According to the theory of biobehavioral synchrony, early caregiving influences the reorganization of mother and infant physiology and behavior (Feldman, Reference Feldman2012). The extent to which mother and infant regulate one another's physiology and behavior over time, or coregulate, is essential for infant adaptation (Feldman, Magori-Cohen, Galili, Singer, & Louzoun, Reference Feldman, Magori-Cohen, Galili, Singer and Louzoun2011). At age 3 months, infants begin to follow their mother's gaze, affect, touch, and vocal patterns (Feldman, Reference Feldman2007); at age 6 months, mother-infant interactions become truly reciprocal (Feldman, Greenbaum, & Yirmiya, Reference Feldman, Greenbaum and Yirmiya1999). Importantly, these coordinated regulatory processes between mother and infant lay the foundation for the development of infants’ stress regulation systems (Feldman, Reference Feldman2007). Specifically, the parasympathetic nervous system plays a central role in regulating stress and helps maintain biological homeostasis during rest as well as facilitates optimal arousal under conditions of stress (Beauchaine, Reference Beauchaine2001). It can be measured by respiratory sinus arrhythmia (RSA) – an index of parasympathetic nervous system activity that reflects the amount of variability in heart rate associated with the respiration rate (Porges, Reference Porges2007).
Studies have shown that RSA is a key transdiagnostic marker of risk and is implicated in self-regulation and the development of later psychopathology (Beauchaine, Reference Beauchaine2015; Beauchaine & Thayer, Reference Beauchaine and Thayer2015; Patriquin, Lorenzi, Scarpa, Calkins, & Bell, Reference Patriquin, Lorenzi, Scarpa, Calkins and Bell2015). During infancy, RSA has been associated with temperamental, behavioral, cognitive, and affective outcomes (Fox, Henderson, Rubin, Calkins, & Schmidt, Reference Fox, Henderson, Rubin, Calkins and Schmidt2001; Haley & Stansbury, Reference Haley and Stansbury2003; Whedon, Perry, Calkins, & Bell, Reference Whedon, Perry, Calkins and Bell2018). Although a large body of research has examined infants’ RSA in the context of early caregiving, knowledge is very limited with respect to understanding the extent to which high-risk caregiving may confer vulnerability for poorer physiological coordination between infants and their mothers. Therefore, the aim of this study was to examine mother–infant coregulation of RSA in relation to factors that may reflect adverse caregiving environments, such as those characterized by child maltreatment and instability in night-to-night sleep patterns of mothers and infants. Specifically, this study advances the knowledge base regarding the Research Diagnostic Criteria (RDoC) framework developed by the National Institutes of Health by investigating dimensional systems that may be implicated in the development of psychopathology across multiple levels of analysis (i.e., physiological and behavioral). Understanding how child maltreatment risk and sleep patterns relate to mother and infant coordination of physiological regulatory processes may help to identify modifiable health-risk factors that could be targeted in future interventions to prevent maladjustment and promote overall health and development in families.
