Introduction
The Bali myna Leucopsar rothschildi was first encountered by a Western scientist at a site on the north coast of Bali, Indonesia, on 24 March 1911 (Stresemann, Reference Stresemann1912). The event was remarkable for three reasons: firstly, the description of a new species of bird on an island with close biogeographical links to Java was unexpected, particularly so late in the exploration of the Indonesian archipelago; secondly, the systematic distinctiveness of the species, requiring its own genus, was even more unexpected; and thirdly, the bird is extraordinarily beautiful (snow white, with a thick floppy crest, black tips to the wings and tail, naked blue face and yellowish bill; Plate 1). Its rarity (Stresemann, Reference Stresemann1913) could explain why the species went undescribed for so long, and its beauty explains why it became even rarer in the remainder of the 20th century.

Plate 1 Bali myna Leucopsar rothschildi displaying its crest. Photo: Panji Gusti Akbar.
From 1911, the species was observed and recorded at a total of 10 sites in or near tracts of deciduous savannah-like woodland around the coast of north-west Bali (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). However, this range and the population decreased over the 20th century and by 1990 the wild population had been reduced to c. 20 individuals, confined to Bali Barat National Park on the 140 km2 Prapat Agung peninsula in the far north-west of the island (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). The decline of the myna has been reflected in its IUCN Red List classification: it was first categorized as Threatened in 1988 and then as Critically Endangered in 1994, a status that remains unchanged (BirdLife International, 2021). The cause of this contraction, although poorly documented before the 1970s, can, with reasonable confidence, be assumed to have been habitat loss in the first half of the 20th century and trapping for trade in the second half (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001; BirdLife International, 2021). A cooperative conservation breeding programme, using already captive stock on Java plus donations from the USA and UK, was initiated in the early 1980s, and releases of captive-bred birds to supplement the surviving population in Bali Barat National Park began in 1988 (van Balen & Gepak, Reference van Balen, Gepak, Olney, Mace and Feistner1994). However, this practice only became annual at the start of the 21st century, with 196 birds released during 2000‒2011 (i.e. a mean of 16.3 releases per year, but with high annual variability in numbers released and little insight gained into the effects of these releases on the free-living population; Sutedi, Reference Sutedi2012).
In 2006, an attempt to establish a self-sustaining population of Bali mynas outside their known range began on the island of Nusa Penida off the south-east coast of Bali, with the release of 25 birds sourced from a non-commercial breeding programme (Dijkman, Reference Dijkman2007). Some 6‒7 years later as many as 100 birds were reported to be free-living there (it is unclear whether this is because of natural increase, continuing releases or a combination of the two; Dijkman, Reference Dijkman2014), but soon afterwards the population suffered major losses that were attributed to trapping (Eaton et al., Reference Eaton, Shepherd, Rheindt, Harris, van Balen, Wilcove and Collar2015; Mattison, Reference Mattison2016) but could have been driven by a combination of dilapidated nestboxes and extreme weather events (D. Donato, pers. comm., 2022.). Numbers are reported to have naturally recovered and there are now c. 20 breeding pairs (D. Donato, pers. comm., 2022.). On Bali itself a programme intended to create a semi-urban population of free-living Bali mynas is ongoing (Asian Species Action Partnership, 2022), with 40 birds released into Bali Safari Park in May 2019 (Bali Safari Park, 2019). Other sites have been proposed for releases, including Besikalung and Pejeng Wildlife Sanctuaries, both in central Bali outside the original known range of the species (Friends of the National Parks Foundation, 2022). Thus, although two smaller extralimital populations have been established and more releases are being considered or are already underway, the programme in Bali Barat National Park remains the only effort actually within the recorded distribution of the myna and is the primary focus of this study.
Recent developments (2010–present)
During the 3 decades up to 2010, based on the steady downward trajectory of census results since 1980 (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001; Sutedi, Reference Sutedi2012), conservation efforts for the Bali myna, implicitly or explicitly aimed at stopping and/or reversing the decline in its numbers, were unsuccessful, and the species was considered in all likelihood extinct in the wild by 2006 (Jepson, Reference Jepson2016). However, an imaginative scheme devised by Asosiasi Pelestari Curik Bali (Bali Myna Conservation Association) allowed the breeding facility of Bali Barat National Park to loan captive birds to local communities for commercial breeding, with the aim of flooding the market with captive-bred birds, thereby reducing their price and in turn the likelihood of poaching within the National Park (Collar et al., Reference Collar, Gardner, Jeggo, Marcordes, Owen and Pagel2012; Jepson, Reference Jepson2016). There have also been changes in management in the National Park, with > 100 nestboxes provided, more birds released, some collaborative enterprises begun and information exchanged through the International Advisory Board of the Bali Myna Conservation Association (Yuni et al., Reference Yuni, Collar, Nugroho, Marsden, Sarmawi and Squires2022).
The conservation breeding programme continues to supplement the free-living population, with the ultimate aim of establishing a viable self-sustaining population of Bali mynas in and around Bali Barat National Park (Bali Barat National Park, 2021). Although the numbers of birds released during 1998–2012 fluctuated widely, since 2013 there have been numerically more consistent annual releases of captive-bred birds (Fig. 1). This has gradually increased from 24 in 2013 to 68 in 2021 (Bali Barat National Park, 2021). Releases have been carried out at four main sites: Brumbun, Lampu Merah, Labuan Lalang and Cekik (Figs 2‒4). The latter two (first used in 2014 and 2015, respectively) were a departure from the previous strategy of the National Park authority of releasing birds on Prapat Agung peninsula, where habitat might be suboptimal (too dry, with a lack of dry-season water sources), to releasing them into the largely anthropogenic habitats around the main road through the National Park. Most releases have been in the wet season when food availability is highest. Following previous recommendations (Collins et al., Reference Collins, Smith, Seibels and Putra1998), backed up by insights from Mauritius and New Zealand (Jones & Merton, Reference Jones, Merton, Ewen, Armstrong, Parker and Seddon2012) and now broadly supported (Resende et al., Reference Resende, Viana-Junior, Young and Azevedo2021), a soft release strategy has been used, whereby birds are kept in an aviary at the release site for a period of acclimatization and with food and water provided year-round for both wild and released birds.
