Choline was recognised by the Institute of Medicine as an essential nutrient in 1998( 1 ). The need for choline increases during periods of rapid growth and development including pregnancy and lactation( Reference Zeisel and da Costa 2 ), as it plays a vital role in fetal development, particularly in brain development( Reference Craciunescu, Albright and Mar 3 – Reference Zeisel 10 ). Choline is transferred by placental transporters( Reference Molloy, Mills and Cox 11 ) with umbilical cord concentrations of choline being approximately three times higher than maternal plasma concentrations( Reference Molloy, Mills and Cox 11 ) and newborn infants have higher serum free choline concentrations compared with their mothers( Reference Ozarda, Uncu and Ulus 12 ). In a rat model, pregnancy has been found to diminish hepatic choline stores compared with those in non-pregnant controls( Reference Zeisel, Mar and Zhou 13 ). When fed a choline-deficient diet, lactating rats have been found to exhibit greater depletion of hepatic choline metabolites compared with lactating animals fed a control diet, suggesting greater sensitivity to choline deficiency during lactation( Reference Zeisel, Mar and Zhou 13 ). Dietary choline intake has been indicated to influence milk composition in a rodent model( Reference Holmes-McNary, Cheng and Mar 14 ). In humans, large amounts of choline are present in breast milk( Reference Holmes-McNary, Cheng and Mar 14 ) and lost from maternal stores through breast-feeding( Reference Ilcol, Ozbek and Hamurtekin 15 ).
Due to the lack of human dietary studies, there are currently no estimated human requirement values for choline, and the Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes( 1 ) has determined adequate intake (AI) values of choline during pregnancy and lactation to be 450 and 550 mg/d, respectively. The AI has been established for total choline, which may comprise six forms of choline including free (unesterified) choline, phosphocholine, glycerophosphocholine, phosphatidylcholine (PC), lysophosphatidylcholine and sphingomyelin( Reference Zeisel, Mar and Howe 16 ). Research in rodents suggests that different forms of choline may be absorbed and metabolised differently( Reference Cheng, Holmes-McNary and Mar 17 ).
Several studies have estimated dietary choline intake in women( Reference Mygind, Evans and Peddie 18 – Reference Yonemori, Lim and Koga 20 ); however, there are a limited number of studies that have estimated dietary choline intake in pregnant and lactating women, in whom choline requirement is increased. A case–control cohort study of pregnant women in the USA has investigated the association between maternal dietary choline intake and infant neural tube defects and found that consuming >290 mg/d of choline is associated with a decreased risk of neural tube defects( Reference Shaw, Carmichael and Yang 4 ). Higher dietary choline intake has been found to be associated with increased use of choline as a methyl donor, which is essential for several fetal development processes that involve DNA methylation( Reference Yan, Jiang and West 21 ).
Currently, the United States Department of Agriculture (USDA) Database for the Choline Content of Common Foods Release 2 is the only available food composition database, containing data on 634 foods, that can be used to estimate choline intake from dietary records( Reference Patterson, Bhagwat and Williams 22 ). The aims of the present study were to estimate dietary choline intake in a cohort of pregnant and lactating women in Alberta, Canada, to identify major food sources of choline and to characterise the contribution of some frequently consumed food sources of choline to overall daily choline intake. The contribution of egg and milk consumption to choline intake was further studied in this population. Eggs are one of the richest dietary sources of choline containing approximately 130 mg of choline per medium (50 g) egg, mainly in the form of PC. Although milk is not a rich source of choline (16 (sd 2) mg of total choline per 100 g of milk, varying depending on milk fat percentage) compared with eggs, milk is consumed in significant quantities by the majority of pregnant( Reference Mannion, Gray-Donald and Koski 23 ) and lactating women( Reference Mannion, Gray-Donald and Johnson-Down 24 ) in North America and therefore could represent a major food source of choline.
Participants and methods
Participants and study design
The Alberta Pregnancy Outcomes and Nutrition (APrON) study is a Canadian cohort study of women and infants that enrolled women during their first or second trimester of pregnancy( Reference Kaplan, Giesbrecht and Leung 25 ). The included participants were pregnant women aged >16 years, at < 27 weeks of gestation, and living in or near Edmonton or Calgary. Participants were excluded if they planned to move outside the region during the time of the study or if they were unable to answer questions in English. Dietary intake information for the first 600 women enrolled in the APrON study was available at the time the present study was conducted. The sample size of approximately 600 participants is a convenience sample size that includes those for whom data were available at the time of writing this manuscript. Women were recruited from Edmonton and Calgary (Alberta, Canada) and surrounding areas between June 2009 and June 2010 through media advertisements and physicians' offices. Women were recruited early in pregnancy and interviewed during each trimester and at 3 months postpartum. Women who were recruited at ≤ 13 weeks of gestation were assessed once during each trimester. Women recruited at 14–27 weeks of gestation were assessed only in the second and third trimesters. Further analysis was conducted at 3 months postpartum (lactation period). Detailed description of the overall study design, recruitment process and data collection methods has been published previously by Kaplan et al. ( Reference Kaplan, Giesbrecht and Leung 25 ). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Alberta Health Research Ethics Biomedical Panel and the University of Calgary Health Research Ethics Board. Written informed consent was obtained from all subjects.
