The perinatal period is an especially vulnerable time in development and epidemiological studies have supported the theory that overweight and obesity begin early in life. During the third trimester, fetal weight can increase four-fold or more when the majority of fetal fat is laid down(Reference Haggarty1). It is also well established that infants born either large or small for gestational age are at increased risk for diseases later in life compared with infants born appropriate for gestational age (2500–4000 g)(Reference Oken and Gillman2–Reference Boney, Verma and Tucker4). In a randomised, double-blinded, clinical trial, 21-month-old infants of mothers who had been supplemented with DHA (22 : 6n-3) had lower BMI than infants of mothers who did not consume supplemental DHA(Reference Bergmann, Bergmann and Haschke-Becher5).
Insulin is an anabolic hormone essential for fetal development. As insulin is unable to cross the placental barrier, the infant produces its own insulin in response to maternal nutrient supply. Increased long-chain PUFA in cellular membranes results in increased membrane fluidity and insulin receptor sensitivity. Insulin sensitivity negatively correlates with the proportion of n-6 and SFA in the plasma(Reference Simopoulos6), pointing to the importance of n-3 fatty acids for maternal diets. DHA in the maternal diet can purportedly increase infant insulin sensitivity, benefiting infant development.
Cord blood insulin concentrations correlate with weight-for-gestational age. Higher cord blood insulin is found in infants born large for gestational age compared with infants with normal in utero growth(Reference Mazaki-Tovi, Kanety and Pariente7). Other studies have shown trends for increased cord blood insulin concentrations in large-for-gestational age infants and significant positive correlations for insulin with weight or length at birth(Reference Tsai, Yu and Hsu8).
Research on the impact of DHA supplementation during pregnancy on intra-uterine growth and adiposity is limited and evidence for an effect comes almost exclusively from animal models. Thus, the objective of the present study was to examine the impact of a DHA-containing functional food (DHA-FF) consumed by mothers during the last half of pregnancy on intra-uterine growth and umbilical cord blood insulin concentration.
Methods
The present study was a randomised, double-blinded, longitudinal design following pregnant women from mid-pregnancy (20–24 weeks) until delivery. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Hartford Hospital and University of Connecticut Institutional Review Boards. Healthy pregnant women were recruited, by direct invitation and flyers placed in the waiting rooms at Hartford Hospital (Hartford, CT, USA), and informed consent was obtained. Women in the present study were representative of the women living in the Hartford, CT area (i.e. similar ethnicity and socio-economic status). Exclusion criteria included the following: parity >5; history of chronic hypertension; hyperlipidaemia; renal or liver disease; heart disease; thyroid disorder; multiple gestations; having been pregnant or lactating in the previous 2 years.
Women were randomised to consume an average of five DHA cereal-based or placebo bars per week. The functional food, produced by Nestec Limited (Vevey, Switzerland), contained either 300 mg DHA (EPA (20 : 5n-3):DHA ratio of 1:8) or maize oil. Subjects in the DHA-FF group consumed an average of 214 mg DHA/d from the DHA-FF. Subjects kept logs to record bar consumption and unconsumed bars were returned. At scheduled visits, 24 h dietary recalls were obtained and analysed using Nutrition Data Systems for Research (Minneapolis, MN, USA), and the study protocol was reviewed with subjects to ensure protocol compliance.
Clinical data, including laboratory results, medications, supplements, health habits, length of gestation, ethnicity, etc., were obtained from hospital records. Infant birth weight, length and head circumference were measured at delivery by trained registered nurses following the hospital protocol. Ponderal index was calculated as weight (g)/length (cm)3 × 100.
Maternal venous blood was collected at 20–24 weeks' gestation. Infant cord blood was collected using a double-clamp procedure at each delivery. Only twenty-three cord blood samples were successfully obtained due to the inability to successfully collect the sample before the blood clotted, parental decision to bank cord blood, etc. Blood was collected into EDTA-containing tubes and plasma was stored at − 80°C until analyses.
