Introduction
Countries have developed healthcare systems in order to ensure people have access to healthcare in a coordinated fashion and ensure the wellness of their nations. The World Health Organization considers health systems as all the organizations, people, and actions that have a primary intent to promote, restore or maintain health including efforts to affect the determinants of health and more directed health-improving tasks (1). Healthcare systems are defined by three main dimensions financing, service provision, and regulation (Reference Böhm, Schmid, Götze, Landwehr and Rothgang2). There are four main types of healthcare systems: the Bismarck model, the Beveridge model, the National Health Insurance (NHI), and the out-of-pocket model (Reference Böhm, Schmid, Götze, Landwehr and Rothgang2;3). Canada primarily uses the NHI model where the healthcare system is funded directly by income tax deductions and the facilities are owned and operated by the government (Reference Böhm, Schmid, Götze, Landwehr and Rothgang2–Reference Husereau4), The Canada Health Act, 1984, was developed to ensure eligible residents have universal access to healthcare services (Reference Husereau4). Delivery of services is determined by provinces and territories that pool funds into general revenue and the federal government contributes to the revenue pools as per the Health Transfer Agreement (Reference Husereau4). Private health insurance can be purchased through employers to cover medical services not covered by the Act (Reference Husereau4).
It was estimated that Canada spent approximately CA$ 305 billion on healthcare in 2020, representing 12.9 percent of GDP with an average of CA$ 7,507 per capita. This is above the Organization for Economic Co-operation and Development (OECD) average of CA$ 5,502 per capita (5). The amount spent on medical devices and technologies in Canada is 3–5 percent of the healthcare expenditure, although these estimates are not systematically tracked (Reference Husereau4). OECD-developed countries are always looking to improve their healthcare systems and are considered early adopters of new technologies that benefit patients.
Surgery is a highly technical specialty that commonly uses advanced devices and technologies to treat patients. The purchase and adoption of these technologies can occur at any time in the technology adoption life cycle from the innovators to the early adopters, to the early majority, to the late majority, and finally to the laggards (Reference Rogers, Singhal and Quinlan6). The initial decision to adopt surgical technology is by the surgeon, who is the primary user of the device. In the early adoption stage and where there is lack of well-established criteria for decision making, surgeons can decide to adopt technologies based on factors such as (i) surgeon’s preference, (ii) beliefs about the benefit of the technology for their patients, (iii) presentations from conferences, and (iv) information from marketing and sales teams. (Reference Perl, Sheth, Shea and Wall7).
Innovation take-up is a dynamic process involving multiple formal/informal decisions by a multitude of interactive factors. In Canada, technology purchase is mainly done through regional health authorities or hospitals via global budgets provided by the provincial health ministries (Reference Husereau4). Some provinces tend to use health technology assessments (HTAs) for devices or drugs, but it is unclear at which stage this assessment is conducted. Surgical devices and technologies are one of the most expensive expenditures of the procurement process. Hospitals commonly create technology assessment committees that act as the gatekeepers for the adoption of these new technologies by assessing their value-added benefit (Reference Perl, Sheth, Shea and Wall7). However, in the early adoption stage, clinical outcomes of the technology are limited and of short duration, making the assessment of value difficult, if not impossible. As there is limited information on clinical outcomes on surgical technologies at the early adoption stage, which is considered within the exploration stage (stage 2b) of the IDEAL framework, informed criteria for decision making, mentoring, and learning curve evaluation would be considered important (Reference McCulloch, Altman and Campbell8).
Understanding the role of provincial and local technology assessment committees and the criteria for decision making will help surgeons better recognize the opportunities and requirements to influence the early adoption of innovative technology for the surgical care of their patients (Reference Perl, Sheth, Shea and Wall7). The aim of this study is to identify the criteria used by surgeons, hospitals, and provincial bodies and characterize the decision-making process for the adoption of new innovative surgical technology in the Canadian healthcare system. The study will also explore the current challenges and opportunities in the Canadian healthcare system to adopt new technologies to highlight opportunities in other healthcare systems.
