Flavonoids include a diverse group of over 6000 compounds(Reference Corradini, Foglia and Giansanti1). There are seven major classes of flavonoids in the human diet, including flavonols, flavan-3-ols, proanthocyanidins, flavones, flavanones, anthocyanidins and isoflavones(2–4). Major dietary sources of flavonoids include tea, fruit and vegetables, chocolate and red wine.
There is increasing evidence that dietary flavonoids generally, and flavonols specifically, contribute to cardiovascular health(Reference Kawaguchi, Matsumoto and Kumazawa5). A number of population studies have investigated the relationships of specific flavonoid classes with CVD risk. Early population studies that assessed flavonol and flavone intakes have indicated a significant reduction in CHD mortality with a higher flavonol intake. A high flavonol intake has previously been associated with a 20 % lower risk of fatal CHD(Reference Huxley and Neil6). More recent studies have investigated the relationships of all seven major classes of flavonoids with CVD outcomes(Reference Mink, Scrafford and Barraj7, Reference Mursu, Voutilainen and Nurmi8). This has been made possible by recent improvements to food composition databases, which have allowed the assessment of all seven major classes of flavonoids(2–4).
Tea is frequently the main source of flavonoids in the diet. It often contributes more than half of the total flavonoids. Tea is particularly rich in flavan-3-ols, but also provides a significant contribution to flavonols, and makes a less important contribution to other flavonoid classes: more than 40 % of flavonols and more than 90 % of flavan-3-ols in the diet(Reference Chun, Chung and Song9). Meta-analyses of population-based studies have indicated that higher tea consumption is associated with a lower risk of CVD(Reference Arab, Liu and Elashoff10, Reference Wang, Zhou and Wang11). The relationships of flavonols specifically derived from tea and non-tea sources with cardiovascular outcomes have yet to be directly explored.
Therefore, the primary objective of the present study was to explore the association of the habitual intake of flavonols from tea and non-tea sources with the risk of atherosclerotic vascular disease mortality in a population of elderly women. The relationships for the intake of other major classes of flavonoids were also investigated.
Subjects and methods
Participants
All participants were women originally recruited to a 5-year prospective, randomised, controlled trial of oral Ca supplements to prevent osteoporotic fractures(Reference Prince, Devine and Dhaliwal12). The women were then invited to take part in an observational follow-up study beginning in 2003: the Calcium Intake Fracture Outcome Age Related Extension Study. All women were older than 75 years at baseline (2003) for the present study. A total of 1063 participants had complete food-frequency and beverage intake data at baseline. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Human Ethics Committee of the University of Western Australia. Written informed consent was obtained from all participants.
Atherosclerotic vascular disease mortality
The primary outcome of interest was atherosclerotic vascular disease mortality. Atherosclerotic vascular disease death data were retrieved from the Western Australian Data Linkage System for each of the study participants from 2003 until 2008. Atherosclerotic events were defined using diagnosis codes from the International Classification of Diseases, Injuries and Causes of Death: Clinical Modification (ICD-9-CM)(13) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM)(14). These codes included IHD including myocardial infarction (ICD-9-CM codes 410–414 and ICD-10-AM codes I20–I25), heart failure (ICD-9-CM code 428 and ICD-10-AM code I50), cerebrovascular disease, excluding haemorrhage (ICD-9-CM codes 433–438 and ICD-10-AM codes I63–I69, G45.9), and peripheral arterial disease (ICD-9-CM codes 440–444 and ICD-10-AM codes I70–I74). The search for atherosclerotic vascular ICD codes included all available diagnostic information that comprised parts 1 and 2 of the death certificate and the principal diagnosis in the inpatient data. All diagnosis text fields from the death certificate were used to ascertain the cause(s) of recent deaths where these data were not yet available from the Western Australian Data Linkage System.
Baseline vascular disease risk assessment
Participants provided their previous medical history which was coded using the International Classification of Primary Care – Plus method(Reference Britt15), as described previously in Ivey et al. (Reference Ivey, Lewis and Hodgson16). Previous atherosclerotic vascular disease was determined using verified hospitalisations from 1980 to 2003 from the Western Australian Data Linkage System. The use of anti-hypertensive medication was also recorded. Because many participants were already on these medications, it was considered that these medications would give a better estimate of prevalent hypertension, a risk factor classically used in calculating cardiovascular risk(Reference D'Agostino, Vasan and Pencina17). Smoking status was coded as non-smoker or ex-smoker/current smoker if they had smoked more than one cigarette per d for more than 3 months at any time in their life.
