Behavior problems, such as internalizing (i.e., anxiety, depression, emotional problems, somatic complaints, and withdrawnness) and externalizing (i.e., aggression and attention problems) behaviors, may manifest during the preschool years, potentially extending over childhood and adolescence (Bartels et al., Reference Bartels, van den Oord, Hudziak, Rietveld, van Beijsterveldt and Boomsma2004). Early identification and deeper understanding of the etiology of these behaviors are important, mainly because children with behavior difficulties are at a greater risk of adverse developmental outcomes, including poor academic performance, conflictual relationships with peers and family members, delinquency, and even early death (Jokela et al., Reference Jokela, Ferrie and Kivimäki2009; Sourander et al., Reference Sourander, Jensen, Davies, Niemelä, Elonheimo, Ristkari and Almqvist2007; Van Der Ende et al., Reference Van Der Ende, Verhulst and Tiemeier2016).
Twin studies have revealed significant genetic influences on internalizing and externalizing behaviors, with common and non-shared environmental effects generally being considered more modest (Bartels et al., Reference Bartels, van den Oord, Hudziak, Rietveld, van Beijsterveldt and Boomsma2004; van der Valk et al., Reference van der Valk, Verhulst, Stroet and Boomsma1998). Among the diverse mechanisms via which the environment can influence twins’ behavior, the prenatal environment is receiving increasing interest (Knopik et al., Reference Knopik, Neiderhiser, de Geus and Boomsma2016). In this context, birth-weight discordance is considered a good proxy of the non-shared environment's effect. In fact, as twins are characterized by the same gestational age, a difference in their birth weight is the result of any factor affecting the growth of each individual twin (Pettersson et al., Reference Pettersson, Sjölander, Almqvist, Anckarsäter, D'Onofrio, Lichtenstein and Larsson2015). These factors could be ascribed to genetic or structural abnormalities, or to adverse intrauterine factors, such as small placental weight, single umbilical artery, excessive velamentous cord insertions (Victoria et al., Reference Victoria, Mora and Arias2001), or to various placental abnormalities (Kent et al., Reference Kent, Breathnach, Gillan, McAuliffe, Geary, Daly and Morrison2012), which may randomly affect only one twin in a pair.
Growth discordance has been associated with impaired cognitive development (Ross et al., Reference Ross, Krauss and Perlman2012), ADHD symptoms (Pettersson et al., Reference Pettersson, Sjölander, Almqvist, Anckarsäter, D'Onofrio, Lichtenstein and Larsson2015), and problem behaviors (Mankuta et al., Reference Mankuta, Goldner and Knafo2010; van Os et al., Reference van Os, Wichers, Danckaerts, Van Gestel, Derom and Vlietinck2001). In particular, van Os et al. (Reference van Os, Wichers, Danckaerts, Van Gestel, Derom and Vlietinck2001) analyzed the association between birth-weight difference and Child Behavior Checklist (CBCL) total score difference in 745 twin pairs from the East Flanders Prospective Twin Survey (EFPTS), observing a positive association between the intrapair behavior-problem difference and the degree of birth-weight discordance.
The aim of the current study was to replicate the previously reported association between the intrapair birth-weight difference and the intrapair difference in behavior problems, measured by the CBCL questionnaire. Our primary interest was to examine whether and how birth-weight difference and other perinatal factors, including gestational age, maternal smoking, and BMI before pregnancy, may affect the development of problematic behaviors. Specifically, we focused on each CBCL subscale and presented the absolute birth-weight differences in order to help parents and clinicians to easily identify the more challenging areas of development. Furthermore, we concentrated on young, preschool-aged twins to reduce as much as possible external, non-controlled for influences.
Materials and Methods
The data collection process has been described previously (Antoniou et al., Reference Antoniou, Fowler, Reed, Southwood, McCleery and Zeegers2014). Briefly, the Twins and Multiple Births Association Heritability Study (TAMBAHS) is a UK, volunteer-based study investigating the psychological development of twins from birth until 5 years of age. Mothers of twins aged 18 months to 5 years were selected for this study. Between July 2008 and May 2010, 492 mothers completed an online questionnaire on their twins’ emotional and behavioral development. Participants’ geographical spread was representative of the twin families’ spread across the UK.