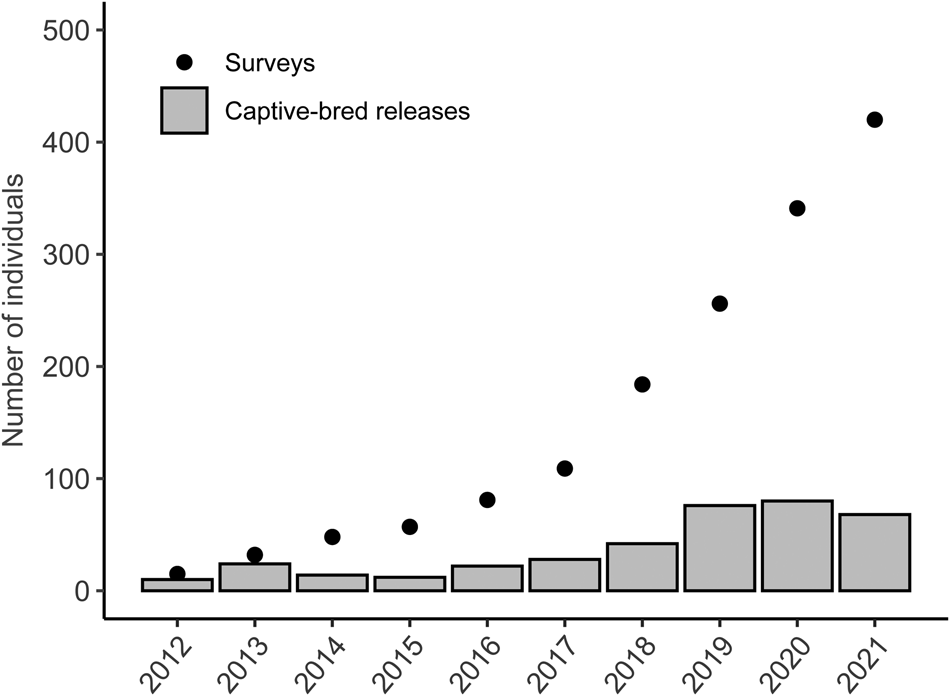
Fig. 1 Bali myna Leucopsar rothschildi population size from annual population survey data and the number of captive-bred individuals released each year during 2012–2021 in Bali Barat National Park, Bali.
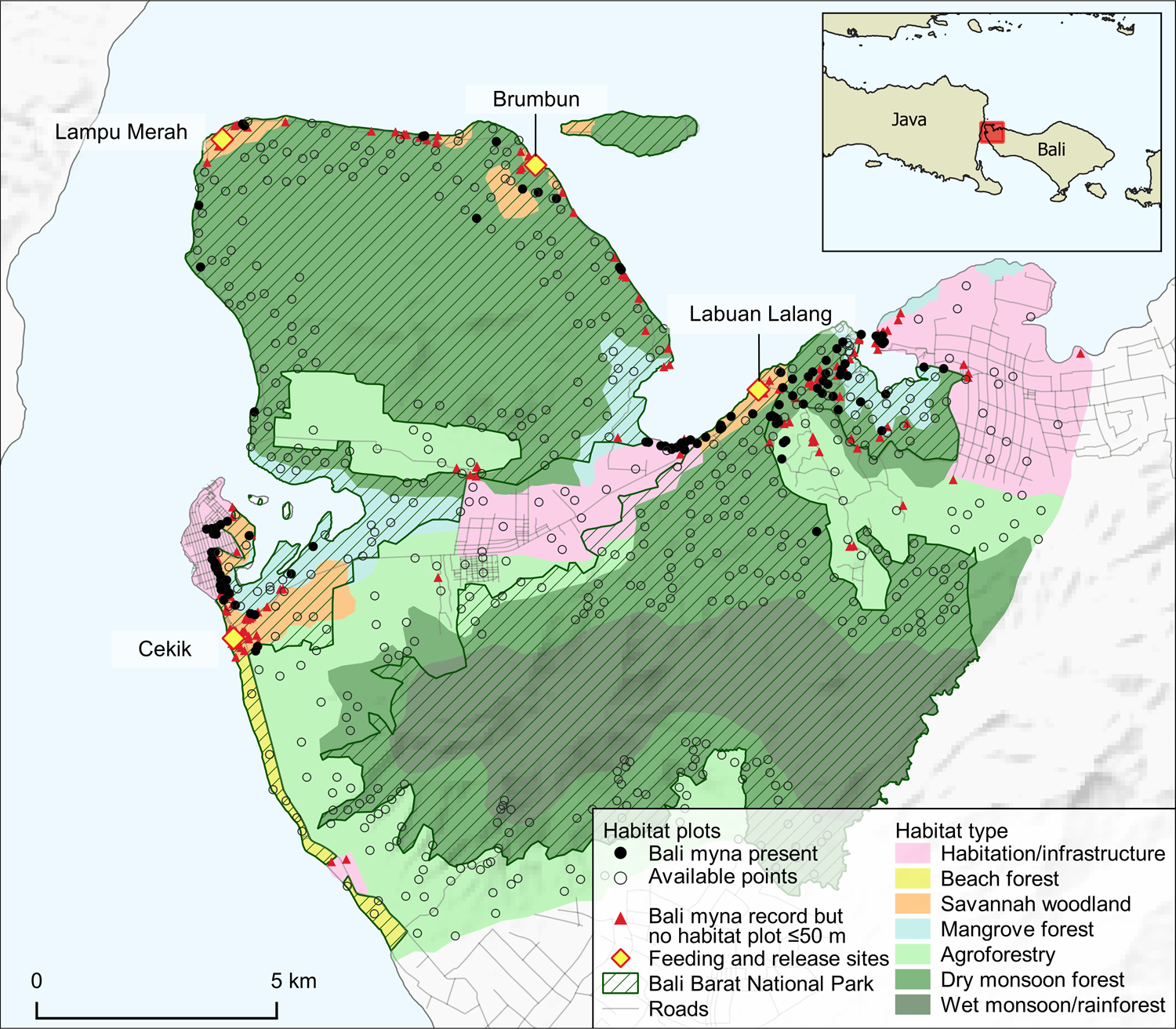
Fig. 2 Distribution of habitat types in Bali Barat National Park, Bali (Table 1; adapted from Bali Barat National Park, 1997). Habitat plots ≥ 500 m away from feeding and release sites (n = 612) were included in the habitat association analysis as a Bali myna ‘presence’ if they were ≤ 50 m from a Bali myna record (n = 114) or as an ‘available habitat point’ if they were > 50 m from a Bali myna record (n = 488). Bali myna records > 50 m from a habitat plot (triangles) were not part of the habitat association analysis (n = 157). (Readers of the printed journal are referred to the online article for a colour version of this figure.)