Physical activity was assessed using a previously validated questionnaire in which the participants reported the time in hours of involvement in up to four sports, recreational activities and other forms of regular physical activity including walking, which were undertaken in the past 3 months. Energy expenditure (kJ/d) for these activities was calculated using published energy costs(Reference McArdle, Katch and Katch18, Reference Pollock, Wilmore and Fox19). Baseline weight was assessed using digital scales with participants wearing light clothes and no shoes. Baseline height was assessed using a stadiometer, and BMI was calculated in kg/m2.
Dietary assessment
A validated semi-quantitative FFQ developed by the Anti-Cancer Council of Victoria was used to assess the baseline (2003) dietary intake(Reference Allison, Amanda and Wendy20). The process of collection was identical, whereby a research assistant supervised the completion of the questionnaire in small groups. Food models, cups, spoons and charts for frequency were provided. Energy and nutrient intakes were estimated based on the frequency of consumption and an overall estimate of usual portion size(Reference Ireland, Jolley and Giles21). Participants also completed a beverage intake questionnaire that quantified habitual beverage consumption during the preceding year. Specifically, they reported average consumption over the past 12 months of the number of cups (250 ml) per d or week of tea and coffee.
Flavonoid intake
Estimates of the flavonoid content of foods in the FFQ and beverage questionnaire were derived from the Flavonoid 2.1(3), Isoflavone 2.0(4) and Proanthocyanidin(2) food content databases developed by the US Department of Agriculture. The method of computing the flavonoid content of foods was similar to that outlined in Mink et al. (Reference Mink, Scrafford and Barraj7). Specifically, for each food, we computed the sum of assessed flavonoids for each flavonoid class by summing the individual compounds of each flavonoid class, with the exception of isoflavones, where the total isoflavone value from the database was used.
The flavan-3-ol content of foods was considered to represent the average of total flavan-3-ol and proanthocyanidin monomer contents(Reference Mink, Scrafford and Barraj7). For foods where only the flavan-3-ol or proanthocyanidin monomer content was available, the single value provided was used to represent the flavan-3-ol content. The proanthocyanidin content of foods was calculated by summing the proanthocyanidin dimers, trimers, 4–6mers, 7–10mers and polymers.
Where multiple varieties of a food listed in the FFQ were reported in the databases, the average flavonoid content of all similar varieties was computed, consistent with the descriptors used in the FFQ output. Foods in the FFQ that were not in the flavonoid databases were assumed to contain no flavonoids.
Intakes of flavonoid classes (in mg/d) were calculated by multiplying the estimated intake (g edible portion/d) from the FFQ and beverage questionnaire, with the flavonoid class content (mg/g edible portion) of each food item on the questionnaire.
Statistics
Before commencing statistical analysis, a pre-specified analytical protocol was produced. SAS (version 9; SAS Institute, Inc.) was used to identify and categorise the mortality data from the Western Australian Data Linkage System. SPSS (version 20; IBM) was then used for all further analyses. OR and 95 % CI for atherosclerotic vascular disease death were obtained using the binary logistic regression of flavonoid intake by standard deviation scores and tertiles of consumption of each flavonoid class.
In the present study, three models were used: unadjusted; age- and energy-adjusted; multivariate-adjusted. This included pre-specified baseline risk factors significantly different in ANOVA and χ2 test stratified by 5-year atherosclerotic vascular disease mortality, including age, energy expended in physical activity, previous atherosclerotic vascular disease, previous diabetes and history of smoking. The multivariate analysis included 1008 participants due to missing data for one or more of the atherosclerotic vascular disease risk factors for fifty-five participants.
Stepwise logistic regression of flavonoid class intake standard deviation score and atherosclerotic vascular disease mortality was performed. Multivariable candidate variables included age, energy intake, BMI, previous atherosclerotic vascular disease, energy expended in physical activity, previous diabetes, anti-hypertensive medication use, history of smoking and intakes of saturated fat, fibre, protein, starch, vitamin C and alcohol at baseline. Sensitivity analysis was performed by repeating logistic regression analysis in participants without previous atherosclerotic vascular disease and diabetes at baseline.
