The early and prodromal stages of psychopathology are marked with the expression of intermediate phenotypes, including subtle, nonclinical psychotic experiences (Dominguez et al., Reference Dominguez, Wichers, Lieb, Wittchen and van Os2009; Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015; Rossler et al., Reference Rossler, Hengartner, Ajdacic-Gross, Haker, Gamma and Angst2011); cognitive impairment (Ahern & Semkovska, Reference Ahern and Semkovska2017; Dominguez et al., Reference Dominguez, Saka, Lieb, Wittchen and van Os2010; Reichenberg et al., Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray and Moffitt2010) and affective dysregulation (Fusar-Poli et al., Reference Fusar-Poli, Nelson, Valmaggia, Yung and McGuire2014; Häfner et al., Reference Häfner, Maurer, Trendler, an der Heiden, Schmidt and Könnecke2005). In the general population, subclinical phenotypes may display low levels of correlation, whereas increased multidimensional psychopathology can be found at the level of psychiatric services (Goes et al., Reference Goes, McCusker, Bienvenu, Mackinnon, Mondimore, Schweizer and Potash2012; Lamers et al., Reference Lamers, van Oppen, Comijs, Smit, Spinhoven, van Balkom and Penninx2011; van Os et al., Reference van Os, Kenis and Rutten2010). Psychotic experiences are more severe in the presence of other dimensional phenotypes such as affective dysregulation (McGrath et al., Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet, Bromet and Kessler2016; Pries et al., Reference Pries, Guloksuz, ten Have, de Graaf, van Dorsselaer, Gunther and van Os2018) and are linked to the severity of psychopathology (Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015; Kelleher et al., Reference Kelleher, Devlin, Wigman, Kehoe, Murtagh, Fitzpatrick and Cannon2014; Navarro-Mateu et al., Reference Navarro-Mateu, Alonso, Lim, Saha, Aguilar-Gaxiola and Al-Hamzawi2017). Furthermore, studies show that the genetic and environmental vulnerabilities that are commonly associated with major mental disorders are also nonspecifically associated with different intermediate phenotypes (Brainstorm Consortium et al., Reference Anttila, Bulik-Sullivan, Finucane, Walters, Bras and Murray2018; Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015; Misiak et al., Reference Misiak, Krefft, Bielawski, Moustafa, Sasiadek and Frydecka2017; Nivard et al., Reference Nivard, Gage, Hottenga, van Beijsterveldt, Abdellaoui, Bartels and Middeldorp2017; van Os et al., Reference van Os, Kenis and Rutten2010; van Os, van der Steen et al., Reference van Os, van der Steen, Islam, Guloksuz, Rutten and Simons2017). In addition, greater exposure to genetic and environmental risks drives greater co-occurrence of phenotypes and increased severity of psychopathology (Guloksuz et al., Reference Guloksuz, van Nierop, Lieb, van Winkel, Wittchen and van Os2015; Pries et al., Reference Pries, Guloksuz, ten Have, de Graaf, van Dorsselaer, Gunther and van Os2018). Based on this, much attention has been given to the study of nonclinical multidimensional psychopathology to understand the development of mental ill health.
