Energy is the most critical nutrient affecting milk production of dairy cows. Feeding supplemental fats and low-fibre diets are common practices to meet the energy requirement of high-producing dairy cows. However, consumption of low effective fibre diet shifts rumen microbial processes, and if diet contains unsaturated fatty acids (UFA), unique trans-fatty acids (FA) isomers (e.g. trans-10 C18 : 1 and trans-10, cis-2 C18 : 2) are formed(Reference Griinari, Dwyer and McGuire1). These FA induce a metabolic challenge manifested by milk fat depression (MFD)(Reference Griinari, Dwyer and McGuire1,Reference Bauman and Griinari2) . Although fat supplementation is beneficial to provide extra energy in the diet, the effects of FA profile can be quite different on milk yield responses, energy partitioning, reproduction and health of dairy cows(Reference Rabiee, Breinhild and Scott3–Reference Moallem5). Research from a variety of animal models has shown that linoleic (C18 : 2n-6) and α-linolenic (C18 : 3n-3) acids or their derivatives (e.g. EPA (C20 : 5n-3), DHA (C22 : 6n-3)) regulate the expression of several genes involved in carbohydrate and lipid metabolism (e.g. PPAR, carbohydrate regulatory element binding) and hormonal responses (e.g. insulin, growth hormone)(Reference Jump6,Reference Palmquist7) . As such, dietary PUFA intake can influence animal energy metabolism and partitioning(Reference Newman, Bryden and Fleck8–Reference Liu, VandeHaar and Lock10). The use of energy for milk fat yield reduces during MFD with feeding PUFA, and this may be associated with an increase in body fat accretion(Reference Bauman and Griinari2). Modifying intakes of n-6 and n-3 FA also regulates the balance of 2- and 3-series PG, which in turn affect physiology of animal and inflammatory responses(Reference Greco, Neto and Pedrico11,Reference Papadopoulos, Maes and van Weyenberg12) . Silvestre et al.(Reference Silvestre, Carvalho and Francisco13) reported that feeding n-6 FA source during transition period followed by n-3 FA after breeding improves milk production as well as fertility of dairy cows. Provision of n-3 (fish or linseed oil) or n-6 (soyabean oil) FA source has also been shown to improve growth performance(Reference Ghasemi, Azad-Shahraki and Khorvash14) and immune system function(Reference Lessard, Gagnon and Godson4), and milk content of beneficial FA(Reference Donovan, Schingoethe and Baer15).
Despite the positive effects of PUFA, feeding unprotected oils may depress milk fat, fibre digestion and performance even at moderate levels of FA supplementation (≥2 %)(Reference Rabiee, Breinhild and Scott3,Reference Donovan, Schingoethe and Baer15) . Hence, rumen inert fat sources (e.g. saturated fats) have been commercially developed to minimise the negative effects of UFA on rumen fermentation. Palmitic acid (C16 : 0) supplementation has been reported to increase milk yield and milk fat concentration without depressing fibre digestibility and DM intake (DMI) in some studies(Reference Mosley, Mosley and Hatch16,Reference Piantoni, Lock and Allen17) . Feeding C16 : 0 supplement has different effects on milk FA profile secretion, generally increasing milk C16 : 0 and reducing concentrations of n-3 and n-6 PUFA(Reference Mosley, Mosley and Hatch16,Reference de Souza, Preseault and Lock18,Reference Lock, Preseault and Rico19) . Also, several studies(Reference Liu, VandeHaar and Lock10,Reference Piantoni, Lock and Allen17,Reference de Souza and Lock20,Reference Bianchi, Macedo and Silva21) have indicated that C16 : 0 may influence metabolic status and nutrient partitioning between milk synthesis and body reserves. For instance, C16 : 0 supplementation lowered body weight (BW) as compared with feeding soyabean oil(Reference Liu, VandeHaar and Lock10) or fish oil(Reference Silvestre, Carvalho and Francisco13), thereby may diminish the benefits on the overall energy efficiency. Therefore, supplementation strategies (feeding rate and FA profile) are necessary to overcome MFD and to take advantage of the benefits of UFA.
Dietary intake and the ratio of n-6 and n-3 FA have the potential to alter feed intake, body fat metabolism, nutrient partitioning and performance(Reference Newman, Bryden and Fleck8–Reference Silvestre, Carvalho and Francisco13). Although adequate consumption of both n-6 and n-3 FA is essential for human health, a lower dietary n-6:n-3 ratio is regarded as healthier(Reference Micallef, Munro and Phang9). The effects of supplementation of C16 : 0(Reference Mosley, Mosley and Hatch16–Reference de Souza and Lock20), n-6 FA(Reference Ammah, Benchaar and Bissonnette22,Reference Petit23) or n-3 FA(Reference Donovan, Schingoethe and Baer15,Reference Ammah, Benchaar and Bissonnette22,Reference Petit23) on lactational performance have been well investigated. However, little attention has been given to the dietary ratio of n-6:n-3 FA or mixture of FA that would optimise their utilisation or improve lactation performance of dairy cows. Indeed, no data exist concerning n-6 and n-3 FA requirements or the ideal ratio in the diet for ruminants. In sows, feeding a lactation diet of a low (2:1) v. high (10:1) ratio of n-6:n-3 FA improved feed intake and was associated with a better metabolic change(Reference Papadopoulos, Maes and van Weyenberg12). Greco et al.(Reference Greco, Neto and Pedrico11) found that decreasing the n-6:n-3 ratio from 6 to 4 attenuated inflammatory response to a lipopolysaccharide challenge in dairy cows. In goats, decreasing the ratio of n-6: n-3 from 10·4:1 to 2·3:1 in the diet improved FA profile of muscular tissues, increased back fat thickness, but did not affect meat quality or growth performance(Reference Ebrahimi, Rajion and Jafari24). In dairy cows, the combination of C16 : 0 with oleic acid (C18 : 1n-9) or feeding either n-3 or n-6 FA improved performance(Reference Donovan, Schingoethe and Baer15,Reference de Souza, Preseault and Lock18) . Palm oil contains a negligible amount of n-3 (<1 %, C18 : 3, C20 : 5 and C22 : 6)(25) and a small concentration of n-6 (<10 %, C18 : 2)(25); thereby, palm FA supplements could not change the intake or the ratio of n-6 and n-3 FA. Hence, there is interest to know whether concurrent supplementation of C16 : 0 with supplements enriched in UFA can prevent MFD in low-fibre diet, enrich milk PUFA and improve the metabolism of dairy cows. Besides, we hypothesised that lowering dietary n-6:n-3 ratio in the diet of high-producing dairy cows may have beneficial effects on energy partitioning, milk production and milk FA. Therefore, the present study was designed to determine the effect of C16 : 0-enriched supplement alone or in combination with altering the ratio of n-6:n-3, high (Ca salts of soyabean oil), intermediate (no n-6 or n-3 supplement) and low (Ca salts of fish oil) in a low-fibre diet on energy metabolism, milk FA profile and performance of high-yielding dairy cows.
