INTRODUCTION
It is well known that bovine tuberculosis (bTB) placed a heavy burden on the British cattle industry in the first half of the twentieth century. In the absence of proper control, some animals reached the most advanced stage of the disease before they were slaughtered and it was common for infected meat and milk to arrive at the market [Reference Atkins and Fenton1, Reference Waddington2]. Being a zoonotic disease, bTB also challenged the human population, usually at non-pulmonary sites of the body. Each year in the early 1930s over 4000 people were infected and 2000 died of bTB infection [Reference Savage3].
It was not until after the Second World War that the Ministry of Agriculture finally faced up to the scale of the problem. Starting in 1950, they followed an area eradication programme of slaughtering cattle ‘reactors’ to the tuberculin test, and where necessary whole herds were eliminated. This policy was so successful that the entire UK was declared attested in 1960 and it was assumed that the few remaining pockets of disease would be dealt with in time. However, in the 1970s a final clearance looked less likely with the discovery of reservoirs of infection in wildlife, particularly the European badger (Meles meles) in the south-west of England. Then in the 1980s there began a slow recrudescence of bTB in British cattle that has accelerated in the last 10 years to the extent that over £150 million annually is now being spent on a variety of control measures, including compensation to farmers for the slaughter of their stock [4]. Despite the Randomised Badger Culling Trial (RBCT) (1998–2007) and an associated scientific literature that (in Britain at least) seems to be sceptical about the benefits accrued from culling badgers to reduce bTB, there remains room for politics [Reference Spencer5]. The farmers’ lobby has continued to call for large-scale culls and the present Coalition government has agreed to facilitate this in England with effect from the summer of 2013 [6].
A feature of both the scientific and popular literatures is the absence of any in-depth historical contextualization of bTB. This is partly because the first infected badger in Britain was not discovered until 1971 but it seems reasonable to assume that epidemiological links between badgers and cattle existed before that, possibly for decades. One related but unanswered question about bTB in wildlife reservoirs is its persistence and whether in badgers it is a maintenance or a spillover disease [Reference Palmer7]. According to Cheeseman et al., bTB ‘is endemic in the British badger population and … the badger is an ideal maintenance host’ [Reference Cheeseman8]. It is the purpose of the present paper to comment on the implications of this claim in as much as the historical evidence allows, particularly with regard to a shift in the centre of gravity of cattle bTB from the north-west of England before 1960 to the south-west at the present day.
We will use novel cartographic sources to show that the distribution of bTB in cattle is very different today from patterns in the past. This is important because the approximate coincidence between the present-day regional peaks of cattle bTB and badger bTB cannot be taken as proof of a long-term causal correlation. Possible explanations for the spatial rupture through time are fourfold:
(1) That one or more of the data sources used in this paper is unreliable.
(2) That the role of the wildlife reservoir in mediating cattle disease was different before 1960.
(3) That regional agricultural practices have changed.
(4) That the ecology of badgers is such that bTB in them is less persistent outside the south-west of England than has hitherto been thought.
CARTOGRAPHIC SOURCES
It is not possible to map bTB in badgers or cattle precisely. For both species we must use surrogate data, such as the tuberculin test in cattle. This checks for an immune response (an allergic swelling) in the animal's skin to tuberculin and so helps to identify ‘reactors’, which then become ‘confirmed breakdowns’ if lesions are identified in the abattoir or if the disease is cultured from tissue samples in the laboratory. Figure 1 a shows the distribution of confirmed TB breakdowns in Britain in 1991, most of which it can be assumed will have been initially detected by use of the single intradermal comparative cervical tuberculin test. This was a decade before the recent rapid increase in the number of reactors. Since then what appeared to be an archipelago of small islands of infection became a series of larger islands that on the map for 2010 look to have merged to form an epidemic mainland (Fig. 1 b). We can be confident that at the regional scale it is indicative of the spatial distribution of bTB in Britain's cattle herds although, having said that, it is important to note, (a) that the tuberculin test is imperfectly sensitive and is thought to detect only about 80% of infected animals [Reference De la Rua-Domenech9, 10], (b) that ethnographic work with veterinarians shows that some do not stick closely to the official testing protocol [Reference Enticott11], and (c) that routine herd test intervals vary from 1 to 4 years in different parishes around Britain according to local TB incidence, but herds will be tested more frequently when contiguous to breakdowns.