Zygosity Determination
The adapted version of the Goldsmith's zygosity questionnaire (Goldsmith, Reference Goldsmith1991) was used to assess the zygosity of the twins included in the TAMBAHS dataset. This questionnaire has been validated against determination by identity of polymorphic DNA markers, reaching an accuracy of verifying zygosity in 95% of cases.
Behavior Problems
The CBCL 1½–5 (Achenbach & Rescorla, Reference Achenbach and Rescorla2000) is a questionnaire developed to obtain a standardized report of children's behavior problems as perceived by their parents. Numerous versions of this questionnaire have been developed to target different age groups. The CBCL 1½–5 was developed to assess children from 18 months to 5 years of age. It contains 99 problem items, split into 7 subscales: emotionally reactive, anxious/depressed, somatic complaints, withdrawn, sleep problems, attention problems, and aggressive behavior, originally derived by factor analyses. The broadband scale ‘Internalizing’ is the sum score of the first four syndrome scales, whereas ‘Externalizing’ is the sum score of attention problems and aggressive behavior. ‘Total problems’ is the sum score of all 99 problem items. Each item is scored 0–2 (not true, somewhat or sometimes true, and very true or often true), based on the preceding 2 months. Good reliability and validity criteria have been reported for this checklist (Achenbach & Rescorla, Reference Achenbach and Rescorla2000).
Statistical Analysis
Twins’ absolute birth-weight difference in grams (i.e., birth weight of the larger twin — birth weight of the smaller twin) and CBCL score discordance (i.e., CBCL score of the larger twin — CBCL score of the smaller twin) were calculated. The association between birth-weight difference and CBCL score difference was investigated by the means of multiple linear regression analysis. The hypothesis under test was that an increase in birth-weight difference between co-twins will be associated with a CBCL score difference. The expected mean CBCL difference was calculated to determine how the score is expected to change with increasing birth-weight difference per 100 g difference. Twins’ gender (male–male, female–female, opposite-sex), age, gestational age, weight difference at the time of survey, zygosity, and maternal prepregnancy BMI, age, and smoking status before, during and after pregnancy were controlled for in the analysis. An interaction term was added to the regressions in order to test the possible interaction of zygosity with birth-weight difference.
Statistical analysis was performed in Stata v.13 (StataCorp, 2013). Alpha was set at 0.05.
Results
Summary Statistics
A total of 960 twins were included in the analysis, of which 202 were monozygotic (MZ) male twins, 152 MZ female twins, 162 dizygotic (DZ) male twins, 166 DZ female twins, and 278 opposite-sex twins. Mean twins’ birth weight, gestational age, and age at survey are presented in Table 1, subdivided by zygosity. MZ twins’ mean gestational age was significantly shorter than that of DZ twins (35.38 ± 2.48 weeks of MZ twins, compared to 36.26 ± 2.69 weeks of DZ twins; p = .0001). Furthermore, MZ twins’ mean birth weight was significantly lower than that of DZ twins (2,326.64 ± 575.86 g compared to 2,487.02 ± 578.78 g for MZ and DZ twins, respectively; p = .0001), while MZ twins’ age at the time of survey was significantly higher than DZ twins’ mean age (37.50 ± 11.63 months and 34.67 ± 11.35 months for MZ and DZ twins, respectively; p = .0003). Table 1 also shows mean CBCL scores for each zygosity group, subdivided by gender and adjusted for age at survey to account for the rapid development that occurs in the age period considered. As can be seen, mean scores were statistically significantly higher in female MZ twins as compared to female DZ twins in total problems (34.69 [1.43] compared to 31.11 [1.02] for female MZ and DZ twins, respectively; p = .042), internalizing problems (8.90 [0.51] and 6.80 [0.36] for female MZ and DZ twins, respectively; p = .001), anxiety/depression (3.17 [0.20] for female MZ twins and 2.20 [0.14] for female DZ twins; p = .001) and withdrawnness (1.82 [0.12] for female MZ twins and 1.27 [0.09] for female DZ twins; p = .001). No statistically significant difference in mean scores was observed among male twins.
TABLE 1 Phenotypic Characteristics of Twins According to their Zygosity

SD = standard deviation; SE = standard error.
aCorrected for twins’ age at survey.
