Some chronic diseases in adulthood can be traced back to early nutritional status, which is called ‘nutrition programming’( Reference Godfrey and Robinson 1 ). Birth weight is a proxy for fetal nutritional status in utero. Epidemiological studies suggest that low birth weight increases the risk of obesity, type 2 diabetes and CVD in adulthood( Reference Harder, Rodekamp and Schellong 2 , Reference Smith, Sterne and Tynelius 3 ). During pregnancy, the mother is the provider of all nutrition, and it is essential for the diet during pregnancy to have sufficient nutrients to allow for adequate fetal growth and development.
At present, research on maternal diet has focused on the specific nutrients or just a part of food intake. However, these studies failed to take into consideration that some nutrients are inter-correlated( Reference Newby and Tucker 4 ). In fact, intake of foods and nutrients are correlated. Dietary patterns are a way to capture the overall dietary characteristics. They evaluate the overall diet and take into account the interactive (i.e. synergistic or antagonistic) effects between nutrients( Reference Cespedes and Hu 5 ). Therefore, it is more intuitive for public health nutrition recommendations.
Another important factor relevant to birth weight is cytokines in maternal/fetal blood( Reference Briana and Malamitsi-Puchner 6 ). Adiponectin (APN) is an adipokine that is secreted by adipose tissue and related to insulin sensitivity and lipid metabolism. Recent reports suggest that fetal APN may promote expansion of adipose tissue and stimulate fetal growth( Reference Aye, Powell and Jansson 7 ). Some studies have reported that, in contrast to adults, cord blood APN is positively associated with birth weight( Reference Chan, Yuan and Chen 8 , Reference Tsai, Yu and Hsu 9 ). Maternal or fetal inflammatory cytokines also play an important role in the regulation of fetal growth( Reference Amarilyo, Oren and Mimouni 10 , Reference Farah, Hogan and Connor 11 ). The Th1/Th2 paradigm indicated that a dynamic balance of Th1 cells (e.g. interferon-γ (IFN-γ)) and Th2 cells (e.g. IL-6) is very important to a successful pregnancy and prevention of premature birth( Reference Mor, Cardenas and Abrahams 12 ). Some researchers have reported that higher maternal or fetal inflammatory cytokines are related to the delivery of small-for-gestational-age( Reference Amarilyo, Oren and Mimouni 10 ), premature( Reference Ahmad, Zaldivar and Iwanaga 13 ) infants or low birth weight( Reference Rogers and Velten 14 ). However, these studies were small and limited.
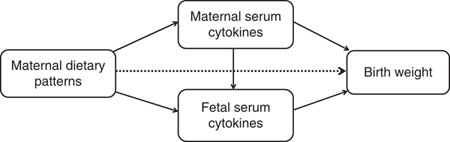
Furthermore, dietary intake can affect serum cytokines levels( Reference Calder, Ahluwalia and Brouns 15 , Reference Barbaresko, Koch and Schulze 16 ). In the context of the association among diet, cytokines and birth weight, we hypothesised that maternal diet may affect birth weight in two ways: directly or indirectly (mediated through cytokines). Normal correlation or regression analysis cannot explain the mediation effects. Path analysis, an extension of regression analysis, simultaneously estimates the linear association between all path variables and allows the estimation of direct, indirect and total effects of each path variable( Reference Fonseca, Severo and Correia 17 ). Thus, the aim of the study was to investigate how maternal dietary pattern and maternal/fetal cytokines are associated with birth weight and whether cytokines mediate the association.
Methods
Study population
This study was based on data from the Study on Prenatal and Postnatal Dietary Intervention of Women in Southern China, a prospective cohort study. Women who attend routine pregnancy diagnosis in hospitals in the Maternal and Children’s Hospital of BaiYun and Yuexiu District in Guangzhou were invited to take part in the cohort, from September 2010 to November 2011. The eligibility criteria for enrolment are as follows: (1) healthy pregnant women, (2) 18–35 years old, (3) in their third trimester and (4) bearing a singleton fetus. We excluded those women who had a history of pre-existing diabetes, chronic hypertension, endocrine disorders or other severe maternal illnesses. The mothers and their full-term babies were recruited.
Justification of sample size
We use the path analysis to explore the relationships between maternal dietary patterns, serum cytokines and birth weight. The path analysis is one type of structural equation modelling (SEM). We followed the indication of MacCallum & Browne( Reference MacCallum and Browne 18 ) to include a minimum of ten participants per covariate in SEM. In consideration of the need to detect the dietary patterns, maternal and fetal characteristics, serum cytokines, etc., about thirty indicators (the covariates) are preliminarily determined. In addition, we consider the rate of blood sample haemolysis (15 %) and withdrawal (10 %) and the sample size of 400 is finally confirmed (N=30×10÷(1–15 %)÷(1–10 %)≈400).
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the local Health Department and the research ethics boards of Tongji Medical College, China (trial registration: ClinicalTrials.gov ID NCT01039051). All participants gave written informed consent.
Data collection
During the study recruitment period, 533 pregnant women who met the inclusion criteria agreed to participate. Some women were excluded from the study as they were unable to provide incomplete questionnaire data (n 38), premature birth (n 20) and prolonged pregnancy (n 6). Hence, a total of 469 eligible mothers and their full-term babies were available for this analysis.
Maternal characteristics
An interviewer-administered questionnaire was used to collect the demographic and historical information including educational status, household income, personal medical and obstetrical history, information regarding the current pregnancy and the maternal pre-pregnancy weight and height.