Several neuropsychological processes have been proposed to index vulnerability and resilience for mental disorders. For example, experiences of subtle alterations in salience attribution in response to environmental information may result in paranoid ideation, hallucinatory experiences and thought interference (Catalan et al., Reference Catalan, Simons, Bustamante, Drukker, Madrazo, de Artaza and Gonzalez-Torres2014; Galdos et al., Reference Galdos, Simons, Fernandez-Rivas, Wichers, Peralta, Lataster and van Os2011). Similarly, experiences of social defeat are highly stressful for young people and increase the risk for psychopathology (Eisenberger et al., Reference Eisenberger, Jarcho, Liebermann and Naliboff2006; Nesdaele & Lambert, Reference Nesdaele and Lambert2007; Sandstrom et al., Reference Sandstrom, Cillessen and Eisenhower2003). Furthermore, stress sensitivity and reward sensitivity, that is, negative affect and positive affect in response to environmental inputs, are crucial mechanisms in both psychotic and nonpsychotic disorders (Delespaul, Reference Delespaul1995; Lataster et al., Reference Lataster, Wichers, Jacobs, Mengelers, Derom, Thiery and Myin-Germeys2009; Myin-Germeys & van Os, Reference Myin-Germeys and van Os2007; Wichers et al., Reference Wichers, Myin-Germeys, Jacobs, Peeters, Kenis, Derom and van Os2007b). On the other hand, experiencing positive emotions such as reward can be protective for psychopathology (Rutten et al., Reference Rutten, Hammels, Geschwind, Menne-Lothmann, Pishva, Schruers and Wichers2013; Wichers et al., Reference Wichers, Myin-Germeys, Jacobs, Peeters, Kenis, Derom and van Os2007a). Therefore, individuals who are particularly skilled in experiencing and seeking such positive experiences can be hypothesized to be less prone to develop a mental disorder than people who are less reward sensitive.
The current cohort was specifically sampled to further evaluate the mechanism of dimensional phenotypes at the early stages of psychopathology. The TwinssCan sample is a unique cohort of a young twin population. It provides deep phenotyping of subclinical symptoms, thorough assessment of putative risk factors (i.e. genetic and nongenetic) and experimental tests to evaluate underlying neuropsychological processes, for example, salience attribution, social defeat, stress sensitivity and reward sensitivity. Therefore, this project is well equipped to investigate not only cross-sectional differences of psychopathology and resilience but also dynamic moment-to-moment variations, as well as long-term trajectories. The primary objective of the project is to investigate risk and protective factors for the development of psychopathology and the role of genetic and nongenetic factors contributing to intermediate phenotypes. Further, it aims to examine whether variations of (1) salience attribution, (2) reward sensitivity, (3) stress sensitivity and (4) the level of subtle psychotic experience and affective dysregulation in daily life are associated with genetic vulnerability, exposure to environmental factors and gene-environment interactions.
Sample Characteristics and Assessments
Participants were sampled from the East Flanders Prospective Twin Survey (EFPTS), a prospective, population-based registry of multiple births in the province of East Flanders, Belgium (Derom et al., Reference Derom, Thiery, Rutten, Peeters, Gielen, Bijnens and Weyers2019). The TwinssCan project was recruited across three waves (baseline and two follow-ups). Baseline data were assessed from April 2010 to April 2014 (Pries, Guloksuz, Menne-Lothmann et al., Reference Pries, Guloksuz, Menne-Lothmann, Decoster, van Winkel, Collip and van Os2017), including male and female twins in the age range of 15–35 years, their singleton siblings and their parents. It included 839 participants: 292 monozygotic twins (MZ), 486 dizygotic twins (DZ), 18 triplets and 43 siblings. Furthermore, data from 363 parents were assessed. At baseline, 60% of the participants were female (MZ: 63%; DZ: 57%; triplets: 61%; siblings: 70%). The mean age was 17.4 (SD = 3.6) years (MZ: 18.0 [SD = 4.2]; DZ: 16.9 [SD = 2.8]; triplets: 16.8 [SD = 2.0]; and siblings: 20.1 [SD = 4.7]), and most participants had an upper secondary education (primary education: 0.1%; lower secondary education: 6%; upper secondary education: 68%; tertiary education: 26%). For the second wave, 60% of the twins and siblings were reassessed. Sequential analysis based on sex, fetal membranes, umbilical cord blood groups, placental alkaline phosphatase and DNA fingerprints was used to determine zygosity (Deromet al., Reference Derom, Thiery, Peeters, Vlietinck, Defoort and Frijns2013).
Participants were included if they clearly understood and were able to verbally assent to the study procedures and when they voluntarily agreed to participate by means of written informed consent. Signed consent of the parent(s) was required when participants were younger than 18 years. Participation was not possible if caregivers indicated the presence of a pervasive mental disorder. Participants were excluded from the sample if the instructor, study coordinator or neuropsychological tester confirmed they were not able to complete testing and gave invalid, unreliable data on questionnaires, structured interviews and experimental tests. The local ethics committee approved the study (Commissie Medische Ethiek van de Universitaire ziekenhuizen KU Leuven, Nr. B32220107766).