DZ twins’ mothers were significantly older than mothers of MZ twins (36.69 ± 4.17 years compared to 35.53 ± 4.69 years; p =.0002), while no difference was observed in maternal prepregnancy BMI (24.52 ± 4.32 kg/m2 of MZ twins’ mothers compared to 24.76 ± 5.11 kg/m2 of DZ twins’ mothers; p = .570). Mothers of MZ and DZ twins did not differ much regarding their ethnicity, with the vast majority of them being white (97.19% and 98.07% of mothers of MZ and DZ twins, respectively). Table 2 presents the mean differences in birth weight and behavior problems according to zygosity group. As can be seen, MZ twins’ mean birth-weight difference was lower than DZ twins’ (246.07 ± 228.48 g compared to 295.65 ± 271.74 g; p = .004). Table 2 also shows mean differences in CBCL scores in MZ and DZ twins, subdivided by gender and adjusted by age at survey. Among female–female twin pairs, statistically significantly larger differences were found in MZ twin pairs in the emotional reactiveness scale (1.40 [0.51] and -0.40 [0.49] for females, MZ and DZ twin pairs, respectively; p = .012). On the other hand, DZ twins showed statistically significantly larger differences in somatic complaints (-0.48 [0.28] for female MZ twin pairs, and 0.67 [0.28] for female DZ twin pairs; p = .004) and withdrawnness (Males: 0.19 [0.13] and -0.34 [0.16] for MZ and DZ twins, respectively; p = .011. Females: 0.13 [0.15] and -0.38 [0.15] for MZ and DZ twin pairs, respectively; p = .016). A marginally significant difference in the mean score was observed in male twin pairs in the sleep problems scale (-0.14 [0.16] and 0.33 [0.18] for MZ and DZ twins, respectively; p = .051). Note that, here, a negative value signifies that the smaller twins at birth scored higher on average than larger birth-weight co-twins, and vice versa.
TABLE 2 Phenotypic Discordance According to Zygosity
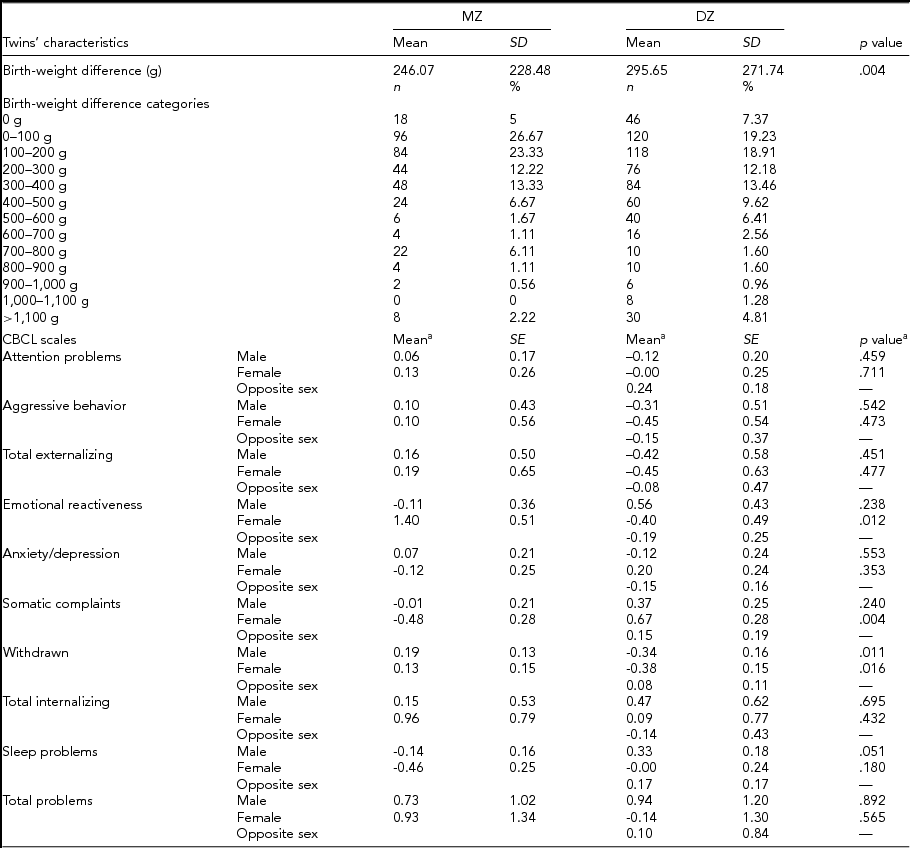
SD = standard deviation; SE = standard error.
aCorrected for twins’ age at survey.