Maternal dietary assessment
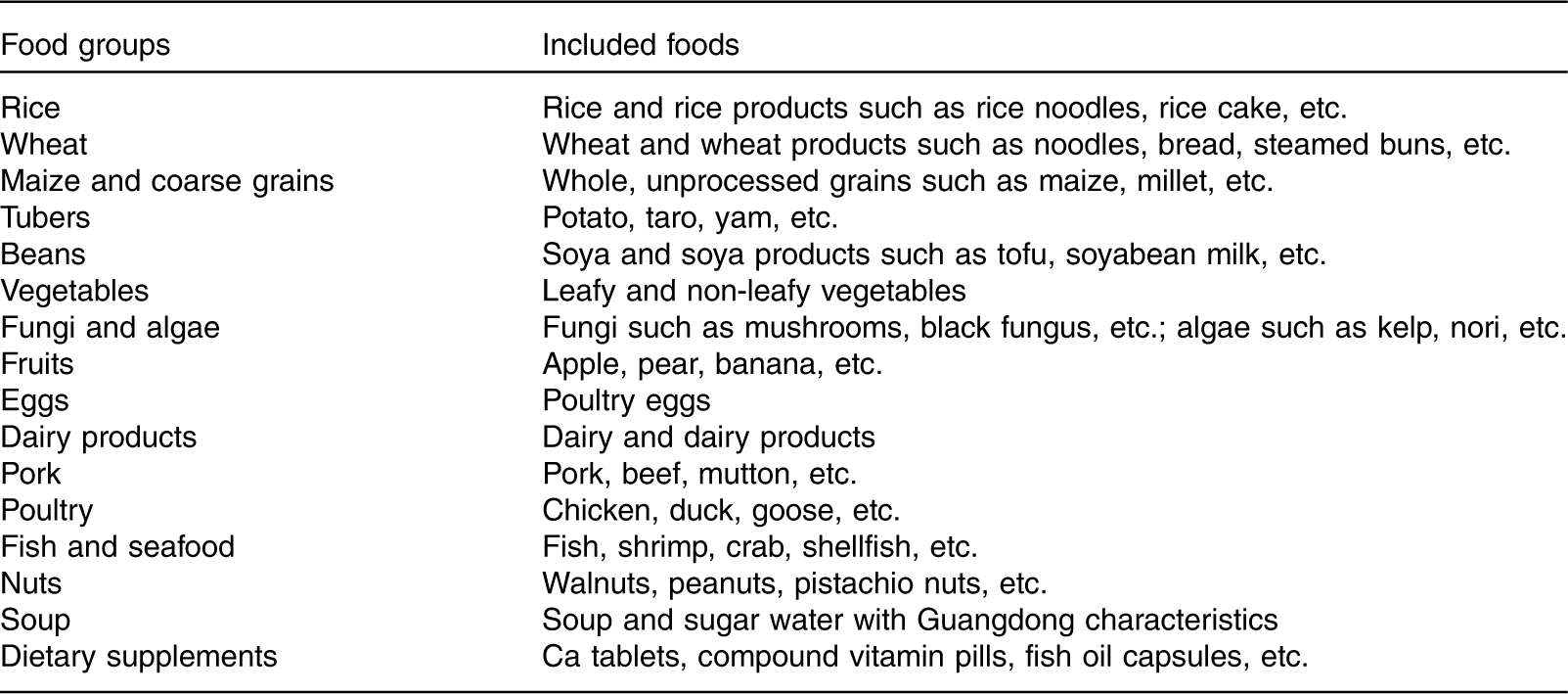
The maternal dietary intake in the third trimester was assessed using three consecutive 24 h dietary recalls. Studies have shown that, compared with early pregnancy, maternal frequency of eating and amount of food intake increases in the third trimester of pregnancy, but the dietary pattern remains unchanged( Reference Crozier, Robinson and Godfrey 19 , Reference Cuco, Fernandez-Ballart and Sala 20 ). Therefore, dietary patterns in the third trimester can represent the maternal dietary pattern throughout pregnancy. The 24 h dietary recalls have good reproducibility and validity( Reference Martin, Siega-Riz and Sotres-Alvarez 21 , Reference Chen, Aris and Bernard 22 ), even though the FFQ is the most common method for analysing dietary patterns. In this study, we used 24 h diet recalls to assess typical dietary consumption. Interviewers were trained on using food models and picture aids to assist women in quantifying their dietary intake. All food and drinks recorded were subsequently grouped corresponding to Chinese dietary habits according to the similarity of nutrient composition and comparable usage (e.g. noodles, bread and steamed buns were grouped into the ‘wheat’ group), resulting in eighteen food groups (Table 1).
Table 1 Classification of food in the dietary pattern analysis
Infant characteristics at birth
Information on gestational age, infant sex and birth order was abstracted from obstetric records. Infant birth weight was measured shortly after birth using an infant digital scale to the nearest 1 g according to standardised techniques.
Serum cytokine measurement
All the 469 maternal venous blood samples were obtained in the morning after overnight fasting before delivery. A total of 334 cord blood samples were successfully collected from the umbilical vein after delivery of the baby and before delivery of the placenta. The samples were centrifuged and then the serum was stored at 80°C until assayed. We measured the serum APN, IL-6 and IFN-γ with a commercially available ELISA kit (APN with R&D Systems; IL-6 and IFN-γ with ExCell Biology). The intra- and inter-assay CV were 7 and 7 % for APN, 9 and 20 % for IL-6 and 12 and 14 % for IFN-γ, respectively.
Statistical analysis
The data were analysed using SPSS (version 18.0; SPSS Inc.) for descriptive statistics. P<0·05 was considered significant. All continuous variables were expressed as mean with standard deviation, or median with interquartile range (IQR). Because the concentrations of APN, IL-6 and IFN-γ were not normally distributed, they were log transformed for further analysis. Pearson correlations were conducted to assess the linear relationship between maternal and fetal cytokine concentrations.
Maternal dietary patterns were identified by exploratory factor analysis. Exploratory factor analysis is an established technique to extract dietary patterns; it reduces the dimension of the data by aggregating correlated variables, resulting in linear combinations (factors) of the included variables. Dietary patterns were derived by principal component extraction with the use of varimax rotation on the food groups. Dietary pattern scores for each participant were then calculated by summing the standardised intake of food groups (g/d) weighted by their factor loadings (correlation coefficients between each food group and the dietary pattern).The adequacy of the data was checked using the Kaiser–Meyer–Olkin (KMO) index and Bartlett’s sphericity test. KMO values ≥0·5 and P≤0·05 on Bartlett’s sphericity test were considered acceptable.
Path analysis is an extension of regression analysis that simultaneously estimates the linear association between all path variables and makes it possible to assess the total, direct and indirect effects of each path variable( Reference Gamborg, Andersen and Baker 23 ). This technique is being increasingly used to decompose and compare the magnitudes of effects between variables with complex interrelations or to test the plausibility of mediation effects. So we use the path analysis to understand the relationships between maternal dietary patterns, maternal/fetal serum cytokines and birth weight. The direct effect is not mediated through other included variables, whereas the indirect effect is the mediating effect operating through other variables on the pathway. The total effect is the sum of the direct and indirect effects( Reference Kirkegaard, Stovring and Rasmussen 24 ). We depicted a theoretical model based on current knowledge (Fig. 1). In this model, we wished to test whether maternal dietary patterns had a direct effect (dotted line) on birth weight in addition to indirect effects (mediated by maternal/fetal serum cytokines) (solid lines). Mediation by maternal and fetal serum cytokines (APN, IL-6 or IFN-γ) were each considered separately. Before the path analysis, we performed the regression analysis of factors affecting birth weight to select the adjustable variables used in the final model. The results showed that infant sex, gestational age, parity, maternal pre-pregnancy BMI and weight gain during pregnancy remain in the equation, in addition to maternal dietary scores (online Supplementary Table S1). AMOS 7.0 statistical software in SPSS was used for the path analysis. The 95 % CI were calculated by bootstrapping. The fit of the model was assessed using these indexes: the root mean square error of approximation, the goodness-of-fit index, the adjusted goodness-of-fit index, the comparative fit index, the Tucker–Lewis index and the incremental fit index( Reference Fonseca, Severo and Correia 17 , Reference Ye, Yao and Liu 25 ).