A broad range of variables were assessed using validated self-report questionnaires, structured interviews and experimental tests. Placenta, blood and saliva samples have been stored at −80°C in biobanks. DNA has been isolated from saliva for all subjects. DNA from placenta and blood is available for the monozygotic twins. A study battery summary of clinical, biological, social-demographics, physical, environmental exposures, cognitive, psychological and experimental measures is reported in Table 1.
Table 1. TwinssCan Study battery summary
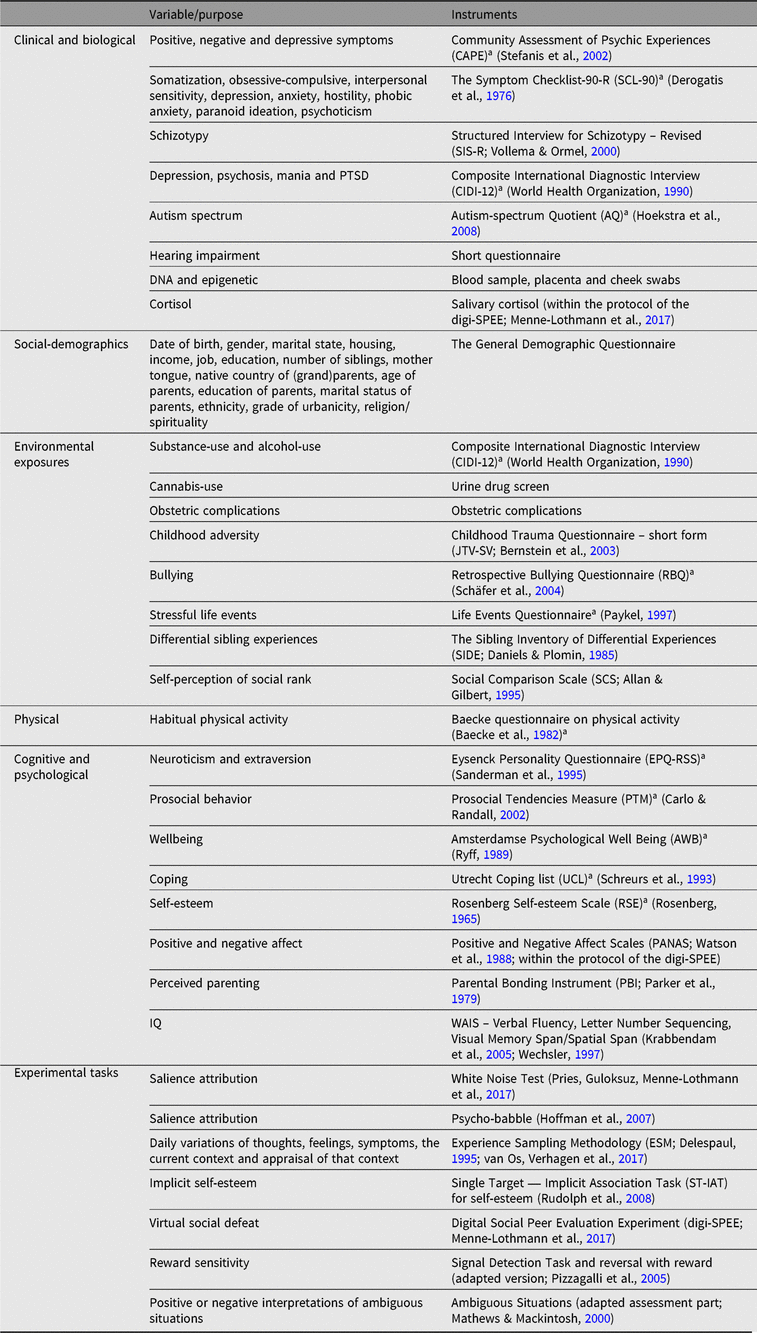
a Reassessed during wave 2 and wave 3.