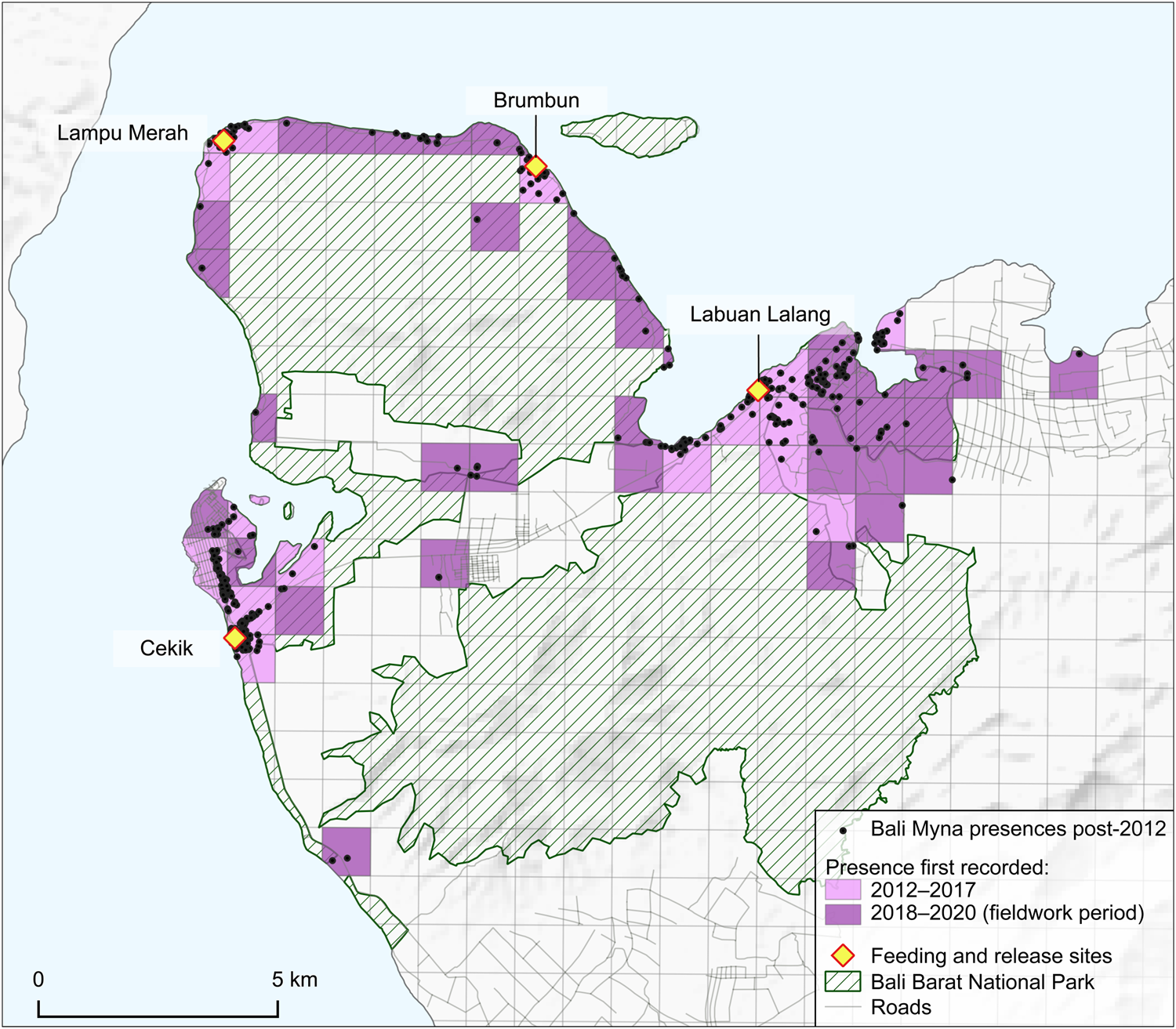
Fig. 3 Records of the Bali myna in and around Bali Barat National Park. Grid squares (1 km2) are shaded according to whether Bali mynas were recorded there before or after 2018 (but note that the grid squares with early records remain occupied). (Readers of the printed journal are referred to the online article for a colour version of this figure.)
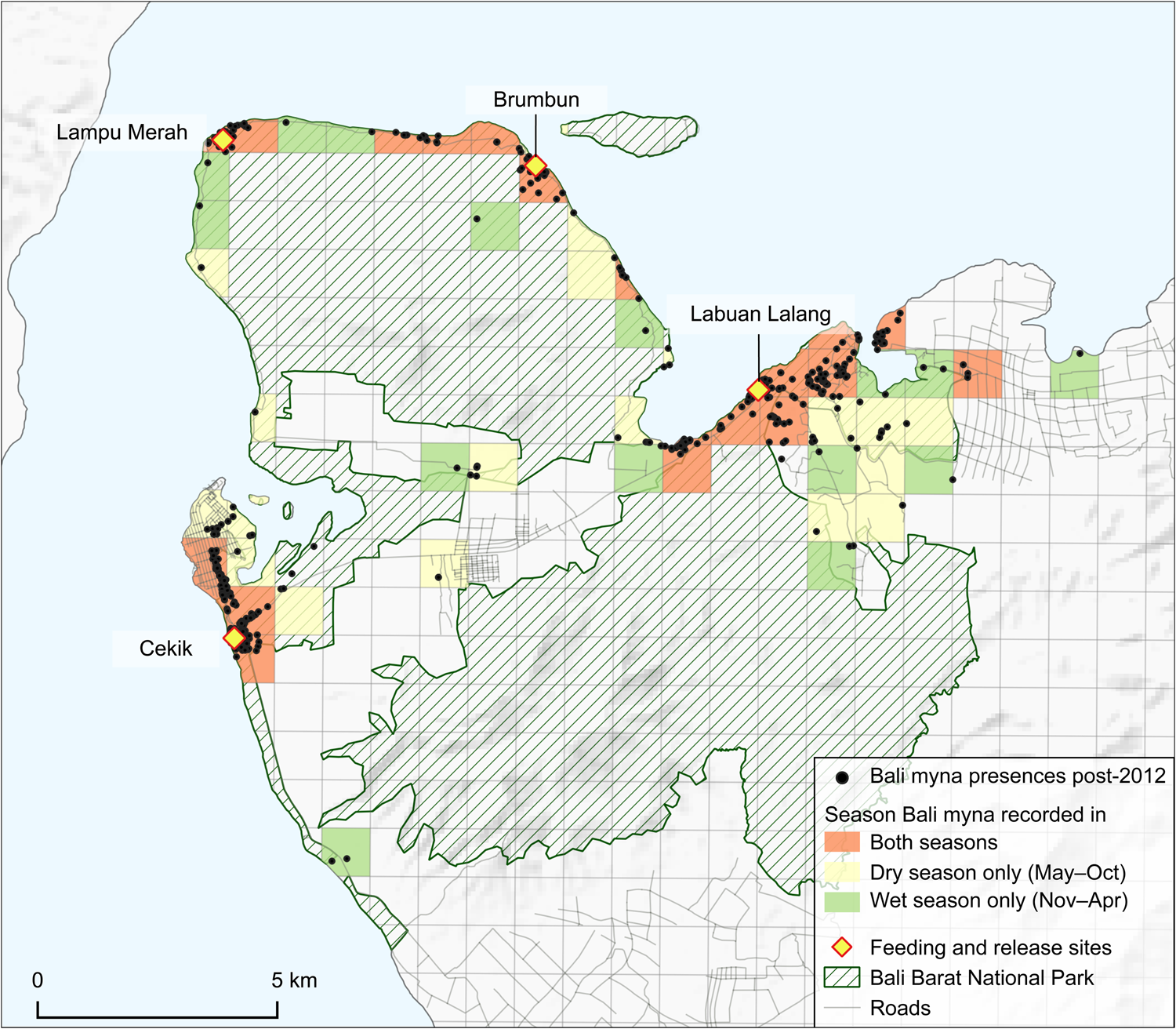
Fig. 4 Seasonality of Bali myna distribution in Bali Barat National Park. (Readers of the printed journal are referred to the online article for a colour version of this figure.)
Here we present evidence for a recent expansion and increase in the population of free-living Bali mynas at the only occupied site within the original known range of the species. Firstly, we present distributional and population survey data from within Bali Barat National Park for 2012‒2021 and put these into context in terms of numbers of birds released during that period and nesting output. We then examine the current distribution of free-living Bali mynas in relation to key habitat features such as canopy openness, understorey density and sward height, both to identify the habitat preferences of the mynas and to discuss which other areas of the National Park could be suitable in the future. Finally, we use these results to make recommendations for future management and research within the National Park and in adjacent areas.
Methods
Bird census and searches
Bali Barat National Park staff conduct a standardized survey to estimate numbers of Bali mynas each year following a protocol established in the early stages of conservation efforts for the species (van Balen et al., Reference van Balen, Dirgayusa, Adi Putra and Prins2000). Annual count data for Bali Barat National Park are available from 1991 onwards (Bali Barat National Park, 2021). The census is made at the end of the dry season (September–October) when birds tend to associate in flocks at roosting sites, which are identified by National Park staff in the weeks leading up to the census. Roost counts lasting 2.5 h are carried out by teams of 4–10 experienced National Park staff at each site over a survey period lasting c. 6 days, depending on the number of sites visited. Most sites are > 5 km apart, and roosts are used repeatedly over periods of months (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001), with movements between them believed to be rare (TMS, pers. obs., 2019.), leaving only a small risk of miscounting. Staff are positioned strategically to cover roosting trees and major flight lines, to tally the number of birds entering and leaving the sites but far enough from roost trees to reduce the risk of double-counting temporarily disturbed birds. Occasional double-counting could slightly inflate population estimates but, as the method is standardized and repeated annually, the relative estimates remain an important population monitoring tool.
