measurements over 90 min, palatability, and time to consume preload in sixteen healthy participants (Mean values with their standard errors)
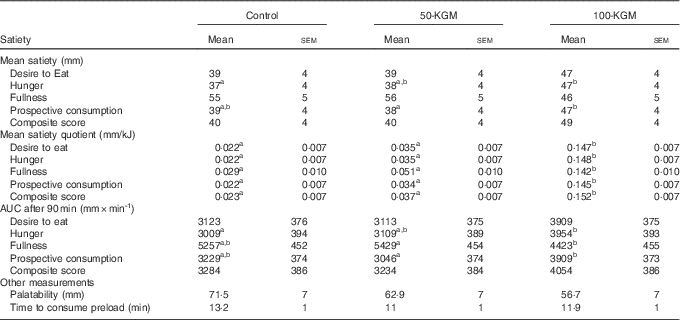
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
When comparing the AUC for satiety measurements, no significant differences were observed for desire to eat (100-KGM v. control: 786·4 (sem 374·9) mm×min−1, P=0·11; 50-KGM v. control: −9·9 (sem 374·6) mm×min−1, P=0·99; 100-KGM v. 50-KGM: 796·2 (sem 371·0) mm×min−1, P=0·10). For hunger, only 100-KGM compared with control increased hunger (100-KGM v. control: 945·6 (sem 358·8) mm×min−1, P=0·03; 50-KGM v. control: 100·1 (sem 346·9) mm×min−1, P=0·96; 100-KGM v. 50-KGM: 845·5 (sem 344·4) mm×min−1, P=0·05). Fullness decreased only when comparing 100-KGM to 50-KGM (100-KGM v. 50-KGM: −1005·5 (sem 394·7) mm×min−1, P=0·04; 100-KGM v. control: −833·8 (sem 389·2) mm×min−1, P=0·10; 50-KGM v. control: 171·7 (sem 384·0) mm×min−1, P=0·90) and prospective food consumption increased when comparing 100-KGM to 50-KGM (100-KGM v. 50-KGM: 862·4 (sem 321·7) mm×min−1, P=0·03; 100-KGM v. control: 679·6 (sem 320·3) mm×min−1, P=0·10; 50-KGM v. control: −182·7 (sem 323·7) mm×min−1, P=0·84). No significant differences were detected for CS AUC (100-KGM v. control: 769·6 (sem 348·3) mm×min−1, P=0·09; 50-KGM v. control: −49·9 (sem 343·3) mm×min−1, P=0·99; 100-KGM v. 50-KGM: 819·5 (sem 341·4) mm×min−1, P=0·06).
The satiating capacity of each preload was also assessed with the SQ, where changes in satiety ratings were expressed as a factor of energy intake in Table 2. The mean SQ was significantly higher for 100-KGM for all satiety measurements when compared with both 50-KGM and control preloads (P<0·0001) and no differences were detected among other measurements between preloads. For desire to eat, the differences in SQ were 0·125 (sem 0·0096) mm/kJ (P<0·01) for 100-KGM v. control, 0·0134 (sem 0·0096) mm/kJ (P=0·35) for 50-KGM v. control, and 0·112 (sem 0·0093) mm/kJ (P<0·01) for 100-KGM v. 50-KGM. For hunger, the differences in SQ were 0·126 (sem 0·010) mm/kJ (P<0·01) for 100-KGM v. control, 0·013 (sem 0·0098) mm/kJ (P=0·41) for 50-KGM v. control, and 0·113 (sem 0·0098) mm/kJ (P<0·01) for 100-KGM v. 50-KGM. For fullness, the differences in SQ were 0·113 (sem 0·012) mm/kJ (P<0·01) for 100-KGM v. control, 0·0218 (sem 0·012) mm/kJ (P=0·17) for 50-KGM v. control, and 0·0911 (sem 0·012) mm/kJ (P<0·01) for 100-KGM v. 50-KGM. For prospective food consumption, the differences in SQ were 0·123 (sem 0·0098) mm/kJ (P<0·01) for 100-KGM v. control, 0·0124 (sem 0·0098) mm/kJ (P=0·42) for 50-KGM v. control, and 0·111 (sem 0·0098) mm/kJ (P<0·01) for 100-KGM v. 50-KGM. For the CS, the differences in SQ were 0·129 (sem 0·0098) mm/kJ (P<0·01) for 100-KGM v. control, 0·0139 (sem 0·0098) mm/kJ (P=0·35) for 50-KGM v. control, and 0·115 (sem 0·0098) mm/kJ (P<0·01) for 100-KGM v. 50-KGM.
