Clinical tuberculosis (TB) is a major public health problem in Greenland(Reference Søborg, Søborg and Pouelsen1). After the Second World War, one of the highest incidences (2300 per 100 000) in the world was found here. Due to significant national efforts, the incidence of TB in Greenland decreased substantially and reached in 1985 the lowest registered level of 25 per 100 000(Reference Søborg, Søborg and Pouelsen1). Since then the incidence has increased again to an average of seventy-three cases per year corresponding to 131 per 100 000 during the period 1998–2007(2).
TB is caused by the intracellular pathogen Mycobacterium tuberculosis which resides predominantly within macrophages. Paradoxically, macrophage activity is the first type of defence against the M. tuberculosis infection(Reference Fenton3). Vitamin D is a modulator of macrophage function and can activate host anti-mycobacterial activity. Its active metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D), may improve the ability of macrophages to inhibit the growth of mycobacteriae(Reference Rockett, Brookers and Udalova4, Reference Liu and Modlin5). Hence, susceptibility to TB may be increased by vitamin D deficiency(Reference Davies6, Reference Wilkinson and Pasvol7). Accordingly, a recent systematic meta-analysis concluded that low serum vitamin D concentrations are associated with higher risk of active TB(Reference Nnoaham and Clarke8). Thus, it is reasonable to suggest that vitamin D supplementation to individuals exposed to M. tuberculosis may be beneficial, especially in populations where vitamin D synthesis in the skin is limited and where vitamin D intake through the diet is scarce.
In Greenland exposure to sunlight is limited during winter and vitamin D synthesis in the skin is therefore likely to be low during this season. Moreover, during the last decades significant cultural changes have occurred in Greenland(Reference Bjerregaard9), resulting in the fact that many Greenlanders rely on a Westernised diet low in vitamin D, contrasting to the Greenlandic traditional diet consisting of vitamin D-rich fish and sea mammals(Reference Keiver, Draper and Ronald10, Reference Rejnmark, Jørgensen and Pedersen11). It has been shown that changes from a Greenlandic to a Westernised diet are associated with a reduced vitamin D status(Reference Rejnmark, Jørgensen and Pedersen11). The present study was implemented to analyse the association between vitamin D and active TB in Greenland to assess the feasibility of population-based vitamin D supplementation.
Subjects and methods
Study design and participants
We performed a case–control study including diagnosed TB patients and matched controls in Greenland. The study was part of a larger investigation which aimed at identifying risk factors of TB. Recruitment of participants took place during 2004–6 at thirteen district hospitals along the west, south and east coasts of Greenland. A total of 216 adults were diagnosed with TB in 2004–6. Due to a rapid turnover of authorised staff and laboratory technicians to take care of blood sampling at some district hospitals, it was not possible to include all. Thus, a subsample of 155 (71 %) patients was asked and accepted to participate. The selection was determined by the availability of staff to take care of the inclusion procedures at the time of diagnosis and not by any patient characteristics. For each patient it was intended to identify four healthy sex- and age- ( ± 5 years) matched population controls from the same hospital district. In Greenland, all individuals have a file at the district hospital containing identification details from the Civil Registration System (CPR) register, which contains unique details on all individuals in Denmark and Greenland. Controls were chosen randomly among these files. For four of the 155 patients no matched controls could be identified, for two patients only two controls were identified, and for one patient three controls were identified. Thus, a total of 599 controls were identified. All patients and controls answered questions about ethnicity, weight and height, smoking, alcohol and diet habits, and social status (employment and income source). Individuals were defined as Inuit if both parents and all grandparents were born in Greenland. For the present study the intention was that all patients and one of their four controls should donate a blood sample for vitamin D (25-hydroxyvitamin D; 25(OH)D) measurement. Among the patients, 148 had four identified controls. The one control who provided a blood sample was selected randomly among the four controls. However, for practical reasons it was not possible to obtain a blood sample from all 148 patients and 148 controls. Successful measurement of 25(OH)D was obtained from seventy-two matched patient and control pairs. Blood samples were collected at all times of the year, and samples from pairs were collected within 1 week to avoid seasonal differences in vitamin D concentration.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by The Commission for Scientific Research in Greenland. Written informed consent was obtained from all subjects.
Tuberculosis diagnosis
The inclusion criteria for patients were a TB diagnosis based on clinical findings in combination with either (1) a positive M. tuberculosis culture, (2) characteristic X-ray abnormalities together with either a positive tuberculin skin test by the Mantoux method or a positive interferon-γ release assay (Quantiferon Gold test) or (3) characteristic histology. The Greenlandic TB registration did not differentiate between lung and pleural TB; this is why all cases in the respiratory tract are stated as pulmonary. Mycobacteriological analyses were provided by the International Reference Laboratory of Mycobacteriology (Statens Serum Institut, Copenhagen, Denmark).