Mother–infant RSA coregulation
During the first year of life, infants experience a progressive change in their ability to regulate biobehavioral states through interactions with their caregivers (Porges & Furman, Reference Porges and Furman2011). Porges’ polyvagal theory (Porges, Reference Porges2007) postulates that vagal regulation of the heart (indexed by RSA) is an important substrate of self-regulatory and social engagement skills. Specifically, vagal tone is associated with social processes that assist individuals in maintaining optimal arousal via parasympathetic influence on cardiac functioning (Porges, Reference Porges2007). Normative patterns of self-regulation show higher RSA or RSA increases (augmentation), contributing to a slowing down of heart rate, during periods of rest or in the absence of stress, and show lower RSA or RSA decreases (withdrawal) when there is a need to actively regulate or cope under conditions of stress (Porges, Reference Porges2007). These patterns are theorized to reflect better self-regulation and social functioning (Thayer & Lane, Reference Thayer and Lane2000). In contrast, lower RSA during periods of rest or higher RSA in the context of stress are implicated in poorer regulation and maladaptive responding during social interaction (Patriquin et al., Reference Patriquin, Lorenzi, Scarpa, Calkins and Bell2015; Porges, Reference Porges2007; Thayer & Lane, Reference Thayer and Lane2000). For example, the still-face procedure is a common experimental task used to observe mother and infant physiological and behavioral regulation during social engagement and disengagement (Tronick, Als, Adamson, Wise, & Brazelton, Reference Tronick, Als, Adamson, Wise and Brazelton1978). Across several studies (e.g., Bazhenova, Plonskaia, & Porges, Reference Bazhenova, Plonskaia and Porges2001; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996), the normative pattern for infants is higher RSA during interactive episodes when mothers provide sensitive and responsive caregiving and infants can rely on their mother for structure and support, and lower RSA during the still-face episode when a stressor – mother disengagement – is introduced. Mothers similarly show higher RSA during interactive episodes and lower RSA when parenting under stress, though findings have been mixed (Skowron et al., Reference Skowron, Loke, Gatzke-Kopp, Cipriano-Essel, Woehrle, Van Epps and Ammerman2011, Reference Skowron, Cipriano-Essel, Benjamin, Pincus and Van Ryzin2013).
During dyadic interactions, research demonstrates that mother and infant RSA are interrelated. Sensitive and well-regulated caregiving behaviors are key functions implicated in attachment theory (Bowlby, Reference Bowlby1969) in which mothers are responsible for providing a safe and secure base for the infant. Thus, a goal of early mother-infant interactions is to engage in coordinated regulation so that infants can develop their own regulatory skills needed to cope with social perturbations or stressful conditions (Conradt & Ablow, Reference Conradt and Ablow2010). With regard to normative patterns of parasympathetic synchrony, mothers and infants show positive coordination of RSA during face-to-face interaction, or concordance of RSA coregulation over time (Feldman et al., Reference Feldman, Magori-Cohen, Galili, Singer and Louzoun2011). Specifically, mothers and infants show increases in RSA concordance during positive dyadic interactions (Feldman et al., Reference Feldman, Magori-Cohen, Galili, Singer and Louzoun2011; Lunkenheimer, Tiberio, Lucas-Thompson, Boker, & Timpe, Reference Lunkenheimer, Tiberio, Lucas-Thompson, Boker and Timpe2015). However, some research suggests there are also differences in individual and dyadic mother–infant regulation among dyads with higher familial risk, which may reflect disrupted regulatory processes in mothers and infants in the context of adverse caregiving environments.
Child maltreatment and regulatory processes
Among the most severe forms of adverse caregiving environments are those characterized by child maltreatment risk and incidence. Early stressful mother-infant interactions associated with child maltreatment may contribute to infants’ altered biobehavioral regulatory systems, which in turn has serious, long-term implications for poor child health and developmental outcomes (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001; Martinez-Torteya et al., Reference Martinez-Torteya, Dayton, Beeghly, Seng, McGinnis, Broderick and Muzik2014; Wismer Fries, Shirtcliff, & Pollak, Reference Wismer Fries, Shirtcliff and Pollak2008). Likewise, impairments in mothers’ ability to self-regulate in the caregiving role is a known risk factor for child maltreatment (Henschel, de Bruin, & Möhler, Reference Henschel, de Bruin and Möhler2014; Holden, Coleman, & Schmidt, Reference Holden, Coleman and Schmidt1995; Skowron, Kozlowski, & Pincus, Reference Skowron, Kozlowski and Pincus2010). For example, mothers at risk of harsh parenting show difficulties in regulating their emotions and behavior as well as greater physiological reactivity (Deater-Deckard, Sewell, Petrill, & Thompson, Reference Deater-Deckard, Sewell, Petrill and Thompson2010; Joosen, Mesman, Bakermans-Kranenburg, & van IJzendoorn, Reference Joosen, Mesman, Bakermans-Kranenburg and van IJzendoorn2013). Therefore, examining the coordination of regulatory processes between mothers and infants may be one critical way to understand the etiology of stressful mother-infant interactions and prevent child maltreatment and its health and developmental consequences.