Methods
The methodology for the study was conducted following the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis (Reference Peters, Godfrey, McInerney, Munn, Tricco, Khalil, Aromataris and Munn9). This study was also reported using the Preferred Reporting Items for Systematic Reviews and Meta-analysis for Scoping Reviews statement guidelines and flowchart (PRISMA-ScR) (Reference Tricco, Lillie and Zarin10).
Search strategy
A literature review was conducted using MEDLINE and EMBASE databases, and Google Scholar searching for grey literature. A medical librarian has been consulted for assistance with the keywords and literature search. Provincial HTA Web sites in Canada were also searched along with federal HTA agencies including the Canadian Agency for Drugs and Technologies in Health (CADTH). Search terms were developed to identify articles for the study, and they included the ten provinces and three territories in Canada, all surgical fields, decision making, opportunities, challenges, adoption, innovators, and health technologies. Medical subject headings (MeSH terms) used were “surgical procedure,” “decision making,” “surgical technologies,” “Canada.” All terms were combined using Boolean terms “And”/“Or.” The search terms used are found in Supplementary Table 1.
Study selection and inclusion and exclusion criteria
The study selection and screening were conducted by two independent reviewers and there were no disagreements. This review only included articles published from inception until December 2021. Articles included were observational studies, randomized trials, HTA’s, case studies, and series. The study included articles focused on the decision-making process for early new surgical technology adoption into clinical practice, articles published in English and French, articles that focus only on the Canadian healthcare system and its thirteen provinces and territories and articles that explore the strengths and weaknesses in the Canadian system for technology adoption. The articles that focused on the decision-making process include whether these were decisions already made to adopt a technology, or decisions yet to be made by physicians. All hospital-based and province-based studies were considered and screened for eligibility according to the inclusion criteria and were then referred for full-text assessment. Articles outside Canada, in languages other than English and French which did not include adoption of technologies were excluded from this study.
Data extraction