Experimental methods
Animal welfare
The experiment was conducted from January to March 2017 at Laverk Teaching and Research Farm of Isfahan University of Technology (IUT, Esfahan, Iran). All the experimental procedures and animal welfare, management and samplings were approved by the Animal Care and Ethics Committee of the Isfahan University of Technology.
Experimental treatments
Twelve lactating Holstein cows (604 (sd 42·7) kg of BW, 2·58 (sd 0·68) parity, 54·9 (sd 3·8) kg/d of milk yield; 69·9 (sd 11·0) days in milk; mean values and standard deviations) were blocked by milk production and days in milk and used in a replicated 4 × 4 Latin square design with 28-d periods: 21 d of adaptation and 7 d of collection. The treatments comprised a basal diet contained no FA supplement (CON) or added with 2·4 % FA supplements as C16 : 0 (735 g/kg of C16 : 0, RumiFat® R100, prilled hydrogenated palm FA distillate; PAL), mixture (2:1, w/w) of C16 : 0 and n-6 FA (PW6, Persia fat n-6, Ca salts of soyabean oil, Kimiya Danesh Alvand Co.) or mixture (2:1, w/w) of C16 : 0 and in n-3 FA (PW3, Ca salts of fish oil, Persia fat n-3; Kimiya Danesh Alvand Co.). The amount of C16 : 0-enriched supplement (2·4 % dietary DM) was chosen based on the recommended value by the manufacture (500–1000 g/cow) and the previous work(Reference Mosley, Mosley and Hatch16). Similarly, the level of supplemental n-6 and n-3 FA sources was selected based on the manufacture (200 g/cow) and to avoid the detrimental effects on DMI and milk yield(Reference Donovan, Schingoethe and Baer15), thus obtaining a high (10·0:1) and a low (2·8:1) n-6:n-3 ratio for PW6 and PW3, respectively. The FA supplements replaced maize grain in the diets (Table 1). Diets contained a low forage to concentrate ratio (35:65) and were balanced for individual nutrients except for energy that was increased in FA-supplemented diets. The FA profile in the three supplements and diets is presented in Table 2. Cows were housed individually in box stalls (4 × 4 m) located in a roofed area with open sides. Each box stall was equipped with a concrete feed bunk and automatic water trough. Clean wood shavings were used for bedding and refreshed daily. Diets were mixed manually and delivered ad libitum (about 5–10 % refusals) as a total mixed ration two times daily at 08.00 and 16.00 hours.
Table 1. Dietary ingredients and nutrient composition of the treatment diets (g/kg DM, unless otherwise stated)
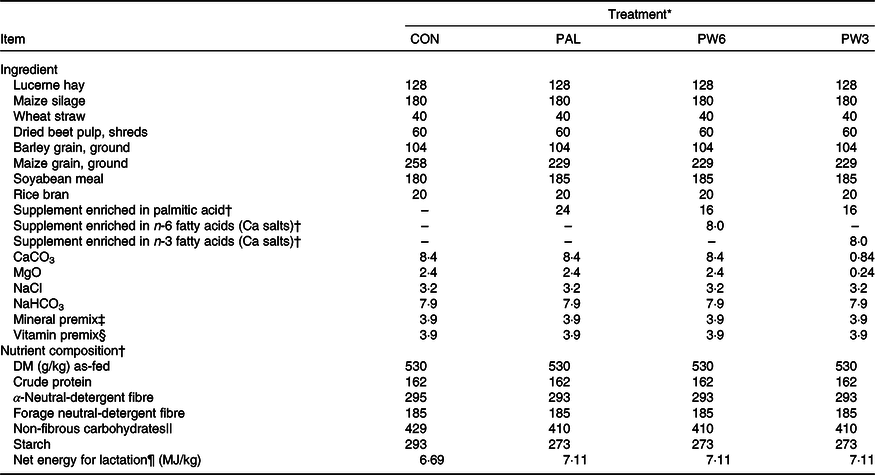
* CON = diet containing no supplement, PAL = diet containing palm supplement, PW6 = diet containing PAL and n-6-enriched fatty acid supplements, PW3 = diet containing PAL and n-3-enriched fatty acid supplements.
† Fatty acid composition is represented in Table 2.
‡ Contained (per kg DM): 245 g Ca, 55 g Mg, 18 g Zn, 13·5 g Mn, 4·5 g Cu, 0·2 g iodine, 0·1 g Co and 0·072 g Se.
§ Contained (per kg DM): 1500 kIU vitamin A, 250 kIU vitamin D3, 15 kIU vitamin E, 2 g organic Zn, 1·5 g organic Mn, 0·5 g organic Cu, 0·008 g organic Se, 3 g monensin, 0·2 g biotin.
‖ Non-fibrous carbohydrate = 100 − (crude protein + ash + neutral-detergent fibre + ether extract), computed according to the National Research Council (2001) model(25).
¶ Based on DM intake of 25 kg (National Research Council, 2001)(25) using SPARTAN (2011).
Table 2. Main fatty acids (FA), ether extract and calcium composition of the supplements and experimental diets
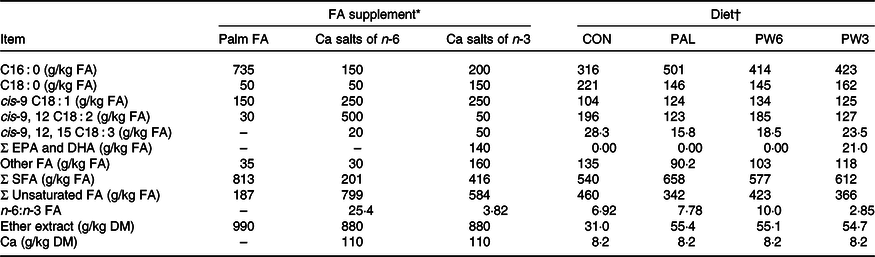
EPA, cis-7,10,13,16,19 C20 : 5; DHA, cis-4,7,10,13,16,19 C22 : 6.
* Palm = RumiFat® R100 (99·5 % DM), prilled hydrogenated palm FA distillate (Malaysia); n-6 FA = Ca salts of soyabean oil (95·5 % DM), Persia fat n-6, Kimiya Danesh Alvand Co.; n-3 FA = Ca salts of fish oil (95·5 % DM), Persia fat n-3, Kimiya Danesh Alvand Co.
† CON = diet containing no supplement, PAL = diet containing palm supplement, PW6 = diet containing PAL and n-6-enriched FA supplements, PW3 = diet containing PAL and n-3-enriched FA supplements.
Data collection and sample analysis
Cows were milked three times a day (08.00, 16.00 and 00.00 hours) in a herringbone milking parlour. Milk yield was recorded during days 22–28 of each period. Milk samples from three consecutive milkings for each cow were collected on days 23, 25 and 27 of each period, preserved (sodium azide) and analysed for fat, protein and lactose using an IR analyzer (MilkoScan 134 BN; Foss Electric). Additional samples were collected without preservatives but composited to form one composite sample per cow per period. Samples were stored at –20°C until further analysis for milk FA composition. All cows were weighed and scored for body condition score (BCS) using the five-point BCS system in 0·25 increments once at the beginning and another on the last day of each period(Reference Ferguson, Galligan and Thomsen26). On the last day of each period, back fat thickness was measured by a portable B-mode ultrasound generator (SonoVet 600V; BCF Technology Ltd) with a linear transducer and frequency 5·0–6·5 MHz. Changes in BW, BCS and back fat thickness were calculated as the difference between values (final − initial).