Measurement of vitamin D
The vitamin D status was assessed by measuring 25(OH)D as previously described(Reference Haderslev, Jeppesen and Sorensen12). We defined vitamin D insufficiency, mild vitamin D deficiency, and severe vitamin D deficiency as serum 25(OH)D concentrations of 50–75 nmol/l (20–30 ng/ml), 25–49 nmol/l (10–19·6 ng/ml) and < 25 nmol/l ( < 10 ng/ml), respectively, according to previously used definitions(Reference Friis, Range and Pedersen13, Reference Vieth14). Serum 25(OH)D concentrations of 76–140 nmol/l (30·4–56 ng/ml) and>140 nmol/l (>56 ng/ml) were characterised as normal and high, respectively.
Statistical analysis
The association between vitamin D and other potential risk factors and TB were evaluated by unadjusted and adjusted OR estimated by conditional logistic regression using PROC LOGISTIC in SAS (SAS Institute, Inc., Cary, NC, USA). Adjusted OR were adjusted for 25(OH)D, ethnicity and alcohol. Adjustment variables were selected by a backward elimination procedure. Due to particular focus on vitamin D we also conducted analyses where only 25(OH)D was in the model while adjusting for all other variables one by one in separate analyses to test for whether these confounded the association between 25(OH)D and TB. Associations between 25(OH)D and other potential risk factors were evaluated by χ2 tests. In an additional analysis the 25(OH)D concentration in cases and controls was compared using the non-parametric Wilcoxon test. Assuming a causal effect of 25(OH)D on TB we estimated the effect of supplementing the low-25(OH)D group ( < 75 nmol/l) to normal concentrations (76–140 nmol/l) using attributable risk. All statistical analyses were performed with SAS version 9.2 software (SAS Institute, Inc.).
Results
Of the seventy-two patients, sixty-seven (93 %) were clinically diagnosed with pulmonary TB. Of these, sixty-one patients (91 %) were confirmed by M. tuberculosis culture and/or sputum microscopy for acid-fast bacilli (twenty-one (31 %) by cultures alone, four (6 %) by sputum microscopy alone, and thirty-six (54 %) by both). Of the patients, five (7 %) were diagnosed with extra-pulmonary TB. In one case (1 %) the TB localisation was not registered. All cases of extra-pulmonary TB were culture-confirmed. The mean time from symptom debut to diagnosis was 116 (sd 119) d. The blood sample was taken before treatment start in 29 %; among the rest the mean time from treatment start to sample collection was 18 (sd 25·6) d. Among controls with low, normal or high 25(OH)D, 36, 54 and 75 % had eaten fish often (P = 0·26), respectively, and 9, 25 and 38 %, respectively, had eaten seal or whale often (P = 0·38). The respective numbers for patients were 36 %, 60 % and 57 % (P = 0·19) and 20 %, 35 % and 43 % (P = 0·26). Multivitamin tablets were taken by one patient and two controls.
The mean age was 39 years and ranged between 8 and 69 years and 8 and 74 years for patients and controls, respectively. Of the patients and controls, 89 and 74 % were Inuit. Non-Inuits had a lower risk of TB (OR 0·1; 95 % CI 0·02, 0·5) compared with Inuits after adjusting for alcohol and 25(OH)D. Individuals with frequent (every day or between one and six times per week) intake of alcohol had a higher risk of TB (OR 3·9; 95 % CI 1·4, 11·2) compared with individuals with a non-frequent (less than once per week) intake after adjusting for 25(OH)D and ethnicity. BMI, smoking, intake of fish, seal and whale meat, social status and concomitant disease (thirteen individuals had hypertension, chronic obstructive lung disease, rheumatic disorders, epilepsy, mental retardation, HIV, psychiatric disorders or eye problems) were not associated with TB.
Fig. 1 shows the distribution of 25(OH)D. Mild deficiency (25–49 nmol/l) was observed in 13 % of the patients and 1 % of the controls, whereas insufficiency (50–75 nmol/l) was found in 22 % of the patients and 15 % of the controls. No individuals had severe deficiency ( < 25 nmol/l). As there was only one control with 25(OH)D < 50 nmol/l, the categories < 50 nmol/l and 50–75 nmol/l were merged into the one category.
