Introduction
In all coffee-producing regions, the coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae), is a significant pest of coffee plants (Rubiaceae), especially the Arabica and Robusta cultivars (Vega et al. Reference Vega, Infante, Castillo and Jaramillo2009). Aristizábal et al. (Reference Aristizábal, Bustillo and Arthurs2016) reported that the losses among coffee producers around the world due to this tiny pest, which is 1.4–1.7 mm long (Barrera Reference Barrera and Capinera2008), amounted to more than $USD 500 million. Because of this, H. hampei’s life cycle biology and ecological observations of the insect have been reported widely (Damon Reference Damon2000; Johnson et al. Reference Johnson, Kellermann and Stercho2010; Fotso et al. Reference Fotso, Touzeau, Grognard, Tsanou and Bowong2023). The adult female coffee berry borer survives about 90–120 days after infesting the coffee cherry, making holes in the cherry to consume the contents and to lay about 30 eggs, which coexist in the cherry through various maturation stages (Damon Reference Damon2000). The infestation reduces the quality of the beans and increases premature bean drop, posing a considerable threat to both small-scale household growers and large producers alike (Barrera Reference Barrera and Capinera2008; Johnson et al. Reference Johnson, Kellermann and Stercho2010). Coffee bean drop increases by 30–35% following coffee berry borer infestation, and because of its impacts on the industry, prevention strategies have focused on combining various approaches in integrated pest management programmes (Johnson et al. Reference Johnson, Kellermann and Stercho2010; Moreno-Ramirez et al. Reference Moreno-Ramirez, Bianchi, Manzano and Dicke2024). Several methods for controlling coffee berry borer have been reported, with a focus on insecticide applications because these provide effective control before coffee berry borer females enter the coffee berry; however, the environmental problems and the effects of insecticide on the nontarget organisms are recorded (Pereira et al. Reference Pereira, Vilela, Tinoco, de Lima, Fantine, Morais and França2012; Aristizábal et al. Reference Aristizábal, Jiménez, Bustillo, Trujillo and Arthurs2015).
Entomopathogenic fungi provide a bio-rational alternative to synthetic insecticides for combating insect pests (Rajula et al. Reference Rajula, Rahman and Krutmuang2020; Wakil et al. Reference Wakil, Kavallieratos, Eleftheriadou, Haider, Qayyum and Tahir2024). Fungal species such as Beauveria bassiana (Cordycipitaceae), Metarhizium anisopliae (Clavicipitaceae), and Isaria fumosorosea (Cordycipitaceae) that are pathogenic to insects have helped control the coffee berry borer and other insect pests (Thaochan and Ngampongsai Reference Thaochan and Ngampongsai2015, Reference Thaochan and Ngampongsai2018; Thaochan and Chandrapatya Reference Thaochan and Chandrapatya2016; Thaochan et al. Reference Thaochan, Benarleea, Prabhakarb and Huc2020). Bustillo et al. (Reference Bustillo, Bernal, Benavides and Chaves1999) and Vera et al. (Reference Vera, Montoya, Benavides and Góngor2011) reported that B. bassiana controlled the coffee berry borer under experimental observation. Cure et al. (Reference Cure, Rodríguez, Gutierrez and Ponti2020) employed B. bassiana and M. anisopliae to target the coffee berry borer, demonstrating that a spore density of 1 × 109 conidia/mL most effectively controlled the pest.
Histopathology is a useful approach to assessing animal health and physiological reactions to environmental stimuli (Lacey et al. Reference Lacey, Lacey and Roberts1988; Ribeiro et al. Reference Ribeiro, Pereira, Freire, de Oliveira, Casotti and Boery2018; Bava et al. Reference Bava, Castagna, Piras, Musolino, Lupia and Palma2022). Several publications indicate that an approach for analysing pest pathology and entomopathogenic fungi has been reported exclusively within the relevant databases: for example, Lacey et al. (Reference Lacey, Lacey and Roberts1988) histopathologically discovered that M. anisopliae could invade the midgut of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae), within 48 hours, resulting in the degeneration and subsequent death of the insect’s intestinal absorptive cells, and Ibrahim et al. (Reference Ibrahim, Alahmadi, Binnaser and Shawer2019) observed histopathological changes in the skin and haemocytes of Zeuzera pyrina (Lepidoptera: Cossidae) larvae after a 3–4-day exposure to B. bassiana. As mentioned above, these methods are useful for assessing existing knowledge of the insect response to entomopathogenic fungi.
Several entomopathogenic fungi, specifically B. bassiana and M. anisopliae, have been shown to be effective against coffee berry borer (De La Rosa et al. Reference De La Rosa, Alatorre, Barrera and Toreillo2000; Haraprasad et al. Reference Haraprasad, Niranjana, Prakash, Shetty and Wahab2001; Vera et al. Reference Vera, Montoya, Benavides and Góngor2011; Greco et al. Reference Greco, Wright, Burgueño and Jaronski2018; Hollingsworth et al. Reference Hollingsworth, Aristizábal, Shriner, Mascarin, de Andrade Moral and Arthurs2020). However, the pathogens’ histopathological sites and apoptotic cell death in hosts remain unmapped and unconfirmed (Green and Kroemer Reference Green and Kroemer2004; Elmore Reference Elmore2007). The objective of the present study was to expand knowledge of entomopathogenic fungi by examining the effects of M. guizhouense on H. hampei through histopathological observations for potential pest management applications.
Methods
Coffee berry borer sampling and study area
Coffee beans infested with coffee berry borer were randomly collected from two sites in Thailand: Thepha Research Station, Faculty of Natural Resources (Thepha District, Songkhla Province), and the Agricultural Career Promotion and Development Centre (Mueang Yala District, Yala Province). The beans were collected in March 2023 at approximate coordinates and at various coffee berry borer life stages – but primarily at adult stages – to ensure a representative sample. Because no significant variation in insect response to inoculation was observed between sites, the collection data were pooled for analysis. The samples were preserved in plastic containers and transported to the laboratory at the Department of Agricultural Innovation and Management, Prince of Songkla University (Songkhla, Thailand), where the coffee berry borers were isolated from the collected berries. Individual specimens were isolated from the samples and placed in 5-cm Petri dishes for species identification. Adults were collected from the infested coffee beans, whereas larvae and pupae were allowed to emerge naturally. In some cases, berries were dissected to obtain specific H. hampei life stages for examination. These specimens were observed and photographed under a Leica S8 APO stereomicroscope (Leica Microsystems Inc., Wetzlar, Germany). The identification process adhered to Smith et al.’s (Reference Smith, Kanowski, Keenan and Phimmavong2021) taxonomic standards.