Samples of total mixed ration, forages, concentrates and orts were collected daily throughout the collection period. For determination of apparent digestibility, samples of faeces were collected every 9 h during 3 d in each period resulting in eight samples per cow per period. Faecal, diet ingredients and orts samples were composited by period for chemical analysis. The DM content was measured at 55°C in a forced-air oven for 72 h. The samples were ground using a Wiley mill (1-mm screen; Arthur H. Thomas) and analysed for ash (by combustion at 550°C for 5 h in a furnace, method 942.05)(Reference Horwitz27), aNDF (neutral-detergent fibre) (using heat-stable amylase and Ankom 220 fiber analyzer)(Reference Van Soest, Robertson and Lewis28), crude protein (using the macro-Kjeldahl method; method 955.04)(Reference Horwitz27) and ether extract (EE) (method 920.39; AOAC)(Reference Horwitz27). The non-fibrous carbohydrate component was calculated as 100 – (crude protein + NDF + EE + ash; National Research Council)(25). Acid-insoluble ash was used as an internal marker to estimate faecal output and nutrient digestibility(Reference Van Keulen and Young29).
Feeds and milk samples were analysed for FA composition. Lipid extraction of the samples was carried out three times with a mixture of chloroform and methanol (2:1, v/v) to a final volume of 100 ml administered under Ar stream as described in Folch et al.(Reference Folch, Lees and Sloane Stanley30). After each extraction, samples were centrifuged at 1800 g for 10 min, and the organic fraction was separated and transferred into a 100 ml volumetric flask. Afterward, samples were treated with anhydrous sodium sulphate, dried using a rotary evaporator and vaporised at 40°C under vacuum. Using mild methanolysis/methylation via methanolic hydrochloric acid (HCl/MeOH), FA methyl esters were prepared by a method explained in Ichihara & Fukubayashi(Reference Ichihara and Fukubayashi31). Milk FA profile of the samples was performed using GC (Agilent 6890, Agilent Technologies) equipped with a RESTEK column for FAME (Rtx®-2330, 105 m × 250 µm×0·2 µm; catalogue no. 10729; serial no. 1525353, Restek Corporation) and a flame ionisation detector. Instrument conditions were an injector temperature of 250°C, a flame-ionisation detector temperature of 255°C, N2 carrier gas at 0·8 ml/min and an injector split ratio of 50:1. The initial column temperature was 70°C for 1 min, programmed at 5°C/min to 100°C for 2 min, and then 10°C/min to 175°C and kept for 35 min, and finally from 4°C/min to 225°C and was kept for 35 min. Based on a FAME standard mix (GLC 463; Nu-Chek Prep Inc.; http://www.nu-chekprep.com/catalog.pdf), individual peaks were specified. Non-adecanoic acid was utilised as an internal standard.
Ruminal fluid samples were collected on day 27 with a flexible oro-gastric tube connected to a vacuum pump at 4 h after feeding. The first rumen fluid sample (about 500 ml) was discarded before taking samples. The sample was strained through two layers of cheesecloth. Then, 4 ml of filtrate was preserved by adding 1 ml of 25 % metaphosphoric acid for volatile FA determination by GC (6820 Gas Chromatograph; Agilent Technologies) using a HP-FFAP capillary column (J&W HP-FFAP GC Column, 30 m, 0·25 mm, 0·25 µm, 7-inch cage; Agilent Technologies). The injector and detector temperatures were set at 250 and 300°C, respectively. The column oven temperature to be increased from 60 to 200°C at 10°C/min and to hold 10 min at the final temperature. The carrier gas was N2 at a flow of 1·0 ml/min. Samples were injected via an autosampler (split ratio 50:1).
Blood samples were collected from the coccygeal vein of each cow using tubes containing EDTA (2·1 mg/ml) at 4 h after feeding on day 27. Samples were centrifuged for 15 min at 2000 g at room temperature. After separation, the supernatant plasma was transferred to centrifuge and frozen at –20°C for later analysis. Commercial kits (Pars Azmoon Co.) were used to determine the concentrations of glucose (GOD – PAP), cholesterol (CHOD-PAP), TAG (GPO-PAP), HDL-cholesterol (immunoinhibition), LDL-cholesterol (direct method), albumin (bromocresol green method), total protein (Biuret method) and urea N (Berthelot method) by an autoanalyzer (BT 1500; Biotecnica SpA) according to the manufacturer’s instructions. Plasma concentration of NEFA was determined by a colorimetric method (Randox Laboratories Ltd) and β-hydroxybutyrate (Randox Laboratories Ltd) was determined by a kinetic enzymatic method based on the oxidation of d-3-hydroxybutyrate to acetoacetate (RB1007; Randox Laboratories Ltd).
Calculations and statistical analysis
Yield of 3·5 % fat-corrected milk (FCM) and net energy of milk (NEL) was calculated using the following equations, respectively: FCM yield = (0·432 × (milk yield (kg/d)) + 16·23 × (milk fat yield (kg/d))); and milk NEL (MJ/d) = milk yield (kg/d) × ((fat % × 0·389) + (true protein % × 0·236) + (lactose % × 0·165)) (NRC)(25). Energy for maintenance (MJ/d) was calculated as NEM = (0·334 × BW0·75)(25). Energy output in body reserves (MJ/d) was estimated according to body reserves = ((2·88 + 1·036 × BCS) × ΔBW) × 4·184, where BCS and ΔBW were the average BCS and BW change, respectively. Energy partitioning (% milk, maintenance or body tissue gain) was predicted based on observed performance. Feed efficiency (kg/kg) was calculated as milk/DMI or FCM/DMI.
Intake of DM and milk yield and composition were averaged for each period before analysis. Data were analysed as a replicated 4 × 4 Latin square design using the mixed model procedure of SAS (SAS Institute Inc.). The model included fixed effects of square, period, and treatment and RANDOM statement of cow within square. The statistical model used for analyses was
where Yijkm = response variable, μ = overall mean, Sm = fixed effect of square m, Pi = fixed effect of period i, C(S)jm = random effect of cow j within square m, Fk = fixed effect of FA supplements k and eijkm = random residual error. Orthogonal contrast statements were used to test the effects of (1) FA supplementation (CON v. SUP), (2) FA combination (PAL v. PW6 and PW3) and (3) FA combination type (PW3 v. PW6). Reported values were least squares means with significance which was declared at P ≤ 0·05.
