It is well established that the presence of mixed protozoa species populations in the rumen have a negative effect on the utilisation of dietary N by ruminants(Reference Veira1). In comparison with defaunated (all existing protozoa eliminated from the rumen) or fauna-free (FF; protozoa never existed in the rumen) ruminants a number of experiments have shown a decreased flow of non-NH3-N (NAN) components from the stomach to the intestinal tract when mixed protozoa populations were present in the rumen(Reference Coleman, Nolan, Demeyer and Leng2–Reference Ivan, Hidiroglou and Petit5). However, the species of the mixed population that contribute negatively to N utilisation and to what extent they do was not known. Although a number of studies involved individual species of protozoa such as Entodinium (EN) and Isotricha (IS)(Reference Jouany and Senaud6–Reference Jouany, Zainab, Senaud, Groliere, Grain and Thivend9), the effect of these species on the duodenal flow of NAN components has not been measured. Therefore, we conducted a series of experiments in this laboratory on N metabolism by individual or combinations of ciliate protozoa species (IS, Dasytricha (DS), Polyplastron (PP), Epidinium (EP), Eudiplodinium (EU), EN and total mixed fauna (TF)). These species were established in the rumen of naturally FF sheep which originated from a sheep flock free of rumen ciliate protozoa for several generations(Reference Ivan, Veira and Kelleher10). The present work concludes the series which commenced with an experimental approach where FF sheep were inoculated stepwise with individual major species of ciliate protozoa and measurements were conducted progressively after each inoculation(Reference Ivan, Neill and Entz11). Since PP cannot coexist with EP and EU in the rumen, and it belongs to a different type of rumen fauna population (type A) than do EP and EU (type B)(Reference Eadie12), concomitant measurements of effects of individual and combinations of the above protozoa species were conducted in both the type A and type B populations(Reference Ivan, Neill and Entz11). The results showed that each species or combination of species decreased the duodenal flow of bacterial N (BN) and of NAN, but to a different degree; the effects on the flow of individual cellulolytic species in the type A (PP) and type B (EP, EU) populations are virtually the same. Owing to the above initial experimental approach of the progressive inoculation the effects of certain species or combinations of species could not be precisely established. Therefore, further work was then conducted with individual ciliate protozoa populations that were established in individual groups of FF sheep that allowed direct comparison of results(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). Thus the magnitude of the effects on components of the duodenal N flow were established for IS, DS, EN and TF-type A in sheep fed different diets(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13), and for EN and PP in sheep fed a single diet(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). It was found that the decreasing duodenal flow of BN and NAN due to EN was much larger than those of IS or DS(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13), but these effects of the cellulolytic PP were similar to those of EN, while there was no duodenal protozoal N flow in PP-monofaunated sheep(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). However, when DS was added to the population of IS monofauna in the initial experiment(Reference Ivan, Neill and Entz11) the flow of duodenal NAN was similar to that of FF sheep and higher than the flow in sheep with IS monofauna or DS monofauna. In the stepwise establishment of individual protozoa species in the rumen protozoa population the existing species might affect the establishment of a new species, and vice versa (Reference Ivan, Neill and Entz11, Reference Williams and Coleman15). It was hypothesised, therefore, that similar to the differences in effects of PP in previous experiments(Reference Ivan, Neill and Entz11, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) the combined effects of IS and DS might be different when these species are established in the same rumen individually (stepwise)(Reference Ivan, Neill and Entz11) than when established simultaneously as a Holotrich (IS plus DS sp.) protozoa population (HT). Because direct comparison of this population of HT with TF and FF sheep was not made in previous experiments(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13), there was a lack of conclusive evidence on the relative effects of HT on the duodenal flow of N components. To alleviate this lack and to complete the overview of the dynamics of effects on the duodenal N flow of individual ciliate protozoa monofaunas and their combinations it was the objective of the present experiment to compare these effects of HT with EN as one of the species affecting N metabolism the most(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14), and with TF and FF sheep as controls.