Fungal preparation
The fungal strain used in this study, M. guizhouense PSUM04, was kept in special storage at the Pest Management Laboratory, Faculty of Natural Resources, Prince of Songkla University, per Thaochan and Chandrapatya (Reference Thaochan and Chandrapatya2016). For propagation, the fungus was placed on sabouraud dextrose agar (Himedia Laboratories, Mumbai, India) in 9-cm diameter Petri dishes. These were then placed in an incubator at 28.0 ± 0.2 °C under dark conditions. Incubation lasted 14 days to ensure that fungal spores developed completely on the agar surface. Following incubation, the fungal spores were harvested by filtration through sterile filter paper or mesh to separate them from the growth medium. The collected spores were then resuspended in sterile water to create a spore suspension. To achieve a concentration of 1 × 109 spores/mL, the suspension was counted using a haemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda–Königshofen, Germany) for subsequent experiments.
Exposure to pathogen, and sampling
Adult coffee berry borers (n = 160) were coated with powdered M. guizhouense PSUM04. The insect specimens were first disinfected externally with 0.1% sodium hypochlorite for one minute and with 70% ethanol for another minute, and then they were washed three times with sterile distilled water. The insects were transferred onto sterile paper (Whatman #1; Cytiva Life Sciences, Marlborough, Massachusetts, United States of America) to dry. The live specimens were immersed in 5 mL of the fungal spore suspension for one minute. The treated H. hampei specimens were transferred to three 9-cm Petri dishes, each containing 50 individuals. The dishes were lined with sterile filter paper (Whatman #1) and sprayed with autoclaved distilled water for moisture. The insects were subsequently kept in a laboratory setting at 28.0 ± 0.2 °C under a 12-hour light:dark cycle. The control group (n = 10, triplicate) was soaked in sterilised distilled water for one minute, following the method described by Rännbäck et al. (Reference Rännbäck, Cotes, Anderson, Rämert and Meyling2015) and Liu et al. (Reference Liu, Smagghe and Liu2023). The control and treatment groups were otherwise kept under identical conditions to ensure comparability, with exposure to the fungal treatment being the only difference between them.
Coffee berry borers infested with M. guizhouense PSUM04 were sampled at 0, 6, 12, 18 24, 30, 36, 42, 48, 54, 60, 72, 84, 96, 122, and 144 hours after the exposure. Ten individuals were collected at each time point (Rännbäck et al. Reference Rännbäck, Cotes, Anderson, Rämert and Meyling2015). These specimens were euthanised using the chilling method described by Wilson et al. (Reference Wilson, Bunte and Carty2009). Three individuals randomly selected from each time point were kept for future observation.
Histological and histopathological examinations
Sampled coffee berry borers were first preserved in Davidson’s fixative (Dietrich and Krieger Reference Dietrich and Krieger2009) for approximately 24 hours. They were then prepared following standard histological procedures outlined by Bancroft and Gamble (Reference Bancroft and Gamble2002) and Suvarna et al. (Reference Suvarna, Layton and Bancroft2013). Briefly, the samples were processed to yield thin sections of about 4 μm thickness. These sections were stained with haematoxylin and eosin for observation of structural and histopathological changes in tissues. Tissue changes were assessed using the histological alteration index. To determine histological alteration indices, some slides were prepared with additional staining techniques, including Masson’s trichrome and periodic acid–Schiff. Kongtueng et al. (Reference Kongtueng, Charoenphon, Senarat and Thaochan2024) described the details of all laboratory materials and kits used, and Wang et al. (Reference Wang, Lai, Wu, Zhang, Huang and Xiao2022) described Grocott methenamine silver stains. These methods facilitate the analysis of tissue chemical composition and the detection of fungal elements within tissues (Bancroft and Gamble Reference Bancroft and Gamble2002; Dietrich and Krieger Reference Dietrich and Krieger2009). In the present study, stained sections were mounted on slides and then observed and recorded using a 3D Histech Pannoramic SCAN 150 (3DHISTECH Ltd., Budapest, Hungary), employing the 3DHISTECH slide scanning method per manufacturer’s instructions. Coffee berry borer samples were also assessed quantitatively. Six different cuticles were measured by using the ImageJ software (J, version 4.0.1, for MS Windows 1998; National Institutes of Health, Washington, DC, United States of America, and University of Wisconsin, Wisconsin, United States of America).
Analysis of apoptotic cell death using TUNEL staining
Terminal deoxynucleotidyl transferase dUTP nick-end labelling staining was employed to identify and localise apoptotic cell death in the coffee berry borer samples (Rojo and Gonzalez Reference Rojo and Gonzalez1998). The TUNEL Assay Kit (HRP-DAB ab206386; Abcam, Cambridge, United Kingdom) was used according to the manufacturer’s protocol. Images of stained tissues were captured for detailed analysis using a standard light microscope. Cell death maps depicting the distribution of apoptotic cells within the tissues were created based on these images. The number of apoptotic cells in tissue sections was counted at 40× magnification from three permanent slides per sample, making a total of 30 permanent slides in the full experimental set. ImageJ was used for the image analysis. A semi-quantitative analysis was also conducted to assess the density of apoptotic cells in tissues. The levels of immunoreactivity in the TUNEL assay were evaluated using the following semi-quantitative classifications: “–” = no immunoreactivity, “+” = weak immunoreactivity, “++” = moderate immunoreactivity, and “+++” = strong immunoreactivity.