In addition to the standardized bird survey, we (TMS, AN, MUS and Panji Gusti Akbar) actively searched for Bali mynas across the Park during December 2018–December 2020 to determine their distribution. In total, we devoted the equivalent of 88 full days to these searches. We also asked National Park staff, Bali bird guides and local people to report sightings to us. For historical records (pre-2018), we interviewed 16 National Park officials and other knowledgeable people to ask where and when they had previously sighted Bali mynas. We geolocated all sightings and visited each site to record the habitat type. We searched for nest sites, whether in natural cavities or nestboxes, at the same time as searching for birds. We monitored nests found during October 2019–September 2020 to measure the breeding productivity of the population over a single 12-month period, thus encompassing one breeding season.
During July–October 2019, we identified the habitat associations of free-living Bali mynas at Bali Barat National Park. We gathered habitat data from all 222 locations where mynas had been recorded up to October 2019 and we positioned a further 491 habitat plots randomly within a stratified sample of broad habitat types in the National Park, based on the most recent Bali Barat National Park land-use map (Bali Barat National Park, 1997; Fig. 2). We limited the number of random habitat plots included to the maximum that we estimated could be covered by the two data recorders in the available sampling period. We excluded plots ≤ 500 m from the 108 release and supplementary feeding sites from the analysis because of the influence supplementary feeding is likely to exert on the distribution of the birds (by restraining many individuals from dispersing more widely). At the end of the fieldwork period (December 2020) we considered all remaining habitat plots ‘presences’ if they were ≤ 50 m from a Bali myna record (n = 114). If more than one plot was ≤ 50 m from a single myna record, we included only the closest habitat plot to the record in the analysis. A total of 157 Bali myna records were > 500 m from the feeding and release sites but not ≤ 50 m from a habitat plot, and we therefore excluded these from the analysis (Fig. 2). We considered the 488 remaining plots as ‘absences’. We cannot be certain these plots were representative of true absences and they might better be termed ‘available habitat points’. However, the habitat plots were randomly assigned and covered a large area (Fig. 2), and the fieldworkers were always alert to the presence of the species during habitat recording. Local people who reported historical sightings are subject to the same biases in terms of their movement, but it is still striking that there were no records in those areas despite the time spent there. Moreover, we undertook a systematic multi-species bird survey using point count distance sampling during 20 June–30 August 2019, with 303 point count locations distributed across most of Bali Barat National Park except for rainforest areas (TMS, unpubl. data, 2019). Amongst 1,815 bird records there were 18 Bali myna observations, with these occurring at 14 of the 303 point count locations (most were at Labuan Lalang and all were ≤ 1 km from the coast). Given our prior knowledge of the ecology of this species and the lack of a single record from these areas obtained either in this study or in previous thorough assessments of the species' range (van Balen et al., Reference van Balen, Dirgayusa, Adi Putra and Prins2000; Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001), we are confident in the accuracy of our assessment of the available habitats that were assumed to be absences. We believe few of these points would turn into ‘presences' with significantly greater sampling effort.