Estimation of choline intake during pregnancy and lactation
Dietary choline intake during each trimester of pregnancy and at 3 months postpartum was estimated by conducting 24 h dietary intake recall interviews. At each study visit, women were asked to describe in detail the quantity and type of food and dietary supplements consumed in the previous 24 h. These 24 h dietary intake recalls were conducted in face-to-face interviews by trained nutrition education research assistants using a ‘multiple-pass method’( Reference Conway, Ingwersen and Vinyard 26 ). Food models were used to help women estimate portion sizes and probes including details regarding cooking method and food brand names were used. Data collected during the 24 h dietary intake recall interviews were entered into Food Processor Standard Query Language (ESHA Research) to estimate macronutrient intake. All the output from the Food Processor was checked for completeness by research assistants who reviewed the data for entry errors, which were corrected by referring back to original paper copies of the 24 h dietary intake recalls. The data were further checked for completeness and cleaned based on outliers in energy intake, and 24 h recalls in which the participants reported intakes >14 654 kJ/d (3500 kcal/d) and < 3349 kJ/d (800 kcal/d) were checked for entry errors to ensure that correct dietary intake data had been entered. If these data were correct, outliers were removed from the analysis, as has been mentioned while discussing the exclusion criteria of the study.
A comprehensive choline database (Alberta database) was developed for use with the 24 h dietary intake recall data to estimate the choline content of foods consumed by the APrON participants. This database contained information on total choline content in foods, as well as on the five most common dietary forms of choline (free choline, glycerophosphocholine, phosphocholine, PC and sphingomyelin) and betaine. The Alberta database was developed by entering the available choline values for food items from the USDA Database for the Choline Content of Common Foods Release 2 (634 foods) by matching the nutrient database number used in the USDA choline database and that used in the USDA National Nutrient Database for Standard Reference( Reference Shaw, Carmichael and Yang 4 , Reference Patterson, Bhagwat and Williams 22 ). Foods not included in the USDA choline database were substituted with nutritionally comparable foods, with substitutions being made based on similar energy and appearance. Overall, 2576 substitutions were made and added to the Alberta database. Recipes were constructed for foods with multiple food items where choline composition data were available for individual ingredients. In total, 105 new recipes were added to the database. In addition, data for twenty-six new foods were added to the database through analysis by liquid chromatography–tandem MS (LC–MS/MS), as described by Xiong et al. ( Reference Xiong, Zhao and Goruk 27 , Reference Zhao, Xiong and Curtis 28 ), including several varieties of pulses (chickpeas, black beans and lima beans), shellfish and seafood (shrimp). Samples were analysed by LC–MS/MS using an Agilent 1200 series LC system (Agilent Technologies) coupled to a 3200 QSTAR mass spectrometer (AB Sciex). Foods that were predicted to contain negligible amounts of choline (e.g. water, hard candies and beverage syrups) were not included in the database. The Alberta database that was developed included choline content values for 2707 foods that were consumed by the APrON participants. Foods that had no appropriate substitution, or for which a recipe could not be formulated, were not included in the analysis (e.g. almond milk, rice milk, hemp hearts and bee pollen). The accuracy of the database in automatically estimating choline content was validated by a comparison of total choline intake values obtained using the automated database and those estimated using manual calculations. Choline intake for eighteen participants selected at random was estimated using 24 h dietary intake recalls and the difference was found to be non-significant using a paired t test. Dietary choline intake was estimated for each trimester of pregnancy and 3 months postpartum (lactation). The number of participants included in the analysis was determined based on the availability of dietary intake information. Every participant who completed a visit at each time point did not complete a 24 h dietary intake recall and in some cases entry of 24 h dietary intake recall data into Food Processor had not been made and therefore these were listed as missing from the analysis of choline intake. We then examined 24 h dietary intake recalls for the representativeness of intake. This was done by excluding from the analysis any recalls where estimated energy, fibre or choline intake was ± 3 standard deviations from the mean. This represented a small number of recalls, and the number is identified as ‘excluded’ in Fig. 1.