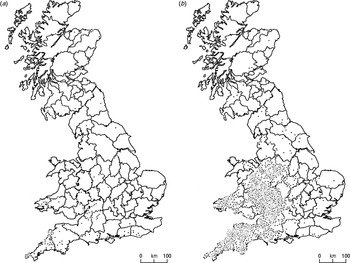
Fig. 1. Confirmed cattle breakdowns for (a) 1991, (b) 2010 (source: Animal Health and Veterinary Laboratories Agency, UK).
Figure 2 and Table 1 relate to badgers rather than cattle. The distribution is of those that were collected from road traffic accidents (RTAs) and then tested post mortem for bTB. The overall distribution is not representative of the national spread of badgers, most likely because members of the public reporting carcases were more aware of the need for testing in some parts of the country than others, introducing potential biases in the data [Reference Krebs12, Reference Abernethy, Urcelay and Pinto13]. Nevertheless, in those regions with coverage we can show the location of tuberculous badgers in relation to those without the disease. According to Goodchild et al., RTAs are informative indicators of the prevalence of bTB in the badger population [Reference Goodchild14], although they cannot be taken as definitive evidence.
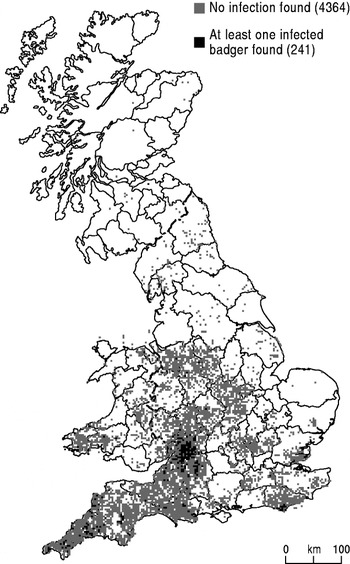
Fig. 2. Badger road traffic accidents, 1972–1990 (source: Krebs, 1997 [Reference Krebs12]).
Table 1. Bovine tuberculosis in badgers collected from RTAs, 1972–1994

RTA, Road traffic accident.
Source: Animal Health and Veterinary Laboratories Agency, UK.
Figures 1 and 2 give an impression of the spatial patterning of bTB in cattle and badgers during the early stages of the disease's resurgence. The situation before 1960 was somewhat different, however, and is best exemplified by Figure 3. This map shows the results of tuberculin testing in 1938 by government veterinarians, when data were compiled ahead of what the then government hoped would be an extensive area eradication scheme for tuberculous cattle, although in the event this final push to slaughter all reactors was postponed by the war until the 1950s. In the late 1930s skin testing had at last been accepted after a period of scepticism among farmers and veterinarians [Reference Waddington15]. In 1938 there were on average 130 reactors per thousand head tested in Britain as a whole, with over 300 in eight counties and a peak of 416 in Cheshire. It is important to note that this testing does not seem to have been undertaken with a view to strict statistical representativeness. Focusing on self-contained herds that bred their own replacements, for instance, will have underestimated disease in regions used to buying in stock, and a few counties were omitted in the survey. One knowledgeable commentator observed that the true figure for Cheshire was probably 600–800 tuberculous dairy cows per thousand and for Derbyshire over 500 [Reference Francis16]. Moreover, post mortems were not performed, so these data will include some of the false positives that are still today a weakness of the skin test.