At each habitat plot we recorded the following within a 10-m radius from the central point of the plot (this radius being a compromise between characterizing habitat over a sufficiently large but manageable area and the available survey effort; Htike et al., Reference Htike, Round, Savini, Tantipisanuh, Ngoprasert and Gale2021): number of trees > 150 mm diameter at breast height (1.3 m); diameter at breast height of the largest tree; height of the tallest tree; mean canopy cover; per cent covers of grass and detritus; and mean sward height from four measurements taken at points 3 m from the centre of the plot in each cardinal direction. At each of these points we also recorded a measure of understorey density by counting the number of times a 1 m pole, held at its end and parallel to the ground at a height of 1.3 m, struck vegetation when rotated through 360°. We chose habitat variables based on a priori knowledge of the habitat associations of the species (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). The species makes use of trees, understorey and the ground, so our variables reflect this habitat variety. Perhaps the most important is openness of habitat, reflected by our tree density and understorey density, but sward height is also believed to be important. Habitat data collection coincided with the end of the local dry season when canopy and understorey cover and other habitat measures such as tree density are expected to be reasonably constant.
Data analysis
Firstly, we ran a principal components analysis (PCA) on the eight habitat variables measured in the plots, using the prcomp function in R 4.0.2 (R Core Team, 2022), and retained the four components with the highest eigenvalues. We excluded wet monsoon forest and rainforest as there was just one record in these extensive habitats (Fig. 2), leaving the focus on those anthropogenic and semi-natural habitats commonly used by the myna.
The four components with the highest eigenvalues extracted using the PCA accounted for 37.3, 14.5, 12.8 and 10.3% of the variability (axes 1–4, respectively), so together they retained 74.9% of the variability from the eight original variables (Fig. 5 shows factor loadings on these four axes). The scores on axis 1 clearly correlate with openness, with grassy areas having negative scores and closed-canopy woodlands having positive scores. Axis 2 appears to be related to increasingly mature natural savannah habitat, with positive scores in areas with few but large-girth trees and with grass cover, and negative scores associated with a dense understorey and increasing detritus cover. Axis 3 appears to be related to grazing pressure, with negative values in areas with a taller sward and therefore lower grazing pressure. The scores on axis 4 are related to woodland maturity, with negative scores for dense immature woodland with a closed canopy and positive scores for areas with fewer trees that are taller and have greater diameter.
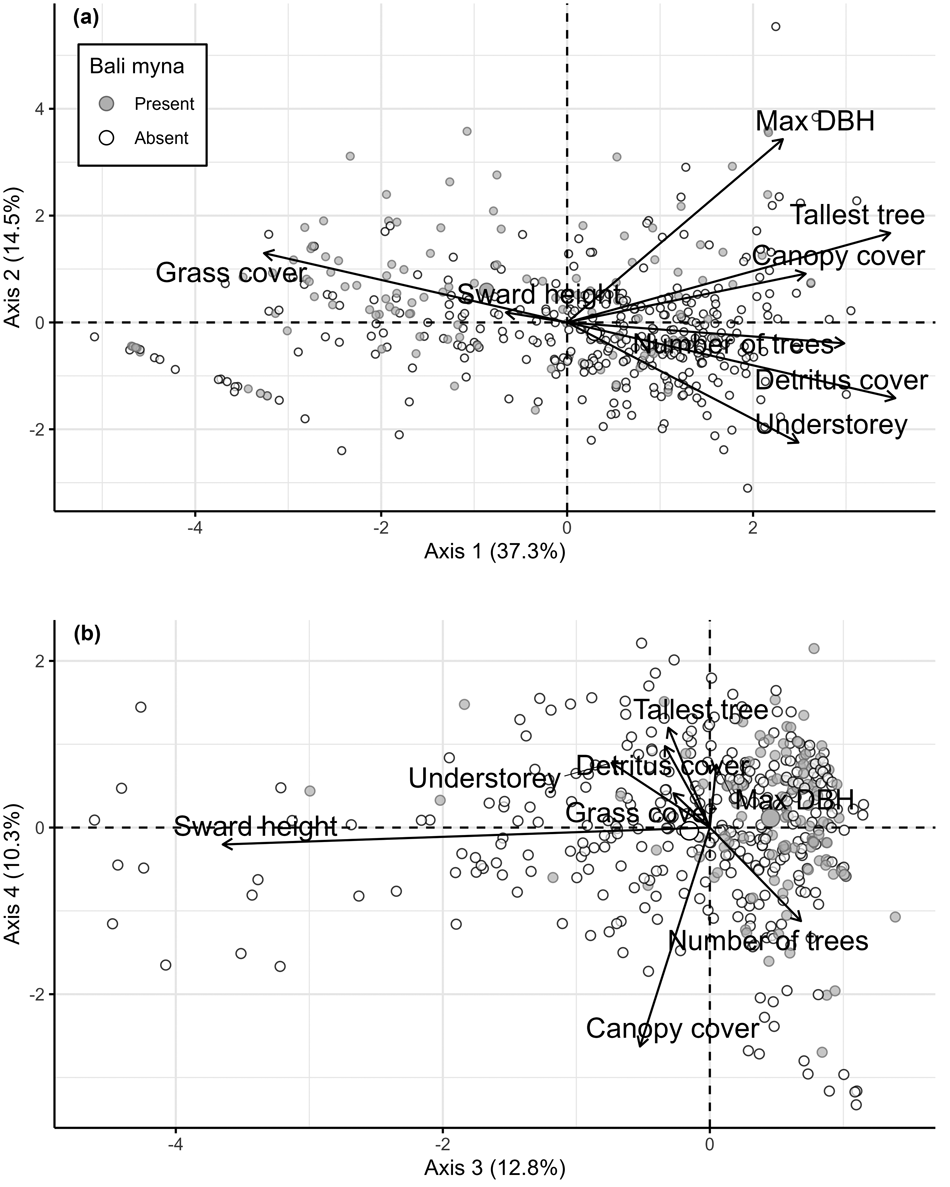
Fig. 5 Biplots showing the positions of habitat plots linked to Bali myna presence and absence in Bali Barat National Park, in relation to (a) axes 1–2 and (b) axes 3–4 of the principal components analysis (Table 2). DBH, diameter at breast height.