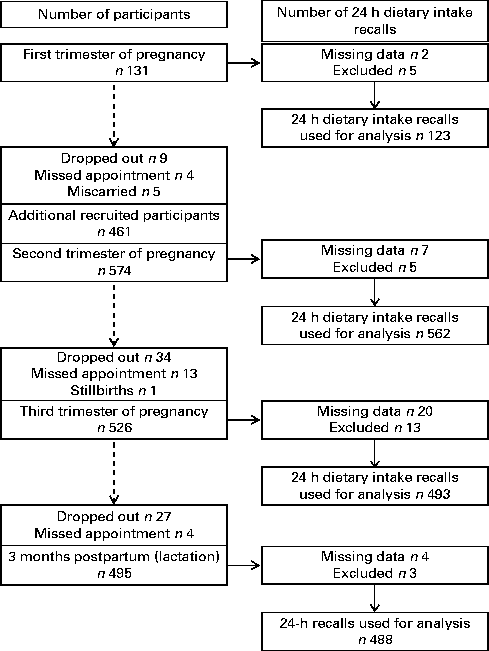
Fig. 1 Study participants enrolled and included in the analysis at each time point.
Estimation of choline supplement intake during pregnancy and lactation
Maternal supplement intake was estimated using a questionnaire that was administered through interviews at visits during each trimester of pregnancy and 3 months postpartum, as described previously( Reference Gomez, Field and Olstad 29 , Reference Leung, Kaplan and Field 30 ).
Determination of major food sources of total dietary choline
To determine important food sources of choline, foods were classified based on the twenty-two food categories listed in the USDA database( Reference Patterson, Bhagwat and Williams 22 ). The ‘Dairy and Eggs’ category was further subdivided into ‘Dairy’ and ‘Egg’ to specifically determine the contribution that foods in each of these groups made to choline intake. The ‘Dairy’ category included all dairy products such as butter, cheese, milk and yogurt. The ‘Meat’ category included the following: ‘lamb, veal and game’; ‘sausages and luncheon meats’; ‘pork and pork products’; ‘beef and beef products’. A total of twenty food categories were used to determine the contribution made by food categories to total choline intake. For each time point, the total amount of choline consumed from foods in each category was reported as the percentage of total dietary choline( Reference Mygind, Evans and Peddie 18 , Reference Cho, Zeisel and Jacques 19 , Reference Subar, Krebs-Smith and Cook 31 ). The ranking of the food categories that contributed to total choline intake was consistent across all the trimesters of pregnancy; therefore, only data obtained during the second trimester of pregnancy and lactation are reported.
Estimation of the contribution of egg and milk consumption to choline intake during pregnancy and lactation
Egg and milk intakes were determined for each trimester of pregnancy, but only those in the second trimester were chosen to represent the intakes during pregnancy. There was no significant difference in milk and egg intakes during each trimester of pregnancy, and similar results were obtained in the first and third trimesters. The second trimester was used to determine egg and milk intakes due to the largest sample size, thereby having greater statistical power.
The contribution of daily egg consumption to choline intake was estimated using reported egg intake from 24 h dietary intake recall data collected during the second trimester of pregnancy and lactation. Based on egg intake, the participants were grouped into two categories: egg consumers (women who reported consuming at least one egg, as a separate food, in a day) and non-egg consumers (women who reported not consuming at least one egg in a day). Total choline intake, choline intake from choline-containing moieties and the proportion of participants meeting the AI recommendation at each time point were compared between the two categories.
The contribution of fluid milk consumption to daily choline intake during the second trimester of pregnancy and during lactation was assessed using 24 h dietary intake recalls. Milk consumers were participants who reported consuming any amount of fluid milk (as a beverage, in cold cereal or in coffee/tea) in a 24 h recall period. Milk consumption included whole milk, 2 % milk, 1 % milk and skimmed/non-fat milk. This analysis included only data on the contribution of fluid milk intake and not those on the contribution made by the intake of any other form of dairy products. The contribution of servings of milk to choline intake was assessed from the total daily reported intake of fluid milk from the 24 h dietary intake recall data that were collected in the second trimester of pregnancy and lactation. The frequency of milk consumption was categorised as follows: < 250 ml/d (includes women who did not report consuming any milk in the 24 h recall period); 250 ml to < 500 ml/d; ≥ 500 ml/d.
Statistical analyses
All data are presented as means and standard deviations, unless otherwise indicated. All data were tested and found to be normally distributed. Differences in choline intake based on egg and milk consumption during pregnancy and lactation were assessed using a two-tailed t test or one-way ANOVA with Bonferroni corrections where appropriate. Differences in choline intake between the milk consumption groups were assessed using one-way ANOVA with a Bonferroni correction or a Kruskal–Wallis non-parametric test and Wilcoxon rank-sum test as appropriate. Multinomial logistic regression was performed to determine the likelihood of meeting the AI recommendation based on egg or milk consumption. Data were analysed using STATA version 11 (StataCorp LP), with a P value < 0·05 being considered to be statistically significant for all analyses.