Maternal plasma lipids were extracted using a modified Folch procedure(Reference Folch, Lees and Sloane Stanley9) as described previously(Reference Cheruku, Montgomery-Downs and Farkas10). Cord blood insulin concentrations were assessed using an ELISA kit (Linco Research, Inc., St Charles, MO, USA). Samples were analysed in duplicate and absorbance was read using a Bio-Rad Benchmark Microplate Reader (Hercules, CA, USA).
Subject characteristics were compared using the independent-samples t test and Mann–Whitney U test for continuous variables and the χ2 test for categorical variables. Multiple linear regression analysis was used to assess associations between DHA-FF consumption and ponderal index and insulin concentration. Insulin concentration was log transformed for multiple regression analysis. Models were developed for each dependent variable using a list of variables that were gathered from previously published work and other variables suspected to have an impact on the dependent variable. A general model containing all pre-specified covariates was chosen and non-significant covariates were eliminated so that only covariates that significantly had an impact on the model were used. The best model was chosen using the adjusted R 2 statistic. SPSS statistical software (SPSS, Chicago, IL, USA) was used for statistical analyses.
Results
A total of forty-seven women and infants were included in the present study. From these subjects, twenty-three cord blood samples were collected at delivery. Maternal and infant characteristics are presented in Table 1. There were no significant differences in maternal characteristics between groups, indicating that women were well randomised. Overall, women had normal, healthy, uncomplicated, full-term pregnancies. The mean BMI, based on self-reported pre-pregnancy body weights, indicates that women were overweight.
Table 1 Maternal and infant characteristics*
(Mean values, standard deviations, number of participants and percentages)

DHA-FF, DHA-containing functional food.
* Independent-variables t test unless otherwise noted.
† Mann–Whitney U test.
‡ Maternal glucose concentrations at 2 h obtained from the oral glucose tolerance test at 24–28 weeks gestation.
§ Dietary intake from usual foods not including the average 214 mg/d consumed in the DHA-containing functional food.
∥ χ2 test.
Ponderal index, an indicator of infant body fatness, was higher in infants born to women consuming the placebo bar when compared with infants of mothers consuming the DHA-FF (Table 2; P = 0·045). Controlling for all other variables in our analyses, a change from intervention to placebo predicted an increase in the infant ponderal index of 0·198. Furthermore, venous umbilical cord insulin concentration in the twenty-three cord samples was significantly higher (P = 0·043) for infants of women consuming the placebo bar v. the DHA-FF during pregnancy (Table 2). Controlling for maternal haematocrit, baseline n-6:n-3 ratio and pre-pregnancy BMI, a change from control to treatment could be predicted to result in a decrease in the insulin concentration of 14·58 pmol/l.
Table 2 Regression models for predicting ponderal index and infant cord blood insulin concentration
(β-Coefficients and P values)
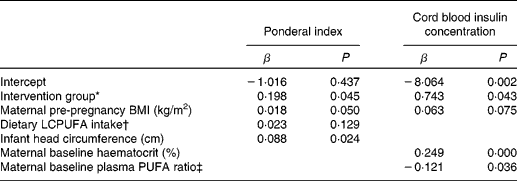
LCPUFA, long-chain PUFA.
* Coded as 0 for the DHA intervention group and 1 for the placebo group.
† Dietary LCPUFA is equivalent to the ratio of 20 : 4n-6 to the sum of 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
‡ PUFA ratio is equivalent to the ratio of the sum of 18 : 3n-6 and 20 : 4n-6 to the sum of 18 : 4n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
Discussion
In the present study, infant ponderal index at birth was lower in the DHA intervention group where infants with similar weights tended to be longer than infants in the placebo group. Observational studies have indicated that DHA increases length of gestation, thus infants are born larger(Reference Hansen and Olsen11). Rump et al.(Reference Rump, Mensink and Kester12) reported that increased weight for gestational age was associated with decreased umbilical cord DHA. In the present study, there was no difference in gestational age between the groups. As ponderal index provides an estimate for infant body fatness, the present finding may point to the antenatal period as an interval that is especially susceptible to dietary intervention with DHA or foods high in DHA. Infants of mothers consuming an additional 214 mg DHA per d from the DHA-FF were approximately 250 g lighter than infants of the same length in the placebo group (Table 2).