Materials and methods
All sheep used in the experiment originated from an FF flock(Reference Ivan, Veira and Kelleher10); thus, ciliate protozoa have never been previously present in the rumen of these sheep. They were cared for according to guidelines of the Canadian Council on Animal Care(Reference Olfert, Cross and McWilliams16), and the experimental protocol was approved by the Research Centre Animal Care Committee.
Sixteen approximately 1·5-year-old FF Canadian Arcott sheep (castrated males) were each surgically fitted with rumen(Reference Hecker17) and re-entrant duodenal(Reference Ivan and Johnston18) cannulas. The duodenal cannula was placed proximal to the common bile and pancreatic duct. The sheep were used in the present experiment approximately 4 months after cannulation. One group of four sheep remained FF, while the other three groups of four sheep were inoculated intraruminally with the HT, EN and TF protozoal populations as described previously(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). Each group of sheep was housed in individual pens in a separate room and measurements were initiated 2 weeks later in a 25 d experiment.
Sheep were fed twice daily (500 g DM in each feeding) a maize silage-based diet at 08.00 and 16.00 hours. Drinking water was available continuously. The diet (DM basis) consisted of (g/kg): maize silage, 853; soyabean meal, 122; Co-iodised salt, 11; monodical (contained 21 % P and 15 % Ca), 4; limestone, 6; vitamin mix, 1 (4 mg retinol and 30 μg α-tocopherol); Cr2O3, 3. The silage was supplemented with soyabean meal to ensure sufficient N for optimal microbial growth. Chromic oxide was used as a flow marker and together with monodical and vitamin mix was mixed into the soyabean meal, which was then again mixed into the silage as a single batch. The mixed feed was bagged and stored in a cold room. The chemical composition of the diet was (g/kg DM): N, 23; acid-detergent fibre, 234; neutral-detergent fibre (NDF), 413; organic matter (OM), 934.
On day 15 the sheep were placed into individual metabolism cages(Reference Ivan and Hidiroglou19). Faeces, duodenal digesta, and rumen fluid were collected and sampled on days 18 to 19, 22 and 23, and 24 and 25, respectively. A small quantity of the diet was collected at each feeding on days 15 to 25 and accumulated as a sample. The samples were freeze-dried. Concentrations of Cr in feed and faecal samples were used for calculation of the daily excretion of faeces (g) = DM intake (g) × (feed Cr (mg/g)/faecal Cr (mg/g)).
Total duodenal digesta were collected on two consecutive days as described previously(Reference Ivan, Neill and Entz11). A 10 % sample was accumulated for each sheep and each sample was subdivided into two parts. One part was centrifuged at 70 000 g and 5°C for 30 min and the other part was freeze-dried. Concentrations of Cr in feed and digesta samples were used for calculation of the daily digesta flow (g) = DM intake (g) × (feed Cr (mg/g)/digesta Cr (mg/g)).
Rumen contents (500 ml) were obtained through the rumen cannulas(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) at 0, 2, 4 and 6 h after the morning feeding. The pH of the rumen contents was measured immediately. Part (150 ml) of the rumen sample was then strained through one layer of cheesecloth. Filtrate (5 ml) was preserved with 5 ml methyl green–formalin–saline solution(Reference Ogimoto and Imai20). Preserved samples from the two collection days were combined and used for protozoal counts as described previously(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). The remainder of the contents was strained through two layers of cheesecloth and a 20 ml portion was centrifuged at 70 000 g at 5°C for 30 min to obtain the soluble cell-free fraction.