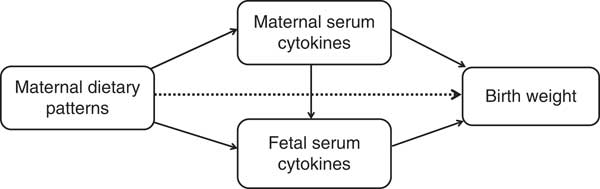
Fig. 1 Theoretical model of the relationship between maternal dietary patterns, maternal/fetal serum cytokines and birth weight. Dotted lines indicate the direct effects of maternal dietary patterns on birth weight, and solid lines indicate the indirect effects (mediated by maternal/fetal serum cytokines).
Results
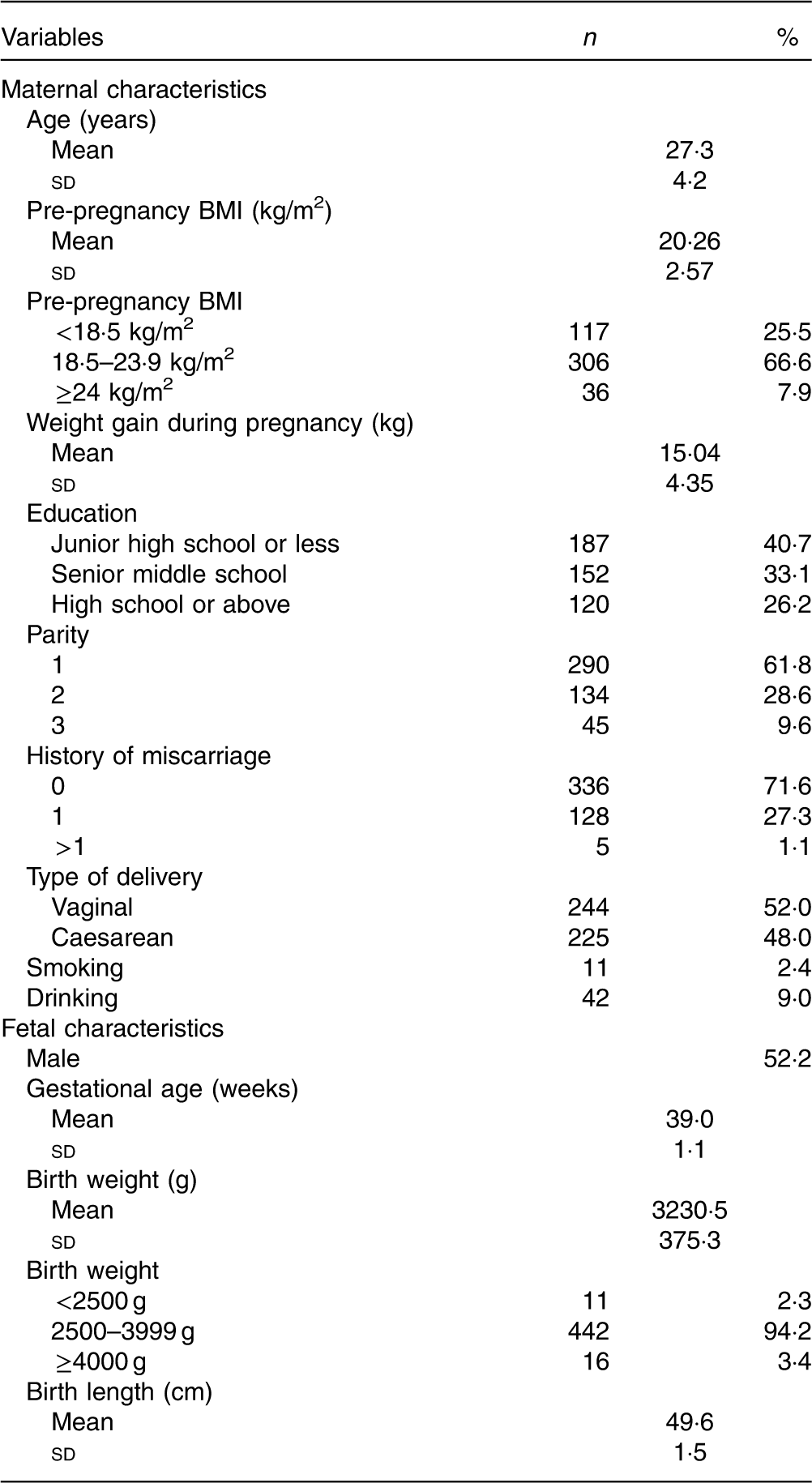
The characteristics of 469 pregnant women and their children are displayed in Table 2. The mean pre-pregnancy BMI of the pregnant women was 20·26 (sd 2.57) kg/m2. A total of 66·6 % of the women had a BMI between 18·5 and 23·9, and 7·9 % had a BMI ≥24 kg/m2. The mean weight gain was 15·04 (sd 4·35) kg. The majority were primiparous, and 52·0 % had a vaginal delivery. In all, 2·4 % reported smoking and 9·0 % reported drinking during pregnancy. In all, 52 % of infants were male. The mean gestational age was 39·0 (sd 1·1) weeks, and the mean birth weight was 3230·5 (sd 375·3) g, with 94·2 % within the normal range.
Table 2 Characteristics of the participants (n 469) (Numbers and percentages; mean values and standard deviations)
Maternal dietary patterns
The factor analysis revealed that four factors (i.e. dietary patterns) were retained based on the break point of the Scree plot (eigenvalues >1·3) and factor interpretability. As KMO=0·526 and Bartlett’s sphericity test P<0·001, values were accepted. The factor loadings associated with each pattern are given in Table 3. The total energy intake was adjusted for each food item using the residual method( Reference Willett 26 ). Each pattern was labelled according to the food groups with high absolute loadings. The fruits, dairy products and poultry (FDP) pattern was characterised by higher intake (high-positive factor loadings) of fruits, dairy products, poultry and maize and coarse grains and lower intake (negative factor loadings) of rice. The vegetable, bean and pork (VBP) pattern had higher intake of vegetables, beans and nuts, fungi and algae and pork. The fish and soup (FS) pattern was characterised by higher intake of fish and seafood, dietary supplements, maize and coarse grains, and soup and lower intake of fungi and algae. The tuber and egg (TE) pattern was characterised by higher intake of tubers and eggs and lower intake of wheat. Factors 1–4 accounted for 12·0, 9·3, 8·7 and 8·7 % of the variability, respectively, and 38·8 % of the overall variance in food intake.