Results
Population characteristics, enrolment and analysis at each time point
The population characteristics of the cohort are given in Table 1. The number of participants enrolled and included in the analysis at each time point is summarised in Fig. 1. The majority of subjects were recruited during their second trimester of pregnancy. During the 3-month postpartum visit, dietary intake information was available for 488 of the 495 women who attended their clinic visit. At 3 months postpartum, 90·5 % of the participants (n 410) reported breast-feeding their infants (54 % exclusively breast-fed), as reported previously( Reference Jessri, Farmer and Maximova 32 ). Participants for whom 24 h dietary intake data were missing or who were excluded from the analysis were aged 31·4 (sd 4·3) years, had a BMI of 22·2 (sd 3·4) kg/m2, 62 % were Caucasian and 45 % had a high family income ( ≥ $100 000/year). The characteristics of the participants for whom dietary intake data were not available (missing) or who were excluded from the analysis of choline intake did not differ from those of the participants who were included.
Table 1 Characteristics of women enrolled in the first cohort of the APrON (Alberta Pregnancy Outcomes and Nutrition) study (Number of participants and percentages)

* Baseline characteristic data collected at the time of study participant enrolment.
† The percentage of each characteristic based on available data does not include the percentage of unknown values, for which data were not collected or obtained from the participants.
‡ Pre-pregnancy BMI was assessed based on self-reported pre-pregnancy weight (kg) and height (m). BMI was categorised using the WHO classifications for underweight ( < 18·5 kg/m2), normal weight (18·5–24·9 kg/m2), overweight (25·0–29·9 kg/m2) and obese ( ≥ 30 kg/m2)( 50 ).
§ Weight gain during pregnancy was assessed using calculated pre-pregnancy BMI and total weight gain during pregnancy (third-trimester visit). Weight gain was classified as inadequate, adequate and excessive based on the recommended range of total weight gain by BMI category from the Canadian Gestational Weight Gain Recommendations( 51 ).
Total choline intake, choline requirements and supplement intake
Before estimating dietary choline intake, the accuracy of the automated choline database for estimating choline intake was validated. Mean total choline intake values obtained using the automated database (378 (sd 194) mg/d) were compared with those obtained using manual calculation (358 (sd 199) mg/d), P= 0·11.
The mean intake values of total choline and choline-containing moieties across pregnancy and lactation are given in Table 2. The estimated daily choline intake value was 340 (sd 148), 349 (sd 154) and 353 (sd 144) mg/d in the first, second and third trimesters, respectively, with 23 % of the participants meeting the AI recommendation for choline intake (450 mg/d) across pregnancy. During lactation, the mean total choline intake value was 346 (sd 151) mg/d and 11 % of the participants met the AI recommendation of 550 mg/d. The major form of choline in the diet of the APrON women was PC, which contributed 48 (sd 2) % of the total estimated choline in pregnancy and lactation (Table 2).
Table 2 Estimated daily intake values of total choline, choline-containing moieties and betaine obtained using 24 h dietary intake recall data across pregnancy and lactation (3 months postpartum) in the APrON (Alberta Pregnancy Outcomes and Nutrition) cohort (Mean values and standard deviations)

AI, adequate intake
A total of thirty-six women reported consuming a supplement that contained choline, at least once during the study period. Choline bitartrate was the most common form of supplemental choline (n 35), providing a mean of 70 (sd 44) mg choline bitartrate (29 (sd 18) mg of choline) and was present in the prenatal multivitamin or B complex vitamin consumed by the participants. The consumption of a PC supplement providing 420 mg of PC (58 mg of choline) during the second and third trimesters of pregnancy was reported by one woman. Supplement intake was included in the estimation of total choline intake (Table 2). However, due to the limited number of participants consuming supplemental choline and the small amounts of choline provided, considering the intake of supplements did not significantly alter the proportion of participants meeting the daily intake recommendations when compared with considering only that of dietary sources. Women in the lowest quintile of dietary choline ingested approximately half of the AI amount for choline in pregnancy or lactation. Only women in the highest quintile met or slightly exceeded the AI recommendation during pregnancy and lactation.
Food sources of choline
Major food categories contributing to total choline intake during pregnancy and lactation are listed in Table 3. The three food categories that made the largest contributions to choline intake in the cohort were dairy products, eggs and meat and accounted for approximately 50 % of total dietary choline. Furthermore, seven other food categories (poultry, vegetables, baked products, fruits, legumes, finfish and shellfish, and mixed dishes (pregnancy) or fast foods (lactation)) contributed 35 % to total choline intake. The remaining ten food groups (not listed) included beverages, snacks, nuts, seeds, fats and oils, which together contributed 15 % to choline intake during pregnancy and lactation.