Results
Nutrient composition
The C16 : 0-enriched supplement was primarily in NEFA form and contained mostly C16 : 0 (735 g/kg; Table 2). The Ca salts of fish oil contained 190 g/kg FA as n-3 (50 g C18 : 3 and 140 g C20 : 5 and C22 : 6), while the Ca salts of soyabean oil comprised mainly from C18 : 2n-6 (500 g/kg FA). Both n-3 and n-6 FA supplements contained a medium level of C18 : 1n-9 (250 g/kg FA), but higher than C16 : 0-enriched supplement (150 g/kg FA). Diets were planned to contain a low level of NDF (about 290 g/kg DM) or forage NDF (about 180 g/kg DM; Table 1). As per the design of the study, experimental diets were isonitrogenous (162 g/kg DM of crude protein), but FA-supplemented diets had greater content of EE (55 v. 31 g/kg DM) and NEL value (7·11 v. 6·69 MJ/kg DM). With the inclusion of FA supplements instead of maize grain, non-fibrous carbohydrate content (mainly starch) was decreased from 429 to 410 g/kg DM. The diets differed in the FA composition, mainly in the proportions of C16 : 0, C18 : 2n-6, C18 : 3n-3, C20 : 5n-3 and C22 : 6n-3 (Table 2). The dietary n-6:n-3 FA ratio was intermediate for CON (6·9:1) and PAL (7·8:1), but it was increased in PW6 (10·0:1) and decreased in PW3 (2·8:1).
Nutrient intake and milk yield
Feed intake was unaffected by FA-supplemented treatments and averaged 28·8 kg/d (Table 3). Although PAL treatment increased (P < 0·01) DMI compared with the FA mixtures, no difference in DMI was found between CON and PW3 or PW6. As expected, feeding PAL, PW6 and PW3 increased (P < 0·01) intake of C16 : 0, n-6 FA and n-3 FA, respectively. Likewise, all FA-supplemented treatments increased intake of C16 : 0 and C18 : 1n-9. Feeding either PW3 or PW6 did not change milk fat content, but PAL increased milk fat concentration (P = 0·04) and tended to increase (P = 0·06) milk fat to protein ratio. All FA-supplemented treatments decreased concentration of milk lactose (P < 0·01) and protein (P < 0·01). Compared with CON, FA-supplemented treatments increased (P < 0·01) milk yield (about 2·1 kg/d, P < 0·01) and 3·5 % FCM yield with the highest value observed in PAL. All FA-supplemented treatments tended to increase milk protein yield (P = 0·07) and increased milk fat yield (P < 0·01). Milk yield efficiency was lower (P < 0·01) in PAL than FA mixtures, regardless of the ratio of n-6:n-3. However, FCM efficiency was similar among FA-supplemented diets and higher than CON (P < 0·01). Nevertheless, no difference was observed in FCM/DMI between CON and PAL.
Table 3. Effect of palm supplementation and altering the dietary ratio of n-6:n-3 fatty acids (FA) on feed and FA intake and milk yield and composition
(Least squares means with their standard errors)
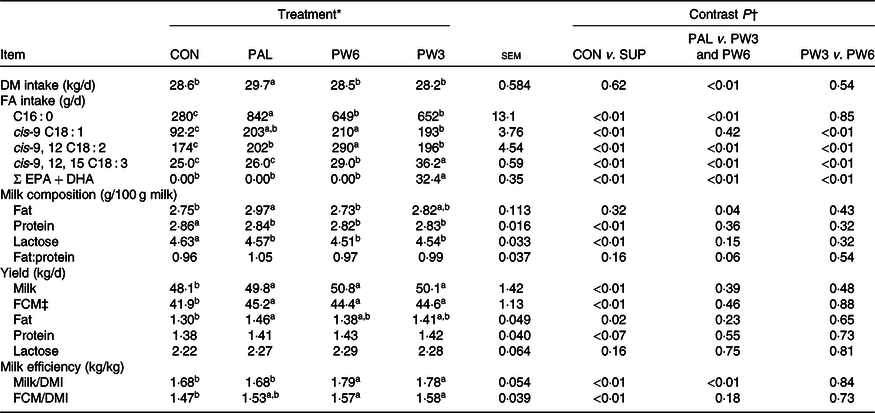
EPA, cis-7,10,13,16,19 C20 : 5; DHA, cis-4,7,10,13,16,19 C22 : 6; FCM, fat-corrected milk; DMI, DM intake.
a,b,c Least squares means within a row with unlike superscript letters were significantly different (P < 0·05) according to the Tukey–Kramer test.
* CON = diet containing no supplemental fat with 6·9:1 n-6:n-3 FA ratio, PAL = diet containing palm supplement with 7·8:1 n-6:n-3 FA ratio, PW6 = diet containing PAL and n-6-enriched FA with 10:1 n-6:n-3 FA ratio, PW3 = diet containing PAL and n-3-enriched FA with 2·8:1 n-6:n-3 FA ratio.
† CON v. SUP = diet containing no supplement v. diets containing 2·4 % FA supplement as PAL or mixture of either PAL and PW6 or PAL and PW3.
‡ FCM yield = (0·4322 × (milk yield (kg/d))+16·23 × (milk fat yield (kg/d))).
Ruminal fermentation and nutrient digestibility
The FA supplementation had no effect on rumen pH, total volatile FA concentration, molar proportion of acetate, butyrate, valerate and isobutyrate or the ratio of acetate:propionate (Table 4). Molar proportion of isovalerate tended (P = 0·07) to be higher in the PW3 than the PW6. Total tract DM, OM and NDF digestibility did not differ between CON and FA-supplemented treatments, while FA-supplemented treatments increased apparent EE digestibility. Compared with PAL, FA mixtures decreased DM (P = 0·02), OM (P = 0·02), NDF (P = 0·02) and EE (P = 0·01) digestibility. The apparent DM and EE digestibility was similar between PW6 and PW3, whereas apparent NDF digestibility (P = 0·03) was lower in PW6 than PW3.
Table 4. Effect of palm supplementation and altering the dietary ratio of n-6:n-3 fatty acids (FA) on rumen fermentation and nutrient digestibility
(Least squares means with their standard errors)
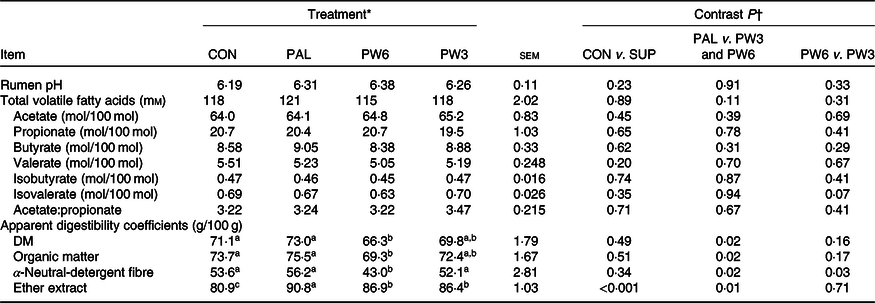
a,b,c Least squares means within a row with unlike superscript letters were significantly different (P < 0·05) according to the Tukey–Kramer test.
* CON = diet containing no supplemental fat with 6·9:1 n-6:n-3 FA ratio, PAL = diet containing palm supplement with 7·8:1 n-6:n-3 FA ratio, PW6 = diet containing PAL and n-6-enriched FA with 10:1 n-6:n-3 FA ratio, PW3 = diet containing PAL and n-3-enriched FA with 2·8:1 n-6:n-3 FA ratio.