A growing literature suggests that disruptions in the mother–child relationship due to child maltreatment are associated with impairments in mother–child biobehavioral coregulation. Mothers with higher risk of maltreatment are more likely to show physiological miscoordination with their child than mothers without such risk (Skowron et al., Reference Skowron, Kozlowski and Pincus2010). On the whole, researchers have found that maltreating mother–child dyads show no or weakened concordance in RSA coregulation over time (Creaven, Skowron, Hughes, Howard, & Loken, Reference Creaven, Skowron, Hughes, Howard and Loken2014; Giuliano, Skowron, & Berkman, Reference Giuliano, Skowron and Berkman2015; Lunkenheimer, Busuito, Brown, & Skowron, Reference Lunkenheimer, Busuito, Brown and Skowron2018; Skowron, Cipriano-Essel, Benjamin, Pincus, & Van Ryzin, Reference Skowron, Cipriano-Essel, Benjamin, Pincus and Van Ryzin2013). For example, Giuliano et al. (Reference Giuliano, Skowron and Berkman2015) found that mother–child dyads characterized by maltreatment showed weakened positive synchrony during a joint puzzle task. Similarly, Lunkenheimer et al. (Reference Lunkenheimer, Busuito, Brown and Skowron2018) reported that, during dyadic tasks, non-maltreating dyads showed positive RSA concordance, but this relationship was not found in dyads with maltreatment.
However, the coregulation of RSA between mother and child may also differ by specific dimensions of child maltreatment, such as maltreatment subtype and severity. For example, it has been shown that physically abusive mother–child dyads demonstrate positive concordance of RSA coregulation overall, whereas neglectful dyads show no association between mother and infant RSA over time (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). Moreover, as physical abuse severity increases, it is associated with lower mean child RSA and a mother-driven dyadic stress response, with mother RSA predicting declines in child RSA (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). In contrast, higher neglect severity is associated with higher mean child RSA and a child-driven dyadic stress response, with child RSA predicting declines in mother RSA (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). Thus, higher severity of child maltreatment is associated with greater discordance in RSA. In sum, the findings tend to show weaker, absent, or discordant coregulation of RSA in maltreating mother–child dyads, but differences may exist depending on the extent and nature of exposure to maltreating behaviors. However, it should also be noted that all of the existing research on RSA coregulation in the context of child maltreatment has been conducted with mothers and preschool-aged children; research is thus needed to determine whether and how child maltreatment influences RSA coregulation in infancy.
Sleep variability and regulatory processes
Infants at risk of maltreatment and their caregivers often live in highly stressful and unpredictable contexts that may differentially impact their daily functioning. Sleep is one process that co-develops with cardiovascular and respiratory parameters (Dahl, Reference Dahl1996; Feldman, Eidelman, Sirota, & Weller, Reference Feldman, Eidelman, Sirota and Weller2002; Groome, Swiber, Atterbury, Bentz, & Holland, Reference Groome, Swiber, Atterbury, Bentz and Holland1997) and may be disrupted in families with high risk, such as those characterized by maltreatment or other household challenges (Alkon, Boyce, Neilands, & Eskenazi, Reference Alkon, Boyce, Neilands and Eskenazi2017). Sleep problems, including insufficient sleep, difficulties falling asleep, and frequent nighttime awakenings, have been reported in infants and young children who have experienced maltreatment (Hash, Oxford, Ward, Fleming, & Spieker, Reference Hash, Oxford, Ward, Fleming and Spieker2020; Zajac et al., Reference Zajac, Prendergast, Feder, Cho, Kuhns and Dozier2020) as well as in adults with a history of adversity (Chapman et al., Reference Chapman, Liu, Presley-Cantrell, Edwards, Wheaton, Perry and Croft2013; Greenfield, Lee, Friedman, & Springer, Reference Greenfield, Lee, Friedman and Springer2011). Given that children's sleep can disrupt parents’ sleep and vice versa, maladaptive parenting and the home environment may contribute to sleep problems in both mothers and infants.