Results
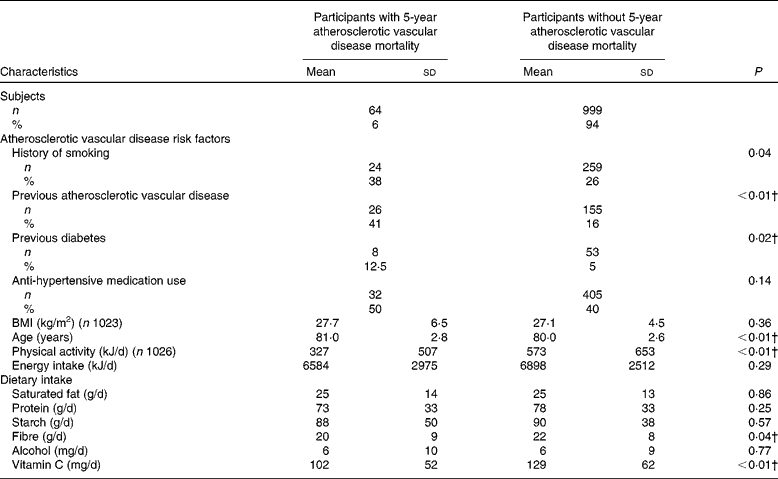
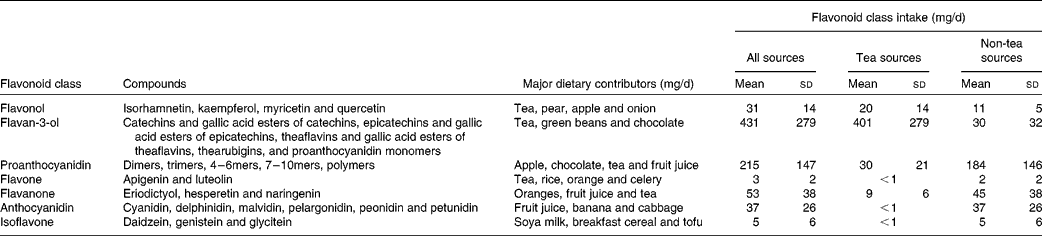
Over a 5-year period, sixty-four women died from atherosclerotic vascular disease. Participants who died from atherosclerotic vascular disease were more likely to have smoked and have a history of atherosclerotic vascular disease and diabetes at baseline (Table 1). The mean total intakes of individual flavonoids, classified according to the chemical structure, from tea and non-tea sources, are shown in Table 2. The mean tea consumption was 2·9 (sd 2·0) cups/d.
Table 1 Baseline characteristics of the cohort stratified by atherosclerotic vascular disease mortality* (Mean values and standard deviations; number of subjects and percentages)

* Using ANOVA or χ2 test where appropriate (n 1063).
† P< 0·05.
Table 2 Baseline flavonoid class intake according to dietary source (Mean values and standard deviations, n 1063)

All-source flavonoid consumption
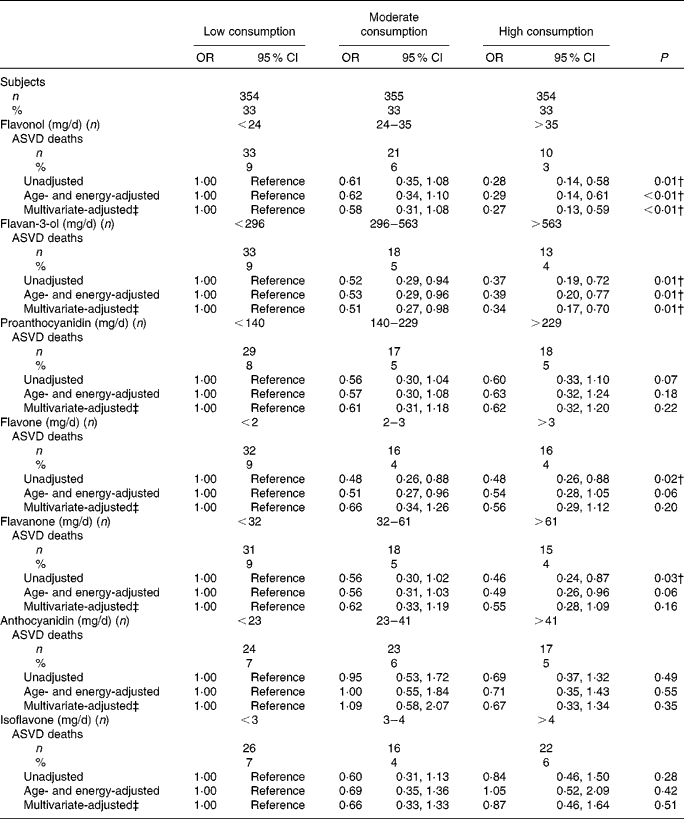
In the unadjusted and fully adjusted models, the risk of atherosclerotic vascular disease mortality was significantly negatively associated with the intake of all-source flavonol (multivariate-adjusted OR 0·54, 95 % CI 0·39, 0·74 per sd), flavan-3-ol (multivariate-adjusted OR 0·62, 95 % CI 0·46, 0·85 per sd), flavone (multivariate-adjusted OR 0·68, 95 % CI 0·48, 0·96 per sd) and flavanone (multivariate-adjusted OR 0·70, 95 % CI 0·50, 0·98 per sd), but not with proanthocyanidin, anthocyanidin and isoflavone intakes (P>0·05).