† CON v. SUP = diet containing no supplement v. diets containing 2·4 % FA supplement as PAL or mixture of either PAL and PW6 or PAL and PW3.
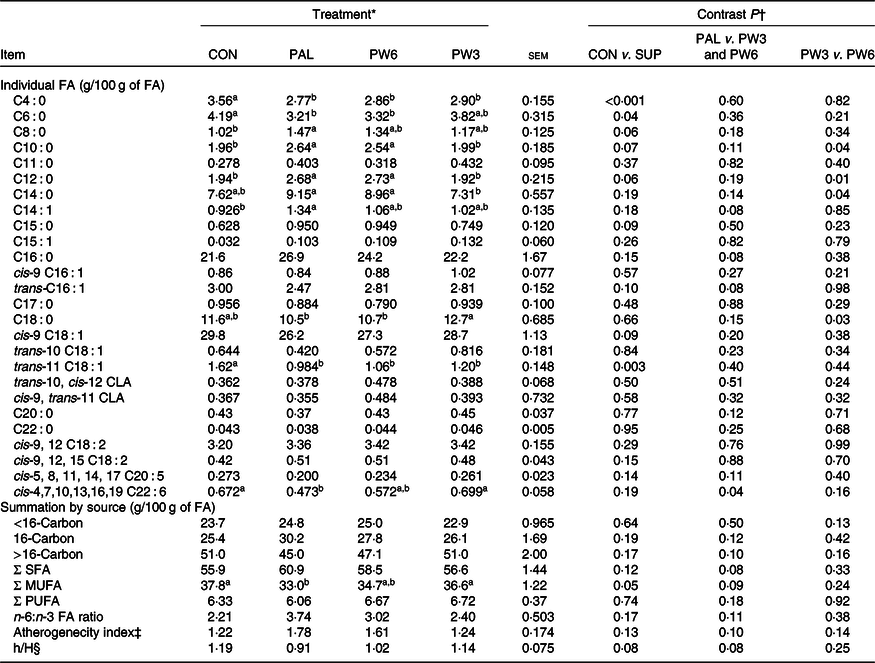
Milk fatty acid composition
In general, feeding PAL generally decreased milk proportion of SCFA (C4 : 0, P < 0·001 PAL v. CON) and tended to increase/increased concentrations of medium-chain FA (C8 : 0, P = 0·06; C10, P = 0·07; C12 : 0, P = 0·06; C14 : 0, P = 0·04; PAL v. CON; and C16 : 0, P = 0·08, PAL v. PW3 and PW6) (Table 5). The concentration of C22 : 6n-3 was lower (P = 0·04) in PAL than PW3 or CON. Moreover, numerically but not significantly (P = 0·11), the concentration of C20 : 5n-3 was lower and the ratio of n-6:n-3 was greater in PAL and PW6 compared with CON and PW3. Milk concentration of C12 : 0 and C14 : 0 was higher and that of C18 : 0 was lower in PW6 than PW3. Milk concentration of trans-10 C18 : 1, trans-10, cis-12 C18 : 2, cis-9 and trans-11 C18 : 2 was similar across the treatments, but milk trans-11 C18 : 1 was greater (P = 0·003) in CON than FA-supplemented treatments. Milk concentration of SFA and PUFA, and atherogenecity index were unaffected by FA supplementation, but PW3 or PW6 tended to decrease milk concentration of SFA (P = 0·08) and atherogenecity index (P = 0·10) and increase the ratio of hypocholesterolaemic:hypercholesterolaemic FA (P = 0·08) compared with PAL. The overall proportion of milk FA < 16 carbon (mammary de novo synthesis), >16 carbon (extraction from plasma) and 16-carbon (mammary de novo synthesis and extraction from plasma) did not differ between CON and FA-supplemented treatments.
Table 5. Effect of palm supplementation and altering the dietary ratio of n-6:n-3 fatty acids (FA) on milk FA profile
(Least squares means with their standard errors)
a,b,c Least squares means within a row with unlike superscript letters were significantly different (P < 0·05) according to the Tukey–Kramer test.
* CON = diet containing no supplemental fat with 6·9:1 n-6:n-3 FA ratio, PAL = diet containing palm supplement with 7·8:1 n-6:n-3 FA ratio, PW6 = diet containing PAL and n-6-enriched FA with 10:1 n-6:n-3 FA ratio, PW3 = diet containing PAL and n-3-enriched FA with 2·8:1 n-6:n-3 FA ratio.
† CON v. SUP = diet containing no supplement v. diets containing 2·4 % FA supplement as PAL or mixture of either PAL and PW6 or PAL and PW3.
‡ Atherogenecity index was calculated = (C12 : 0 + 4(C14 : 0) + C16 : 0)/(MUFA + PUFA).
§ Hypocholesterolaemic to hypercholesterolaemic fatty acids = (cis-9 C18 : 1 + cis-9, 12 C18 : 2 + cis-9, 12, 15 C18 : 3 + cis-5, 8, 11, 14, 17 C20 : 5+ cis-4, 7, 10, 13, 16, 19 C22 : 6)/(C14 : 0 + C16 : 0).
Energy-related parameters
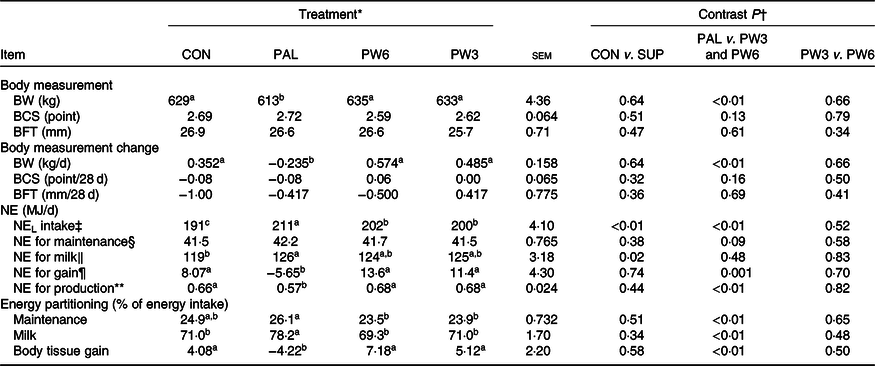
BW was unaffected by the inclusion of supplemental FA but differed among FA-supplemented treatments (Table 6). Feeding PAL decreased mean BW (613 v. 634 kg; P < 0·01) and daily BW gain (−0·235 v. 529 g/d; P < 0·01) compared with PW3 and PW6. BCS and backfat thickness value or changes remained unchanged among the treatments. All FA-supplemented treatments enhanced NEL intake with the highest value (P < 0·01) observed for PAL, followed by PW6 and PW3, and then CON. Milk NEL output was affected by FA supplementation (P = 0·02), in which all FA-supplemented treatments increased milk NEL output. Feeding PAL decreased (P < 0·01) net energy for gain and the percentage of energy stored as body reserves compared with CON or PW3 and PW6. No difference was observed for energy partitioning between CON and PW3 or PW6 or between PW3 and PW6. Moreover, the amount of NEL intake to be converted to milk energy and tissue gain did not differ between CON and FA-supplemented treatments, but the value was lower (P < 0·01) in PAL (0·57) than PW3 and PW6 (0·67).