Table 3 Most commonly reported food categories* contributing to total dietary choline intake during pregnancy and lactation in the APrON (Alberta Pregnancy Outcomes and Nutrition) cohort
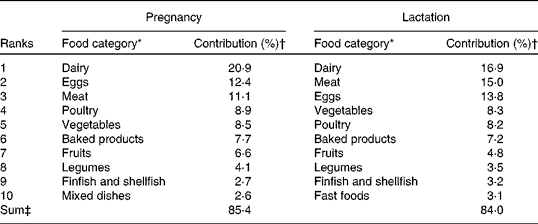
* Food categories are based on the United States Department of Agriculture Database for the Choline Content of Common Foods Release 2. Food categories include Dairy (butter, cheese, milk and yogurt), Eggs (egg and egg products), Meat (lamb, veal and game, sausages and luncheon meats, pork and pork products, and beef and beef products), Poultry (chicken and turkey), Vegetables (vegetables and vegetable products), Baked products (bagels, bread, crackers and muffins), Fruits (fruits and fruit juices including bananas, orange juice, oranges, berries and apples), Legumes (peanuts, pulses and soya products), Finfish and shellfish (crab, salmon and tuna), Mixed dishes (stew, chilli and lasagne), and Fast foods. The remaining food categories (not shown) include Sugars and sweets, Soups, sauces and gravies, Beverages, Cereal grains, Pastas and snacks, Breakfast cereals, Snacks, Nut and seed products, Fats and oils, and Spices and herbs.
† The percentage of contribution was calculated by summing the total amount of choline consumed for each food category and dividing by total choline from all food categories for all the participants. These values were then multiplied by 100 to generate the percentage of contribution to total dietary choline intake by each food category( Reference Mygind, Evans and Peddie 18 , Reference Cho, Zeisel and Jacques 19 , Reference Subar, Krebs-Smith and Cook 31 ) at each time point. Only data obtained during second trimester of pregnancy and lactation are reported.
‡ Sum represents the total percentage of contribution to total choline intake by the top ten food categories consumed during pregnancy and lactation.
Contribution of egg and fluid milk consumption to daily choline intake
During pregnancy and lactation, egg consumption in a 24 h recall period was found to significantly affect total choline intake per day. During the second trimester of pregnancy and lactation, consuming at least one egg, as a single food item, was found to be associated with significantly (P< 0·001) higher daily intake of choline (Table 4). This increase in daily choline intake was due to a significantly higher content of PC and sphingomyelin in the diet (Table 4). During pregnancy, women who reported consuming one or more eggs during the 24 h recall period were found to more likely meet daily choline intake recommendations compared with those who did not consume eggs (OR 8·1, 95 % CI 5·2, 12·6; Table 4). During lactation, women who reported consuming one or more eggs during a 24 h recall period were found to more likely meet daily choline intake recommendations compared with women who did not consume eggs (OR 10·8, 95 % CI 5·7, 20·7).
Table 4 Association between egg consumption and mean intake of total choline and choline-containing moieties in a 24 h recall period during pregnancy and lactation in the APrON (Alberta Pregnancy Outcomes and Nutrition) cohort (Mean values and standard deviations)
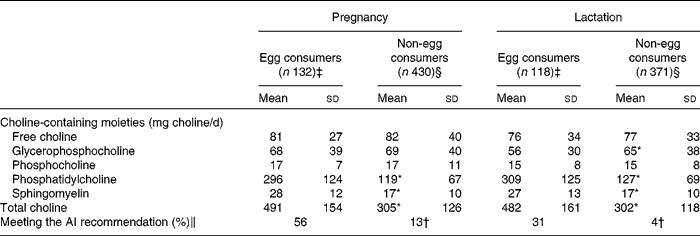
AI, adequate intake.
* Mean values were significantly different from those of egg consumers (P< 0·05). Independent-sample t tests were used to test differences in choline intake between the groups.
† Mean values were significantly different from those of egg consumers (P< 0·05). A χ2 test was used to test for differences in the proportion of participants meeting the daily recommendations in each group.
‡ Defined as participants who reported consuming at least one egg (as a single food item) in a 24 h recall period. Egg consumption included those prepared using various methods (fried, poached, scrambled and boiled).
§ Defined as participants who did not report consuming an egg in a 24 h recall period.
∥ Percentage meeting the AI recommendation for the 24 h recall day for each time point, 450 mg/d during pregnancy and 550 mg/d during lactation.
During pregnancy and lactation, milk consumption in a 24 h period was found to be associated with significantly (P< 0·05) higher daily choline intake (Table 5). This higher daily choline intake in a 24 h recall period in participants reporting milk consumption was due to a significantly higher intake of free choline, glycerophosphocholine and phosphocholine in the diet (Table 5).