Table 3 Factor loading matrix of the four dietary patterns (after rotation) in pregnant women (n 469)*
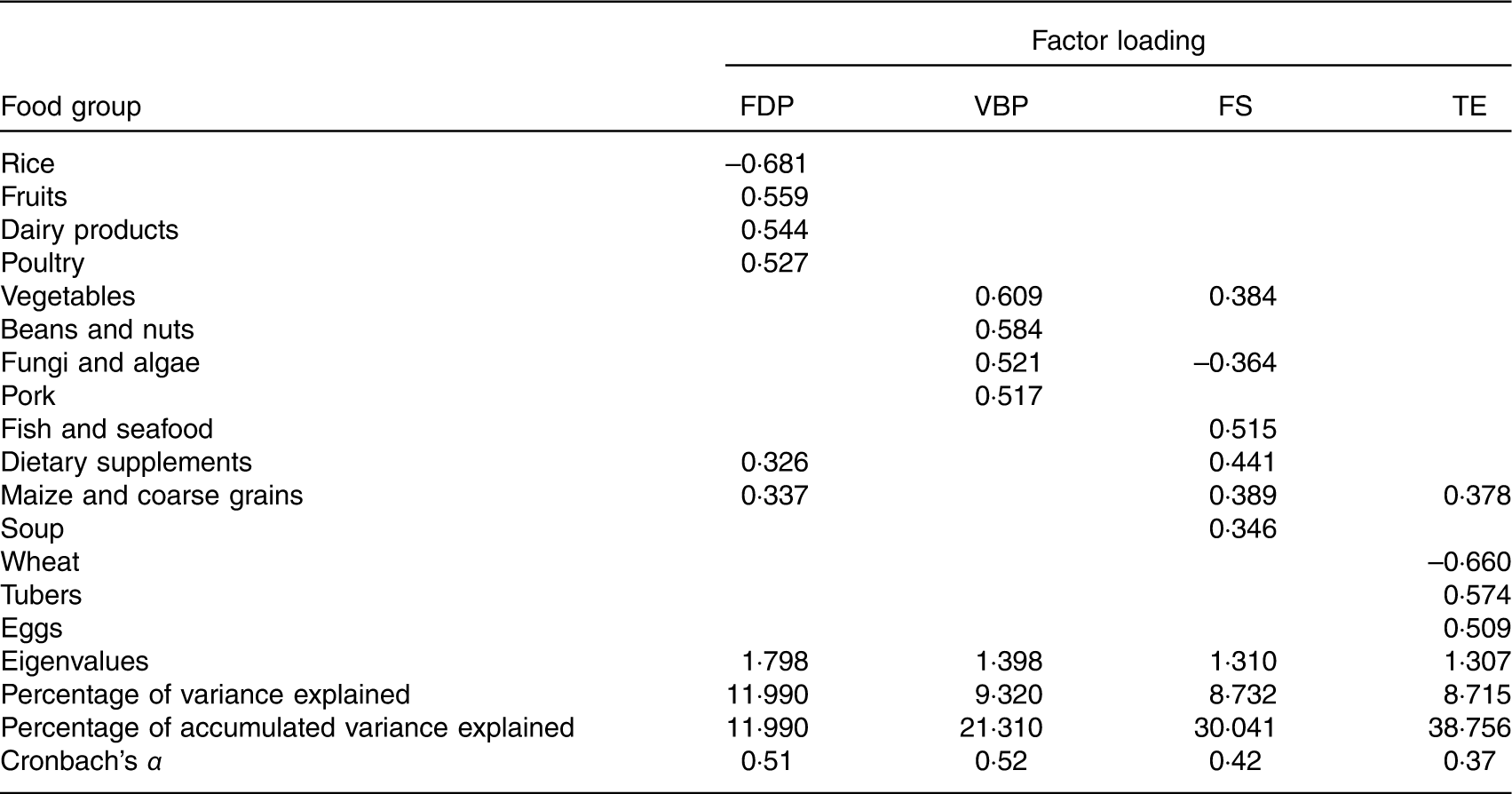
FDP, fruits, dairy products and poultry pattern; VBP, vegetables, beans and pork pattern; FS, fish and soup pattern; TE, tuber and egg pattern.
* Absolute values <0·30 are not listed for simplicity.
The association of dietary patterns with nutrient intake is shown in Table 4. Pregnant women with higher scores for the FDP pattern had higher intake of twelve types of nutrients such as protein, fat and Ca and lower intake of energy and carbohydrates. Higher scores for the VBP pattern were associated with higher intake of energy and eighteen types of nutrients such as protein, fibre and Zn. Higher scores for the FS pattern were associated with higher intake of ten types of nutrients as well as lower intake of n-3 PUFA. The TE pattern score was positively associated with intake of only four nutrients and negatively associated with carbohydrate and Fe. Regarding the comparison of nutrient intake of the four dietary patterns (online Supplementary Table S2), the VBP pattern has the highest intake of energy and eighteen nutrients such as protein, while the FDP and TE patterns have the lowest intake of energy. The TE pattern also has the lowest intake of almost all the nutrients such as protein, Ca and Fe.