We computed intrapair correlations by using Pearson's correlation coefficients between the standardized residuals of birth-weight difference, adjusted for gestational age and sex of the pair (i.e., male–male, female–female, or opposite-sex), and CBCL score differences, adjusted for age at survey and sex of the pair (Table 3). All residuals were standardized to a mean of zero and a standard deviation of 1. MZ correlations were higher than DZ correlations only in 4 out of 10 scales (i.e., externalizing and internalizing problems, attention problems, and emotional reactiveness), while in 3 scales, MZ twins’ standardized correlations were below the mean value of zero (i.e., sleep problems, somatic complaints, and withdrawn). However, when we computed intrapair twin correlations of raw CBCL scores, MZ twins’ correlations were always higher than those of DZ twins (Table 3). Specifically, MZ twins’ correlations ranged from 0.136 to 0.921, with most of them being higher than 0.8, while DZ twins’ correlations ranged from -0.093 to 0.639. In both cases, the lowest correlations were associated with emotional reactiveness.
TABLE 3 Intrapair Correlations for Each CBCL Scale, Subdivided Into Zygosity Group
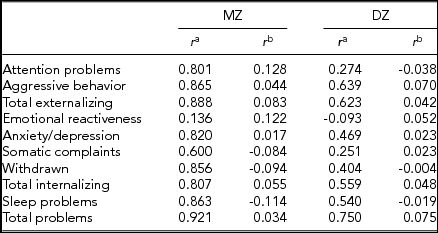
r = Pearson's correlation coefficient.
aIntrapair correlations of raw CBCL scores.
bCorrelations of standardized residuals. Birth-weight difference was adjusted for gestational age and sex, while CBCL scales score differences were adjusted for age at survey and sex.
Multiple Linear Regression
Multiple linear regressions were performed first with the absolute birth-weight difference as a continuous variable, and second, after subdividing it into categories of 100 g difference. No statistically significant association was found when treating birth-weight difference as a continuous variable. However, when subdividing it into birth-weight difference categories, statistically significant associations were found in total problems (β = -5.95; 95% CI [-11.08, -0.82]; p = .023), internalizing behavior (β = -4.17; 95% CI [-7.65, -0.69]; p = .019) and emotional reactiveness scales (β = -2.70; 95% CI [-5.23, -0.17]; p = .036) in MZ twins analyzed as a separate group. Moreover, a borderline statistically significant association was found in sleep problems in MZ twins (β = -0.84; 95% CI [-1.69, 0.01]; p = .052). No statistically significant association was found neither in MZ and DZ twins combined nor in DZ twins analyzed separately.
We then computed expected mean differences for every birth-weight category, subdivided by CBCL scale, in MZ and DZ twins combined (Table 4), MZ twins (Table 5), and DZ twins (Table 6) analyzed separately; p values relative to the whole multiple linear regressions are shown.