Articles found were imported into Endnote X9 reference manager software where duplicates were removed and the filtration process for all studies took place. There were no disagreements between authors. The data was then extracted into a spreadsheet created in Microsoft Excel (Table 1). The data extracted included information based on the author and year the article was published, the level of evidence and study type, the geographic location, surgical specialty, the surgical device (technology), decision-making framework and criteria, challenges, opportunities, and general applicability. Data were also extracted from provincial Web sites identifying the criteria used and the responsible HTA agency (Table 2).
Table 1. Data extraction from database search

HTA, health technology assessment; NA, not applicable.
Table 2. Data extraction from provincial Web sites
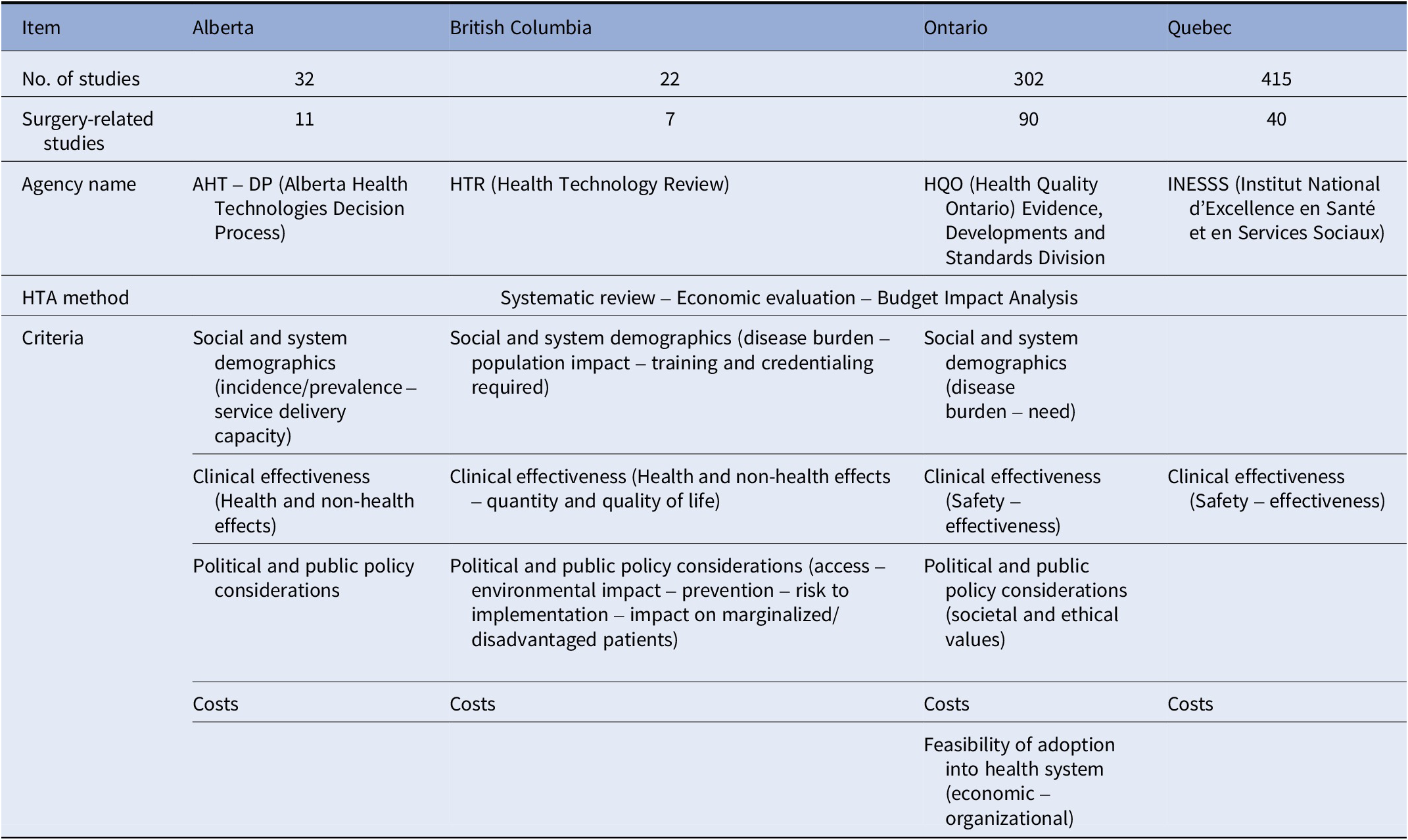
HTA, health technology assessment.
Data synthesis and analysis
Articles found were grouped into hospital-based and province-based studies. Criteria that were used by physicians in the decision-making process for technology adoption were collected and reported. The frequency of reporting of each criterion was also collected. The criteria were then grouped and classified based on a thematic categorization of all the criteria and guidance sought from previous studies (Reference Almeida, Mines and Nicolau11;Reference Husereau, Boucher and Noorani12). Finally, the surgical technologies identified in the studies were grouped into surgical fields along with when they were adopted.
Results
The search strategy for this study yielded a total of 4,966 articles (4,195 from the database search and were hospital-based; and 771 from provincial Web sites). After duplicates were removed and screening was done, the searches identified 155 articles that met the inclusion criteria (Figure 1). Of these, 148 were HTA reports from provincial Web sites, four were case studies and three were policy review articles. A total of ninety-three articles were from Ontario, forty were from Quebec, thirteen were from Alberta, seven were from British Columbia, one was from Nova Scotia and one was a national study. The technology assessed included surgical devices for cardiothoracic surgery, general surgery, obstetrics and gynecology, orthopedics, and ophthalmology. None of the articles indicated in which stage of the technology adoption life cycle the technology was in at the time of its review. Figure 2 shows all criteria and subcriteria found from the search strategy.

Figure 1. PRISMA (scoping review) flowchart.

Figure 2. Frequency of criteria reporting from studies.