Findings
Genetic and Nongenetic Risk Factors
Several studies examined how childhood adversity (CA) and genetic liability for psychopathology may affect mental ill health in the TwinssCan cohort. Lecei et al. (Reference Lecei, Decoster, De Hert, Derom, Jacobs, Menne-Lothmann and Wichers2019) explored whether gene-environment correlation may explain the association previously found between CA and psychopathology. In other words, they tested whether genetic liability for psychopathology made individuals also more likely to experience CA, which would result in a mechanism referred to as genetic confounding of the association between CA and psychopathology. Within-twin differences of CA were regressed on within-twin differences of psychopathology (assessed with the Symptom Checklist-90-R; SCL-90; Derogatis et al., Reference Derogatis, Rickels and Rock1976) in MZ twins. As MZ twins have identical DNA, the researchers argued that associations between within-twin differences indicate that the association between CA and psychopathology cannot be attributed only to genetic predisposition. CA in the whole sample as well as within-twin differences in CA was associated with psychopathology. These results suggest that at least part of the association between CA and psychopathology is independent from genetic predisposition and therefore genetic confounding cannot explain the association between CA and psychopathology.
Following this, Pinckaers et al. (Reference Pinckaers, Rotee, Krolinski, Nwosu, Smeets, Gülöksüz and Drukker2019) examined whether CA interacted with proxy genetic liability and affected psychopathology in the TwinssCan cohort. Genetic liability was approximated by estimating the co-twins’ psychopathology scores on the SCL-90. Genetic vulnerability moderated the association between CA and the negative dimension of the Community Assessment of Psychic Experiences (CAPE; Stefanis et al., Reference Stefanis, Hanssen, Smirnis, Avramopoulos, Evdokimidis, Stefanis and Van Os2002). The association with the total CAPE score approached near significance, whereas neither of the other associations, that is, with the subscales positive and depression symptom dimension, showed a significant interaction effect. The results suggest that genetic vulnerability complicated by CA affects subthreshold expression of psychosis, especially in the negative symptom dimension.
While much attention is given to macro levels of psychopathological changes, which can occur over periods of years or months, recent concepts of psychopathology acknowledge that the development of mental disorders is best understood when also looking at micro, complex, moment-to-moment dynamic changes (van Os, Verhagen et al., Reference van Os, Verhagen, Marsman, Peeters, Bak, Marcelis and Lataster2017). Therefore, Pries, Klingenberg et al. (Reference Pries, Klingenberg, Menne-Lothmann, Decoster, van Winkel, Collip and Guloksuv2019) investigated whether molecular genetic risk for schizophrenia interacted with CA and daily life stressors to influence moment-to-moment variations of mental states (i.e., negative affect, positive affect and subtle psychosis expression) and stress sensitivity. Molecular genetic vulnerability was expressed through polygenic risk score (PRS) for schizophrenia, which was calculated by summing weighted trait-alleles (EUGEI investigators, 2014; Purcell et al., Reference Purcell, Wray, Stone, Visscher, O’Donovan, Sullivan and Sklar2009; Ripke et al., Reference Ripke, Neale, Corvin, Walters, Farh, Holmans and O’Donovan2014). Momentary mental states were assessed using a structured diary technique, the experience sampling methodology (ESM; Delespaul, Reference Delespaul1995; van Os, Verhagen et al., Reference van Os, Verhagen, Marsman, Peeters, Bak, Marcelis and Lataster2017). The results were that exposure to early life events showed a statistical interaction with PRS for schizophrenia, leading to increased psychosis expressions, negative affect, stress-sensitivity and decreased positive affect. However, daily life stressors did not significantly interact with genetic markings for the same outcome variables.