These relationships were explored by dividing the population according to tertiles of the major flavonoid classes (Table 3). The mortality rate in the highest tertile of all-source flavonol intake was 3 %, compared with 9 % in the lowest tertile of consumption. A similar relationship was observed across the tertiles of all-source flavan-3-ol intake.
Table 3 Relationship between total flavonoid class intake groups and 5-year atherosclerotic vascular disease (ASVD) mortality* (Odds ratios and 95 % confidence intervals, n 1063)

* From logistic regression models.
† P< 0·05.
‡ Multivariate-adjusted model adjusted for age, previous CVD, previous diabetes, energy expended in physical activity and history of smoking.
Consumption of tea and non-tea flavonoids
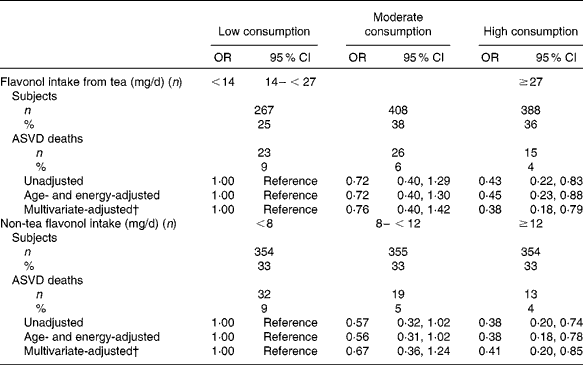
Tea contributed 59 % of total flavonoid intake, but was a major contributor to only two classes of flavonoids: flavonols (65 %) and flavan-3-ols (93 %) (Table 2). The intake of flavonols derived from tea and non-tea sources was associated with a significantly reduced risk of atherosclerotic vascular disease mortality (multivariate-adjusted OR 0·63, 95 % CI 0·46, 0·86 per sd and multivariate-adjusted OR 0·58, 95 % CI 0·41, 0·81 per sd, respectively). These relationships were explored by dividing the population according to the tertiles of consumption (Table 4). Participants in the highest tertile of flavonol intake from either tea or non-tea sources had a significantly lower risk of atherosclerotic vascular disease mortality compared with those in the lowest tertile. The intake of flavonols from tea and non-tea sources was not significantly correlated (r − 0·02, P= 0·48).
Table 4 Relationship between tea and non-tea flavonol intake groups and 5-year atherosclerotic vascular disease (ASVD) mortality* (Odds ratios and 95 % confidence intervals, n 1063)

* From logistic regression models.
† Multivariate-adjusted model adjusted for age, previous CVD, previous diabetes, energy expended in physical activity and history of smoking.
Non-tea flavan-3-ol intake was not significantly associated with the risk (multivariate-adjusted OR) of atherosclerotic vascular disease mortality (multivariate-adjusted OR 0·89, 95 % CI 0·64, 1·26 per sd).