Table 4 Pearson correlations of dietary pattern scores and nutrient intake in pregnant women (n 469)
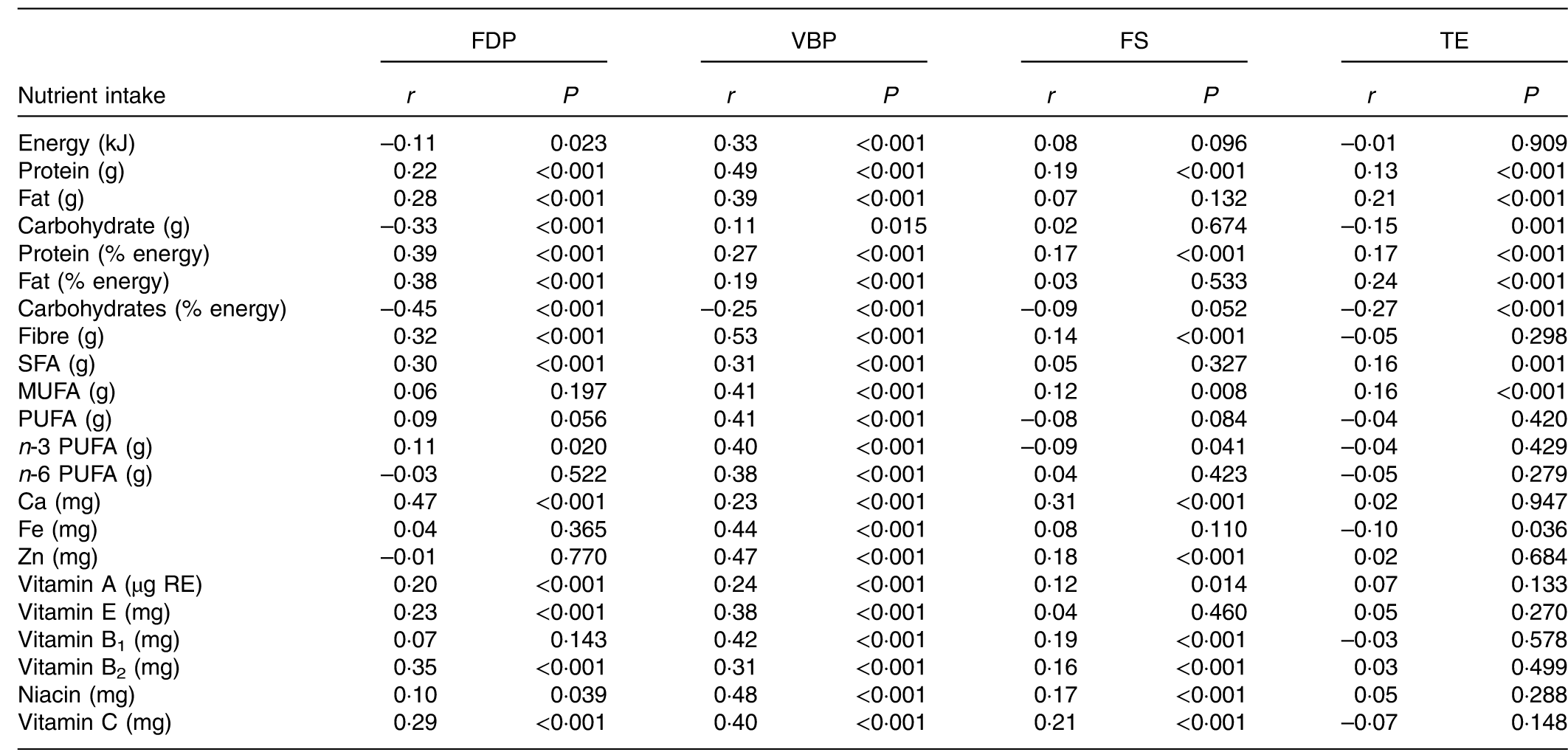
FDP, fruits, dairy products and poultry pattern; VBP, vegetables, beans and pork pattern; FS, fish and soup pattern; TE, tuber and egg pattern; RE, retinol equivalents.
Maternal and fetal cytokines
The maternal and fetal cytokine (APN, IL-6 and IFN-γ) concentrations in serum are shown in Table 5. The fetal concentrations of APN and IFN-γ were higher than the maternal concentrations. Maternal cytokine concentrations were positively correlated with corresponding fetal cytokine concentrations (Table 6). There was no correlation between APN, IL-6 and IFN-γ concentrations (Table 6).
Table 5 Maternal and fetal cytokine concentrations (Medians and interquartile ranges (IQR))
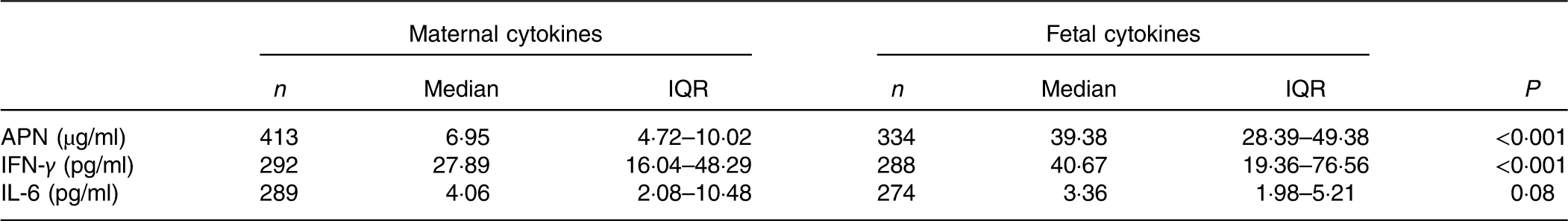
APN, adiponectin; IFN-γ, interferon-γ.
Table 6 Matrix of the Pearson correlations of maternal and fetal cytokine concentrations
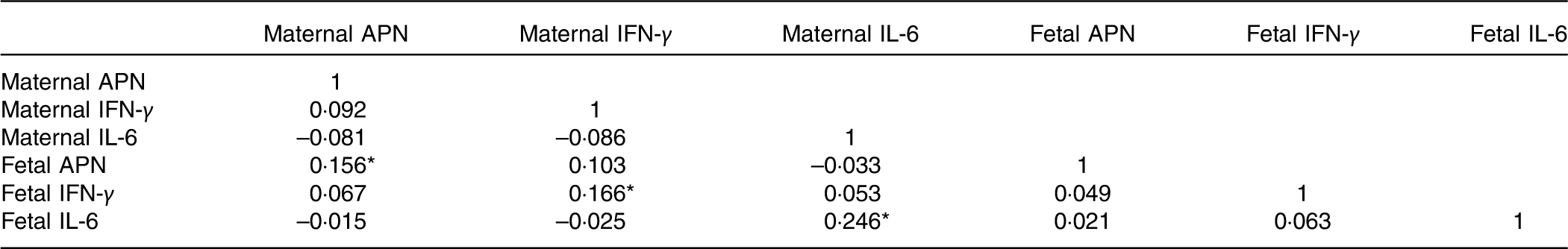
APN, adiponectin; IFN-γ, interferon-γ.
* P<0·05.
Path analysis
The path diagram represents respective standardised β coefficients for all direct effects between dietary pattern scores, maternal/fetal serum cytokines and birth weight as well as the relationships between each variable within the pathways explored (Fig. 2). In agreement with our hypothesis, maternal dietary patterns had direct and indirect effects on fetal birth weight (dotted line indicating the direct effect and solid line indicating the indirect effect). Cytokines acted as the mediator of the association between dietary patterns and birth weight. Of the three maternal/fetal cytokines, we only identified APN and IL-6 as being associated with birth weight. The association between maternal/fetal IFN-γ and birth weight was not statistically significant (the path in the path diagram was omitted). Maternal/fetal APN had a positive association but maternal IL-6 had a negative effect on birth weight. All variables in the path analysis explained 33·6 % of the variation in birth weight.