Table 6. Effect of palm supplementation and altering the dietary ratio of n-6:n-3 fatty acids (FA) on energy partitioning and efficiency
(Least squares means with their standard errors)
BW, body weight; BCS, body condition score; BFT, back fat thickness; NE, net energy; NEL, NE for lactation.
a,b,c Least squares means within a row with unlike superscript letters were significantly different (P < 0·05) according to the Tukey test.
* CON = diet containing no supplemental fat with 6·9:1 n-6:n-3 FA ratio, PAL = diet containing palm supplement with 7·8:1 n-6:n-3 FA ratio, PW6 = diet containing PAL and n-6-enriched FA with 10:1 n-6:n-3 FA ratio, PW3 = diet containing PAL and n-3-enriched FA with 2·8:1 n-6:n-3 FA ratio.
† CON v. SUP = diet containing no supplement v. diets containing 2·4 % FA supplement as PAL or mixture of either PAL and PW6 or PAL and PW3.
‡ NEL intake = diet NEL × kg of DM intake.
§ NE for maintenance (MJ/d) = 0·334 MJ/kg × BW (kg)0·75.
‖ NE for lactation (MJ/d) = milk yield (kg/d) × ((fat % × 0·389) + (true protein % × 0·236) + (lactose % × 0·165)).
¶ NE for gain (MJ/d) = ((2·88 + 1·036 × BCS) × ΔBW) × 4·184.
** NE for production = (NE for lactation + NE for tissue gain or loss)/NEL intake.
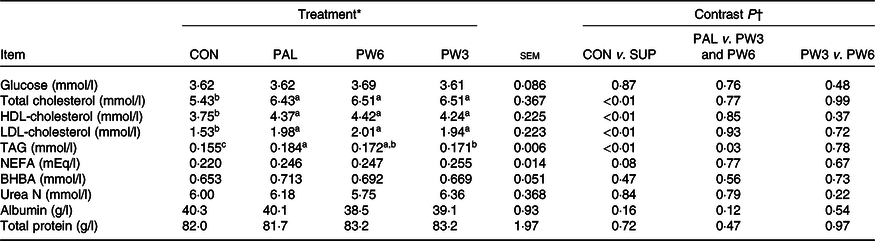
Concentrations of plasma glucose, urea N, total protein, albumin and β-hydroxybutyrate were similar across the treatments (Table 7). Nevertheless, PAL treatment or the combination increased concentrations of TAG (P < 0·01), total cholesterol (P < 0·01), HDL-cholesterol (P < 0·01) and LDL-cholesterol (P < 0·01) and tended (P = 0·08) to increase NEFA concentration. The extent of TAG rise was higher (P = 0·03) for PAL v. PW3 or PW6. No other differences were observed for blood energy metabolites between PAL and PW3 or PW6 or between PW3 and PW6.
Table 7. Effect of palm supplementation and altering the dietary ratio of n-6:n-3 fatty acids (FA) on plasma energy and nitrogen metabolites
(Least squares means with their standard errors)
BHBA, β-hydroxybutyrate.
a,b,c Least squares means within a row with unlike superscript letters were significantly different (P < 0·05) according to the Tukey test.
* CON = diet containing no supplemental fat with 6·9:1 n-6:n-3 FA ratio, PAL = diet containing palm supplement with 7·8:1 n-6:n-3 FA ratio, PW6 = diet containing PAL and n-6-enriched FA with 10:1 n-6:n-3 FA ratio, PW3 = diet containing PAL and n-3-enriched FA with 2·8:1 n-6:n-3 FA ratio.
† CON v. SUP = diet containing no supplement v. diets containing 2·4 % FA supplement as PAL or mixture of either PAL and PW6 or PAL and PW3.
Discussion
Feeding low-fibre diet and supplemental PUFA shifts rumen biohydrogenation pathways that cause MFD(Reference Griinari, Dwyer and McGuire1). Previous studies(Reference Liu, VandeHaar and Lock10,Reference Piantoni, Lock and Allen17) indicated that C16 : 0 supplementation had positive effects on milk fat concentration and yield but negative effects on milk PUFA content. Along with this, there is some evidence that C16 : 0-enriched supplement may affect energy partitioning generally associated with a decrease in BW gain in dairy cows(Reference Liu, VandeHaar and Lock10,Reference Bianchi, Macedo and Silva21) , goats(Reference Teh, Trung and Jia32) and sheep(Reference Capper, Wilkinson and Mackenzie33). This can compromise the overall efficiency of energy use. In the present study, we formulated a low-fibre basal diet which resulted in low milk fat contents (<3 %) and fat:protein ratio (about 1) across all cows. Moreover, a portion of C16 : 0-enriched supplement was substituted by either n-6 or n-3 to increase PUFA intake and change the dietary ratio of n-6:n-3. This change was made to understand whether providing n-3 and n-6 FA or their ratios would alleviate the adverse impacts of C16 : 0-enriched supplement as concerned above. Our results showed that feeding FA mixtures, regardless of the ratio of n-6:n-3 FA, did not adversely affect milk fat and DMI but improved milk yield efficiency.
In the present study, PAL alone increased DMI by 1·1 kg/d, while C16 : 0-enriched supplement in combination with n-6 or n-3 FA did not alter DMI as compared with CON. None of the FA-supplemented treatments significantly changed ruminal pH or volatile FA concentrations. NRC(25) cites that Ca salts of or hydrogenated FA have minimal effects on rumen fermentation compared with unprotected UFA. The mechanisms by which fat can improve DMI are unknown but may involve positive effects on diet acceptability(Reference Grummer, Hatfield and Dentine34), nutrient digestibility(Reference Weld and Armentano35) and/or an increase in nutrient requirement driven by an increased milk yield(25,Reference Allen36,Reference Mathews, Rico and Sprenkle37) . In the present study, the DM and NDF digestibility was higher with PAL alone as compared with the other FA-supplemented treatments. This effect may partially explain the increased DMI with PAL. Feeding prilled fat improved nutrient digestibility in Behan et al.(Reference Behan, Loh and Fakurazi38). These authors reported that SFA increased population of total cellulolytic bacteria and decreased protozoa. Also, results from a meta-analysis(Reference Capper, Wilkinson and Mackenzie33) of fat supplementation and total tract digestibility showed an increased NDF digestibility (1·3 %) and DMI (0·66 kg/d) with adding saturated fat (3%). Moreover, the more energy output in milk by feeding PAL may contribute to an increase in nutrient requirement and subsequent hyperphagia. Weiss & Pinos-Rodríguez(Reference Weiss and Pinos-Rodríguez39) observed that prilled fat increased DMI in early lactation, but the same fat reduced DMI in late lactation. Cows in our study had high DMI (about 29 kg/d) and milk yield (about 50 kg/d) and were almost in mid-lactation (days in milk from 70 to 182). During this period, increasing energy density by supplemental fat may not reduce DMI because of high milk production(Reference Allen36). Mosley et al.(Reference Mosley, Mosley and Hatch16) found an increase in DMI by cows in mid-lactation fed low (478 g/d) or moderate (888 g/d) levels of C16 : 0-enriched supplement with no difference between unsupplemented and supplemented diets at a higher level of PAL (1275 g/d). Comparable to Mosley et al.(Reference Mosley, Mosley and Hatch16), the actual intake of palm supplement based on DMI in our study was approximately 700 g/cows.