Statistical analysis
The following data were statistically analysed: the percentage of insect infestation, skin thickness, and the number of apoptotic cells. The mean and standard deviation of each parameter were calculated to summarise the central tendency and variability of data. Using SPSS Statistics, version 22.0 (IBM, Armonk, New York, United States of America), one-way analysis of variance, two-way analysis of variance, and a post hoc test were employed to assess the significance of the differences observed between experimental groups. The significance threshold was 0.95.
Results
Colony morphology and histopathological observation
Metarhizium guizhouense PSUM04 formed distinct colonies on the culture media (Fig. 1). Colonies were not observed in the cuticle or adipose tissue of the H. hampei specimens (Fig. 2A–C) until six hours post-inoculation. At 12 hours post-inoculation, fungal conidia were present on the outer shell of H. hampei and had breached the surface layers (Fig. 2D and F). A distinct germ tube emerged, indicating where the fungus had made its entrance. At the early stage of infection, the fungal body was bound tightly to the cuticle, sending hyphae into the protective layer beneath (Fig. 2F). At 24 hours and 48 hours post-inoculation, a profusion of hyphal bodies was observed on the external shell layer (Fig. 2G–L). Fungal filaments extended through muscles and other tissues. At the end of this period, the hyphae had pervaded nearly the entire adipose tissue and integument (Fig. 2K and L).
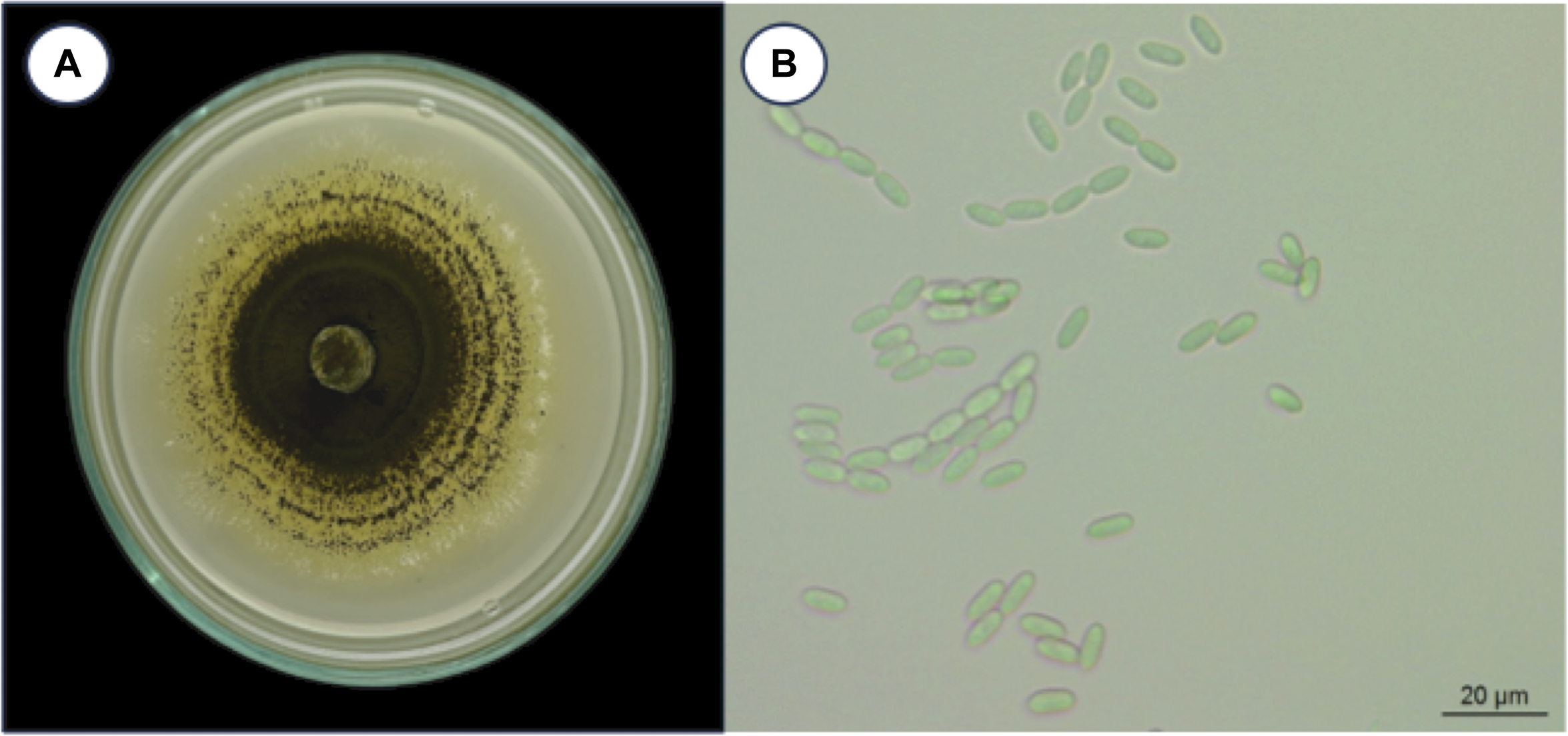
Figure 1. Metarhizium guizhouense PSUM04. A, colony morphology, and B, conidia on culture media.
