To the Editor—Influenza is a seasonal respiratory orthomyxoviral infection that causes major morbidity and mortality in elderly and patients with chronic conditions such as end-stage renal disease. The virus is transmitted via small-particle aerosols and large droplets.Reference Brankston, Gitterman, Hirji, Lemieux and Gardam1 These transmission modes are highly relevant in the context of a hemodialysis unit because patients stay in the same room for several hours and healthcare workers can facilitate the spread through inadequate hand hygiene. Serological studies indicate an impaired immune response in hemodialysis patients to influenza vaccination, even after a booster vaccine dose, and its real effect on clinical outcomes and mortality is uncertain.Reference Liao, Xu, Liang, Xiong, Chen and Ni2, Reference Remschmidt, Wichmann and Harder3 Oseltamivir is a neuramidase inhibitor licensed for the treatment of and prophylaxis for influenza. It is mainly renally eliminated and is cleared by hemodialysis (molecular mass 312,4 g/mol). The optimal dose for prophylaxis in hemodialysis patients in unknown. A population pharmacokinetic study suggested 30 mg every other hemodialysis session,Reference Kamal, Lien and Robson4 whereas another clinical study on influenza A/H1N1 used 75 mg every 5 days.Reference Choo, Hossain, Liew, Chowdhury and Tan5 These studies, however, have been performed in the era of low-flux filters, while hemodiafiltration (HDF) and high-flux dialyzers are the current standard of care.
In the 2017–2018 influenza season, we started oseltamivir 30 mg after each hemodialysis session in our dialysis unit because of an emerging outbreak, even though all our patients had been vaccinated with a standard-dose quadrivalent vaccine. More precisely, 5 new infections occurred in week 8 (Figure 1). Additionally, the number of patients with influenza in our hospital, a 1,182-bed acute- and tertiary-care hospital, was rising sharply (Figure 1). In patients with influenza-like symptoms, testing for influenza A and B on combined nasopharyngeal-throat swabs was performed using real-time PCR on Taqman array cards. Of the 137 patients in our dialysis unit, 130 (mean age, 73.1 ± 12 y; 36% female) provided informed consent to start the prophylactic treatment. Prophylaxis was administered from week 10 up to week 14, given the dropping number of influenza cases in the hospital. Of these 130 patients, 7 (5%) stopped prophylaxis early because of their own decision (n = 3) or reported adverse effects: stomachache (n = 1) and muscle aches (n = 3). During the period of prophylaxis, no case of influenza was detected in our dialysis unit (Figure 1). On average, the number of patients screened for influenza was 2.58 per week during the period of prophylaxis, and it had been 2.34 per week during the 11 previous weeks, indicating continued screening of patients with influenza-like symptoms. One week after the end of prophylaxis, 1 case of influenza occurred in our unit.
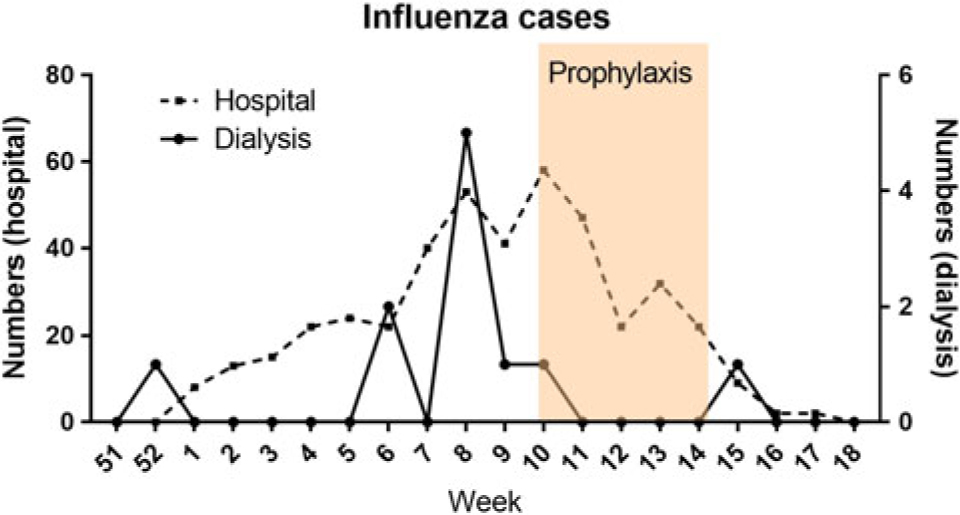
Fig. 1. The number of influenza cases per week is shown for the hospital (left axis) and the hemodialysis unit (right axis). The red box denotes the period the prophylaxis was administered to hemodialysis patients.
Our observational study shows it is feasible and safe to administer prophylaxis for influenza with oseltamivir in hemodialysis patients. To overcome potential enhanced elimination by high-flux dialysis and HDF, we chose 30 mg after each hemodialysis session in contrast to dosing every other hemodialysis session, as indicated in the packet insert and as suggested by a population pharmacokinetic approach.Reference Kamal, Lien and Robson4 Dosing after hemodialysis also allowed for optimal compliance. No major adverse effects were observed, and 95% of the patients continued the prophylaxis during the predetermined period. Although no control group was included, the absence of new influenza cases in our hemodialysis, despite the continuing intensive influenza epidemic in our hospital, indicates the effectiveness of this strategy.
In light of recent findings that vaccination remains a suboptimal strategy to prevent influenza in hemodialysis patients,Reference Liao, Xu, Liang, Xiong, Chen and Ni2, Reference Remschmidt, Wichmann and Harder3 we propose to administer oseltamivir prophylaxis during influenza season as an additional protective measure. Further studies should confirm the effectiveness of this strategy and explore its cost-effectiveness.
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