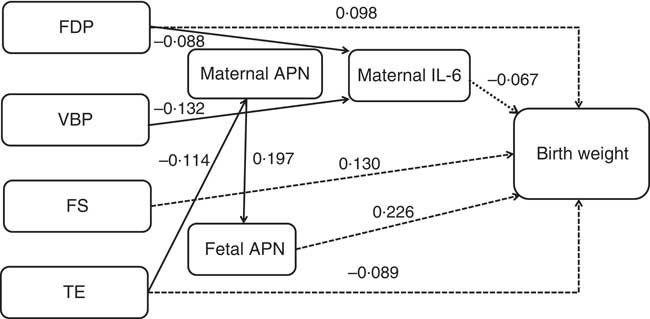
Fig. 2 Path diagram of the relationships among maternal dietary patterns, maternal/fetal serum cytokines and birth weight (n 469). Dotted lines indicate the direct effects of maternal dietary patterns on birth weight, and solid lines indicate the indirect effects. We adjusted for infant sex, gestational age, parity, maternal pre-pregnancy BMI and weight gain during pregnancy. All of the covariates and residual errors have been omitted to simplify the path diagram for illustration. All of the standardised β coefficients in the path were statistically significant (P<0·05). The cytokine levels were log transformed. In the model, R 2=0·336, x 2=63·834, df=47, P=0·05. FDP, fruits, dairy products and poultry pattern; VBP, vegetables, beans and pork pattern; FS, fish and soup pattern; TE, tuber and egg pattern; APN, adiponectin.
Tables 7 and 8 show the total, direct and indirect effects of dietary patterns or maternal/fetal cytokines on birth weight. Of the four dietary patterns, the FS pattern had the greatest effect on birth weight (total effect β=0·130, 95 % CI 0·064, 0·198). For each sd increase in the FS pattern, birth weight increased by 0·130 g. All of the effect (100 %) was direct. The FDP pattern had the second greatest effect (total effect β=0·109, 95 % CI 0·047, 0·188), and 10·1 % of the effect was mediated through decreased maternal IL-6, with 89·9 % of the effect being direct. We also observed that only the TE pattern was negatively associated with birth weight (total effect β=–0·094, 95 % CI –0·171, –0·019); other patterns were positively associated with birth weight. For each sd increase in the TE pattern, birth weight decreased by 0·094 g. In addition, 5·3 % of the effect was mediated through maternal and fetal APN. All of the effect (100 %) of the VBP pattern on birth weight was mediated through decreased maternal IL-6 (total and indirect effect β=0·046, 95 % CI 0·012, 0·080).
Table 7 Effects of maternal dietary patterns on birth weight by path analysis* (β-Coefficients and 95 % confidence intervals)
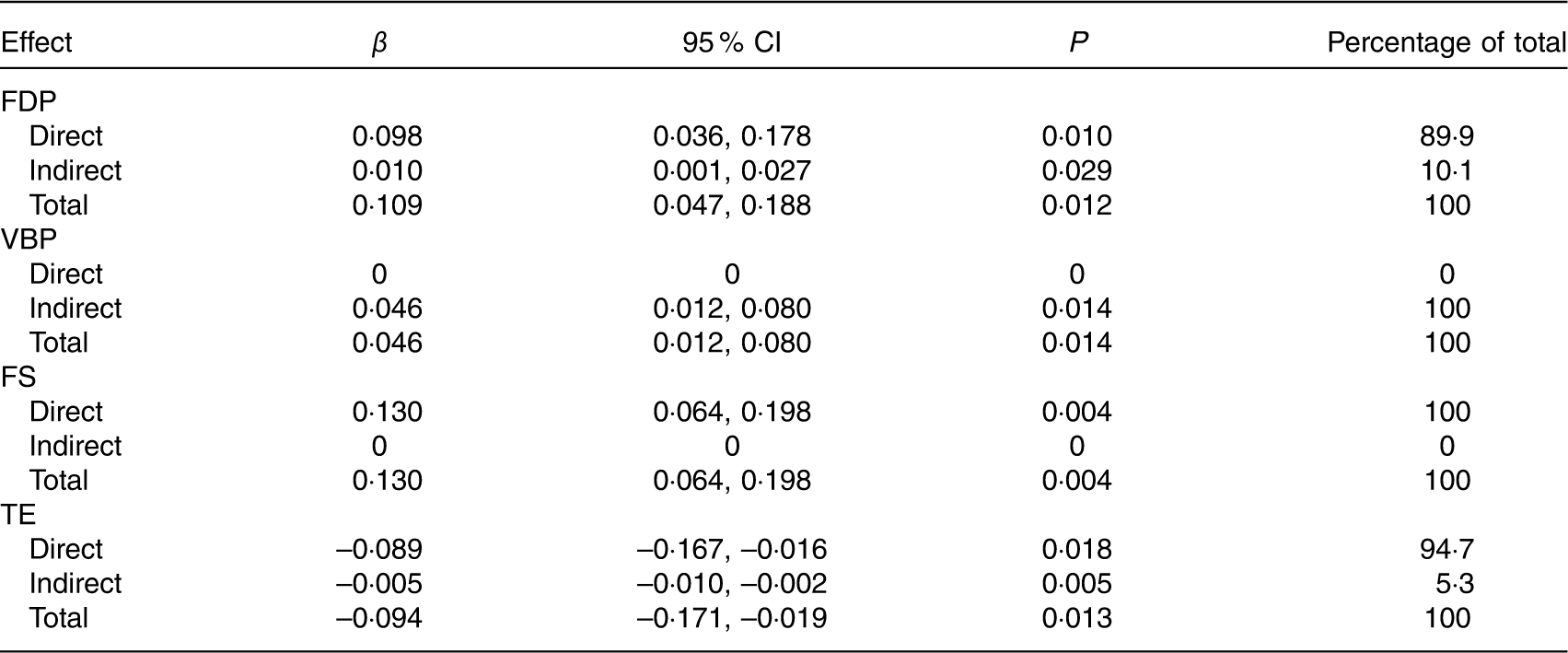
FDP, fruits, dairy products and poultry pattern; VBP, vegetables, beans and pork pattern; FS, fish and soup pattern; TE, tuber and egg pattern.
* n 469. Values were adjusted for infant sex, gestational age, parity, maternal pre-pregnancy BMI and weight gain during pregnancy.