As compared with PAL, the reduction of DMI with the inclusion of FA mixtures can be attributed to differences in FA intake. The intake of C16 : 0 was about 30 % less, while that of PUFA (n-3 and n-6 FA) was 28 % more for FA mixtures as compared with PAL. It has been suggested that the hypophagic effect of oils increases as the degree of UFA in the diet increases(Reference Harvatine and Allen40). Increasing intake of UFA may increase the secretion of gut peptides (i.e. cholecystokinin and glucagon-like peptide-1), which are known to depress gut motility and DMI(Reference Allen36). Our results agree with the study of de Souza et al.(Reference de Souza, Preseault and Lock18) who found that feeding SFA alone or in combination (C16 : 0 and C18 : 0) increased DMI in mid-lactation cows, whereas the blend of SFA with UFA (C16 : 0 plus C18 : 1n-9) decreased DMI. Moreover, the apparent digestibility of NDF declined with FA mixtures, in particular PW6, and this might be responsible for less DMI. The depression of NDF digestibility with PW6 could be because it contained the highest UFA concentration (Table 2). The high proportion of UFA is toxic to rumen microbial populations and particularly to cellulolytic bacteria(Reference Behan, Loh and Fakurazi38).
The PAL treatment increased milk yield 1·7 kg/d and FCM yield 3·3 kg/d when compared with the CON. Supplementation with FA mixtures, regardless of the ratio of n-6:n-3 FA, increased both milk yield (2·4 kg/d) and FCM (2·6 kg/d) to the same extent. A previous meta-analysis(Reference Bauman and Griinari2) reported different FA-supplemented treatments increased milk yield ranged from 0·306 to 3·07 kg/d. In Mosley et al.(Reference Mosley, Mosley and Hatch16), adding 500–1500 g/d C16 : 0 supplement increased milk yield by 2·2 kg/d. de Souza et al.(Reference Newman, Bryden and Fleck8) showed that milk yield was increased to the same extent (2·0 kg/d) by supplementation of C16 : 0 alone or in combination (C18 : 0 or C18 : 1n-9). Increased milk yield or a tendency for increased milk protein yield by the inclusion of FA supplements can be explained by an improvement in energy density and intake. FA can be used as an energy source for body tissues (except brain and erythrocytes); thus, they may spare the use of other fuels, resulting in a simultaneous increase of glucose and amino acids available for milk yield and protein synthesis(Reference de Souza, Preseault and Lock18). Additionally, feeding C16 : 0 might cause insulin resistance shifting glucose utilisation towards the mammary gland and increasing milk production(Reference Mathews, Rico and Sprenkle37). In contrast, PUFA, in particular n-3 FA, may increase hepatic expression of the gluconeogenic enzymes which would probably favour the synthesis of lactose in the mammary gland and, potentially, yield of milk(Reference Palmquist7,Reference Bilby, Jenkins and Staples41) . The constant DMI together with increased milk production in PW3 and PW6 resulted in a greater milk yield efficiency as compared with PAL or CON. Other studies have also found that PUFA supplements enhanced the efficiency of growth(Reference Ghasemi, Azad-Shahraki and Khorvash14) and milk yield(Reference Donovan, Schingoethe and Baer15). PUFA have critical roles in modulating cell function and metabolism and the action of hormones (e.g. insulin and IGF-I) independent of their energetic effects(Reference Jump6).
Milk fat content was improved significantly with PAL, whereas it remained unaffected in both PW3 and PW6. Fat sources with different FA profiles have been reported to affect positively or negatively milk fat content(Reference Rabiee, Breinhild and Scott3). Fat supplements can affect milk fat synthesis by providing FA precursors for milk fat and/or modulating the lipogenesis process in mammary glands. Approximately half of the FA esterified at sn-1 and sn-2 of milk TAG are medium- and long-chain SFA(Reference Koch and Lascano42). The increase in the availability of C16 : 0 with PAL may increase the activity of glycerol-3-phosphate acyltransferase esterifying FA at the sn-1 position, hence increasing milk fat synthesis(Reference Tzompa-Sosa, Van Aken and Van Hooijdonk43). On the other hand, FA mixtures provide PUFA which at low ruminal pH could partially be hydrogenated to intermediates such as trans-10 C18 : 1 or trans-10, cis-12 C18 : 2. These intermediates decrease de novo synthesis of milk FA(Reference Bauman and Griinari2) and may be the reason for the lowered milk fat content in the FA mixtures. The overall results suggest that the combination of C16 : 0 with either n-3 or n-6 FA may neutralise their negative effects on milk fat synthesis resulting in similar milk fat concentration between CON and FA mixtures. In our study, milk protein and lactose contents decreased with all FA-supplemented treatments. These changes in milk composition relating to fat supplementation have often been reported in dairy cows(Reference Rabiee, Breinhild and Scott3). Changes in protein percentage may result from changes in dietary protein and carbohydrate fraction rather than from a direct effect of FA. Reduction of degradable carbohydrates mainly starch as decreased in our study may lower milk protein percentage because of decreased ruminal available energy for microbial growth. Wu and Huber(Reference Wu and Huber44) speculated that reduction in milk protein percentage with FA feeding can be attributed to increased milk production, reduced somatotropin or even development of insulin resistance.
The PAL treatment did not affect milk FA concentration synthesised de novo (<16 C) but tended to decrease preformed FA and increase C16 : 0. Earlier studies(Reference Mosley, Mosley and Hatch16,Reference Piantoni, Lock and Allen17,Reference Mathews, Rico and Sprenkle37) have demonstrated that C16 : 0 supplement increases milk content of C16 : 0 (27–49 %) and decreases C18 : 1n-9 (9–18 %). However, the responses of short- and medium-chain FA to C16 : 0 supplement were different across the studies. In our study, PAL decreased the content of milk SCFA (C4 : 0) and increased that of medium-chain FA (C6 to C12). This is in general agreement with some(Reference Weiss and Pinos-Rodríguez39) but not all(Reference Mosley, Mosley and Hatch16,Reference Piantoni, Lock and Allen17) studies. As compared with PAL, the concentration of milk SFA tended to decrease and that of MUFA tended to increase following FA mixtures supplementation. Feeding n-3 or n-6 FA resulted in increased intake of relative FA which has previously been shown to increase the concentrations of these FA in body tissues(Reference Ebrahimi, Rajion and Jafari24,Reference Bilby, Jenkins and Staples41) and milk(Reference Donovan, Schingoethe and Baer15,Reference de Souza, Preseault and Lock18) . The supplementation of PAL combined with a low n-6:n-3 FA ratio had a tendency effect on milk n-3 FA, in which the highest concentration of n-3 FA (C20 : 5 and C22 : 6) and the lowest ratio of n-6:n-3 FA were observed in PW3. A lower ratio of n-6:n-3 FA is more desirable for reducing the risk of many chronic diseases such as atherosclerosis, hypertension, diabetes, autoimmune diseases and many cancers(Reference Simopoulos45). In terms of the health-related indices, the atherogenecity index tended to decrease and the hypocholesterolaemic:hypercholesterolaemic FA ratio tended to increase with feeding C16 : 0 supplement in combination of a low n-6:n-3 ratio. However, there were no differences between CON and PW3. Collectively, our results confirmed that PAL increased milk fat concentration but adversely affected milk FA profile as reported by others(Reference Piantoni, Lock and Allen17,Reference Weiss and Pinos-Rodríguez39) . Incorporation of specific FA into milk fat can help to prevent or promote atherosclerosis based upon their effects on LDL-cholesterol concentrations(Reference Ulbricht and Southgate46). Some FA, for example, n-3 PUFA, n-6 PUFA and MUFA are anti-atherogenic, while SFA such as C12 : 0, C14 : 0 and C16 : 0 FA are atherogenic.