To examine the habitat associations of the Bali myna, we plotted the presences and presumed absences on biplots of axes 1–4 derived from the PCA. We then ran a binary logistic generalized linear model (GLM), with the response variable being the known presence or presumed absence of Bali mynas at the plot and the four axes retained from the PCA. In this analysis, we added the distance from the nearest myna release and supplementary feeding site in an attempt to determine its importance in limiting the myna's distribution.
Results
Distribution and abundance of free-living Bali mynas
Annual population surveys undertaken by the National Park staff indicate there has been an increase in the Bali myna population from 15 individuals in 2012 to 420 in October 2021 (Fig. 1). The supplementation of the population with releases of captive-bred birds has amounted to 376 individuals over 2012–2021, with the number of birds released showing an increasing trend over this period.
During the 2018–2020 fieldwork, we accumulated 813 records of the Bali myna, of which 112 were gathered through interviews (44 of these pre-dated our fieldwork period) and 701 were recorded directly by us (Fig. 3). Although, as expected, records were clustered around the four main release sites, there was also some significant dispersal from all of them. This was most pronounced in the eastern Labuan Lalang feeding and release site, from which birds dispersed west nearly 5 km along the coast and even more widely both inland and along the coast to the east. These latter areas include the tourist resort concessions at Menjangan and the agricultural lands outside the National Park nearly 7 km away. Dispersal away from the Cekik release site in the west has mainly been towards the outskirts of Gilimanuk town, where birds can be easily seen at the roadside, but notably also c. 4.5 km to the south into coconut plantations. Mynas, presumably from the two peninsula release sites, appear to have dispersed reasonably well along the coasts (from Brumbun better than the western Lampu Merah site), such that there are at least scattered records over much of the coastline away from the west. Most areas where mynas were found provided records from both the wet and dry seasons, and places where birds were only seen in one of the seasons were often within 1 km of locations where the species was only recorded in the other season (Fig. 4).
Effort to locate dispersing Bali mynas was relatively low before the formal surveys of 2018–2020 and any records then made might not have been geolocated. Despite this limitation, we have at least established that all 1-km2 squares where birds were recorded prior to 2018 had records after that time, and 36 were added to the range of the species post-2018. The seemingly new Bali myna records along the coast of the Prapat Agung peninsula suggest that the birds have radiated out from Brumbun and Lampu Merah, but this is probably an artefact of increased survey effort.
Nest locations and productivity
A total of 31 Bali myna nesting sites were located during the fieldwork (Fig. 6) including 26 in artificial nestboxes, four in natural cavities and one in a bee box intended to harvest honey. A total of 76 Bali mynas fledged from the 54 nesting attempts we monitored during the 2019–2020 breeding season (Yuni et al., Reference Yuni, Collar, Nugroho, Marsden, Sarmawi and Squires2022).
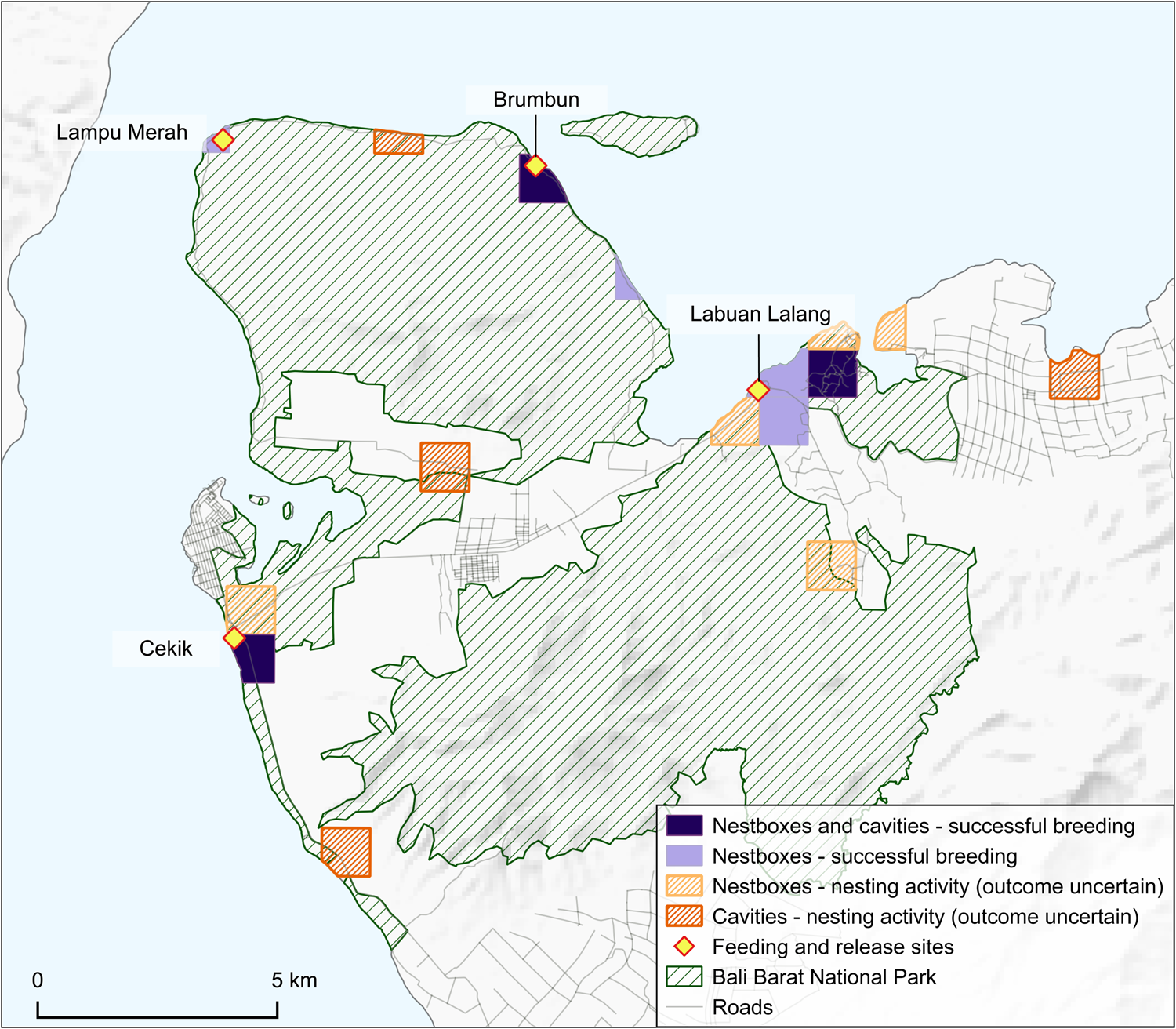
Fig. 6 Records of Bali myna nesting attempts in artificial nestboxes and natural cavities in and around Bali Barat National Park in 2019–2020. (Readers of the printed journal are referred to the online article for a colour version of this figure.)