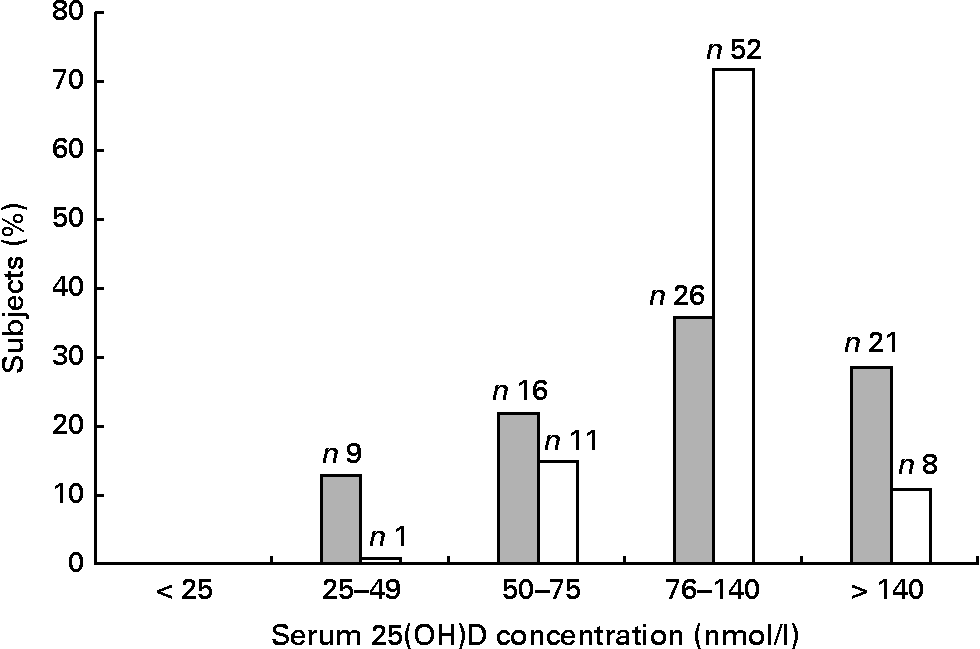
Fig. 1 Distribution of 25-hydroxyvitamin D (25(OH)D) (nmol/l) among the seventy-two tuberculosis (TB) patients (![]() ) and seventy-two controls (□). The overall mean, median, standard deviation, minimum and maximum of the serum 25(OH)D concentration were 108, 93, 60, 28 and 370 for the TB patients and 110, 107, 38, 39 and 260 for the controls.
) and seventy-two controls (□). The overall mean, median, standard deviation, minimum and maximum of the serum 25(OH)D concentration were 108, 93, 60, 28 and 370 for the TB patients and 110, 107, 38, 39 and 260 for the controls.
Table 1 shows the distribution of patients and controls in groups of low ( < 75 nmol/l), normal (76–140 nmol/l) and high (>140 nmol/l) 25(OH)D concentrations together with adjusted and unadjusted OR and 95 % CI. After adjusting for ethnicity and alcohol, the association between 25(OH)D and risk of TB was U-shaped, with an OR of 6·5 (95 % CI 1·8, 23·5) among individuals with a 25(OH)D concentration < 75 nmol/l, and an OR of 6·5 (95 % CI 1·9, 22·2) among those with a concentration>140 nmol/l, when compared with individuals presenting concentrations between 76 and 140 nmol/l. The OR did not change considerably when adjusting for ethnicity, BMI, smoking, alcohol, intake of fish, seal and whale meat, social status or concomitant disease. Restricting the analysis to Inuit pairs only (n 47) resulted in a similar association pattern. We observed no associations between 25(OH)D and any of the above variables. Analyses with alternative cut-off points showed that the U-shaped association pattern was not dependent on the chosen categorisation.
Table 1 Unadjusted and adjusted (for alcohol and ethnicity) risk for tuberculosis according to serum hydroxyvitamin D (25(OH)D) (nmol/l) groups
(Odds ratios and 95 % confidence intervals)

* Test for homogeneity according to 25(OH)D (nmol/l).
† Test for homogeneity between>140 nmol/l and 76–140 nmol/l: P = 0·02 (unadjusted) and P = 0·03 (adjusted).
An estimation of the effect of supplementing individuals with low 25(OH)D ( < 75 nmol/l) to normal concentrations (76–140 nmol/l) showed that the number of TB cases could be reduced by 29 % when assuming a causal effect.
Discussion
The present study demonstrated that active TB was associated with both low and high concentrations of vitamin D.