Table 8 Effects of maternal or fetal cytokines on birth weight by path analysis* (β-Coefficients and 95 % confidence intervals)
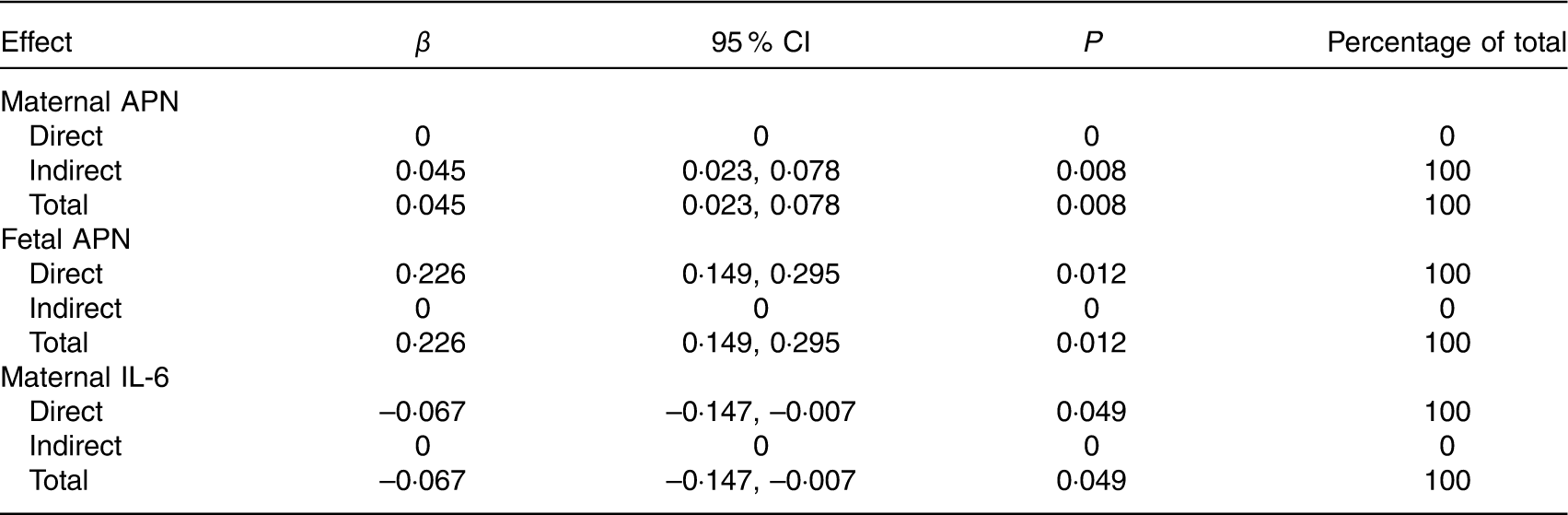
APN, adiponectin.
* n 469. Values were adjusted for infant sex, gestational age, parity, maternal pre-pregnancy BMI, and weight gain during pregnancy.
Of the three cytokines, fetal APN had the greatest effect on birth weight (total effect β=0·226, 95 % CI 0·149, 0·295). Maternal APN was associated with birth weight mediated through fetal APN (total and indirect effect β=0·045, 95 % CI 0·023, 0·078). Maternal and fetal APN mediated the association between the maternal TE pattern and birth weight. Maternal IL-6 had a direct effect on birth weight (total and direct effect β=–0·067, 95 % CI –0·147, –0·007) and acted as a mediator in the association between maternal FDP and VBP patterns and birth weight.
The model modification result is shown in Table 9. All of the indices had high goodness of fit. The model was reasonable.
Table 9 Goodness-of-fit indices for the model
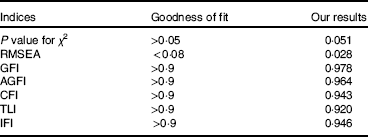
RMSEA, root mean squared error of approximation; GFI, goodness-of-fit index; AGFI, adjusted goodness of fit index; CFI, comparative fit index; TLI, Tucker–Lewis index; IFI, incremental fit index.
Discussion
Maternal diet is an important modifiable risk factor that could be an intervention strategy to reduce the incidence of non-communicable diseases in offspring during adulthood. Cytokines are another factor that regulates offspring health in the short- and long-terms. Results in the available literature suggest that a perturbation of inflammatory cytokines contributes to various adverse pregnancy outcomes such as low birth weight( Reference Rogers and Velten 14 ), whereas APN positively affects birth weight by modulating fetal glucose and lipid metabolism( Reference Mantzoros, Petridou and Alexe 27 ). However, the definite relationships among maternal nutrition, cytokines and birth weight are not fully known. The present study therefore aimed to systematically investigate the above relationships using dietary pattern analysis and pathway analysis in Chinese singleton pregnant women and their full-term infants.
According to the factor analysis, the dietary patterns of subjects in the present study could be divided into FDP (high intake of fruit, dairy products, poultry and coarse grain), VBP (high intake of vegetables, beans, nuts, fungi, algae and pork), FS (high intakes of fish, seafood, dietary supplements, coarse grains and soup) and TE (high intakes of potatoes and eggs) patterns. These four patterns accounted for approximately 38·76 % of the variability, which was similar to results reported by Coelho et al. ( Reference Coelho, Cunha and Esteves 28 ). The classification and nomenclature of dietary patterns are generally different in current studies. For instance, three dietary patterns were identified by a cohort study in pregnant women in Singapore and were termed ‘VFR’ (high consumption of fruit, vegetables and white rice), ‘SfN’ (high intake of noodles, seafood and soya sauce-based gravies) and ‘PCB’ (high consumption of pasta, cheese, bread and butter) patterns( Reference Chen, Aris and Bernard 22 ). However, in another study, the dietary patterns of pregnant women in Japan were defined as the ‘meat and eggs’, ‘wheat products’ and ‘rice, fish and vegetables’ patterns( Reference Okubo, Miyake and Sasaki 29 ). Differences in race, age, educational status, socio-economic condition, lifestyle and especially the dietary behaviours of the subjects are considered the main reasons contributing to various dietary patterns.
The Th1/Th2 balance plays a critical role in the modulation of immune function in both the mother and fetus during pregnancy( Reference Mor, Cardenas and Abrahams 12 ). The median concentrations of IFN-γ (a classical Th1 cytokine) in maternal serum and umbilical cord blood were 27·89 (IQR 16·04–48·29) and 40·67 (IQR 19·36–76·56) pg/ml, respectively, in the present study, which was inconsistent with previous results obtained by An et al. ( Reference An, Nishimaki and Ohyama 30 ) who showed that serum IFN-γ levels were hard to detect. The discrepancy is probably due to different methods: ELISA in the present study and cytometric bead array in An et al. In addition, the concentrations of IL-6 (a classical Th2 cytokine) in the present study were lower than most available data( Reference Guven, Coskun and Ertas 31 , Reference Mardini, Rohde and Cereser 32 ). Different from those research studies, this study was conducted in healthy pregnant women and their babies rather than subjects with pathological conditions such as pre-eclampsia. Finally, correlation analysis demonstrated that positive correlations existed between mother and offspring in IFN-γ and IL-6.