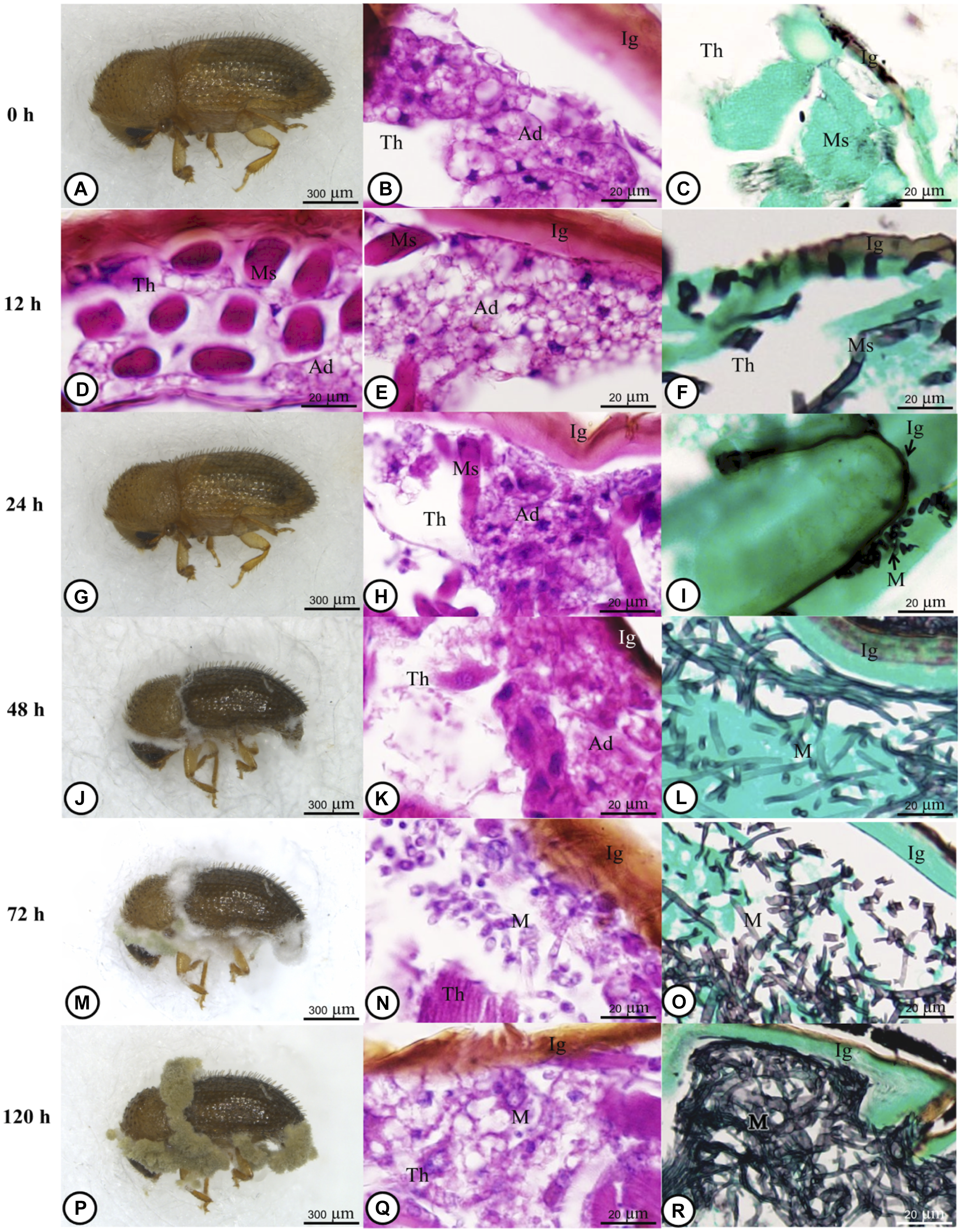
Figure 2. Representative light microscope images of Hypothenemus hampei infected with Metarhizium guizhouense PSUM04. A–C, Adult H. hampei before infection: A, external morphology; B, H&E staining; C, GMS staining of the integument tissue. D–F, At 12 hours post-inoculation: D and E, H&E staining shows hyphae invading the fat body underlying the cuticle; F, GMS staining. G–I, At 24 hours post-inoculation, hyphae formation was similar to that at 12 hours post-inoculation: G, external morphology; H, H&E staining; I, GMS staining. J–L, At 48 hours post-inoculation, hyphae had invaded adipose tissue under the cuticle: J, external morphology; K, H&E staining; L, GMS staining. M–O, At 72 hours post-inoculation, the area of hyphae had increased significantly: M, external morphology; N, H&E staining; O, GMS staining. P–R, At 120 hours post-inoculation: P, External morphology; Q, H&E staining; R, GMS staining. H&E, haematoxylin and eosin; GMS, Grocott methenamine silver stain; h, hour; Ig, integument; Th, thorax; Ad, adipose tissue; Ms, muscle; M, Metarhizium guizhouense PSUM04.
From 72 to 120 hours post-inoculation, the fungus spread out in muscle and adipose tissues (Fig. 2M–R). Significant cellular alterations were observed. Adipose cells exhibited deteriorating cytoplasm and membranes, and hyphae penetrated muscle tissues. In some tissue sections, muscles were altered to the extent that they could not be identified clearly. During this phase, the insect specimens’ digestive systems exhibited signs of fungal encroachment. Periodic acid–Schiff staining showed spore formation with prominent hyphae at 120 and 144 hours post-inoculation (Fig. 3A–F).