Freeze-dried feed, duodenal and faecal samples were ground to pass through a 1 mm diameter sieve (Wiley Mill model 4; Thomas Scientific, Swedesboro, NJ, USA) before determining analytical DM, OM, acid-detergent fibre, NDF, Cr and amino acids (AA). Analytical DM was determined by drying samples at 135°C in an oven for 2 h and followed by hot weighing. All chemical analyses were performed in duplicate in each sample. The OM content was calculated as a difference between 1000 and the g/kg DM of ash(21). The NDF was determined as described by Van Soest et al. (Reference Van Soest, Robertson and Lewis22), and acid-detergent fibre was determined according to the procedure of the Association of Official Analytical Chemists(21). The Kjeldahl procedure was used for determination of N. The NH3-N content in strained rumen fluid and centrifuged duodenal digesta was quantified by the phenol-hypochlorite reaction(Reference Weatherburn23). The NAN content in rumen fluid and duodenal digesta was determined by subtraction of NH3-N from total N. Determination of volatile fatty acids in rumen fluid was as described by Erfle et al. (Reference Erfle, Mahadevan and Sauer24). Amino acid concentrations in freeze-dried duodenal digesta were measured by an AA analyser (Beckmen Instruments, Palo Alto, CA, USA) after hydrolysis in 6 m-HCl(Reference Gehrke, Wall, Absheer, Kaiser and Zumwalt25). Concentration of diaminopimelic acid was used for the determination of duodenal flow of BN as described by Ivan et al. (Reference Ivan, Neill, Forster, Alimon, Rode and Entz13). The Cr concentration in dry feed, duodenal digesta and faeces was measured by air and acetylene flame atomic absorption spectrometry after digestion of samples with a mixture of nitric and perchloric acids.
The data were statistically analysed as a completely randomised design using PROC MIXED from SAS (SAS Institute, Inc., Cary, NC, USA)(26) with treatment as the fixed effect. When the treatment effect was significant, an LSD test was used to separate the means. The UNIVARIATE procedure was used to test the data for normality and for obvious outliers. Differences were declared significant at P < 0·05.
Results
Protozoa and rumen fermentation
There were no protozoa present in any of the sheep of the FF group (Table 1). Similarly, no other than the inoculated protozoa species were found in other groups of sheep, indicating that there was no cross-contamination of protozoa among the experimental groups of sheep. The HT group averaged approximately 0·8 × 104 cells per ml rumen fluid of each the IS and DS species, while the EN group of sheep averaged more than twice as many EN cells (approximately 3 million) compared with the TF group of sheep (approximately 1·5 million), in which the number of EN species formed 98 % of the size of the total mixed protozoa population.
Table 1 Protozoal population in sheep with different types of rumen fauna (number of cells/ml rumen fluid×104)
(Mean values with their standard errors)
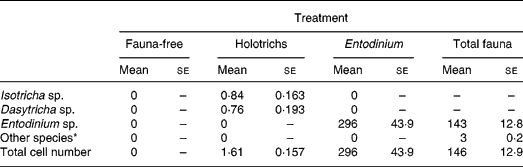
* Includes Isotricha sp., Dasytricha sp., Polyplastron sp., Diplodinium sp., etc.
The mean rumen pH (Table 2) was not affected by the protozoa treatments (P>0·05). The concentration of NH3-N in the rumen fluid of the FF group was lower (P < 0·05) than that of the HT group, while the concentrations in the fluid of the EN and TF groups were similar (P>0·05) but higher (P < 0·05) than in the fluid of the other groups (FF and HT). When the NAN concentration was calculated as a percentage of total N it ranged between 93·6 (FF) and 86·2 (TF). The NAN concentration in the decreasing order was FF > HT > EN > TF; the differences were significant between the TF and the other protozoa treatments (FF, HT, EN), and between the EN and FF treatments. The solubility of N in rumen fluid was highest (16·6 %; P < 0·05) in the EN group and lowest (8·9 %) in the FF group, but the differences between the FF and HT and between the HT and EN groups were not significant. The proportion of α-amino-N in the total NAN was not affected (P>0·05) by the protozoa treatments. The concentration of total volatile fatty acids and acetate:propionate ratio were not affected (P>0·05) by the protozoa treatments, while the proportions of acetic acid were higher and those of butyric acid were lower for the FF and HT groups than for the EN and TF groups (P < 0·05). No specific pattern in proportions of other individual volatile fatty acids was apparent.