Habitats used by free-living Bali mynas
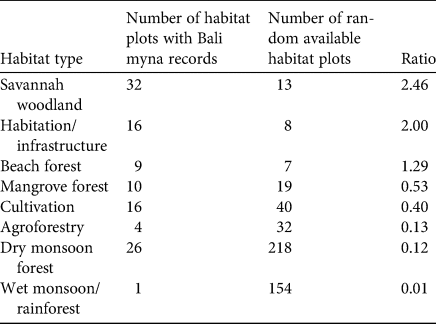
The greatest number of records were from savannah woodland and dry monsoon forest, although the latter is more extensive (as reflected in the number of random plots within that habitat) than the former (Fig. 2, Table 1). However, apart from wet monsoon forest and rainforest, which had only two records despite covering a large area of the National Park, all habitat types, including mangrove forest, beach forest and agricultural land, were well used. Moreover, based on its relative frequency in the area, human habitation, with its associated infrastructure, was the second most preferred habitat (Table 1). There was a clear difference in the proportions of habitat plots used across these different habitats (χ 27 = 163.1, P < 0.001).
Table 1 Numbers of habitat plots with Bali myna Leucopsar rothschildi records (2012–2020; > 500 m from release site) and randomly distributed available habitat plots in broad habitat types in Bali Barat National Park, Bali (Fig. 2). Habitats are ordered by ratio of presences to random points. Proportions of records in each habitat differed significantly (χ 27 = 184.7, P < 0.001).
The habitat plots associated with Bali myna presence and presumed absence are mapped onto each of the four axes in Fig. 5. Areas where Bali mynas were recorded tended to be in places with features typical of savannah, namely extensive grass cover and trees with large diameters, whereas the birds were mostly absent from areas with an increased number of trees and a dense understorey. The spread of presences along the axes highlights that Bali mynas can be found in a wide range of open and semi-open habitats but not in dense woodland, rainforest or wet monsoon forest, which were excluded from this analysis.
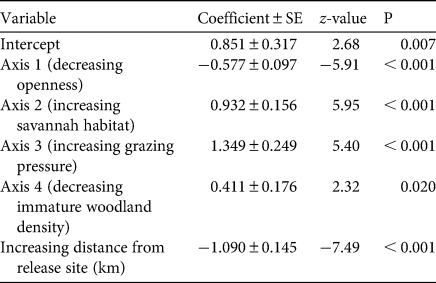
Although there appears to be much suitable but unoccupied Bali myna habitat in Bali Barat National Park, the results of the GLM were striking (Table 2). Myna presence was, as expected, significantly associated with proximity to release site, but it was also significantly related to all four PCA axes. Axis 1 (increasing canopy closedness) deterred myna presence; axis 2 (increasing savannah habitat) and axis 3 (increasing grazing pressure) promoted myna presence; and there was a weaker positive association with high scores on axis 4 (decreasing immature woodland density).
Table 2 Coefficients ± standard errors and significance of principal components analysis axes and intercept in binary logistic generalized linear models of presence records of Bali mynas versus randomly placed ‘available habitat’ plots across Bali Barat National Park, Bali (rainforest and wet monsoon forest, which were almost never used, were excluded; Fig. 5).
Discussion
After decades of evident difficulty in maintaining a reintroduced population of Bali mynas in Bali Barat National Park, recent adjustments to the management of releases within the National Park appear to be achieving positive results. The population is increasing, its area of occupancy within the National Park is expanding and its breeding output could be sufficient to allow this expansion to continue (Yuni et al., Reference Yuni, Collar, Nugroho, Marsden, Sarmawi and Squires2022). Population viability analysis suggests that numbers will continue to increase even when supplementation is phased out and even if some trapping or nest poaching takes place (Squires, Reference Squires2022). Offspring of released birds (largely unringed individuals) are dispersing well away from release sites, and a pair has recently been found breeding outside the eastern boundary of the National Park, c. 7 km from the nearest release site (HK, pers. obs., 2020.). In the context of the Asian songbird crisis (Eaton et al., Reference Eaton, Shepherd, Rheindt, Harris, van Balen, Wilcove and Collar2015), which has seen numerous once-common species driven to the edge of extinction by excessive trapping, this evidence of a recovery of the Bali myna is important in two respects: firstly, as an indication that even the status of a species in critical condition can be improved; and secondly, as a caution that the conservation of any of the other songbird species affected by trade is unlikely to be straightforward, quick or low-cost.
As the free-living population of Bali mynas increases and expands within Bali Barat National Park, it is important to consider the key factors most likely to be limiting the species and to determine the potential for addressing them through conservation management both inside and outside the National Park. One key factor is the availability of appropriate habitat and another is trapping pressure; addressing them together will allow continued population growth. Determining what constituted the original habitat of the Bali myna is, however, made difficult by the lack of records from before the species became confined to part of Bali Barat National Park. As case studies have shown (Moore et al., Reference Moore, Uzabaho, Musana, Uwingeli, Hines and Nichols2021) and as has been observed previously (Caughley, Reference Caughley1994, p. 229), it is safer to hypothesize that a declining species finds its last stronghold ‘not in the habitat most favourable to it, but in the habitat least favourable to the agent of decline’; and, in the case of the myna, it seems probable that the National Park provided at least some protection against trapping, allowing the species to survive there longer than elsewhere.