Criteria elicitation – HTA reports from provinces
All 148 provincial HTA reports used the same methodology which included a systematic review, an economic evaluation, and a budget impact analysis of the technology. Provinces used a set of criteria that were determined by each province and were only standardized across the HTA reports that they used (13). Table 2 shows a summary of the information gathered from the provincial Web sites. The price of the technology and its clinical effectiveness (safety and effectiveness) were the most important criteria used in the decision-making process in all the provinces (Reference Böhm, Schmid, Götze, Landwehr and Rothgang2–Reference Rogers, Singhal and Quinlan6). In three provinces, political and public policy considerations, as well as social and system demographics (incidence and prevalence of the condition) were used to guide their decisions and they were considered additional criteria (13–17). The political and public policy considerations include access to the technology, environmental impact, prevention of diseases, risk of implementing the technology, and impact on marginalized/disadvantaged patients. One of the provinces also used societal and ethical values in considering which technologies to adopt along with the feasibility of adoption into the healthcare system (13–17). Cost, safety, efficacy, economic impact, and feasibility of implementation of the technology were the most frequently reported criteria across all studies. This is in line with the provincial priorities on what guides them to adopt new technologies into their hospitals.
Thematic groupings and criteria elicitation (from the seven articles)
Seven articles from the database search identified the priority criteria that surgeons use in their decision-making process to adopt a new surgical device (Reference Borowski, Brehaut and Hailey18–Reference Urquhart, Porter, Sargeant, Jackson and Grunfeld24). All the criteria were gathered systematically by the authors using structured methodologies from the JBI Manual for Evidence Synthesis (Reference Peters, Godfrey, McInerney, Munn, Tricco, Khalil, Aromataris and Munn9). Overall, thirty-three criteria were identified as influencing surgeons, and other healthcare professionals, in adopting a new technology. The criteria were extracted from Table 1. The methodologies used include using different qualitative frameworks with questionnaires designed to ask surgeons what is considered important in their decision-making process. These frameworks are the Alberta Heritage Foundation for Medical Research Framework, the Accountability for Reasonableness, the Socio-technical dimensions and features, the Calgary Health Region HTA, and the Organizational Framework of Innovation Implementation.
The thirty-three criteria had recurrent themes and could be categorized by thematic categorization into seven distinct groups of criteria (Table 3). Group 1 includes all the criteria that relate to the economics of the technology. Group 2 includes the hospital-specific criteria and refers to how this device fits into the hospital’s ecosystem, integration, and workflow. Group 3 includes the technology-specific criteria and refers to features that define the device and its specifications. Group 4 is the relevance to patients and the public and these criteria refer to the usability of the technology/device to the overall population and their feedback. The Group 5 criteria are related to clinical outcomes from the clinician’s perspective. Group 6 is policies and procedures criteria and refers to regulations in the country/hospital that facilitate integration and ease of usability of the technology. Finally, Group 7 are criteria that are physician-specific and refers to how the physician interacts with the technology. It is worth noting that there was no weighting of any of the thirty-three criteria in considering which is more important among the studies.
Table 3. Categorization of criteria for decision making

Challenges
Three of the studies identified challenges with the criteria used by surgeons to adopt novel technology. First, there is expressed uncertainty about whether or not these criteria were generalizable for all technologies in all surgical specialties (Reference Goeree, Levin and Chandra20). Second, there was potential bias in the surgeon’s criteria, thereby limiting its applicability as these criteria were prioritized by physicians in large hospitals, and may not be generalizable nor applicable to smaller hospitals with smaller budgets and limited access (Reference Danjoux, Martin and Lehoux19). Third, Canada’s healthcare system presently lacks a universal strategic system with a guide on how to adopt new technologies (Reference Lehoux, Hivon, Williams-Jones, Miller and Urbach21). Furthermore, provincial HTAs only assess technology based on cost-effectiveness, budget impact, and clinical outcomes.” Two of the studies found that there might be a recall bias where some physicians could not recall the last time, they decided on how or why should a new technology be adopted (Reference Sharma, Danjoux, Harnish and Urbach23;Reference Urquhart, Porter, Sargeant, Jackson and Grunfeld24).