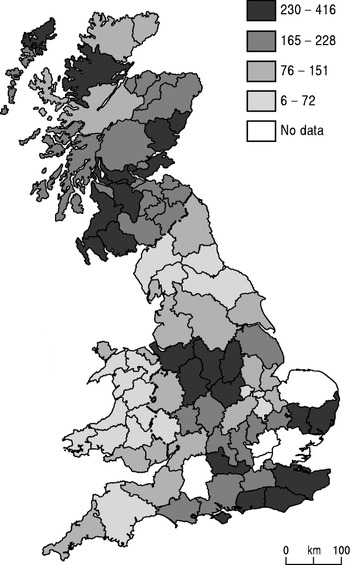
Fig. 3. Reactors per thousand cattle tested, 1938, quartiles (source: Francis, 1947 [Reference Francis16]).
Figure 4 depicts the regional distribution of cattle slaughtered under the Tuberculosis Orders in the period 1926–1940. Once again, Cheshire was unenviably close to the top of this list and, with its contiguous counties, formed the largest cluster of infection in cattle in the period. The data for this series were the result of diagnoses by veterinarians, largely based on their inspections of udders for signs of disease. This picked up only advanced cases and other tuberculous cows were missed when they were withheld from the test due to a calculation by some farmers that a diseased cow that still produced milk was worth more than the compensation that was likely to be paid out by the government under the Tuberculosis Orders. In short, this map under-represents both the scale and extent of the problem, especially in the dairy districts.
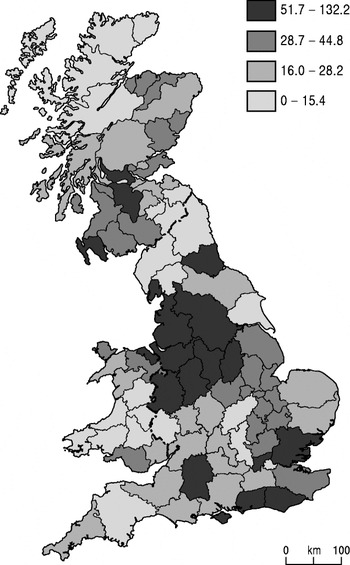
Fig. 4. Cattle slaughtered per 1000 head under TB Order, 1926–1940, quartiles (source: Francis, 1947 [Reference Francis16]).
ANALYSIS
Figure 1 a is a representation of the early phase of the return of bTB in cattle. It focuses on three principal areas: Cornwall and Devon; Gloucester, Hereford and Worcester; and south Wales, with a scattering of other breakdowns, for instance in Staffordshire, Sussex and Dorset. Similarly the pattern of infected badgers in the RTA data (Fig. 2) has concentrations south of the Bristol Channel in the Gloucestershire and Wiltshire Cotswolds, Devon, Cornwall and Sussex. Because Figure 2 was compiled over an extended period (1972–1990), before the collection of the cattle confirmed breakdown data in Figure 1 a, it could be argued that it was prefiguring what was to come. Badgers were collected and analysed in this way for over 20 years and the core areas of infection were stable over this period.
Research on badgers from the 1980s onwards has found strong links between infection in the two species, including evidence of spatial correlation. Most of this research has tended to be observational and no one has yet proved definitively which direction the infection travels and which species is the maintenance host. Nevertheless, the RBCT organized by the Independent Scientific Group on Cattle TB (ISG) provided a rare opportunity to gather data and some of the analysis by its members is indicative of interwoven worlds of infection within a radius of 1–2 km [Reference Woodroffe17].
In the light of the patterns discussed above, Figures 3 and 4 are markedly similar to each other (but different from Fig. 1 a, b). The two maps have much in common at the regional level, and John Ritchie (Chief Veterinary Officer, 1952–1965) commented that ‘the weight of infection as revealed by the Tuberculosis Orders figures very closely conforms with the test survey figures’ [Reference Ritchie18]. The regional patterns also correlate broadly with the extensive qualitative commentaries of agricultural journalists, veterinarians and civil servants in the press, parliamentary inquiries and official reports [Reference Atkins19]. It seems unlikely that they are an artefact of the enthusiasm for control of certain local authorities or the result of the pre-war scarcity of statistical resources.