Other measures
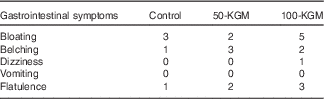
Palatability ratings between the preloads (100-KGM v. control: −14·8 (sem 8·3) mm, P=0·19; 50-KGM v. control: −8·6 (sem 8·3) mm, P=0·56; 100-KGM v. 50-KGM: −6·2 (sem 8·3) mm, P=0·73) and the time taken to consume the preloads (100-KGM v. control: −1·3 (sem 1·6) min, P=0·72; 50-KGM v. control: −2·1 (sem 1·6) min, P=0·41; 100-KGM v. 50-KGM: 0·8 (sem 1·6) min, P=0·87) were not significantly different (Table 2). All preload interventions were well tolerated. The frequency of gastrointestinal symptoms was not statistically different between preloads (Table 3).
Table 3 Presence of gastrointestinal symptoms over 90 min in sixteen healthy participants
Discussion
To our knowledge, the present study is the first to investigate substituting KGM-gel food analogues into a meal on satiety and subsequent food intake. KGM-gels are made by dispersing a small amount of KGM fibre into water and adding a mild alkalizing agent to induce gelation. As a result, the composition of KGM-gels is approximately 97 % water, giving it a very low ED. We hypothesised that substituting energy-dense pasta with KGM-gel noodles would lead to greater energy compensation in a dose-dependent manner at the subsequent meal due to the large differences in energy and macronutrient content between the preloads. Satiety was not different between the control and 50-KGM, but 100-KGM significantly increased hunger when compared with control and lowered fullness when compared with the 50-KGM. Contrary to our hypothesis, no differences in subsequent food intake at 90 min were detected amongst all preloads. This led to an overall reduction in cumulative energy intake across the treatment period.
One possible explanation for the comparable subsequent energy intake, despite the energy disparity between the preloads is the amount, type, and physical form of dietary fibre present among the preloads. Several reviews have suggested that increased fibre intake is associated with greater satiety and reduced energy intake when provided in an isoenergetic setting( Reference Clark and Slavin 15 – Reference Wanders, van den Borne and de Graaf 17 ). In particular, viscous soluble fibres appear to be the most effective as they can increase the viscosity of the digestive contents to promote satiety by lowering the gastric emptying rate and modifying nutrient kinetics( Reference Kristensen and Jensen 4 ). In a previous study using a similar preloading design, we demonstrated that a 5 g mixture of high viscosity fibres containing KGM in a powdered form did not affect satiety, but modestly reduced energy intake by 418 kJ (100 kcal) at 90 min compared with an insoluble fibre control when provided in energy-matched preloads( Reference Vuksan, Panahi and Lyon 13 ). In contrast, gelled fibres similar to those presently used may exert their satiating effects through different mechanisms, as the viscosity and gelation occurs outside of the stomach. Evidence from other gel-forming fibres, such as pectin and agar, suggest that fibre-gels can increase subjective ratings of fullness without reductions in subsequent energy intake( Reference Clegg and Shafat 18 , Reference Wanders, Feskens and Jonathan 19 ). However, the preloads used in other fibre-gel studies are often energy-matched; thus an energy-deficient fibre-gel such as that used presently may not produce the same satiety effect.
Matching for volume between the preloads may have also contributed to comparable energy intakes during the subsequent meal. Food volume has been observed to be an independent predictor of short-term energy intake in studies of similar design. Increasing meal volume leads to greater gastric distention that triggers afferent vagal signals that promote satiety( Reference Ello-Martin, Ledikwe and Rolls 20 ). This claim is supported by studies investigating satiety and food intake after ED manipulations in energy-matched preloads and from intragastric infusion studies that bypass oro-sensory cues of satiety( Reference Rolls, Bell and Waugh 21 – Reference Rolls and Roe 23 ). Although this may explain the comparable energy intakes, it is insufficient to explain the differences in satiety observed after 100-KGM.