Table 5 Intake values of total choline and choline-containing moieties among women who consumed or did not consume liquid milk during pregnancy and lactation in the APrON (Alberta Pregnancy Outcomes and Nutrition) cohort (Mean values and standard deviations)
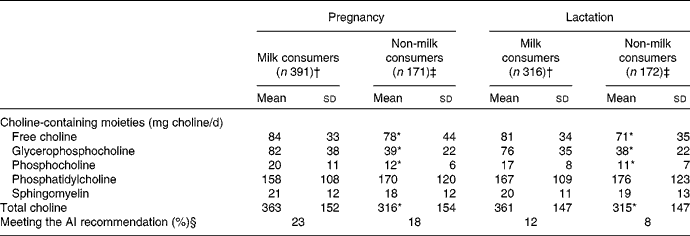
AI, adequate intake.
* Mean values were significantly different from those of milk consumers (P< 0·05). Independent-sample t tests were used to test differences in choline intake between the groups.
† Defined as participants who reported consuming any amount of fluid milk (as a beverage, in cold cereal or in coffee/tea) in a 24 h recall period. Milk consumption included whole milk, 2 % milk, 1 % milk and skimmed/non-fat milk.
‡ Defined as participants who did not report consuming milk in a 24 h recall period.
§ Percentage meeting the AI recommendation for the 24 h recall day for each time point, 450 mg/d during pregnancy and 550 mg/d during lactation.
In Fig. 2, the contribution of servings of fluid milk to total daily choline intake and glycerophosphocholine intake recorded in 24 h dietary intake recall interviews during the second trimester of pregnancy is shown (n 562). Compared with women who did not consume milk and who consumed < 250 ml of milk, those who consumed ≥ 500 ml of milk, equal to two servings of milk per day( 33 , 34 ), were found to 2·8 times more likely meet daily choline intake recommendations (95 % CI 1·7, 4·8). This higher total choline intake was due to a significantly higher intake of glycerophosphocholine (P< 0·05). During lactation, women who consumed ≥ 500 ml of milk, equal to two servings of milk in the 24 h recall period, were found to 3·1 times more likely meet daily choline intake recommendations (95 % CI 1·5, 6·2).
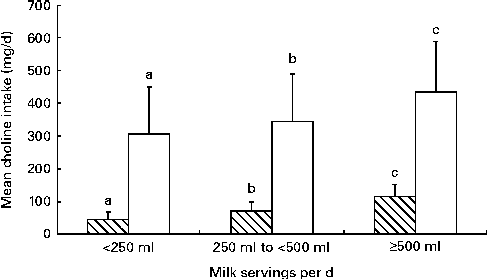
Fig. 2 Contribution of servings of fluid milk to total daily choline intake and glycerophosphocholine intake recorded in 24 h dietary intake recall interviews during the second trimester of pregnancy (n 562: < 250 ml, n 261; 250–500 ml, n 170; ≥ 500 ml, n 131). a,b,cMean values within either the total choline □ or glycerophosphocholine ![]() datasets with unlike letters were significantly different (P< 0·05).
datasets with unlike letters were significantly different (P< 0·05).
Discussion
The increased demand for choline during pregnancy and lactation has been demonstrated( Reference Zeisel and Niculescu 9 , Reference Zeisel 35 ). We found that the mean choline intake was below the current daily recommendations and less than one-quarter of the participants met the choline intake recommendations in pregnancy and only 10 % of women met the increased intake recommendations during the lactation period. Although there was no difference in total choline intake during pregnancy and lactation, the proportion of women meeting the daily intake recommendations during lactation was less than half that during pregnancy. This is due to the change in recommendation, which is 100 mg/d higher during lactation than during pregnancy. The percentage of participants meeting the daily intake recommendations was consistent with that reported by other studies in non-pregnant women( Reference Yonemori, Lim and Koga 20 ) and pregnant women( Reference Shaw, Carmichael and Yang 4 ). In a previous study in adults with non-alcoholic fatty liver disease( Reference Guerrerio, Colvin and Schwartz 36 ), the authors defined less than one-half of the AI to be deficient intake. Using this criterion, approximately 20 and 40 % of the participants, during pregnancy and lactation, respectively, were found to be consuming a diet possibly deficient in choline. The proportion of participants meeting the choline intake recommendations must be interpreted with caution as the current recommendations for choline intake may not be appropriate. The AI values were established based on a metabolic study conducted in men, which were then extrapolated to non-pregnant and then pregnant and lactating women. Despite considering the increased requirement for choline during pregnancy and lactation, there are concerns as to the accuracy of the methods used to determine AI( Reference Yonemori, Lim and Koga 20 ). Nevertheless, individuals with dietary choline intake significantly below the recommendation levels (e.g. less than one-half of the recommended daily intake) may be at the highest risk of choline insufficiency. Implications for women's and their infants' health requires further study to determine whether the current recommendation is appropriate for healthy pregnant and lactating women. An intervention study examining the effects of dietary choline during pregnancy has reported that choline metabolism is altered during pregnancy, as the circulation of choline-derived methyl donors (betaine) is much lower than that in non-pregnant women. Higher maternal choline intake (930 mg/d) has been found to increase the use of choline as a methyl donor compared with low choline intake (480 mg/d)( Reference Yan, Jiang and West 21 ). This could have major health implications for the fetus and infant as a supply of methyl groups is essential for development, as they are necessary to support cell proliferation and DNA methylation.