Table 2 The pH, concentrations of nitrogen components and volatile fatty acids (VFA) in rumen fluid of fauna-free (FF) sheep and of sheep inoculated intraruminally with Holotrich protozoa, Entodinium monofauna or total fauna-type A
(Mean values with their standard errors)

NAN, non-NH3-N.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Duodenal flow
The duodenal flow of N components was not different (P>0·05) between the FF group and the HT group (Table 3). There were also no differences (P>0·05) between the EN group and the TF group. However, the BN flow was higher (P < 0·05) for the FF and HT treatments than for the other treatments (EN, TF), while the difference between the FF and the HT treatments were not significant. The flow of non-bacterial NAN was higher (P < 0·05) for the TF than for the FF treatment, but the differences were not significant among the other treatments (FF, HT, EN). As a percentage of NAN the BN flow ranged between 92·3 (FF) and 63·9 (TF) in the decreasing order FF > HT > EN > TF.
Table 3 Duodenal flow of nitrogen in fauna-free (FF) sheep and in sheep inoculated intraruminally with Holotrich protozoa, Entodinium monofauna or total fauna-type A (g/kg organic matter intake)
(Mean values with their standard errors)
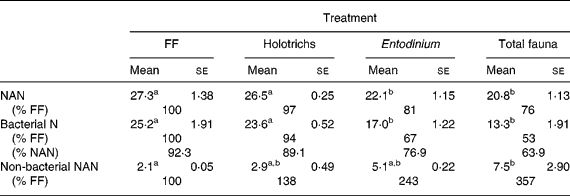
NAN, non-NH3-N.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The duodenal flows of total AA were similar (P>0·05) for the FF and HT treatments and also for the EN and TF treatments, but the flows for the FF and HT were higher (P < 0·05) than those for the EN and TF treatments (Table 4). There were various significant differences among treatments in the flow of individual AA (see Table 4), but no appreciable differences due to treatments were apparent when the flow of individual AA was calculated as a percentage of flow of total AA.
Table 4 Duodenal flow of total and selected individual amino acids (AA) in fauna-free (FF) sheep and in sheep inoculated intraruminally with Holotrich protozoa, Entodinium monofauna or total fauna-type A (g/kg organic matter intake)
(Mean values with their standard errors)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Digestibility
The coefficient of apparent digestibility of OM in the stomach was higher (P < 0·05) for the EN treatment than for the HT treatment, but the differences among the other treatments were not significant (Table 5). The differences among treatments in the apparent digestibility of acid-detergent fibre were not significant, but the apparent digestibility of NDF for the EN treatment (0·506) was higher (P < 0·05) than that for the FF (0·334) and HT (0·343) treatments. The differences among other NDF coefficients were not significant. Except for the non-significant difference between the EN and FF treatments in the total tract digestibility of OM, all the digestibility coefficients (OM, acid-detergent fibre, NDF) were higher (P < 0·05) for the EN and TF treatments than for the FF treatments.