The north-west and north Midlands of England were the main hotspots, along with the south coast of England and central and north-east Scotland. Note how low the south-west of England and south Wales stand in this inter-war period. There are a few discrepancies, for instance Ross and Cromarty being in the top quartile in Figure 3 but in the bottom category in Figure 4. This is probably a small-numbers problem, with only 500 cattle tested there in 1938, the second lowest for the counties in Scotland.
The spatial clustering of counties in the top quartile and the consistency between the two maps suggests a real pattern that has to be taken seriously. There is also further, corroborative evidence to support this claim drawn from the bTB that was identified in human surgical and pathological samples collected and typed from 1943 to 1945 (Table 2). These were mainly taken from consumers of infected milk. We would not expect a perfect geographical match with the cattle disease because milk was transported from the specialist producer districts to distant cities such as London, and so the disease spread beyond its place of origin. Even so, the north Midlands and north-west were the worst affected and, since these regions were largely self-sufficient in milk, we can assume that people there were picking up a local infection [Reference Wilson20].
Table 2. Bovine tuberculosis in English human non-respiratory samples, 1943–1945

Source: Wilson, et al., 1949, [20, p. 339].
* These are modified Registrar General's regions.
The spatial disjuncture between bTB at present and 50 years ago poses an important question. How is it that there is so little evidence of continuity in wildlife disease in those regions where cattle in the past were so heavily infected? One would have expected a proportion of badgers in, say, Cheshire still to have been tuberculous in the 1970s and 1980s, within 10–20 years of the eradication of bTB in cattle in that county (1960) for one of two reasons. If wildlife was a cause of disease in cattle before attestation then the slaughter of all tuberculous cattle would have made little difference to the level of infection in badgers. Or, if the pathway of contagion was in the opposite direction, badgers would undoubtedly have been challenged because, with over 40% of local cattle being tuberculous and some in an advanced stage of the disease that is never seen today, there would have been a greater shedding of bacteria on the pastures here than anywhere else in Britain at any time in history. Much modern research indicates close parallels between disease development in the two species, even to the point of them sharing the same strain (spoligotype) within a radius of 1–2 km [Reference Woodroffe21, Reference Woodroffe22]. Why then is it that only one of 389 badgers collected from RTAs in Cheshire during 1972–1990 proved to be tuberculous?
DISCUSSION
Some writers have argued that bTB is endemic in British badgers [Reference Cheeseman8]. Anderson & Trewhella [Reference Anderson and Trewhella23] suggested that under favourable circumstances an equilibrium of 10–20% prevalence will be reached in an infected community after 30–40 years and others have run similar simulation models over several decades or in one case for 100 years [Reference White and Harris24]. A minimum badger group size of 6–8 is usually said to be required for disease persistence [Reference Smith25], although this and other assumptions about the relationship between group size and bTB incidence have been questioned recently [Reference Woodroffe26]. It is now thought, for instance, that badger mortality from bTB is relatively low and this has implications for the disease's basic reproductive rate.
One difference between the present and the 1930s is the increase in badger numbers since the main protective legislation of 1973, 1981 and 1992. Before that badger numbers were kept down by gamekeepers, farmers and sett diggers. Smaller numbers of badgers before the war meant a lower risk of them catching bTB from cattle but the sheer weight of disease in dairy herds meant the maximum possible exposure of any local wildlife. Even if badgers were naive of the disease at the beginning of the twentieth century, it seems very likely that some infection would have leaked along this route by the 1930s.