Individual dietary flavonols
To investigate the components of the relationship between flavonol intake and atherosclerotic vascular disease mortality, we repeated logistic regression analysis using the major flavonol compounds: quercetin (mean 18 (sd 8) mg/d), myricetin (mean 6 (sd 4) mg/d), and kaempferol (mean 7 (sd 4) mg/d). Tea contributed 56 % of total quercetin intake, and contributed over 70 % of daily myricetin and kaempferol intakes. Higher total quercetin (multivariate-adjusted OR 0·52, 95 % CI 0·37, 0·71 per sd), myricetin (multivariate-adjusted OR 0·59, 95 % CI 0·43, 0·81 per sd) and kaempferol (multivariate-adjusted OR 0·58, 95 % CI 0·42, 0·79 per sd) intakes were significantly associated with a reduced risk of atherosclerotic vascular disease mortality. A similar relationship was observed with the consumption of quercetin (multivariate-adjusted OR 0·58, 95 % CI 0·42, 0·82 per sd) from non-tea sources.
Dietary confounders
To account for diet-related potential confounders, a stepwise logistic regression model of atherosclerotic vascular disease mortality including all the baseline atherosclerotic vascular disease risk factors and dietary variables, outlined in Table 1, was performed. For all-source flavonol intake, the most parsimonious model consisted of flavonol intake standard deviation score (P< 0·01), previous atherosclerotic vascular disease (P< 0·01) and age (P< 0·01). The most parsimonious model for non-tea flavonol consumption consisted of non-tea flavonol intake standard deviation score (P< 0·01), previous diabetes (P= 0·02), previous atherosclerotic vascular disease (P< 0·01) and age (P= 0·01). The most parsimonious model for flavonol intake from tea sources consisted of flavonol intake from tea (P< 0·01), previous atherosclerotic vascular disease (P< 0·01), age (P< 0·01), vitamin C intake (P< 0·01) and previous diabetes (P= 0·05).
For all-source flavan-3-ol intake, the most parsimonious model consisted of flavan-3-ol intake standard deviation score (P< 0·01), previous atherosclerotic vascular disease (P< 0·01), age (P< 0·01), vitamin C intake (P= 0·01) and previous diabetes (P= 0·05).
Sensitivity analysis
Multivariate sensitivity analysis was performed after excluding the participants with previous atherosclerotic vascular disease and/or diabetes at baseline (n 839; thirty-three (4 %) deaths from atherosclerotic vascular disease). In this analysis, consumption of both all-source flavonols and non-tea flavonols remained significantly associated with mortality risk (data not shown). Although the estimates of risk reduction were similar for most of the other flavonoid classes, the relationships were attenuated such that they were no longer significant. This is likely to be due to the reduced number of deaths in this population.
Discussion
A higher consumption of flavonols from all, tea and non-tea sources and flavan-3-ols from all sources was associated with a reduced risk of atherosclerotic vascular disease mortality. These relationships remained after adjustment for baseline atherosclerotic vascular disease risk factors, and were independent of dietary factors. These results are consistent with the benefit of flavonols and flavan-3-ols on cardiovascular health.
Participants with habitual flavonol consumption of ≥ 36 mg/d had a 72 % lower risk of atherosclerotic vascular disease mortality than low flavonol consumers. All three major dietary flavonols contributed to this association. A number of population studies over the past decade have now investigated flavonol intake in relation to cardiovascular outcomes(Reference Mursu, Voutilainen and Nurmi8, Reference Keli, Hertog and Feskens22). These studies have indicated that a high flavonol intake is related to a lower risk of CVD(Reference Huxley and Neil6).
The recent availability of improved food composition databases has allowed the investigation of the relationships of all seven classes of flavonoids with cardiovascular outcomes. We found that participants with high flavan-3-ol consumption had a 59 % lower risk of atherosclerotic vascular disease mortality. However, in many populations, it is difficult to explore this relationship independent of the benefits of tea consumption, which often supplies almost all of the flavan-3-ols in the diet(3).
To our knowledge, this is the first study to investigate the relationship between tea and non-tea flavonoid sources and atherosclerotic vascular disease mortality. The association of flavonol intake with atherosclerotic vascular disease mortality remained when excluding tea-derived flavonols from the analysis. We cannot rule out the collinearity between non-tea food sources of flavonols, such as apples, and dietary and lifestyle factors linked with CVD. However, because consumption of non-tea flavonols was not correlated with tea intake in our cohort, it appears that the cardiovascular benefits of flavonol consumption are independent of any benefits ascribable to tea, and that flavonols may contribute to the cardiovascular benefits of tea consumption. The benefits of non-tea flavonols are also supported by the results of randomised controlled trials that have assessed the effects of flavonols or flavonol-rich foods on mechanisms and risk factors associated with CVD(Reference Perez-Vizcaino, Duarte and Andriantsitohaina23–Reference Edwards, Lyon and Litwin25).