APN is an adipokine secreted by adipocytes with anti-inflammatory, insulin-sensitising and body-weight-controlling properties in adults( Reference Lopez-Jaramillo 33 ). However, in early life, APN is reported to promote fetal growth and development with positive modulations of birth weight, birth height and skinfold thickness( Reference Tsai, Yu and Hsu 9 , Reference Inami, Okada and Fujita 34 , Reference Sivan, Mazaki-Tovi and Pariente 35 ). In accordance with the findings of Ballesteros et al. ( Reference Ballesteros, Simon and Vendrell 36 ), the median concentrations of APN in umbilical cord blood (39·38 (IQR 28·39–49·38) µg/ml) were significantly higher than maternal serum (6·95 (IQR 4·72–10·02) µg/ml) in this study. Previous studies demonstrated that brown adipose tissue, which is widely distributed in newborns but not in adults, was more effective than white adipose tissue in secreting APN( Reference Sivan, Mazaki-Tovi and Pariente 35 ). Furthermore, other tissues or organs such as skeletal muscle and the small intestine in the fetus were also shown to be capable of synthesising and secreting APN( Reference Corbetta, Bulfamante and Cortelazzi 37 ). Hence, APN in newborns is always higher than in the mother, but the relationship between them is undetermined. A positive association between maternal and umbilical APN, which was observed by a published research study( Reference Ballesteros, Simon and Vendrell 36 ), was also detected in the present study, indicating that maternal APN may promote fetal APN in an indirect way because APN cannot pass through the placenta( Reference Mazaki-Tovi, Kanety and Pariente 38 ).
The average birth weight of infants in the present study was 3230·5 (sd 375·3) g, and 94·2 % fell within the normal range according to the Chinese criteria (2500–4000 g). Path analysis showed that the order of effects of dietary patterns on birth weight was FS>FDP>TE>VBP, among which the FS, FDP and VBP patterns were positively associated with birth weight and the TE pattern was negatively associated with it. The opposite effect possibly depended, to some extent, on the distinct nutrient intake in each pattern. As shown in Table 3, different from the other three patterns, the TE pattern was not significantly associated with the dietary intake of several minerals and vitamins including Ca, Zn, vitamins A, B1, B2, C, E and niacin and had lower intake of energy and almost all the lowest nutrients in the four patterns. A review has reported that those patterns labelled ‘nutrient dense’, ‘protein rich’, ‘health conscious’ and ‘Mediterranean’ were positively associated with birth weight; those patterns labelled ‘Western’, ‘transitional’ were opposite( Reference Kjøllesdal and Holmboe-Ottesen 39 ). In our study, the TE pattern contained tubers and eggs and was close to the patterns labelled ‘transitional’. The FS pattern contained more seafood and was close to the patterns labelled ‘Mediterranean’. This may account for the different association between the four dietary patterns and birth weight.
In addition, changes in serum cytokines like APN, leptin and insulin caused by maternal diet were also proposed to influence fetal birth weight( Reference Mantzoros, Sweeney and Williams 40 ). This concept was reinforced by the present study showing that approximately 10·1 and 5·3 % of the effects of the FDP and VBP patterns on birth weight were mediated by decreases in maternal serum IL-6, whereas the effect of the TE pattern was entirely mediated by changes in maternal and umbilical serum APN. All of these findings indicate that maternal dietary patterns affect birth weight in direct and indirect ways, and cytokines may be the link between maternal nutrition and fetal health.
The association between serum IL-6 and birth weight has been controversial. Although umbilical IL-6 in preterm newborns was remarkably higher than that in full-term newborns, no statistical relationship between cord blood IL-6 and birth weight was observed by Lo et al. ( Reference Lo, Tsao and Hsu 41 ). In another paper, the elevated level of serum IL-6 in pre-eclamptic women was reported to be linked to lower fetal birth weight( Reference Guven, Coskun and Ertas 31 ). Partially consistent with the aforementioned research, serum IL-6 in the mother rather than the infant was negatively related to birth weight in the present study, but the underlying mechanism is not completely understood. The results obtained by Wilson & Messaoudi( Reference Wilson and Messaoudi 42 ) provide a plausible explanation suggesting that placental inflammation characterised by overexpression of pro-inflammatory factors like IL-6 and macrophage infiltration may lead to a direct impact on fetal growth and development through regulating nutrient transport in the placenta.
A positive association between cord blood APN and birth weight was detected using pathway analysis in the present study, which was in line with the findings of Sivan et al. ( Reference Sivan, Mazaki-Tovi and Pariente 35 ) APN in the fetus was shown to promote expansion of adipose tissue as well as transcription of lipogenic genes( Reference Aye, Powell and Jansson 43 ), indicating that fetal APN plays an important role in the enhancement of birth weight. Interestingly, maternal APN seems to have opposite effects on fetal growth and development against fetal APN because a long-term infusion of full-length APN in pregnant mice was reported to significantly limit fetal growth in utero via the inhibition of placental amino acid transport and insulin signalling( Reference Rosario, Schumacher and Jiang 44 ). However, our results demonstrated that maternal serum APN could positively regulate birth weight alone or through the modulation of umbilical cord blood APN. There is no reasonable explanation for this discrepancy, which needs further study.
Limitations and strengths
We are aware that the present study has several limitations. First, dietary patterns may vary among studies because of diverse race, age, educational status, socio-economic condition or lifestyles. It is difficult to compare the findings of the present study with those of others. Second, not all of the subjects consented to the assessment of serum cytokine. Thus, the results may be subject to potential bias. Third, maternal dietary intake in this study was assessed using a 24 h recall, which may not reliably evaluate the usual dietary intake. Despite these limitations, our study differs from most previous work in that we used path analysis to reveal the complex relationships between maternal dietary pattern, maternal/fetal cytokines and birth weight. Path analysis allows researchers to test theoretical models and quantify the relative influence of each factor. It allows estimation of direct, indirect and total effects of maternal diet on birth weight. Thus, path analysis produces easily interpretable results, and it has more statistical power compared with standard regression methods( Reference Gamborg, Andersen and Baker 23 ). Furthermore, we demonstrated that some cytokines act as the mediator in the association between maternal diet and birth weight, thus generating improved understanding of how nutrition in early life ‘programs’ the offspring’s growth and development.
Conclusion
The order of effects of maternal dietary patterns in pregnancy on birth weight was FS>FDP>TE>VBP (β=0·130, 0·109, –0·094 and 0·046, respectively). Only the TE pattern’s effect was negative. Maternal and fetal APN were positively associated birth weight and they mediated the association between the TE pattern and birth weight. Maternal IL-6 was negatively associated with birth weight and mediated the association between maternal FDP and VBP diet patterns and birth weight. The results suggested that maternal dietary patterns in pregnancy are associated with birth weight and mediated directly and indirectly through some maternal/fetal serum cytokines.
Acknowledgements
The authors gratefully acknowledge all the participants for their support.
This study was jointly supported by the National Natural Science Foundation of China (no. 81273072), the National Key Technology R&D Program of China (2008BAI58B07).
L. M. analysed the data and wrote the paper. Q. L., J. O., J. H., S. H., C. J. and Z. Z. enrolled participants and collected data. L. M. (corresponding author) was responsible for designing the study and performing data analyses. All the authors contributed to the interpretation of data and critical revision and approval of the article. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000382