Nevertheless, the accumulated records, including an aversion to rain, suggests the Bali myna is a savannah bird rather than one of wetter formations (van Helvoort, Reference van Helvoort1990; Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). Our evidence supports this, showing that free-living birds tend to shun the densely wooded areas of Bali Barat National Park, preferring more open landscapes, including savannah-type habitats and areas with short grasses. Although the Bali myna, unlike the black-winged myna Acridotheres melanopterus (Squires et al., Reference Squires, Collar, Devenish, Owen, Pratiwi, Winarni and Marsden2022), might not associate directly with ungulates such as banteng Bos javanicus, deer and livestock when feeding, grazing promotes the savannahs and short swards that it prefers for feeding (Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). There could have been a significant decline in the extent of savannah on Bali with the loss of the large wild ungulate prey of the now-extinct tiger Panthera tigris sondaica on the island. The myna has also been found in the more open areas of the National Park, and at habitat boundaries between, for example, forest and savannah or savannah and cropland (Sutomo & van Etten, Reference Sutomo and van Etten2021).
If these findings are correct, Bali Barat National Park possesses considerable additional myna habitat that is as yet unoccupied. This includes various agricultural habitats along the main road, some savannahs and pastures that retain large trees or palms, open plantations and even the grounds of residential areas and hotels. Many of these places could be more suitable for the myna than much of the dry woodland on the peninsula, the central block of deciduous woodland on the peninsula and certainly the wetter forest to the south of the main road. The dry forests lack surface water or even a grass layer, from which mynas sip dew (van Helvoort, Reference van Helvoort1990), and are likely to be inhospitable during the dry season; dry-season movements of mynas south from these areas to the landscape around the main road might be natural (Jepson et al., Reference Jepson, van Balen, Soehartono and Mardiastuti1997; Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). It is possible that mynas will occupy the southern areas year-round in the future but expand into northern areas to breed.
However, there are multiple issues to consider in planning or proposing measures to encourage the further spread and increase of the free-living population of Bali mynas. It can reasonably be assumed that savannah offers the species shelter against inclement weather and predators. It could also offer the optimal mix of foraging substrates amongst leafy branches, on tree-trunks and in short grass (Yuni et al., Reference Yuni, Collar, Nugroho, Marsden, Sarmawi and Squires2022). Nevertheless, there is no information on the proportion of the population that lives wholly independently of the supplementary food provided at release sites or by local people keen to have the birds visit their gardens. In anthropogenic quasi-savannah habitats such independence could prove to be much lower. Equally, quasi-savannah habitats might host higher levels of predators because of the presence of various kinds of anthropogenic detritus (Fleming & Bateman, Reference Fleming and Bateman2018). Moreover, natural cavities in which to breed safely could be limited in quasi-savannah habitats, requiring the widespread provision of nestboxes to allow the birds to reproduce successfully.
All of these ecological factors require attention as conservation biologists consider the options for increasing the security of the species, particularly when assessing the risks and benefits of using hotel gardens and lushly vegetated suburbs for future releases. However, the most pressing issue is the control of trapping. To date, levels of trapping or nest poaching in the landscapes into which Bali mynas have recently expanded seem to be low. A population viability analysis recently developed for Bali Barat National Park has suggested that the current trajectory of the population is sufficiently positive to withstand a small amount of trapping in the near future, especially if trapping takes the form of taking chicks from nests rather than mist-netting adults (Squires, Reference Squires2022). Nevertheless, it is essential that levels of trapping do not increase further. Therefore, work by the National Park authority and other agencies that promote Bali myna conservation amongst local communities needs to be continued, seeking to provide livelihood opportunities that help ensure trapping becomes an unnecessary income option and that local ecological conditions are increasingly favourable to the species.
We hope that the evidence presented here will encourage both old and new sponsors and stakeholders to engage with Bali myna conservation efforts within and around Bali Barat National Park to secure the species. In this endeavour we regard the following activities as high priorities: (1) Support local communities in both commercial breeding of the Bali myna and the creation of alternative livelihoods. (2) Establish Bali myna and National Park-based activities for children, and their families, from schools in and around Bali Barat National Park. (3) Develop a scheme in which individual properties and communities host nestboxes for the mynas, creating a sense of local pride and ownership. (4) Extend the release programme to the tourist resort of Pemuteran, east of Bali Barat National Park, as the area has a quasi-savannah habitat (scattered trees and palms), many lawns and water sources, security, a throughflow of Western tourists and a population with an increasing environmental awareness (there have been no wild Bali mynas in this area of Bali for c. 50 years; Collar et al., Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). (5) Extend ecological monitoring within Bali Barat National Park to accurately estimate the productivity and survival of Bali mynas and to examine factors that will ultimately affect carrying capacity within the National Park.
Acknowledgements
We thank the Indonesian Government for permission to undertake this research (Ristekdikti Research Permit 51A/E5/E5.4/SIP.EXT/2019, KLHK/KNP SIMAKSI S.1832/T.16/TU/Kons/11/2018, S.2257/T.16/TU/Kons/12/2019, S.356/T.16/TU/Kons/3/2020 and S.671/T.16/TU/Kons/6/2020); and Chester Zoo and Manchester Metropolitan University for funding TMS's fieldwork in and around Bali Barat National Park. Additional fieldwork by AN and MUS, visits to Bali Barat National Park by TMS and SJM, and other collaborative activities, were funded by the European Association of Zoos and Aquaria through their Silent Forest campaign. Woodland Park Zoo provided additional funding. Visits by AO and SSN were funded by Chester and Lincoln Park Zoos, respectively. We thank Lauren Florisson, Simon Bruslund and David Jeggo for help with grants; Panji Gusti Akbar, Muhammad Arif Romadlon, Aldina Rahmadhani and Dewi Sasmita for their contributions during fieldwork; all of the Bali Barat National Park staff who contributed records of Bali mynas as well as other assistance during the fieldwork; and the Editor and a reviewer for comments that improved this article.
Author contributions
Study conception, funding acquisition: NJC, SJM; study design: TMS, NJC, SJM; data collection: TMS, AN, MUS; fieldwork supervision: HK; data analysis: TMS, SJM; writing: TMS, ANKK, NJC, LPEKY, AO, SSN, NLW, SJM; supervision: NJC, LPEKY, NLW, SJM; revision: all authors.
Conflicts of interest
TMS, NJC, LPEKY, AO, AN, MUS, SSN, NLW, SJM: none. ANKK and HK are employed by Bali Barat National Park.
Ethical standards
This research abided by the Oryx guidelines on ethical standards and did not involve human subjects, experimentation with animals and/or collection of specimens.
Data availability
The full dataset is not freely available because of the threatened status of the study species, but is available from the authors upon reasonable request from bona fide researchers.