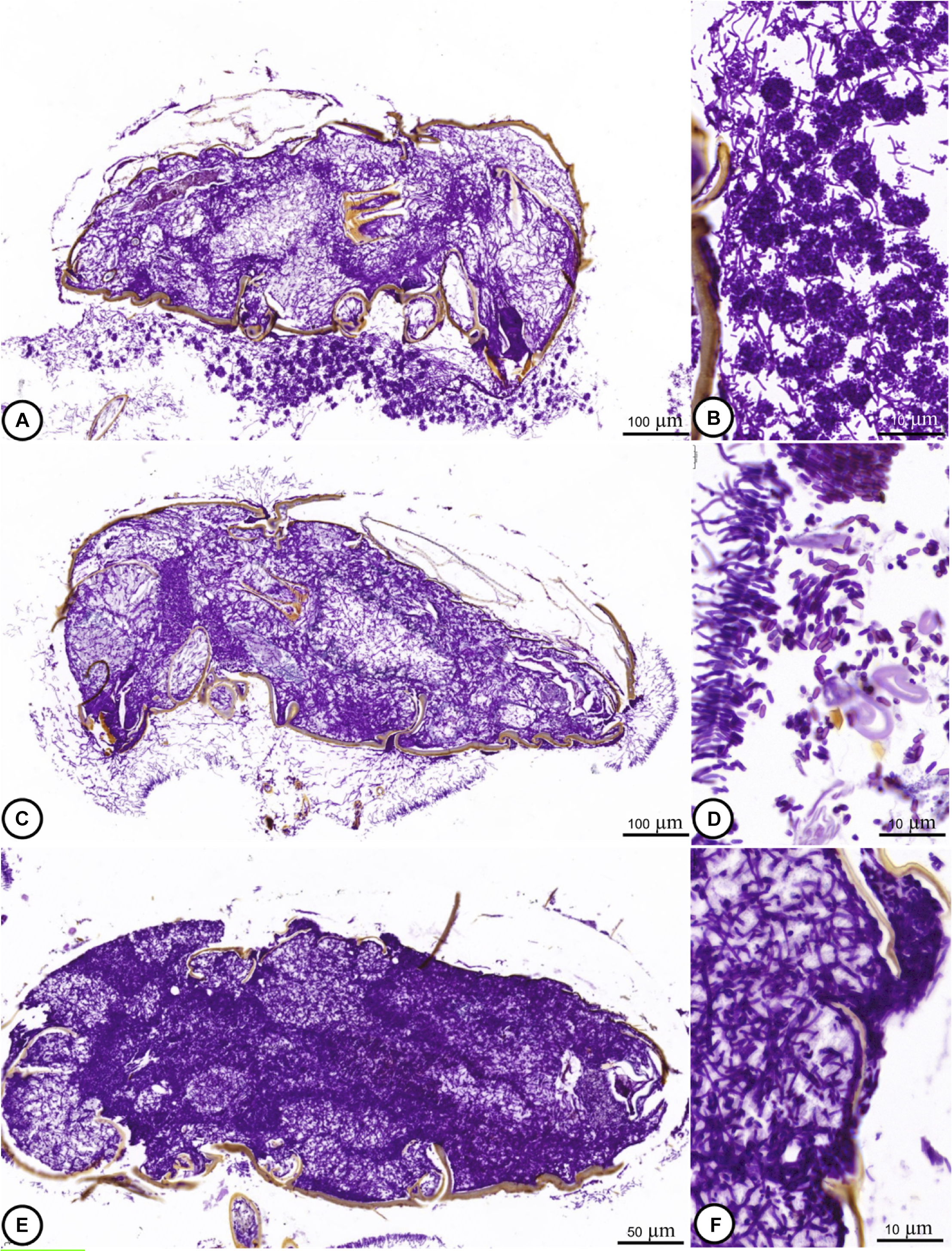
Figure 3. Light microscope images showing an adult of H. hampei infected with M. guizhouense PSUM04: A–D, at 120 hours post-inoculation, and E–F, at 144 hours post-inoculation. The prominent hyphae and spore formation of the infected samples were clearly observed.
Fungal area
Grocott methenamine silver staining successfully visualised the conidia and hyphae within the coffee berry borer tissues, allowing us to quantify the extent of fungal proliferation within the abdominal region (Fig. 4A). The area of fungal infection continuously increased in a time-dependent manner from 12 hours to 144 hours post-inoculation (Fig. 4A). A slight increase was observed at 48 hours post-inoculation, showing statistical significance (P < 0.05) compared to the baseline (0 hours post-inoculation). A substantial rise at 84 hours post-inoculation (P < 0.001) indicated significant fungal colonisation. The highest levels of fungal proliferation were recorded at 120 and 144 hours post-inoculation, when it reached peak growth (P < 0.001; Fig. 4A).
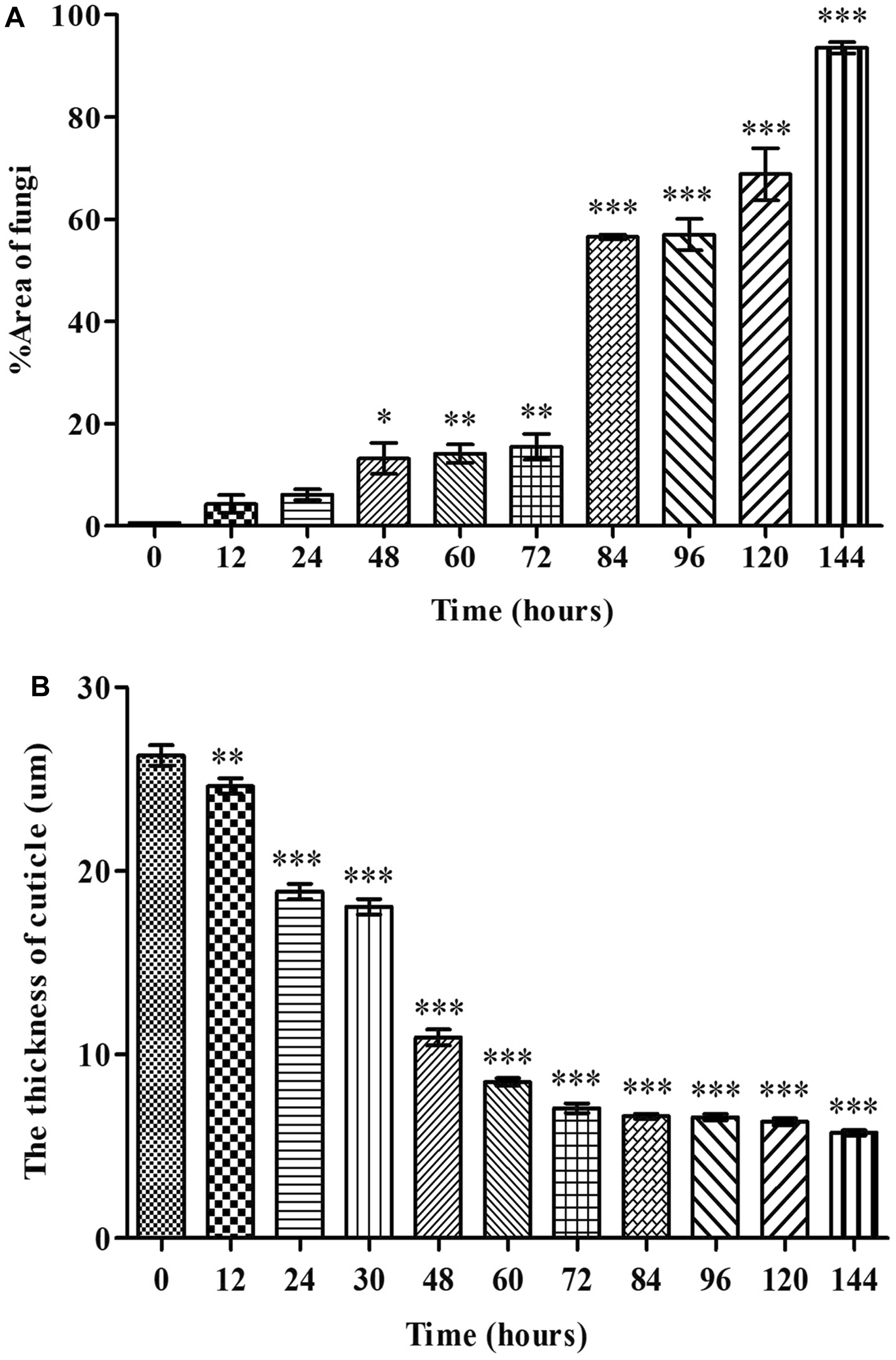
Figure 4. A, The percent area of fungal infection, and B, the thickness of H. hampei cuticle infected with M. guizhouense PSUM04. Values are expressed as mean ± standard error of the mean, ∗P < 0.05, ∗∗P < 0.01, compared with baseline at 0 hours.