Investigations utilising MRI have reported greater delays in gastric emptying by increasing the energy content of a meal by as little as 418 kJ (100 kcal), regardless of the volume administered( Reference Kwiatek, Menne and Steingoetter 24 ). Delays in gastric emptying are consistently associated with increased satiety and suggest that the low satiety response presently observed is driven by the lack of macronutrients rather than the volume of the preloads. This may explain the significantly lower satiety rating of the 100-KGM preload compared with the other preloads of approximately 8–10 mm, which is clinically meaningful for appetite regulation( Reference Blundell, de Graaf and Hulshof 10 ). In this context, it would be more appropriate to assess the satiating capacity of the preloads per energy intake than the absolute effect on satiety, as the KGM-gel noodles have almost no energy content and energy intake is the primary concern for weight management( Reference Green, Delargy and Joanes 12 ). The present study showed that the SQ, which assesses the change in satiety as a function of energy consumed, was significantly higher for 100-KGM than control or 50-KGM. This suggests that satiety increases at a faster rate for 100-KGM than the other preloads when energy intake is matched. Although the evidence on the clinical importance of the SQ is sparse, the SQ for fullness has been suggested to predict energy intake in free-living individuals( Reference Drapeau, King and Hetherington 1 , Reference Drapeau, Blundell and Gallant 25 ).
It has also been proposed that firm fibre-gels, such as KGM-gel, may possess unique properties that can assist in satiety and energy regulation. Marciani et al.( Reference Marciani, Gowland and Fillery-Travis 8 ) used MRI on agar fibre-gels of varying strengths and found that a firm gel exceeding 0·65 N in facture strength was retained in the stomach longer and was correlated with a greater feeling of fullness compared with fibre-gels of a lower strength. The authors concluded that 0·65 N likely exceed the force exerted during mechanical digestion, leading to incomplete gel digestion and greater retention within the stomach. This can increase gastric distention and is likely a unique property of certain fibre-gels as most foods are susceptible to acidic degradation, reducing firmness. Although the strength of the KGM-gels in the present study was not directly measured, data on commercially prepared KGM-gels report a strength ranging between 0·8–1·6 N, which may explain the lack of energy compensation( Reference Herranz, Tovar and Solo-de-Zaldívar 26 , Reference Luo, He and Lin 27 ). Alternatively, this high gel strength may have altered the oro-sensory attributes between the preloads, leading to greater oral exposure that affects satiety and food intake( Reference Clark and Slavin 15 , Reference Mela 28 ). However, this effect in the present study may be small as palatability and mean eating time between preloads were not significantly different.
As the energy intakes at the subsequent meal were similar, the energy deficit between the preloads was maintained when the cumulative energy intake was calculated (approximately 841 kJ (201 kcal) for 50-KGM and approximately 1761 kJ (421 kcal) for 100-KGM). Comparing across various preloading methods, the cumulative reductions observed between 100-KGM and control preloads were higher than those seen with viscous dietary fibre supplementation of higher meal volumes, or preloads with a similar volume and ED (approximately 1·26 kJ/g (0·3 kcal/g) in approximately 300 ml) achieved through fat reduction or increased vegetable consumption( Reference Vuksan, Panahi and Lyon 13 , Reference Williams, Roe and Rolls 29 ). In the form currently administered in the study, the noodle shape allows for discretionary replacement of a commonly consumed high-carbohydrate food to decrease mealtime energy intake. Taking into consideration that palatability was not significantly different between the preloads, KGM-gels may potentially be a dietary tool to reduce daily energy intake without affecting the type or amount of consumed, which may be a key factor to maintaining compliance in weight management diets.