Although the day–night circadian patterning of sleep is often consolidated at about 6 months of age (i.e., infants are sleeping more consistently throughout the night) (Galland, Taylor, Elder, & Herbison, Reference Galland, Taylor, Elder and Herbison2012; Henderson, France, Owens, & Blampied, Reference Henderson, France, Owens and Blampied2010), contextual factors that may disrupt sleep in households with more challenges, such as inconsistent routines, sleeping arrangements, or maternal responses to infant cues, may place families at greater risk of sleep problems (Taylor, Donovan, & Leavitt, Reference Taylor, Donovan and Leavitt2008). Much of the extant literature has used average sleep parameters to characterize sleep disturbance in vulnerable populations (Becker, Sidol, Van Dyk, Epstein, & Beebe, Reference Becker, Sidol, Van Dyk, Epstein and Beebe2017). However, emerging research denotes that unpredictable and variable sleep not only reflects adverse caregiving environments, but may serve as an early risk marker for later psychopathology. For example, irregular sleep patterns, or night-to-night sleep variability, are associated with lower socioeconomic status, less regulated households, worse daytime functioning, negative mood, and depression (Acebo et al., Reference Acebo, Sadeh, Seifer, Tzischinksky, Hafer and Carskadon2005; Bagley, Kelly, Buckhalt, & El-Sheikh, Reference Bagley, Kelly, Buckhalt and El-Sheikh2015; Bei, Wiley, Trinder, & Manber, Reference Bei, Wiley, Trinder and Manber2016; Kjeldsen et al., Reference Kjeldsen, Hjorth, Andersen, Michaelsen, Tetens, Astrup and Sjodin2014; Meltzer, Sanchez-Ortuno, Edinger, & Avis, Reference Meltzer, Sanchez-Ortuno, Edinger and Avis2015; Suh et al., Reference Suh, Nowakowski, Bernert, Ong, Siebern, Dowdle and Manber2012).
Variability in sleep is also associated with adjustment and self-regulation difficulties (Bates, Biken, Alexander, Beyers, & Stockton, Reference Bates, Biken, Alexander, Beyers and Stockton2002; Scher, Hall, Zaidman-Zait, & Weinberg, Reference Scher, Hall, Zaidman-Zait and Weinberg2010; Ward, Gay, Alkon, Anders, & Lee, Reference Ward, Gay, Alkon, Anders and Lee2008). For example, Molfese et al. (Reference Molfese, Rudasil, Prokasky, Champagne, Holmes, Molfese and Bates2015) examined relations between sleep characteristics and temperament in toddlers and found that higher variability in nighttime sleep is associated with lower soothability, which may reflect difficulties in children's capacity to regulate. Moreover, disturbances in physiological regulation have been found in infants and toddlers with more variable sleep, such that higher sleep variability is linked to higher morning cortisol levels and a steeper change in cortisol levels from bedtime to awakening (Scher et al., Reference Scher, Hall, Zaidman-Zait and Weinberg2010). With regard to parasympathetic regulation, in particular, El-Sheikh, Erath, and Bagley (Reference El-Sheikh, Erath and Bagley2013) found that older children with more disrupted sleep (e.g., more frequent long wake episodes) have lower resting RSA and lower RSA during challenge, suggesting poorer physiological regulation among children with disrupted sleep. While extant research demonstrates that sensitive parenting has been shown to help regulate infant sleep–wake organization (Sadeh, Tikotzky, & Scher, Reference Sadeh, Tikotzky and Scher2010) and infants are able to fall and stay asleep more easily when they experience consistent care (Teti, Kim, Mayer, & Countermine, Reference Teti, Kim, Mayer and Countermine2010), less is known about the influence of variable sleep patterns on the dynamic coupling of mother and infant physiological regulation. Moreover, although infant sleep has been shown to predict mother–infant behavioral synchrony in normative samples (de Graag, Cox, Hasselman, Jansen, & De Weerth, Reference de Graag, Cox, Hasselman, Jansen and De Weerth2012; Feldman, Reference Feldman2006), to our knowledge no studies have examined the relationship between sleep variability in relation to RSA coregulation among mother–infant dyads with high familial risk.