In another study on momentary mental states, Vaessen et al. (Reference Vaessen, van Nierop, Decoster, Delespaul, Derom, de Hert and Myin-Germeys2017) used the TwinssCan cohort to evaluate whether sensitivity to daily life stress predicts onset or persistence of psychopathology. The authors used ESM data at baseline to assess affective responses to daily life stress (i.e., stress sensitivity) and found, contrary to previous work, that stress sensitivity was associated with neither persistence nor onset of psychopathology.
Finally, using the network approach, Hasmi et al. (Reference Hasmi, Drukker, Guloksuz, Menne-Lothmann, Decoster, van Winkel and van Os2017) investigated whether CA and proxy genetic liability for psychopathology (measured through co-twin scores on the SCL-90) were associated with network structures of affective regulation in daily life. The researchers compared regression coefficients, density and centrality indices of different networks and found that individuals with low genetic liability showed higher overall and negative affect density between network elements, whereas CA was associated with increased positive affect density and overall density.
Experimental Tests
Three studies used the TwinssCan cohort to validate experimental tasks: the digital social peer evaluation experiment (digi-SPEE) and the white noise test. Menne-Lothmann et al. (Reference Menne-Lothmann, Decoster, van Winkel, Collip, Rutten, Delespaul and Wichers2017) used the digi-SPEE to investigate how negative virtual social evaluation of peers impacts on participants’ implicit self-esteem, cortisol levels and positive and negative affects. For this experimental study, participants were assessed twice. During the first session, baseline assessments were collected. Furthermore, participants were told that they would be coupled with other twins for the next session, based on a rating system. They were instructed to rate other participants’ profiles on intelligence, appearance and congeniality and were told their profile would be rated as well. During the second session, participants were informed that they were rated too low to be allocated to a group, after which follow-up measures were collected. The findings showed that negative affect and cortisol levels were increased after mild negative evaluations, and positive affect as well as self-esteem were reduced. The findings indicate that the digi-SPEE can be used to study important mechanisms of psychopathology and manipulate biological and implicit as well as explicit mental changes.
Following this, Klippel et al. (Reference Klippel, Reininghaus, Viechtbauer, Decoster, Delespaul, Derom and Wichers2018) evaluated the influence of environmental (i.e., prenatal stress, CA, bullying, and subjective social status) and proxy genetic factors on sensitivity to peer evaluation on the digi-SPEE. Genetic factors and gene-environment interaction did not significantly influence implicit self-esteem, negative affect and positive affect after negative peer evaluation. However, bullying was associated with increased negative affect, and low subjective social status was associated with decreased self-esteem as well as positive affect after peer evaluation.
Pries, Guloksuz, Menne-Lothmann et al. (Reference Pries, Guloksuz, Menne-Lothmann, Decoster, van Winkel, Collip and van Os2017) tested whether subclinical expression of psychotic symptoms was associated with experiencing speech illusions that were assessed using the white noise speech illusion task. To detect speech illusions, participants were exposed to white noise and instructed to indicate whether they heard voices and speech fragments. Subtle expressions of psychotic symptoms were measured through the Structured Interview for Schizotypy — Revised (SIS-R; Vollema & Ormel, Reference Vollema and Ormel2000) and the CAPE. For this purpose, two methodological approaches, as published previously in the literature (Catalan et al., Reference Catalan, Simons, Bustamante, Drukker, Madrazo, de Artaza and Gonzalez-Torres2014; Galdos et al., Reference Galdos, Simons, Fernandez-Rivas, Wichers, Peralta, Lataster and van Os2011), were applied. However, neither method revealed an association between speech illusions and subclinical psychosis expressions in the general population. The findings indicate that contrary to findings in clinical populations, white noise speech illusion may not be associated with psychosis proneness in the adolescent/young adult general population.