We did not observe a relationship between non-tea flavan-3-ols and mortality risk. However, it is still possible that flavan-3-ols contribute to cardiovascular health. In the present population of older women, 93 % of flavan-3-ol intake was derived from tea sources. It is therefore difficult to dissociate flavan-3-ol consumption from the consumption of tea in this population, and the ability to observe a relationship with non-tea flavan-3-ol intake is limited. There is indirect evidence that flavan-3-ols from non-tea sources may contribute to cardiovascular health. We(Reference Lewis, Prince and Zhu26) and others(Reference Buitrago-Lopez, Sanderson and Johnson27) have demonstrated an inverse association of chocolate intake with cardiovascular outcomes. The main flavonoids present in chocolate are flavan-3-ols and proanthocyanidins(Reference Steinberg, Bearden and Keen28).
The present results suggesting that flavonols and flavan-3-ols contribute to cardiovascular health are further supported by the results of randomised controlled trials investigating potential mechanisms and pathways. There is now strong evidence that flavonols, flavan-3-ols, and foods and beverages rich in these compounds, including tea(Reference Ras, Zock and Draijer29), chocolate/cocoa(Reference Hooper, Kroon and Rimm30) and apples(Reference Bondonno, Yang and Croft31), can improve endothelial function and augment NO status(Reference Loke, Hodgson and Proudfoot32, Reference Schroeter, Heiss and Balzer33). There is also evidence that these effects result in lowered blood pressure(Reference Egert, Bosy-Westphal and Seiberl24, Reference Edwards, Lyon and Litwin25, Reference Brown, Lane and Coverly34). Although less robust, there is also evidence that these compounds can influence inflammation, oxidative stress and platelet function(Reference Loke, Hodgson, Croft and Fraga35). We did not observe consistent associations for the other flavonoid classes. This may be due to the limited intake of particular flavonoid classes and structural differences between the different flavonoid classes. Apparently, a minor structural difference in flavonoids can have a large impact on their bioactivity(Reference Loke, Proudfoot and Stewart36).
It should be noted that the causality of the relationship between flavonol consumption and atherosclerotic vascular disease mortality cannot be established due to the observational nature of the study. Also, despite the inclusion of baseline, dietary and lifestyle risk factors into the statistical models, residual or unmeasured confounders cannot be ruled out. Specifically, the potential impact of the collinearity of flavonoid intake with other potential dietary confounders such as Na and K cannot be quantified, and, as such, further investigations of this relationship are warranted. Identification of causality is further limited by the complexity of flavonol compounds and the variability of the flavonol content of foods. In particular, the database used for the estimation of the flavonoid content of foods is based on US data. As such, the regional variation of the flavonoid content of foods was not accounted for in the present investigation. However, the strength of the association is such that despite these factors, the association remains significant even after adjustment for baseline, dietary and lifestyle risk factors.
In this cohort of elderly women, total flavonol and flavan-3-ol intakes were associated with a reduced risk of atherosclerotic vascular disease mortality. This provides further support for the suggestion that flavonols and flavan-3-ols can contribute to cardiovascular health. The cardioprotective benefits of flavonols appear to be independent of the benefits ascribable to tea consumption, suggesting that a habitual diet high in flavonols may play a role in stroke and coronary artery disease prevention. Ultimately, in order to make public health recommendations regarding flavonol intake, further observational and intervention trials are necessary to establish the clinical benefits of flavonol consumption, independent of tea.
Acknowledgements
The present study was supported by research grants from the Healthway Health Promotion Foundation of Western Australia, the Sir Charles Gairdner Hospital Research Advisory Committee and by the project grants 254627, 303169 and 572604 from the National Health and Medical Research Council of Australia. The salary of J. R. L. is supported by a Raine Medical Research Foundation Priming Grant. J. M. H. acknowledges the support of a National Health and Medical Research Council Senior Research Fellowship. None of these funding agencies had any input into any aspect of the design and management of the present study.
The authors' responsibilities were as follows: K. L. I., J. M. H., J. R. L. and R. L. P. designed the research, analysed the data and wrote the manuscript.
There are no conflicts of interest reported.