The current study
Given that mother and infant RSA may serve as a transdiagnostic marker of developmental and health risk (Beauchaine & Thayer, Reference Beauchaine and Thayer2015), the overall objective of this study was to understand how contextual factors that may reflect adverse caregiving environments, such as child maltreatment and variability in mother and infant sleep, may impact dynamic relations between mother and infant RSA across time. The goals of the current study were twofold. First, we examined whether higher levels of maltreatment severity influenced mother–infant RSA coregulation in dyads oversampled for high familial risk, thereby extending existing research examining RSA synchrony between maltreating mothers and their young children (e.g., Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018; Skowron et al., Reference Skowron, Cipriano-Essel, Benjamin, Pincus and Van Ryzin2013). Second, we examined whether mother and infant night-to-night sleep variability influenced mother–infant RSA coregulation, which, to our knowledge, has yet to be examined among high-risk mother–infant dyads. We hypothesized that mother–infant dyads with higher levels of self-reported maltreatment severity would show weakened, absent, or discordant coregulation of RSA. However, given the limited research on sleep variability in relation to mother and infant RSA synchrony, we made no specific hypotheses about the nature of differences in mother–infant RSA coregulation by mother and infant night-to-night sleep variability.
Method
Participants
Families involved with public welfare and departments of human services in the rocky mountain region of the United States were recruited by the research team to participate in a longitudinal study aimed at examining parent–child coregulation of sleep and physiology among families experiencing adversity. The data used in the present study were collected from two cohorts of families at baseline. Both English- and Spanish-speaking families with an infant between the ages of 6 and 14 months were invited to participate in the study. The participants were 47 mother–infant dyads. Of the 59 mother–infant dyads initially screened and eligible for the study, 12 mother–infant dyads did not complete assessments on maltreatment severity and cardiac physiology due to administrative or technological error (n = 7) or the infant becoming too fussy during the visit (n = 5). Two mothers chose to complete the study questionnaires in Spanish, which were administered by a bilingual and bicultural research assistant.
The sample characteristics of the final 47 mother–infant dyads are shown in Table 1. The sample was low-income, with approximately 85% receiving financial assistance (e.g., Temporary Assistance for Needy Families; Women, Infants, and Children). Mothers ranged in age from 17 to 40 years (M = 29.82, SD = 6.33) and identified as White (51.1%), Hispanic/Latinx (21.2%), Black/African American (12.8%), American Indian/Alaskan Native (6.4%), more than one race (6.4%), or Asian (2.1%). The majority of mothers were married or partnered (59.5%) and roughly half had a high school diploma/general educational diploma or below level of education (44.7%). On average, there were approximately two children in the household. Infants were 6–14 months old (M = 8.47, SD = 2.39) and were equally identified as male (51.1%) or female (48.9%). The majority of infants were identified as White (38.3%), followed by more than one race (27.7%), Hispanic/Latinx (19.1%), Black/African American (12.8%), and American Indian/Alaskan Native (2.1%).
Table 1. Mother and infant sample characteristics (N = 47)

Note: Sleep duration was calculated by averaging actigraphy and sleep diary data over seven consecutive 24-hr periods. RSA = respiratory sinus arrhythmia.