Our findings on total choline intake during pregnancy and lactation are consistent with those of a study of a cohort of primarily Caucasian (42·7 %) pregnant women in California (n 864) carried out between 1989 and 1991( Reference Shaw, Carmichael and Yang 4 ). The main aim of that study was to examine the association between neural tube defects and dietary choline intake, and choline intake was assessed using an interviewer-administered FFQ( Reference Shaw, Carmichael and Yang 4 ). However, choline intake estimates obtained in the present study are higher than much of what has been reported for the female population enrolled in the Atherosclerosis Risk in Communities (ARIC) study( Reference Bidulescu, Chambless and Siega-Riz 37 ) and a cohort of women of child-bearing age in New Zealand( Reference Mygind, Evans and Peddie 18 ). The ARIC study and the research carried out in New Zealand did not comment on the socio-economic status (SES) of the study populations. In Canada, food insecurity and low SES have been found to be associated with other nutrient inadequacies( Reference Dachner, Ricciuto and Kirkpatrick 38 – Reference Kirkpatrick and Tarasuk 40 ). However, over half of the participants of the APrON study had a high family income ( ≥ $100 000/year), which limits the applicability of the results of the present study to populations with lower incomes. As the participants of the present study appear to be of high SES, we may hypothesise that they may have higher dietary choline intake compared with a population of lower SES. An additional limitation of the present study is the lack of ethnic diversity (87 % Caucasian) within the large study population. The intakes of both choline( Reference Yonemori, Lim and Koga 20 ) and milk and milk products( Reference Fulgoni, Nicholls and Reed 41 ) have been shown to vary based on ethnicity. Hence, the interpretation of the results of the present study may not be directly applicable to multiethnic populations, including data reported previously( Reference Yonemori, Lim and Koga 20 ).
The methodologies used to collect dietary intake data can also influence the estimation of choline intake. A study conducted by Fischer et al. (42) has suggested that under-reporting during the use of self-administered dietary questionnaires may lead to the underestimation of dietary choline intake. In the present study, the 24 h dietary intake recalls were conducted in face-to-face interviews by trained study personnel using food models, which may have helped reduce the chance of missing foods or underestimating portion sizes. In the present study, the problem of portion sizes is supported by the energy-adjusted choline intakes across pregnancy (0·041 mg/kJ) and lactation (0·043 mg/kJ) that are consistent with previous reports in a New Zealand population of women of reproductive age( Reference Mygind, Evans and Peddie 18 ) and a population of healthy individuals in the USA( Reference Fischer, Scearce and Mar 42 ), in whom the absolute choline intake was lower.
In the present study population, 50 % of choline in the diet was provided by dairy products, eggs and meat. Previous studies( Reference Mygind, Evans and Peddie 18 , Reference Cho, Zeisel and Jacques 19 , Reference Bidulescu, Chambless and Siega-Riz 37 ) have also shown these food categories to be among the top contributors to dietary choline. The consumption of eggs or milk significantly increased the daily choline intake. Eggs are one of the richest sources of choline( Reference Patterson, Bhagwat and Williams 22 ), with an average whole medium egg containing approximately 130 mg choline, according to the USDA database( Reference Patterson, Bhagwat and Williams 22 ) and Y-Y Zhao, Y Xiong and JM Curtis (unpublished results). The difference in total choline intake between egg and non-egg consumers was due to a significantly higher intake of PC and sphingomyelin. The results of the most recent National Health and Nutritional Examination Survey III (NHANES III) have revealed that nearly 20 % of the American population are egg consumers (subjects who reported consuming an egg or egg product in the 24 h recall period)( Reference Song and Kerver 43 ). The egg intake of Albertan women during pregnancy and lactation was consistent with this estimation, with 23 and 24 %, respectively, reporting egg intake in their 24 h recalls. The NHANES III study reported that egg consumers had greater daily nutrient intake (except vitamin B6 and fibre and nor did they report choline intake) compared with non-egg consumers( Reference Song and Kerver 43 ). In addition to being a rich source of choline, eggs are a good source of vitamins B12, D, and A, folate, P; an excellent source of riboflavin, vitamin K, and Se( Reference Applegate 44 ); and an inexpensive source of high-quality protein( Reference Herron and Fernandez 45 ). In the present study, for participants in whom choline intake was suboptimal, egg consumption was found to be associated with higher mean total choline intake, and for those in whom egg consumption was not highly prevalent, the promotion of egg consumption may assist in increasing daily choline intake during a period when choline demand is high. The promotion of egg consumption may also confer other nutritional benefits during a period of rapid growth and development.