Opportunities
All seven studies, including the two studies that utilized provincial HTA criteria, identified specific opportunities that could help improve the Canadian healthcare system in procuring new technologies at the early adoption stage. These opportunities addressed the process at both the provincial and hospital level. First, surgeons with well-defined criteria to adopt a new technology would help in providing a useful evidence-based framework for decision making. This primary data collection is considered a supplement to the evidence available for formal HTA reports (Reference Goeree, Levin and Chandra20). Second, criteria gathered from surgeons would help enhance the strength and availability of evidence while enabling decision making to balance what is needed for policy formulation (Reference Borowski, Brehaut and Hailey18). Third, such criteria collected would help in triggering hospitals to better develop a structure that would involve wider stakeholders for more input and prompt the development of a comprehensive appeals mechanism for addressing challenges to decisions made (Reference Danjoux, Martin and Lehoux19). Fourth, such criteria would help manufacturers create Web sites for these products that would bolster the surgeons’ expectations and needs by answering their questions based on the criteria already gathered a priori (Reference Lehoux, Hivon, Williams-Jones, Miller and Urbach21). This would help create a more transparent platform for surgeons to make more informed decisions. Fifth, HTA programs available locally and nationally help bridge gaps where evidence is lacking to support surgeons’ knowledge of a new technology for more informed decisions (Reference Poulin, Austen, Kortbeek and Lafrenière22). Sixth, public representation along with physician’s expertise to ensure public and patient insights are taken into consideration (Reference Sharma, Danjoux, Harnish and Urbach23). Finally, the adoption of technologies should involve internal multilevel stakeholders’ such as administrators, other health professionals and hospital decision makers early in the process to facilitate uptake and adoption as there are regulations that can either prompt or hinder adoption apart from surgeons’ needs (Reference Urquhart, Porter, Sargeant, Jackson and Grunfeld24).
Applicability
The views on the applicability of using these criteria amongst all provinces and hospitals differed between authors. In two studies, it was contended that the observations in one hospital and community might not be generalizable because of the diverse structure and socio-political context of healthcare systems in different jurisdictions (Reference Sharma, Danjoux, Harnish and Urbach23;Reference Urquhart, Porter, Sargeant, Jackson and Grunfeld24). In addition, this is further confounded by a wide range and diversity of stakeholders, complex governance structures, resource arrangements, and high degrees of professional autonomies (Reference Urquhart, Porter, Sargeant, Jackson and Grunfeld24). Two of the studies felt that the inclusion of the surgeons in the decision-making process made the adoption assessment more applicable and universal. Goeree et al. (Reference Goeree, Levin and Chandra20) speculated that when physicians’ criteria for adoption are supplemented by HTA reports outcomes, it could help create more informed decisions that would be applicable to different settings. In addition, applicability to other systems and feasibility of such criteria could be possible when decisions include several stakeholders, especially from the government, so that there is a balance from a multitude of factors including the regulatory environment (Reference Borowski, Brehaut and Hailey18).
Discussion
The aim of this study was to identify the criteria used to determine the decision making for new technology adoption. Thirty-three criteria were identified and grouped into seven categories named: economic, hospital-specific, technology-specific, patient-specific, clinical outcomes, policies and procedures, and finally physician-specific. In the Canadian healthcare system, there is no standardization of decision-making criteria in technology adoption. Although there is some overlap between the criteria felt to be important by surgeons and the provincial/hospital committees, the provincial and hospital committees focus primarily on the cost of the technology and its clinical effectiveness. This limits the opportunity for the adoption of innovative technology in the early adoption stage as there is only limited outcomes information available from the innovators. However, this study identified multiple opportunities to help improve the Canadian healthcare system in procuring new technologies at the early adoption stage.
Prioritization criteria in Canada
CADTH, created in 1989, is the main agency that coordinates an approach for all HTAs to produce evidence-informed recommendations that will assist decision makers and benefit patients (25). CADTH has identified priority-setting criteria for new technology assessment and adoption based on the EUR-ASSESS project and then conducted a multiple-criteria decision making (MCDA) to weigh the criteria and identify priorities based on the weights after consultation with selected committees (Reference Husereau, Boucher and Noorani12;Reference Noorani, Husereau, Boudreau and Skidmore26). The assessment was based on all new technologies and drugs; and the selected committee members were mainly representatives from federal, provincial, and territorial publicly funded drug plans and pharmacists working for the ministries of health (Reference Husereau, Boucher and Noorani12). No surgeons/physicians were included in these committees who are considered the ultimate users of these technologies. The CADTH study revealed that the clinical impact of technologies carries the highest weight for decision makers, followed by (in descending priority order): the burden of disease, the economic impact, budget impact, availability of evidence, and alternatives for the technology (Reference Husereau, Boucher and Noorani12). The process for device use in Canada requires that the product receive Health Protection Branch of Canada (HPB) approval or HPB approval for a batch release to conduct a clinical trial.