Another possible explanation of macro-spatial differences between the 1930s and the present may relate to farm practices. Herd sizes and cattle density have increased, for instance, both acknowledged risk factors for bTB [Reference Brooks-Pollock and Keeling27], although not necessarily connected to the wildlife reservoir. It is true that during the twentieth century there was a gradual intensification of cattle husbandry in the west of England as against the arable eastern counties, but the south-west did not benefit disproportionately vis-à-vis the north-west [28]. Second, from almost nothing before 1960, maize has become an important forage and grain crop, with farmers in the south-west of England the most enthusiastic innovators. Since maize is both nutritious and palatable for badgers, this may be an important new factor, especially on farms where biosecurity is weak and badgers can gain access to storage clamps [Reference Roper29]. Third, cattle marketing has changed, facilitated in recent years by electronic communications. Since 2001, movements have been recorded in the British Cattle Movement Service's Cattle Tracing System database which shows that the greatest concentration of on-movements is currently in the south-west and Midlands of England, and in south-west Wales [Reference Mitchell30]. According to Robinson et al. [Reference Robinson, Everett and Christley31], Britain's cattle network is becoming more cohesive with the result that risk of disease spread is enhanced, and it is generally agreed that cattle-to-cattle transmission is important in the spread of bTB [Reference Gilbert32, Reference Green33].
Figures 3 and 4 show a concentration of cattle bTB between the wars in the north-west of England and in parts of Scotland, but the lack of badger TB here in the 1970s and 1980s indicates one of two possible conclusions. The first is that badgers were never infected in these regions. This seems unlikely, although it should be acknowledged that this early epidemic in bovines may have been mainly the result of cattle-to-cattle infection. The second is that the epidemiologists and ecologists are mistaken and that bTB in badgers is a spillover rather than an endemic disease and therefore does not persist over lengthy periods [Reference Corner34, Reference Nugent35]. It may be that the reproduction of bTB requires an average badger group size that is larger than that present in Cheshire and may only be achieved in a relatively few favourable ecological niches [Reference Allen36]. Two censuses of badgers [Reference Cresswell37, Reference Wilson38] indicate that peak numbers (among the highest anywhere in Europe) lie in the south-west of England although this should not be taken a priori as a statement of risk because, in high densities, badgers are territorial and therefore unlikely to pass infection from group to group [Reference Böhm39]. Hotspots of badger disease are most dangerous in this region when perturbation causes wider ranging than would otherwise occur, or where infected cattle are moved, thus posing a disease challenge to naive badger groups.
In the light of the analysis and discussion in this paper, we conclude on our four introductory questions:
(1) That while the historical and present-day data sources face questions of precision and representativeness, our considered view is that they do indicate a significant change in the spatial pattern of cattle bTB.
(2) That it is unlikely that badger numbers were great before 1960 in those counties where bTB was at its peak in both cattle and human disease. Bovine TB was probably therefore more of a cattle-to-cattle infection in the past than it is said to be today.
(3) That the intensification of cattle husbandry, increase of herd size, frequent movement of animals, and cultivation of new crops such as maize have all played their part in restructuring the industry. Whether this is enough to explain the heightened risk of bTB infection in the south-west is a question that requires further investigation.
(4) That our present assumptions about the endemicity and persistence of bTB in badger groups in lower density regions need to be revisited. We challenge the idea that bTB has historically been persistent in enough wildlife pockets around the country, flaring up recently as badger numbers have increased, to account for the epidemic of the last 10 years in cattle.
Overall, we hope that our introduction of a historical perspective will encourage further research on long-term bTB disease dynamics. Potentially there is a new agenda here, not least because policy-makers have structured their interventions on evidence from the voluminous literature of the last 20 years, largely ignoring the long and difficult history of bTB governance [Reference Grant40].
ACKNOWLEDGEMENTS
Philip Robinson thanks the Department of Agriculture and Rural Development, Northern Ireland, for his doctoral scholarship. The 1991 and 2010 bTB locational data were kindly provided by Barbara Wibberley of the AHVLA and are protected by the terms of an Open Government Licence.
DECLARATION OF INTEREST
None.