Cuticle thickness
The average thickness of the cuticle showed a significant reduction (P < 0.001) from 12 hours to 144 hours post-inoculation in comparison with the baseline at 0 hours post-inoculation (Fig. 4B). The most noteworthy decrease in cuticle thickness, from 24.62 ± 0.43 µm to 18.88 ± 0.41 µm, occurred between 12 and 24 hours post-inoculation.
Histological scoring index
The histological scoring was based on three criteria: loss of adipose tissue thickness (Fig. 5A), muscle tissue degeneration (Fig. 5B), and gastrointestinal epithelial deterioration (Fig. 5C). All assessed parameters showed a significant increase after inoculation. At 144 hours post-inoculation, loss of adipose tissue thickness (Fig. 5D), muscle tissue breakdown (Fig. 5E), and gut epithelial integrity all received the maximum severity rating of “3” (Fig. 5F).
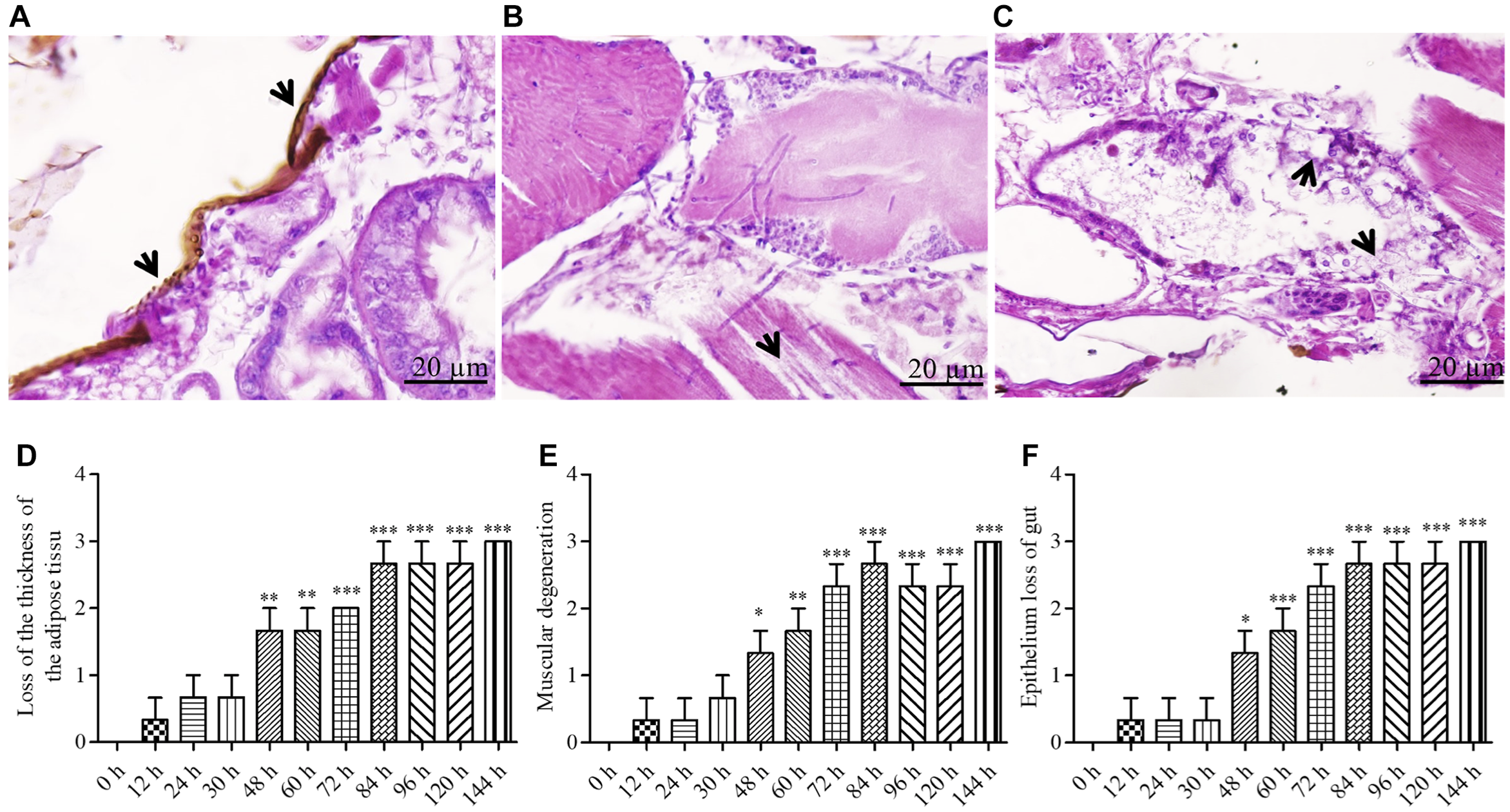
Figure 5. Representative light microscopic level showing the histopathology and histopathological alteration indexes of adult Hypothenemus hampei infected with Metarhizium guizhouense PSUM04: A and D, loss of adipose tissue thickness (arrows); B and E, muscular degeneration (arrows); and C and F, loss of gut epithelium (arrows). Values are means ± standard error (***P < 0.01).