Procedure
Families participated in two home-based visits over a 1-week period. During the first visit, families completed assessments that captured mother and infant sociodemographic characteristics, sleep behaviors, experiences of adversity, maltreating behaviors, and infant development. In addition, mothers and infants were fitted with an actigraph (Motionlogger Micro Watch, Ambulatory Monitoring Inc., Ardsley, NY). Between the two home-based visits, mothers and infants wore an actigraph for seven consecutive days to collect data on sleep onset (sleep start time), sleep offset (sleep end time), and sleep duration (sleep onset to sleep offset). During the second visit approximately 1 week later, mothers and infants participated in a semi-structured still-face procedure in which cardiac physiological data were collected for 8 min. Specifically, infants were placed in a portable infant booster seat across from their mother. Mothers and infants sat across from each other for a 2-min baseline and then mothers were asked to play with their infants with toys for 2 min (face-to-face play episode). Mothers were then instructed to keep a still, expressionless face while looking at their infants and refrain from smiling, vocalizing, or touching their infants for 2 min (still-face episode). Finally, the last condition involved a reunion in which mothers were instructed to resume normal play and interaction with infants for 2 min (reunion episode). If the infant became fussy for more than 20 s during the procedure, the interaction was stopped, and the infant was soothed by their mother. The interaction was stopped for two families due to infant distress and physiological data were subsequently missing for these families following the initial face-to-face play episode. Families were excluded from the study if infants had a chronic illness or developmental disorder that would interfere with sleep or physiological assessment.
Measures
Child maltreatment
Child maltreatment presence and severity were measured from maternal reports of maltreatment behaviors toward her child on the neglect, physical abuse, and psychological aggression items of the Conflict Tactics Scale Parent–Child version (CTSPC) (Straus, Hamby, Finkelhor, Moore, & Runyan, Reference Straus, Hamby, Finkelhor, Moore and Runyan1998). The CTSPC assesses the presence and severity (0 = this never happened, 1 = 1 time, 2 = 2 times, 3 = 3–5 times, 4 = 6–10 times, 5 = 11–20 times, 6 = more than 20 times, 7 = not in the past year, but it happened before) of disciplinary actions used by a parent with their children during the last 12 months. To capture the incidence of maltreatment in the past year, response options for each item were recoded, as recommended by Straus et al. (Reference Straus, Hamby, Finkelhor, Moore and Runyan1998), using the midpoint of ranges and a value of 25 for responses of “more than 20 times” (1 = 1, 2 = 2, 3 = 4, 4 = 8, 5 = 15, and 6 = 25). Responses of “this never happened” and “not in the past year, but it happened before” were recoded as 0. The five items for neglect, 13 items for physical abuse, and five items for psychological aggression were then summed to create a composite maltreatment severity scale. Example items included: In the past year, how often have “you spanked your child on the bottom with your bare hand” (physical abuse), “you burned or scalded your child on purpose” (physical abuse), “you called your child dumb or lazy or some other name like that” (psychological aggression), “you said you would send your child away or kick him or her out of the house” (psychological aggression), “you were not able to make sure your child got the food he or she needed” (neglect), or “you were so drunk or high that you had a problem taking care of your child” (neglect). Mothers’ maltreatment scores ranged from 0 (14 families) to 67, with higher scores indicating more severe maltreating behaviors. Cronbach's alpha for the maltreatment scale was .74.
Sleep patterns
Sleep patterns were measured from activity-based sleep monitoring (actigraphy) and daily sleep diaries. Specifically, mothers wore a Motionlogger actigraph on their non-dominant wrist and infants wore a Motionlogger actigraph on their ankle for seven consecutive 24-hr periods. Actigraphy data were recorded in 1-min epochs (Action-W software; Ambulatory Monitoring Inc., 2002). Sleep duration variables were measured in minutes between scored sleep onset (sleep start time) and sleep offset (sleep end time), and subsequently computed into hours for each 24-hr period. Action-W software was used to score the data based on a validated sleep–wake scoring algorithm for infants (Sadeh, Acebo, Seifer, Aytur, & Carskadon, Reference Sadeh, Acebo, Seifer, Aytur and Carskadon1995) and adults (Cole, Kripke, Gruen, Mullaney, & Gillin, Reference Cole, Kripke, Gruen, Mullaney and Gillin1992). Mothers also kept a daily sleep diary used to compute sleep duration when actigraphy data was missing due to noncompliance. As such, 7 days of sleep data were scored for 80.9% (n = 38) of mothers and 89.4% (n = 42) of infants, 6 days of sleep data were scored for 17.0% (n = 8) of mothers and 10.6% (n = 5) of infants, and 5 days of sleep data were scored for 2.1% (n = 1) of mothers.