Feeding PAL increased NEL intake (20·0 MJ/d) but led to more BW loss during the experiments (about −0·23 kg/d). Feeding PAL lowered energy availability for BW reserves by increasing energy partitioning towards milk fat yield (12%). Indeed, milk fat content is the most energetically expensive milk component to synthesise(25). However, approximately 60% of extra NEL intake with PAL could not be accounted for by increases in milk energy output. Decreases in BW gains or BW reserves were observed with PAL relative to other treatments in several studies(Reference Liu, VandeHaar and Lock10,Reference Piantoni, Lock and Allen17,Reference de Souza, Preseault and Lock18,Reference Bianchi, Macedo and Silva21,Reference Teh, Trung and Jia32,Reference Capper, Wilkinson and Mackenzie33) , but not all(Reference Mathews, Rico and Sprenkle37). Compared with CON, supplementation with PW3 and PW6 increased both energy intake and milk energy output to the same extent (5 %). However, compared with PAL, we observed feeding FA mixtures increased partitioning of energy towards body tissue gains instead of milk fat synthesis. These results agree with the studies of Liu et al. (Reference Liu, VandeHaar and Lock10) and Silvestre et al. (Reference Silvestre, Carvalho and Francisco13), who found that feeding n-6 or n-3 FA had positive effects on body reserves. Ruminants evolved to eat forage not concentrate diets which provide more intake of PUFA, in particular n-3 FA(25). This is while high-producing dairy cows are sometimes fed high concentrate diets and supplemented with PAL to increase milk fat concentration. The greater energy efficiency of cows fed FA mixtures, regardless of the ratio of n-6:n-3 FA, relative to PAL, can be a consequence of different FA intake. Feeding PW6 and PW3 increased intake of n-6 FA (66% in PW6 and 13% in PW3) and n-3 FA (16% in PW6 and 174% in PW3) in addition to C16 : 0 (130%). In goat, decreasing the dietary ratio of n-6:n-3 FA linearly increased back fat thicknesses but did not affect subcutaneous and intermuscular fat(Reference Bianchi, Macedo and Silva21). Although energy status did not differ between PW3 or PW6, the high intake of PUFA, especially with a low n-6:n-3 FA ratio, has the potential to alter metabolic status via modulating hormones(Reference Papadopoulos, Maes and van Weyenberg12) or gene expression(Reference Jump6). This, in turn, might decrease energy spilling (e.g. heat increment), as indicated in higher energy efficiency ((NE gain + NE milk/NEL intake)) in PW3 and PW6 as compared with PAL. Liu et al. (Reference Liu, VandeHaar and Lock10) reported that, compared with C16 : 0 supplement, n-6 PUFA feeding increased both plasma insulin and trans-10, cis-12 C18 : 2 concentrations and more energy towards body tissue gain instead of milk synthesis. Similarly, the abomasal infusion of C18 : 1n-9(Reference Laguna, Gonzalez and Prom47) or combination of C16 : 0 supplement with C18 : 1n-9(Reference de Souza, Preseault and Lock18) was shown to enhance adipose tissue insulin sensitivity and improved body reserves. Mathews et al. (Reference Mathews, Rico and Sprenkle37) reported that feeding C16 : 0 supplement results in increased plasma NEFA and insulin resistance and reduced glucose stimulated FA disappearance, as an indicator of increased lipolytic activity. In the present study, all FA-supplemented treatments increased the levels of blood lipids (TAG and total, HDL- and LDL-cholesterols). However, the FA profiles can be expected to differ for C16 : 0 supplement alone or in mixtures. Higher concentrations of n-3 and lower n-6:n-3 ratio in blood have been previously reported for cows fed sources of n-3 FA(Reference Petit23). After feeding, insulin enhances the activity of lipoprotein lipase which hydrolyses the TAG of chylomicron releasing NEFA. Therefore, the elevated concentration of NEFA with FA-supplemented treatments, in the present study, can be a consequence of the general effect of fats on chylomicron secretion and its mobilisation rather than lipolysis or negative energy balance because other indices (β-hydroxybutyrate and glucose) were not affected. The overall results indicate a preferential partitioning of PAL towards milk energy output, while PUFA addition appeared to favour energy partitioning towards BW gain. Future research will need to assess the dietary effects of long-term C16 : 0 supplement in comparison with n-6 and n-3 FA on BW and health at different lactation stages.
Conclusion
Fat supplements are added in diets of dairy cows as a means to increase milk yield, modulate concentrations of milk fat and FA profile, reduce excessive BW loss and improve health and fertility. The use of C16 : 0 supplement (2·4 % of diet) increased feed intake, milk yield and milk fat concentration. However, the overall changes resulted in decreases in BW and energy partitioning to body reserves. Our data showed no differences in lactation performance and energy partitioning with feeding mixtures of C16 : 0-enriched supplement and n-6 or n-3 FA or their ratios in high-producing dairy cows. However, regardless of the ratio of n-6:n-3 FA, the FA mixtures increased energy intake and milk yield but did not affect milk fat concentration compared with CON. Furthermore, the provision of n-6 or n-3 FA prevented BW loss without increasing feed intake which may be beneficial in view point of reproduction and feed efficiency. Lastly, to lower milk SFA, atherogenecity index and the ratio of milk n-6:n-3, an ideal supplement would appear to be a combination of palmitic acid with supplement containing more n-3 and less n-6 FA.
Acknowledgements
The authors are grateful to Dr Mehdi Dehghan-Banadaky (University of Tehran) for the provision of FA supplements, Dr Hamed Khalilvand (University of Urmia) for FA analysis and Saman Rashidi and the staff of the Isfahan University of Technology Lavark Teaching and Research Center for their assistance in this experiment.
This work was financially supported by Isfahan University of Technology (IUT, Isfahan, Iran) and Kimiya Danesh Alvand Co. (Tehran, Iran).
E. G. designed the experiment; A. P. and D. G. carried out the experiment; D. G., A. P. and E. G. analysed experimental samples and data; E. G. wrote the paper and has primary responsibility for the final content.
The authors have no conflicts of interest.