TABLE 4 Expected Mean Difference Score for Increasing Birth-Weight Difference
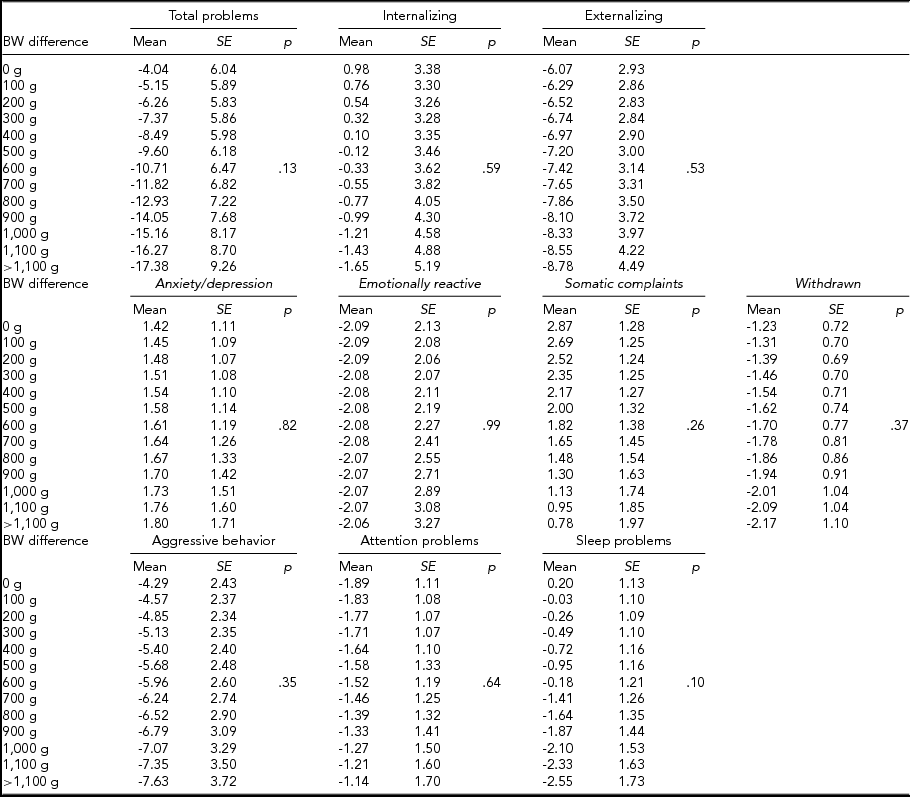
TABLE 5 Expected Mean Difference Score for Increasing Birth-Weight Difference in Monozygotic Twins

TABLE 6 Expected Mean Difference Score for Increasing Birth-Weight Difference in Dizygotic Twins
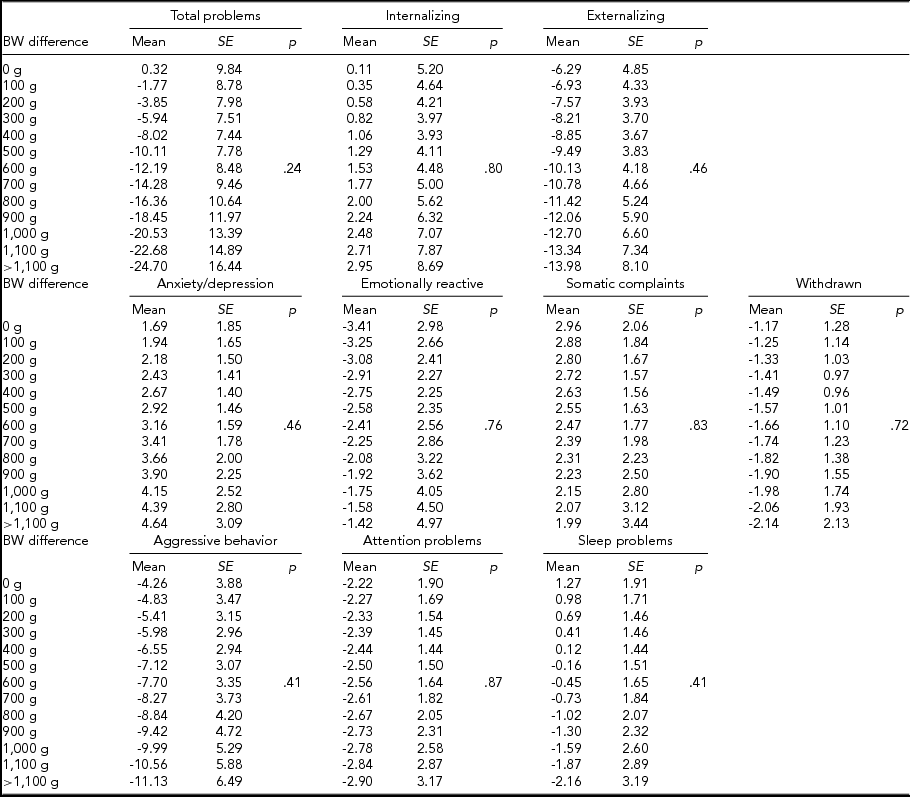
In MZ twins analyzed separately, the expected means for the intrapair difference in total problems score ranged from 15.11 (12.03) to -59.29 (25.14). Furthermore, the expected means for internalizing score ranged from 11.27 (8.16) to -38.80 (17.05), while this figure ranged from 7.31 (5.92) to -25.15 (12.38) for emotional reactiveness.