RSA
During the still-face procedure, mother and infant cardiac physiology was measured with the Bittium Faros Sensor 180, which is a portable, wearable, and externally applied electrocardiograph (ECG) recorder and wireless transmitter for ECG measurement and R–R interval data measurement. Three disposable wet gelled pediatric and adult electrodes were placed in a three-lead position with one-channel on the distal ends of the right and left clavicle and lower left abdomen for infants and mothers, respectively. Heart rate data were measured with an ECG sampling frequency of 1000 Hz.
Three programs were used to synchronize, edit, and analyze the ECG data for cardiac rhythms. First, CardioPeak and Segmenter software (Brain-Body Center for Psychophysiology and Bioengineering, 2019a) was used to extract the R–R peaks from the ECG data and segment the data by condition. Second, CardioEdit software (Brain-Body Center, 2007) was used to review and edit the resulting heart period (interbeat interval) files for artifacts. Editing consisted of adjusting incorrect R-peak identifications and/or integer arithmetic (i.e., dividing intervals between heart beats when detections of R-peak from the ECG were missed or adding intervals when spuriously invalid detections occurred). Third, CardioBatch Plus Synchrony software (Brain-Body Center for Psychophysiology and Bioengineering, 2019b) was used to analyze the synchronized edited interbeat interval files between mother–infant dyads. RSA was calculated using the Porges–Bohrer method (Porges, Reference Porges1985), which quantifies the amplitude of RSA with age-specific parameters that are sensitive to the maturational shifts in the frequency of spontaneous breathing.
The Porges–Bohrer method, as applied in CardioBatch Plus Synchrony, includes the following steps: (a) R–R intervals are timed to the nearest millisecond to produce a time series of sequential heart periods; (b) sequential heart periods are both resampled to 5 Hz to maintain synchrony within the dyad when producing time-based data; (c) the time-based series is detrended by a cubic moving polynomial (53-point for adults; 21-point for infants) (Porges & Bohrer, Reference Porges, Bohrer, Cacioppo and Tassinary1990) that is stepped through the data to create a smoothed template and the template is subtracted from the original time-based series to generate a detrended residual series; (d) the detrended time series is bandpassed to extract the variance in the heart period pattern associated with spontaneous breathing (0.12–0.40 Hz for adults; 0.3–1.3 Hz for infants); (e) the natural logarithm of the variance of the bandpassed time series is calculated as the measure of the amplitude of RSA (Riniolo & Porges, Reference Riniolo and Porges1997). These procedures are statistically equivalent to frequency domain methods (i.e., spectral analysis) for calculation of the amplitude of RSA when heart period data are stationary (Porges & Byrne, Reference Porges and Byrne1992). RSA and heart period were quantified during each sequential 30-s epoch within each condition of the still-face procedure. Due to the additional data required by the Porges–Bohrer method, only the first 90 s of data (i.e., the first three complete 30-s epochs) in each condition were used in analyses. Thus, physiological data used in this study were available for a total of 6 min of dyadic interaction.
Analytic approach
First, the variables were assessed for normality, and descriptive statistics were calculated for mother and infant sample characteristics. Outliers were identified on the CTSPC. Thus, in order to preserve the sample and minimize the influence of an extreme outlier (n = 1), the value was replaced by the next lower value plus one, following the statistical approach recommended by Winsor (Dixon & Yuen, Reference Dixon and Yuen1974).