Semi-quantitative analysis on TUNEL-positive cells
At 0 hours post-inoculation, tissues of coffee berry borer were largely intact, showing only negligible immunoreactivity (Fig. 6A). By 12 hours post-inoculation, a small increase in TUNEL-positive apoptotic cells was observed (Fig. 6B). A notable escalation in the number of apoptotic cells was observed by 48 hours post-inoculation (Fig. 6C), which continued up to 144 hours post-inoculation (Fig. 6D–F). Table 1 provides a semi-quantitative evaluation of TUNEL-positive cell counts. These observations suggest that cell death accounted for the toxic effect of M. guizhouense on coffee berry borer.
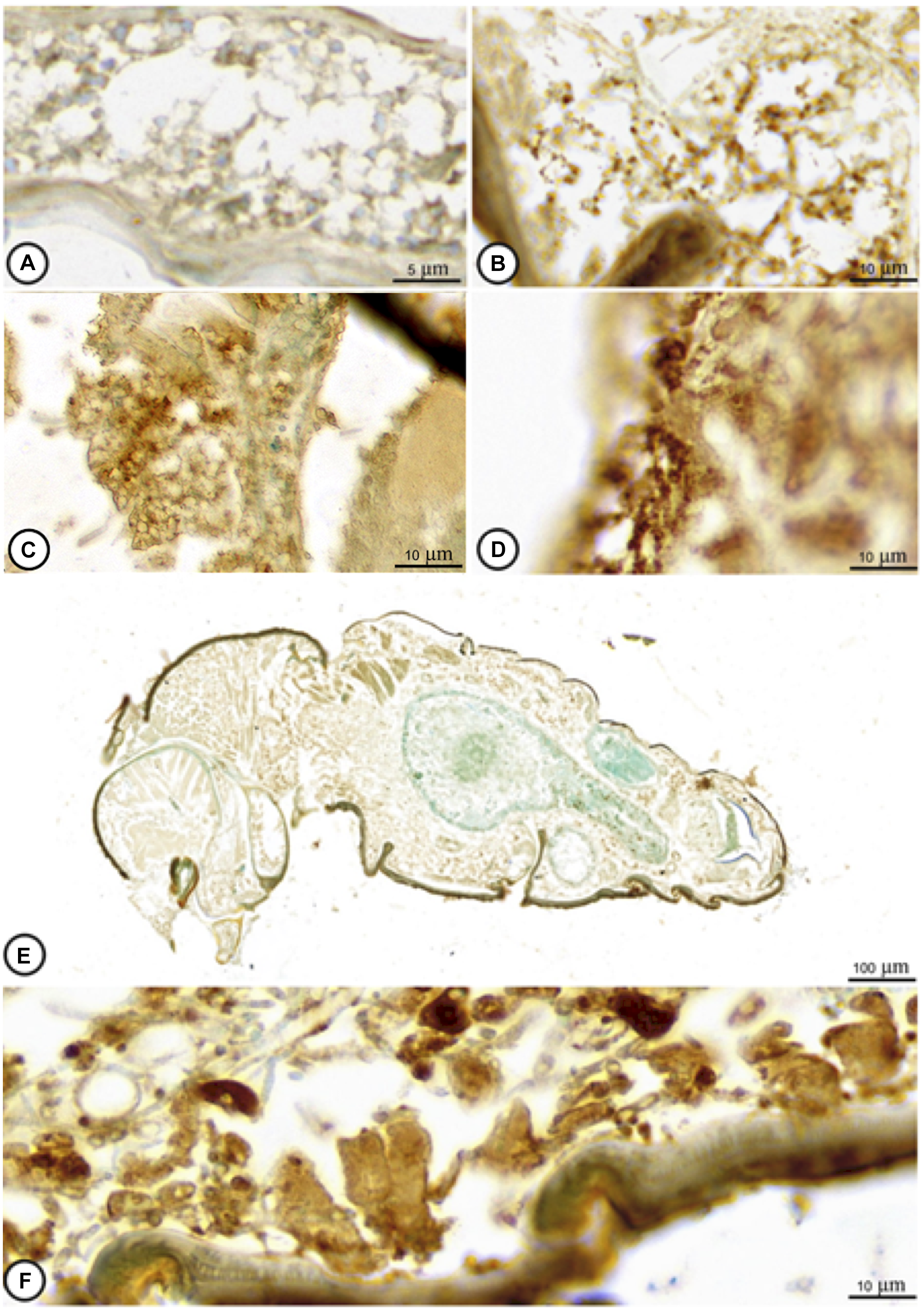
Figure 6. Representative light microscope observation of apoptotic cells in adult Hypothenemus hampei infected with Metarhizium guizhouense PSUM04, at different times post-inoculation, such as A, 0 hours, B, 12 hours, C, 48 hours, and D–F, 144 hours.
Table 1. Representative semi-quantitative analytical score of the TUNEL-positive cell numbers each time. TUNEL, terminal deoxynucleotidyl transferase (TdT) dUTP (deoxyuridine triphosphate) nick-end labelling
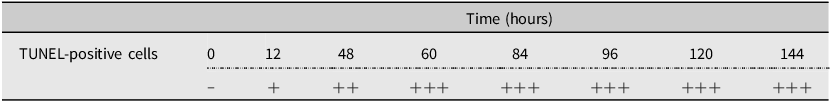
Semi-quantitative analytical scale: “−” = no immunoreactivity; “+” = weak immunoreactivity; “++” = moderate immunoreactivity; and “+++” = strong immunoreactivity.