Post-Hoc and Sensitivity Analysis
Among the independent variables included in the multiple regressions, gestational age was given special attention because of its possible association with childhood neurodevelopment. For this reason, all multiple regressions were repeated without controlling for gestational age. The results were not significantly affected by the exclusion of gestational age (results not shown).
Furthermore, we repeated all the analysis after removing all twin pairs (n = 2 and n = 9 for MZ and DZ twins, respectively) with at least one extremely low birth-weight (ELBW) twin (i.e., with a birth weight ≤ 1,000 g, according to the World Health Organization's definition) from the sample. Again, no difference in the results was observed (results not shown).
Discussion
The aim of the current study was to replicate the previously reported association between the intrapair birth-weight difference and the intrapair difference in behavior problems, measured by the CBCL questionnaire. As MZ twins share 100% of their genotype and 100% of their common environment, if birth-weight discordance in MZ twins predicts a discordance in CBCL score, then it is plausible to assume a causal association between the two (Vitaro et al., Reference Vitaro, Brendgen and Arseneault2009).
The associations between the birth-weight difference and behavior problem scales were first investigated in MZ and DZ twins combined, but no significant association was identified. The analysis was then repeated in MZ and DZ twins separately. The regressions of total CBCL, internalizing problems, and emotional reactiveness score differences over birth-weight difference were significant in MZ but not in DZ twins analyzed separately. These results, and especially the very large and clinically significant expected mean differences ranges, suggest that intrauterine factors affecting birth weight are involved in the early development of behavior problems. In fact, as twins are characterized by the same gestational age, a difference in their birth weight is the result of any factor affecting the growth of each individual twin (Pettersson et al., Reference Pettersson, Sjölander, Almqvist, Anckarsäter, D'Onofrio, Lichtenstein and Larsson2015). Modifiable risk factors of birth-weight discordance include low weight gain during pregnancy, advanced maternal age, artificially induced pregnancy, smoking, and hypertensive disorders (Puccio et al., Reference Puccio, Giuffré, Piccione, Piro, Malerba and Corsello2014).
Note that all statistically significant beta coefficients are negative, implying a rise in CBCL scores in smaller twins (or a reduction in the larger ones) with the increasing birth-weight difference. This is in line with previous studies that have explored the effect of birth-weight discordance on behavior, cognition, and psychopathology. In fact, the smaller twins in discordant, prematurely born pairs showed lower IQ performances at 3 years of age (Ross et al., Reference Ross, Krauss and Perlman2012) and higher ADHD symptoms at 9 and 12 years (Pettersson et al., Reference Pettersson, Sjölander, Almqvist, Anckarsäter, D'Onofrio, Lichtenstein and Larsson2015) compared to their larger co-twins. Van Os and colleagues examined the association between birth-weight discordance and discordance in CBCL scores in 6- to 17-year-old twins (van Os et al., Reference van Os, Wichers, Danckaerts, Van Gestel, Derom and Vlietinck2001), reporting a statistically significant, positive association with the total CBCL score. Two aspects differentiate the present study from van Os et al. (Reference van Os, Wichers, Danckaerts, Van Gestel, Derom and Vlietinck2001), the first being that they analyzed only the influence of birth weight on the total CBCL score, while our aim was to determine which specific subscale(s) might be influenced the most by birth weight. The second aspect is that van Os’ research group analyzed the linear regressions of relative differences, as opposed to the present study, in which the absolute CBCL score differences were regressed over the absolute birth-weight difference. Although this differential approach limits the comparability between the two studies, our choice of presenting expected mean differences for 100 g birth-weight difference was led by the purpose of presenting more interpretable results for clinicians and parents.