Milk has also been reported to be a major contributor to total choline intake in other populations( Reference Mygind, Evans and Peddie 18 , Reference Cho, Zeisel and Jacques 19 , Reference Bidulescu, Chambless and Siega-Riz 37 ). Milk is not considered a rich source of choline, containing an average of 16 (sd 2) mg of choline per 100 g. However, milk was consumed by the majority of participants, with 69 % reporting fluid milk consumption in their 24 h recalls. Women who consumed ≥ 500 ml of milk/d within a day had a higher mean daily choline intake compared with women who consumed < 250 ml of milk (including no milk) the day before their study visit. According to Eating Well with Canada's Food Guide( 33 ) and the USDA MyPlate( 34 ), 250 ml of milk (preferably skimmed or low fat) equal one serving of milk and alternatives, indicating that two servings of milk (500 ml) per day can affect choline intake. The difference in total choline intake was due to a higher dietary content of free choline, glycerophosphocholine and phosphocholine. Similar to egg consumption, milk consumption has been shown to positively affect nutrient intake and is an important source of vitamin D, Ca, K and Mg in the American diet( Reference Subar, Krebs-Smith and Cook 31 , Reference Rice, Quann and Miller 46 – Reference Nicklas, O'Neil and Fulgoni 48 ).
Although the present study utilised a large Canadian cohort to determine dietary choline intake and the contribution of egg and milk consumption to intake, there are various limitations that should be discussed. Despite a reasonable sample size, the present study population was fairly homogeneous in ethnicity, education and SES, all of which influence nutritional status, and therefore the results of the study may not be directly applied to populations with differing characteristics. The use of an AI as a recommendation may also affect result interpretation. Due to limited data, the establishment of AI for choline during pregnancy and lactation was done based on a metabolic study carried out in men. In the present study, dietary intake was estimated using one 24 h recall and was not validated with a biomarker. Unfortunately, there is no good biomarker of choline status as plasma choline is homeostatically regulated( Reference Fischer, daCosta and Kwock 49 ). There are also limitations associated with the collection method used, as 24 h recalls may not fully capture dietary patterns as they only represent dietary intake in a 24 h period. This could present a problem for foods that are less frequently consumed, including eggs, which may not be consumed every day, but a person may consume them on a regular basis. Not considering eggs present in other dishes and not including lysophosphatidylcholine from eggs (absent in the USDA database) may have resulted in an underestimation of the contribution of eggs to total choline intake in the present study population. Similarly, the present study assessed only the importance of consuming fluid milk and not that of consuming other dairy products (yogurt and cheese) or dairy alternatives, limiting our ability to determine the contribution of all dairy products to choline intake.
In conclusion, this is the first study to assess dietary choline intake in a Canadian population. Our findings indicate that choline intake is below the recommendation levels in this population, during a period when choline demand is high. The consumption of eggs and fluid milk increases daily choline intake and the promotion of both egg and milk consumption may assist in meeting the daily dietary choline intake recommendations. In addition, progress should be made to expand the current database of food choline content values to more accurately estimate the dietary intake of this essential nutrient.
Acknowledgements
The authors thank Cora Anderson, Kyla Gallant-Sova, Marisa Salon, Martina Sung, Nour Watter, Teresa Jungwirth, Victoria Okrusko, Viane Faily, Sarah Loehr, Anne Gilbert and Marg Fedyna for their contributions to the development of the Alberta database and analysis of the 24 h recall data. They also thank Dr Patricia Biondo for editorial assistance. They are extremely grateful to all the families who took part in the study and the whole APrON team (www.ApronStudy.ca), including the research assistants, graduate and undergraduate students, volunteers, clerical staff and managers.
The present study was supported by Alberta Livestock and Meat Agency/Alberta Innovates Biosolutions/Egg Farmers of Alberta under the Quality Food for Health programme (JMC, grant number QFH-11-019); an Alberta Livestock and Meat Agency/Alberta Advanced Education and Technology/Alberta Egg Producers grant (JMC, grant number 2009F67R); and the Dairy Farmers of Canada in their 2012 operating competition (CJF, grant number RES0014464). The APrON cohort was established by an interdisciplinary team grant from Alberta Innovates – Health Solutions. E. D. L. was a recipient of the Women and Children's Health Research Institute (WCHRI) Graduate Studentship and the Queen Elizabeth II Graduate Scholarship. None of the mentioned funders had a role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: C. J. F., R. C. B., L. J. M., J. M. C. and R. L. J. designed the research; E. D. L. conducted the research and analysed the data; F. B. S. assisted with the statistical analyses; J. M. C., C. J. F. and R. C. B. obtained specific funding for analysis on the APrON study; E. D. L., R. L. J. and C. J. F. wrote the manuscript; C. J. F. had primary responsibility for the final content of the manuscript. All authors read, edited and approved the final manuscript.
None of the authors has any conflicts of interest to declare.