Table 5 Apparent digestibility of organic matter, acid-detergent fibre and neutral-detergent fibre in fauna-free (FF) sheep and in sheep inoculated intraruminally with Holotrich protozoa, Entodinium monofauna or total fauna-type A (g/g intake)
(Mean values with their standard errors)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
It was previously shown in one of the experiments(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13) in the series conducted in this laboratory(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) that irrespective of the diet the number of EN cells was much higher in the EN monofaunas than in the TF populations. In the present experiment and as previously reported(Reference Koenig, Ivan, Teferedegne, Morgavi, Rode, Ibrahim and Newbold27) the number of EN cells was approximately double in the EN monofauna compared with in the TF population. Results from an additional experiment in the series(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) established that the quantitative duodenal flow of bacterial N for the EN monofauna and the PP monofauna receiving the same diet is virtually identical. This indicates that both ciliate species populations ingest rumen bacteria, and their predatory activity on the bacteria is equal. These species differ, however, in cell size and metabolic activities. Thus PP is much larger and digests cellulose, while EN is a much smaller starch-utilising protozoon(Reference Williams and Coleman15). It would appear that the main competition of the two ciliate species is that for the bacterial protein. Since in the EN monofauna of the present experiment there was an obvious absence of PP as compared with the TF type A that contains this protozoon, there was probably a double amount of bacterial protein available for ingestion by the EN, hence the double number of this protozoon in the EN monofauna as compared with the TF type A. Since the rumen bacteria predatory activity for other cellulolytic ciliate species (EP, EU) is similar to that of PP(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14), there is probably a reciprocal competition for the rumen bacterial protein and resulting dynamics of the population growth between EN and all the cellulolytic protozoa in both type A and type B rumen protozoa populations. This is further substantiated by the fact that there were much lesser cell numbers of the cellulolytic protozoa species (PP or EP plus EU) when EN was present in the type A or B complete populations compared with partial populations of both types containing the cellulolytic species but not EN(Reference Ivan, Neill and Entz11). It should also be emphasised that PP may ingest EN together with bacteria(Reference Williams and Coleman15) and in this way may contribute to the reduction in the proportion of EN in the TF population.
In the present experiment, the pH, concentrations of total volatile fatty acids and actate:propionate ratio in rumen fluid indicated no major effect on the rumen fermentation by the protozoal populations tested. However, the concentration of NH3-N was higher in all three fauna populations (HT, EN, TF) than in FF sheep. Similarly, higher concentrations of NH3-N were observed for IS, DS and their combinations in the previous experiments(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13). This is in agreement with other reports(Reference Jouany, Zainab, Senaud, Groliere, Grain and Thivend9, Reference Christiansen, Kawashima and Burroughs28), but contrary to reports from in vitro and in sacco experiments(Reference Jouany, Sénaud, Toillon, Ben Salah, Bohatier and Prensier8, Reference Jouany, Ivan, Papon and Lassalas29, Reference Jouany30). These reports noted that the monofaunation of a defaunated rumen in sheep with IS decreased concentrations of NH3-N in rumen fluid and the deaminating activity of digesta compared with defaunated or refaunated sheep; it was suggested that IS may increase the by-pass of dietary proteins or peptides from the rumen(Reference Jouany31). The differences are probably due to variable negative effects(Reference Jouany, Demeyer and Grain32) of defaunation technique(Reference Jouany and Senaud33) on rumen bacteria population in the in vitro and in sacco experiments(Reference Jouany, Sénaud, Toillon, Ben Salah, Bohatier and Prensier8, Reference Jouany, Ivan, Papon and Lassalas29, Reference Jouany30) compared with the use of naturally FF sheep in the present series of experiments. Indeed reported (J-P Jouany, poster presentation, Stara Lesna, Slovakia, 1994) rumen fluid bacteria counts (number × 109/ml) were 3·4, 1·9 and 1·6 for defaunated, defaunated and refaunated with IS, and defaunated and refaunated with TF-type B, respectively.