The “value” in decisions
Value is broadly known as the ratio of quality to cost, but this varies among healthcare stakeholders (Reference Perl, Sheth, Shea and Wall7). The global landscape view on value has challenged leaders to explore new models to engage clinicians for shared risk and rewarding successful adoption for improved patient outcomes. Such value committees are growing today more than ever due to the pressing global challenges from natural threats, industrialization, globalization, economic pressures, and changing patients’ needs. In Canada, there has not been a comprehensive study that explores the prioritization criteria for decision making for surgical technology early adoption from the surgeon’s perspective. As well, the criteria presently used for technology adoption are most applicable during and after the early majority stage, when clinical outcomes and longer-term follow-up become available. They do not specifically address the criteria to adopt technology in the early adoption phase, when there is limited outcome data from the innovators, that only make up 2.5 percent of the users. Involving surgeons, the end-users, and making them part of such decisions, or even developing a criteria framework based on surgeon’s decisions in the evaluation of new technologies, would be a more tailored approach that would eventually benefit patients (Reference Perl, Sheth, Shea and Wall7). The IDEAL framework has proposed the assessment of surgical innovation based on a five-stage description of the surgical development process; innovation, development, exploration, assessment, and long-term study (Reference McCulloch, Altman and Campbell8). Early adopters can be involved in the development and improvement of the technology but are primarily involved very early in the exploration phase. This phase uses early and limited prospective and collaborative cohort studies to focus on the learning curve, the indications for the innovation, and its quality. These criteria are some of the assessment tools identified in our review, specifically in the categories of clinical outcomes, physician specific and technology specific. This can prompt the development of controlled trials in the exploration stage where the learning curve can affect surgeons’ involvement in these studies as they can identify relevant outcome measures (Reference McCulloch, Altman and Campbell8). These measures would be crucial for research databases and trials and would include technical, clinical and patient-reported outcomes to help provide further information about the technologies used and guide other surgeons for making informed decisions (Reference McCulloch, Altman and Campbell8).
The limitations of this study would help prompt further research in criteria prioritization. There was a lack of any quantitative metrics for criteria weighting based on the results we found. This makes it challenging to identify which criteria are considered a priority over another. Another limitation is that the results found may not represent all of Canada as most of the results found were attributed to only four provinces’ HTA reports. Most of the studies and reports did not factor in the surgeon’s perspective and priorities in technology adoption. In addition, many of the studies in the literature are older and it is unclear how well, or if they are reflective of current practice. However, it does indicate the need for further studies that explore the changing dynamics of health systems and patients’ needs. More research is needed to challenge and validate the criteria using quantitative metrics to weight and prioritize them for guiding surgeons with informed decision making for the early adoption of new surgical technologies in the Canadian healthcare system. As well, the relative weight of each criterion may vary by geographic region, healthcare system, and hospital.
Conclusion
The economic and clinical impact of new technologies is the two most important criteria for technology adoption in healthcare in Canada. The findings of the scoping review have also highlighted some of the deficiencies in the present literature. Value assessment committees should include surgeons in the decision-making process and more research is needed for a comprehensive study that would explore the surgeon’s perspective in criteria prioritization for technology adoption. Further studies are needed from other provinces to help have a representative set of weighted criteria that would be applicable to the entire country. Specific criteria for decision making in the early adoption stage of novel technologies are lacking. These criteria need to be identified, standardized, and applied in order to provide innovative, and the most effective healthcare to Canadians.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0266462323000363.
Funding statement
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interest
The authors have no competing interest.