Previous heritability analysis showed that internalizing and externalizing problem behaviors in young twins are influenced by genetic and environmental factors, with the vast majority of the observed variance explained by the genetic factors (van der Valk et al., Reference van der Valk, Verhulst, Stroet and Boomsma1998). The analysis of discordances in MZ twins allows one to focus on unique environmental factors only, which are thought to explain 25–32% of the observed variance in internalizing and externalizing problems (van der Valk et al., Reference van der Valk, Verhulst, Stroet and Boomsma1998), and about 22% of the variance in negative emotionality (Schumann et al., Reference Schumann, Boivin, Paquin, Lacourse, Brendgen, Vitaro and Booij2017). However, when we analyzed MZ twins alone, the values of R 2 indicate that each of the association considered accounted only for about 8–11% of the variance. At the same time, the expected mean differences in CBCL scores at 0 g birth-weight difference strongly diverged from the expected value of zero. These results could signify that other factors are likely to influence twins’ behavioral development. For example, it is possible that non-reported intrauterine growth restriction affected the smaller twins’ psychological development independently from birth weight, although a sensitivity analysis removing all pairs with ELBW twins did not show different results. Alternatively, other risk factors able to affect the individuals’ behavior independently from their co-twins (i.e., increasing the difference between the two) could have an influence on the birth-weight difference behavior problems association.
Among the environmental factors that might affect the behavioral development of children, low birth weight (LBW) has been frequently highlighted. As Drvaric and colleagues discussed in their review (Drvaric et al., Reference Drvaric, Van Lieshout and Schmidt2013), extremely low birth-weight (ELBW) infants are at increased risk of emotional regulation issues, internalizing and externalizing behavior problems, and lower IQ, compared to normal-weight infants. In the present study, we did not observe any influence of ELBW on the results of the linear regression analyses, probably due to the low number of twin pairs with at least one ELBW twin (i.e., 11 out of 480). With regards to the association between birth weight and neurologic soft signs, Breslau et al. (Reference Breslau, Chilcoat, Johnson, Andreski and Lucia2000) reported a higher risk of soft signs, along with internalizing, externalizing, and attention problems in children born with a LBW. Finally, analyzing infants’ temperament, Riese (Reference Riese1994) reported that larger twins were more irritable, more difficult to soothe, and more active while awake and during sleep, compared to their smaller co-twins. Although these results seem to deviate substantially from the present and previously published articles, this could be due to the relatively small sample size and the twins’ age, that is, only a few days, which may have influenced infants’ response to the experimental stimuli.
The present study comes with some limitations. Data were collected by means of an online, maternally reported questionnaire, although a commonly used and validated one. This has precluded the collection of any data regarding chorionicity and intrauterine growth. Furthermore, because of the volunteer-based nature of the study, it was susceptible to selection bias, in that mothers of twins with problematic behaviors might have been more interested in participating in the research. However, although parental ratings are subject to several types of limitations, they are still considered a critically important source of information in behavioral genetics research. In fact, even though psychologists and psychiatrics would provide more detailed and less biased description of children's behavior, their observations would involve stressful, standardized situations, and a laboratory setting in which observed responses and reactions may not reflect children's usual behavior. On the opposite, parents’ ratings, while not perfect, are thought to better summarize children's normal reactions to everyday stimuli, especially during the preschool years (Saudino et al., Reference Saudino, Cherny and Plomin2000). Furthermore, we had no data regarding parenting behaviors. It is possible that differential parenting practices toward co-twins might naturally derive from birth-weight discordance, as parents might tend to provide more attention to their smaller, weaker child, which might in turn differentially influence twins’ behavior (Asbury et al., Reference Asbury, Dunn, Pike and Plomin2003). Other externalizing and internalizing risk factors (i.e., familial socio-economic status, maternal education levels, substance abuse, and psychopathology) reported by Carneiro et al. (Reference Carneiro, Dias and Soares2016) were not available for the current study. However, these factors would probably not have affected the results in the current research design, as they are factors common to the co-twins and, therefore, likely to increase their similarities instead of their differences.
In conclusion, this study reports a clinically significant association of MZ twins’ absolute birth-weight difference with total and internalizing problems and emotional reactiveness score differences, calculated with the CBCL 1½–5. This clearly indicates that unique environmental factors (i.e., those causing the different birth weight) are involved in young twins’ psychological and behavioral development. However, we cannot exclude the role of other non-shared environmental influences, such as chorionicity and differential parental treatment between co-twins. Future studies should include these data for elucidating their role in the psychological development of young twins born discordant for birth weight.
Acknowledgments
The authors wish to thank all participating families. The study was supported by the University of Birmingham (grant number: GAS1168).
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the University of Birmingham Ethics Committee.
Informed Consent
Informed consent was obtained from all individual participants included in the study by the University of Birmingham Ethics Committee.