Future Directions
Over recent years, the TwinssCan project resulted in important findings on the effects of genetic and nongenetic exposures on psychopathology. Additionally, researchers evaluated the applicability of the digi-SPEE task (Klippel et al., Reference Klippel, Reininghaus, Viechtbauer, Decoster, Delespaul, Derom and Wichers2018; Menne-Lothmann et al., Reference Menne-Lothmann, Decoster, van Winkel, Collip, Rutten, Delespaul and Wichers2017) and the white noise speech illusion task (Pries, Guloksuz, Menne-Lothmann et al., Reference Pries, Guloksuz, Menne-Lothmann, Decoster, van Winkel, Collip and van Os2017) measuring social defeat and salient attribution, respectively. These studies add valuable knowledge that contributes to our current understanding of the complexity of subclinical multidimensional psychopathology, as well as the nonspecific effects of exposures. They highlight the role of gene-environmental interaction focusing both on macro and micro levels of psychopathological changes, that is, changes occurring over month and years (Lecei et al., Reference Lecei, Decoster, De Hert, Derom, Jacobs, Menne-Lothmann and Wichers2019; Pinckaers et al., Reference Pinckaers, Rotee, Krolinski, Nwosu, Smeets, Gülöksüz and Drukker2019; Vaessen et al., Reference Vaessen, van Nierop, Decoster, Delespaul, Derom, de Hert and Myin-Germeys2017) and from moment to moment (Hasmi et al., Reference Hasmi, Drukker, Guloksuz, Menne-Lothmann, Decoster, van Winkel and van Os2017; Pries, Klingenberg et al., Reference Pries, Klingenberg, Menne-Lothmann, Decoster, van Winkel, Collip and Guloksuv2019; Vaessen et al., Reference Vaessen, van Nierop, Decoster, Delespaul, Derom, de Hert and Myin-Germeys2017), respectively.
It is increasingly acknowledged that the development of pleiotropic psychopathology depends on a complex network of environmental exposures, that is, the exposome (Guloksuz et al., Reference Guloksuz, van Os and Rutten2018; Pries, Lage Castellanos et al., Reference Pries, Lage Castellanos, Delespaul, Kenis, Luykx, Lin and Guloksuz2019), and polygenic vulnerability (Guloksuz et al., Reference Guloksuz, Pries, Delespaul, Kenis, Luykx, Lin and van Os2019), which affect individuals throughout their life. Similarly, resilience is thought to dynamically change and can only be understood by prospectively evaluating different biological and psychological processes (Kalisch et al., Reference Kalisch, Baker, Basten, Boks, Bonanno, Brummelman and Kleim2017; Rutten et al., Reference Rutten, Hammels, Geschwind, Menne-Lothmann, Pishva, Schruers and Wichers2013; Snijders et al., Reference Snijders, Pries, Sgammeglia, Al Jowf, Youssef, de Nijs and Rutten2018). By benefitting from these recent developments, we recently calculated the exposome score (Pries, Lage-Castellanos et al., Reference Pries, Lage Castellanos, Delespaul, Kenis, Luykx, Lin and Guloksuz2019), which we aim to apply to the TwinssCan population. Further, new features are under way. The third wave of the TwinssCan project will soon be processed and provided to the researchers. Future work will include evaluation of epigenetic information in combination with genome-wide molecular data. As the multiples are recruited from the EFPTS, stored placenta samples will be used to compare early life epigenetic variations to markings later in life. As the literature highlights the role of epigenetic variations for psychopathology (Pries, Gülöksüz, & Kenis, Reference Pries, Gülöksüz, Kenis and Delgardo-Morales2017; Rutten & Mill, Reference Rutten and Mill2009) as well as resilience (Rutten et al., Reference Rutten, Vermetten, Vinkers, Ursini, Daskalakis, Pishva and Boks2018; Snijders et al., Reference Snijders, Pries, Sgammeglia, Al Jowf, Youssef, de Nijs and Rutten2018), these approaches are valuable future targets for investigations aiming to better understand factors and processes underlying mental ill health.
Acknowledgments
The authors thank Jill Ielegems, Katrien Lyssens, Davinia Verhoeven and Debora op’t Eijnde for data collection. Further, the authors would like to acknowledge that the East Flanders Prospective Twin Survey (EFPTS) is partly supported by the Association for Scientific Research in Multiple Births and that the TwinssCan project is part of the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI).
Conflict of interest
None.