The present results clearly show that in comparison with the FF sheep the rumen HT population (IS plus DS) decreased the duodenal flow of NAN and total AA only by 2 and 3 % (not significant statistically), while decreases by the EN monofauna and the TF population were 19 and 16 %, and 24 and 23 %, respectively. The fact that the presence of TF(Reference Coleman, Nolan, Demeyer and Leng2–Reference Veira, Ivan and Jui4) or EN monofauna(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) decrease the duodenal flow of NAN and/or AA considerably is well known. Previous studies established that ingestion of bacteria and the proteolytic activities of protozoa in the rumen decrease the efficiency of protein utilisation by the ruminant host(Reference Veira, Ivan and Jui4, Reference Jouany, Ivan, Papon and Lassalas29). Of the ciliate protozoa species in TF, EN are always predominant(Reference Jouany and Senaud6) and ingest bacteria at a high rate(Reference Coleman, Nolan, Demeyer and Leng2). But the recent evidence suggests that the decreased protein utilisation by the host is mainly and almost equally due to the presence in TF of both cellulolytic and EN species(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14). In the present experiment the duodenal flow of NAN, BN, non-bacterial-NAN and total AA was similar for EN monofauna and the TF population and in agreement with previous results(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13).
The present results on the HT reduction of the NAN flow are in general agreement with the results obtained when DS was superimposed on the existing monofauna of IS(Reference Ivan, Veira and Kelleher10). It is, however, evident that these results are similar to those of the IS monofauna, but not to those of the DS monofauna which showed virtually no difference from the FF sheep(Reference Ivan, Neill, Forster, Alimon, Rode and Entz13). This appears to be due to the lower bacterial predation, if any, by the DS monofauna compared with the IS monofauna(Reference Ivan, Veira and Kelleher10, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13). It is evident, therefore, that the HT rumen protozoa population consisting of both IS and DS species will result in virtually the same bacterial predation and the duodenal NAN flow as the IS monofauna. It is also evident from the present results that the duodenal flows of total AA for all treatments were similar to those obtained for the flow of NAN, and none of the proportions of the individual AA in the total AA flow were affected by the protozoa treatments (FF, HT, EN, TF). Therefore, bacterial predation by rumen ciliates and the contribution of the ciliate protein do not appreciably affect the AA composition of the duodenal NAN flow. It should be noted, however, that this flow might not contain protein originating from the cellulolytic protozoa species, even though these species are present in the rumen protozoa population(Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14).
Considering the present results and those from other experiments in the above noted series(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) it can now be definitively concluded that the HT protozoa engulf only a very small amount of rumen bacteria and, consequently, negatively affect the duodenal flow of NAN to only a small extent. Similarly, the HT protozoa had no appreciable effect on the fibre digestion in the rumen. These protozoa ingest starch grains and soluble carbohydrates and convert them into storage polysaccharide(Reference Coleman34). This action prevents alternative bacterial fermentation of these unconverted compounds that would otherwise decrease pH and increase the onset of lactic acid acidosis. However, these beneficial actions might be limited because lactic acid is also the main endproduct of carbohydrate metabolism by the HT protozoa(Reference Williams and Coleman15). Furthermore, HT ciliates are significant indirect contributors to the rumen methanogenesis(Reference Williams and Coleman15). It would appear, therefore, that the presence of the HT protozoa in the rumen ciliate protozoa population is of no value to ruminant production, unless high-carbohydrate diets are used in the feeding system, in which case the presence of the HT protozoa could be beneficial.
The results of the present concluding experiment together with those of the other experiments in the above noted series(Reference Ivan, Neill and Entz11, Reference Ivan, Neill, Forster, Alimon, Rode and Entz13, Reference Ivan, Koenig, Morgavi, Rode, Newbold and Entz14) established the dynamics of effects of the major rumen ciliate protozoa species on rumen fermentation, digestibility, and duodenal flow of NAN components. The knowledge on these effects is essential to the understanding of how protozoal populations and their major species affect the nutrition of the ruminant host. They are also important in the development of the rumen protozoa-reducing technology to advance the efficiency and to alleviate the environmental impact of ruminant production.
Acknowledgements
The author wishes to thank T. Entz for the professional statistical analysis of experimental data, L. Neil for technical assistance and R. Moore for the care of experimental animals. All other work associated with the paper was performed by the sole author. The research work was conducted utilising the institutional budgetary financial resources allotted to the author. There are no conflicts of interest.