Discussion
The entomopathogenic fungus, Metarhizium guizhouense, has been employed as a biological control agent against many insect pests (Thaochan and Chandrapatya Reference Thaochan and Chandrapatya2016; Thaochan and Ngampongsai Reference Thaochan and Ngampongsai2018; Thaochan et al. Reference Thaochan, Benarleea, Prabhakarb and Huc2020; Theary et al. Reference Theary, Akter, Prabhakar and Thaochan2020; Wei et al. Reference Wei, Wang, Nima, Chen, Song and Chen2023). In the present study, we evaluated the effects of M. guizhouense infection on the coffee berry borer. At 12 hours post-inoculation, M. guizhouense penetrated the integument of the beetle, and at 24 hours post-inoculation, it had entered the body. Lesions were formed after 12 hours, followed by cell death and integument weakening. Eventually, the hyphae colonised the entire body of coffee berry borer. These observations are consistent with earlier reports by Thaochan and Ngampongsai (Reference Thaochan and Ngampongsai2015, Reference Thaochan and Ngampongsai2018), Thaochan and Chandrapatya (Reference Thaochan and Chandrapatya2016), and Thaochan et al. (Reference Thaochan, Benarleea, Prabhakarb and Huc2020). Wu et al. (Reference Wu, Meng, Merchant, Zhang, Li, Zhou and Wang2021) explored the effect of entomopathogenic fungi on the gut microbiome of the termite Odontotermes formosanus (Blattodea: Termitidae), which may provide valuable insights for future studies on the coffee cherry borer: specifically, investigating how entomopathogenic fungi affect the gut microbiome of H. hampei could offer additional understanding of its infection process and potential impacts on host physiology.
Three possible mechanisms have been proposed to explain mortality following entomopathogenic fungal infection, primarily based on studies of M. anisopliae. The first mechanism suggests that conidia germinate inside the larvae and form hyphae that clog the respiratory syphon, causing asphyxia and death. The second mechanism involves the production of the toxic peptide, destruxin. The third mechanism suggests that extracellular proteases produced by the fungus damage the host’s tissues (Butt et al. Reference Butt, Greenfield, Greig, Maffeis, Taylor and Piasecka2013; Kirubakaran et al. Reference Kirubakaran, Haripriya, Naveenraj and Thirumalaivasan2015; Aw and Hue Reference Aw and Hue2017). In the present study, the TUNEL assay revealed a significant increase in apoptotic cells within coffee berry borer adipose tissue and integument, providing strong evidence for the involvement of toxin-induced cell death and protein damage. These findings suggest that the mortality observed is more likely linked to the cytotoxic effects of destruxin and proteases, rather than simply loss of physical function such as asphyxia. Destruxin is known to induce Bcl2-dependent apoptosis in human cells (Wu et al. Reference Wu, Chen, Liu, Wu, Chen and Tzeng2013), and the observed increase in apoptosis in coffee berry borer tissues in the present study supports the relevance of this mechanism in the insect’s death. The presence of apoptotic cells and the lack of evidence for significant physical blockage of respiratory structures further suggest that the mode of death is largely due to toxin-induced cellular damage and not asphyxia.
Arias-Aravena et al. (Reference Arias-Aravena, Altimira, Gutiérrez, Ling and Tapia2022) reported that fungi produce digestive enzymes, such as chitinase, lipase, and protease, which can cause direct damage to host cells. The spread of entomopathogenic fungi involves enzymatic breakdown, as well as physical force (Kaczmarek et al. Reference Kaczmarek, Struszczyk-Swita, Li, Szczęsna-Antczak and Daroch2019). Indeed, in the present study, the coffee berry borer displayed inadequate movement, which is consistent with observations made of other infected beetles (Aw and Hue Reference Aw and Hue2017; Reddy et al. Reference Reddy, Padmavathi and Zachariah2022). In the present study, H. hampei clearly suffered damage at the cellular level, as evidenced by the significant increase in apoptotic cells observed in adipose tissue and integument during the TUNEL assay. This cellular damage is consistent with the known effects of entomopathogenic fungi, which are often characterised by the induction of apoptosis, necrosis, and tissue disruption. For example, in similar histological studies, Butt et al. (Reference Butt, Greenfield, Greig, Maffeis, Taylor and Piasecka2013) and Kirubakaran et al. (Reference Kirubakaran, Haripriya, Naveenraj and Thirumalaivasan2015) reported extensive cellular damage in insects infected with M. anisopliae, where conidial germination and subsequent hyphal growth led to the destruction of host tissues and induction of apoptosis. In addition, Aw and Hue’s (Reference Aw and Hue2017) work on B. bassiana infection in other insect species demonstrated comparable tissue damage and immune responses, further supporting the idea that entomopathogenic fungal infection leads to significant histological changes, primarily through toxin-induced cellular damage. The findings of the present study add to the growing body of literature suggesting that entomopathogenic fungal infection causes systemic cellular damage, and our results highlight the importance of examining the cellular mechanisms underlying entomopathogenic fungus–induced mortality in H. hampei.
Conclusion
Histological studies revealed that H. hampei is susceptible to infection by M. guizhouense PSUM04, with fungal penetration observed approximately 12 hours after inoculation. The observed timing of infection aligns with similar observations in other host tissues, indicating a consistent pattern of fungal interaction. Although the fungal infection demonstrated notable histological effects on the insect, including tissue damage and apoptosis, conclusions about population suppression should be drawn with caution, because the current study focused primarily on fungal penetration and cellular responses. Further research in field conditions is necessary to assess the broader potential of M. guizhouense PSUM04 as a biological control agent.
Acknowledgements
The authors thank the Microtechnique Laboratory (MIC-LAB), Division of Biological Science, Faculty of Science, Prince of Songkla University, for the technical support and equipment used in this experiment. This research was funded by the Center of Excellence in Agricultural and Natural Resources Biotechnology (CoE-ANRB), phase 3, Faculty of Natural Resources, Prince of Songkla University. The authors also thank the Pest Management Laboratory, Faculty of Natural Resources, Prince of Songkla University, for the supporting facilities. The research is supported by the Center of Excellence on Agricultural Biotechnology, Office of the Permanent Secretary, Ministry of Higher Education, Science and Innovation (AG-BIO/MHESI). The experimental protocol was approved by the Animal Care and Use Committee, Prince of Songkla University (protocol number 2023-NAT02-006 (AI019/2023)). Special thanks to Dr. Tommy Coyne for the grammatical structure and suggestion.