Maternal DHA supplementation during pregnancy and infant growth was examined previously. In a Norwegian study in which women were supplemented with cod-liver oil (approximately 1000 mg DHA/d) during pregnancy and lactation, there was no effect of DHA supplementation on infant birth weight or length(Reference Hellend, Saugstad and Smith13). It is important to recognise that usual average daily seafood intake, high in DHA, is much greater in Norway than for the US population studied and reported here. The findings of a German study demonstrated that supplementation of women with 200 mg DHA/d (as fish oil) during pregnancy and lactation resulted in decreased body weight and BMI of children at 21 months of age(Reference Bergmann, Bergmann and Haschke-Becher5). Conflicting findings to date regarding the effect of DHA on infant size or adiposity at birth may be attributed to differences in study populations and design, including the infant evaluations or estimates made. In light of the finding reported here, it is notable that a difference in childhood BMI as an outcome of DHA intervention during pregnancy has been reported by others.
Future studies to assess the influence of DHA during pregnancy on infant size at birth and throughout the first years of life should be carried out in populations with typical Western diets, i.e. low in DHA, and supplementing with various amounts of DHA, including amounts that bring the daily intake close to the Institute of Medicine's current recommendation of two servings of DHA-rich fish per week (200–300 mg DHA/d)(Reference Nesheim and Yaktine14). Body composition assessments would permit determination of the antenatal influence of DHA on infant adiposity. Infant ponderal index is similar to BMI in adulthood as it is an indicator of infant fatness, and therefore infants with lower ponderal indices may be at decreased risk for future diseases, including heart disease and diabetes mellitus which are occurring at increasingly higher rates in both youths and adults(15). This should be explored in future studies.
The cord blood plasma insulin concentration of infants born to mothers consuming the DHA-FF was lower compared with infants of women consuming the placebo. Previous research indicates that long-chain n-3 PUFA, specifically EPA and DHA, influence cellular insulin sensitivity(Reference Lardinois16, Reference Baur, O'Connor and Pan17). Min et al.(Reference Min, Lowy and Gebremeskel18) reported that lower maternal erythrocyte phospholipid DHA during pregnancy was associated with decreased maternal insulin sensitivity. Furthermore, research from our laboratory has demonstrated previously that umbilical cord blood phospholipid DHA weight percentage is compromised in mothers with gestational diabetes mellitus(Reference Wijendran, Bendel and Couch19). Thus, our finding that the consumption of a DHA-FF during pregnancy was associated with a decreased concentration of insulin in umbilical cord blood in the present study complements previous reports indicating that a greater tissue content of n-3 long-chain PUFA is associated with increased insulin sensitivity. Unfortunately, we were unable to control for factors such as maternal stressors, e.g. length of delivery, and their impact on umbilical cord insulin concentrations. Future studies should examine maternal delivery as well as umbilical and maternal glucose concentrations to substantiate the impact of DHA supplementation during gestation on infant insulin concentration and glucoregulation.
While the present results are intriguing, there is the need for further research in a larger number of subjects with higher amounts of DHA supplementation to assess the impact of DHA on these outcome variables. As ponderal index is an indicator of infant fatness, future research should examine more closely the impact of DHA on infant lean v. fat mass.
Infant size at birth is a topic of current interest, as it has been associated with future risk for developing comorbidities, including the metabolic syndrome. We report here that DHA supplementation during normal pregnancy is associated with lower infant ponderal index at birth and also decreased umbilical cord insulin concentrations compared with infants of mothers consuming a placebo. Further examination of the impact of maternal DHA supplementation on infant body composition and later development of disease is warranted for a more complete understanding of the effect of DHA during pregnancy on infant size at birth.
Acknowledgements
The study was supported in part by the US Department of Agriculture Initiative for Future Agriculture and Food Systems; Nestec Limited, Switzerland; and the National Fisheries Institute. There are no conflicts of interest. C. J. L.-K. secured funding; A. B. C. and C. J. L.-K. were involved in the study design; A. B. C. and C. J. L.-K. conducted the research; A. B. C. and O. H. analysed the data; A. B. C. wrote the manuscript draft.