Low vitamin D concentrations being associated with a higher risk of TB has been observed previously from different parts of the world(Reference Nnoaham and Clarke8, Reference Friis, Range and Pedersen13, Reference Chan15, Reference Wilkinson, Llewelyn and Toossi16). The association may reflect a causal relationship, but the direction cannot be determined from the present design. However, given that the active metabolite of vitamin D, 1,25(OH)2D, modulates macrophage function and improves the ability of macrophages to inhibit the growth of the mycobacteria(Reference Liu and Modlin5, Reference Chan15), it is likely that low vitamin D concentrations increase the susceptibility to TB(Reference Chan15). Once an individual has become infected and developed active TB, less exposure to sunlight(Reference Holick and Chen17) while confined more to indoor life, and impaired appetite as a consequence of the infection(Reference Toth, Fackelmann and Pigott18), may contribute to low 25(OH)D concentration through reduced vitamin D synthesis in the skin and reduced food intake.
An association between high serum 25(OH)D and TB in a human population has, to our knowledge, not been described previously. However, the phenomenon of high (and low) 25(OH)D has been associated with prostate cancer(Reference Tuohimaa, Tenkanen and Ahonen19), although other limits for low ( ≤ 19 nmol/l) and high ( ≥ 80 nmol/l) concentrations were used. The authors suggested that high plasma and intracellular 25(OH)D stimulates intracellular 24-hydroxylase which rapidly degrades 1,25(OH)2D to the inactive 1,24,25-trihydroxyvitamin D resulting in low concentrations of intracellular 1,25(OH)2D(Reference Tuohimaa, Tenkanen and Ahonen19, Reference Miller and Stableton20) which, again, results in increased proliferation of malignant cells. This would result in increased prostate cancer risk.
A similar mechanism may apply in the present study. High 25(OH)D concentration may lead to stimulation of 24-hydroxylase, which degrades 1,25(OH)2D and 25(OH)D to their 24-hydroxylated inactive forms. This would result in low 1,25(OH)2D concentrations and a probably increased risk of TB. Alternatively, increased 1,25(OH)2D3 concentrations might lead to down-regulation of vitamin D receptor expression resulting in defective vitamin D receptor signalling as previously suggested(Reference Selvaraj, Prabhu Anand and Harishankar21).
We speculate that the association between high vitamin D and TB could be confounded by n-3 fatty acids which are largely gained from the same dietary sources as vitamin D. Experimental studies(Reference Bonilla, Fan and Chapkin22, Reference Jordao, Lengeling and Bordat23), although presenting contradictory findings, indicated that n-3 fatty acids impair the immune response against M. tuberculosis. A recent study showed that transgenic mice enriched in n-3 fatty acids were more susceptible to M. tuberculosis than wild-type mice(Reference Bonilla, Fan and Chapkin22). Thus, high levels of n-3 fatty acid may play a role in increasing the risk of TB.
Vitamin A is another factor that might affect the risk of TB. Individuals in the group of high vitamin D level may also have an excessive level of vitamin A as a result of a high intake of traditional food, possibly including liver from sea mammals. Vitamin A supplementation in animal studies has shown that a chronic excess of vitamin A may depress cellular and humoral immune responses(Reference Chen, Zhuo and Yuan24). Accordingly, it has been hypothesised that vitamin A supplementation of children with adequate vitamin A stores might cause a temporary immune dysregulation and lead to increased susceptibility to infectious diseases(Reference Grotto, Mimouni and Gdalevich25). This concurs with the finding that vitamin A supplementation reduced the incidence of acute lower respiratory tract infections in children with poor nutritional status, but increased it in children with normal nutritional status(Reference Chen, Zhuo and Yuan24). Thus, concomitant excess of vitamin A might have contributed to the high risk of TB.
The U-shaped association between vitamin D and TB suggests that population-based vitamin D supplementation might not be suitable in Greenland, as supplementation of non-deficient individuals may result in a vitamin D concentration which could give a higher risk of TB. However, when assuming a causal effect, our data indicate that supplementation of deficient individuals would have beneficial effects, as increasing the vitamin D concentration from low to normal was estimated to result in a 29 % reduction in the number of TB cases. Larger and prospectively designed studies are needed to determine the direction of a possible cause–effect relationship between vitamin D and TB.
Acknowledgements
We thank the study participants for their collaboration, and the staff at the district hospitals for valuable assistance. Medical student Melanie Veber is thanked for assistance in the creation of the database. The study was supported by a grant from The Greenland Home Rule Health Department.
K. L. and T. S. designed and conducted the research. B. S. was involved in the creation of the database. N. O. N. wrote the paper and had the primary responsibility for the final content. M. A. and J. W. analysed the data. A. K. and M. M. participated in the interpretation of the results. All authors read and approved the final manuscript.
The authors have no conflicts of interest.