Based on the present results, it is unclear whether the energetic deficit introduced from the preloads will be sustained beyond the subsequent meal. A reduction in energy intake introduced by low ED foods have been shown to be sustained over several days in healthy individuals( Reference Bell, Castellanos and Pelkman 30 ). Repeated consumption of fibre-gels may have a similar effect but available clinical evidence is sparse. One study supplemented pectin fibre-gels for 15 d and only observed a modest increase in fullness with no reduction in overall energy intake( Reference Wanders, Mars and Borgonjen-van den Berg 31 ). However, the pectin gel was energy matched with the control and was likely more susceptible to mechanical digestion, suggested by the low gel strength of approximately 0·36 N reported in the study. Alternatively, the dietary fibres found in fibre-gels may increase production of SCFA through fermentation by the gut microbiota, which can influence satiety hormones such as PYY and GLP-1( Reference Chambers, Morrison and Frost 32 ). As KGM-gels have been shown to be fermentable in vitro with a profile similar to the un-gelled form, KGM-gels have the potential for long term energy management given the fermentation profile and high gel strength( Reference Chen, Lin and Wang 33 – Reference Matsuura 35 ). Additional benefits of extended KGM consumption may include reductions in LDL-cholesterol, systolic BP, and improvements in glycaemic control in individuals with T2DM, metabolic syndrome and healthy individuals( Reference Jenkins, Jenkins and Wolever 36 – Reference Vuksan, Jenkins and Spadafora 39 ). However, these metabolic benefits have only been attributed to the viscous, powdered form and future studies should investigate whether these benefits extend to KGM-gels.
Several limitations to the present study should be acknowledged. First, the sample size calculation was based on a large difference in energy content (628 kJ (150 kcal)) as we hypothesised that individuals would compensate for the missing energy content from KGM-gels. As the study was only powered to assess this difference, detection of smaller differences and confidence in secondary outcomes, such as satiety and palatability, was limited by the small sample size of the study. Second, while the dessert ad libitum meal was chosen to represent a commonly consumed snack food, the sweet taste and high palatability may have promoted overconsumption and overrode satiety signals in the control and 50-KGM preload. Although a high palatability food has been observed to increase ad libitum energy intake beyond habitual amounts, this wider range of energy intake is suggested to be more sensitive to satiety manipulation and more applicable in a controlled setting( Reference Deighton, Frampton and Gonzalez 40 ). Likewise, in similarly palatable meals, difference in ad libitum energy intake between sweet and savoury foods appears to be negligible in healthy individuals, suggesting that the use of a dessert meal may not impact the present findings to a significant degree( Reference Griffioen-Roose, Mars and Finlayson 41 ). Third, a negative control was not used to determine ad libitum food intake in the absence of a preload(10). Although several studies have reported reduced ad libitum food intake after preloading, the addition of the preload energy content resulted in higher energy intake than the no preload condition. However, as the KGM-gel contributes negligible energy content to the preload, the total energy intake of the 100-KGM condition is unlikely to exceed a negative control. Lastly, information on dietary restraint was not collected, limiting the interpretation of the subsequent meal, as restrained eaters would be less likely to eat to satiation.
Conclusions
In conclusion, KGM-gel noodle substitution resulted in a dose–response reduction of cumulative energy intake without altering meal palatability. Due to the soluble fibre content, very low ED, and its ability to replace common high-carbohydrate foods such as pasta without changing meal volume, partial substitution of KGM-gels noodles holds great promise for satiety and food intake regulation and may potentially introduce a new tool for body weight regulation.
Acknowledgements
The authors would like to thank Johnny Fung from Wellbond Import Export Inc. for the donation of the konjac glucomannan study product.
V. V. currently holds grant support for dietary fibre and ginseng research from the Canadian Diabetes Association and for ginseng research from the National Institute of Horticultural & Herbal Science, RDA, Korea. Wellbond Import Export Inc. donated the study material. Wellbond Import Export Inc. had no role in the design, analysis or writing of this article.
E. J.: acquired the data, conducted the research, and analysed the data; F. A.-Y.: analysed the data and drafted the manuscript; V. V.: had primary responsibility for the final content; A. L. J., A. Z., H. V. T. H. and V. V.: critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
V. V. holds an American (no. 7,326,404 B2) and Canadian (no. 2,410,556) patent for use of a powdered viscous fibre blend in diabetes, metabolic syndrome and cholesterol lowering. At the time of the study, V. V. was a partial owner of Glycemic Index Laboratories (Toronto, ON., Canada) and has since retired from the organisation (April, 2015); A. L. J. is a VP and partial owner of Glycemic Index Laboratories, a clinical research organisation. F. A.-Y. is a contract research assistant for Glycemic Index Laboratories. All other authors have no conflicts of interest to declare.