Second, because sleep onset and offset differed each night across families, we calculated mothers’ and infants’ night-to-night sleep variability to obtain an index of sleep instability, which has been shown to be a robust predictor of poor health outcomes (e.g., Meltzer et al., Reference Meltzer, Sanchez-Ortuno, Edinger and Avis2015; Suh et al., Reference Suh, Nowakowski, Bernert, Ong, Siebern, Dowdle and Manber2012). Specifically, we computed a mean square successive difference (MSSD) (Jahng, Wood, & Trull, Reference Jahng, Wood and Trull2008) by calculating differences among adjacent sleep observations within the same participant (e.g., infant sleep duration for Night 2 minus infant sleep duration for Night 1, infant sleep duration for Night 3 minus infant sleep duration for Night 2, etc.). To account for negative values, these differences were squared and subsequently averaged. Mothers’ and infants’ MSSDs were used as predictors in the following analyses.
Third, separate multilevel coupled autoregressive models were conducted to examine differences in mother–infant coregulation of RSA by maltreatment severity and by mothers’ and infants’ night-to-night sleep variability, which varied as a function of time (RSA; Level 1) and person (maltreatment severity, mothers’ night-to-night sleep variability, or infants’ night-to-night sleep variability; Level 2). Models specifying the effects of mother-on-infant and infant-on-mother were performed separately. Models addressed how mother and infant mean RSA, self-regulation of RSA (the prediction of one's current RSA from one's own previous RSA at 30-s lags – i.e., RSA at time t regressed on RSA at time t − 1 in the time series), concurrent coregulation in RSA (i.e., prediction of one's current RSA from the partner's concurrent RSA), and lagged coregulation in RSA at a 30-s lag (i.e., prediction of one's current RSA from the partner's previous RSA at a 30-s lag) were predicted by (a) maltreatment severity, (b) mothers’ night-to-night sleep variability (MSSD), and (c) infants’ night-to-night sleep variability (MSSD). Thus, in total, six analytic models were conducted. Missing data were managed using full information maximum likelihood estimation, which accommodates missing data by estimating each parameter using all data from incomplete and complete cases (Enders, Reference Enders2010). Although the sample size of 47 was relatively small, statistical power was robust given that there were 498 person-by-time observations in each model (Maxwell & Delaney, Reference Maxwell and Delaney2004).
Results
Child maltreatment severity and mother–infant RSA
We examined whether mothers’ and infants’ mean RSA, self-regulation, and coregulation of RSA were predicted by differences in maltreatment severity (Table 2). Model intercepts indicated that there were positive effects of one's own mean RSA and self-regulation of RSA (autoregressive effects – i.e., the effect of one's RSA at time t regressed on one's RSA at t − 1) on current RSA in both mothers and infants. The Level 1 effects of coregulation of RSA were not significant, indicating that there were no time-dependent relations between mother and infant RSA on average in the sample overall (no concordance). In addition, maltreatment severity was not a significant predictor of mother or infant self-regulation of RSA. However, at higher levels of maltreatment severity, a different pattern emerged for RSA coregulation. Higher maltreatment severity was associated with weakened concordance in coregulation. Specifically, at a one-unit increase in maltreatment severity, the parameter for RSA coregulation was adjusted by b = −.031 (going from b = .037 to b = .006) for mother-to-infant concordance and adjusted by b = −.047 (going from b = .052 to b = .005) for infant-to-mother concordance. In other words, higher maltreatment severity was associated with a particular pattern of weaker concordance via the combination of higher mother RSA with lower infant RSA. This pattern suggests that the higher the maltreatment severity, the more divergent their RSA, specifically such that infants are more distressed or challenged while mothers are less distressed. Figure 1 shown as an illustration of differences in model-predicted individual mother and infant RSA over time by maltreatment severity. As shown in Figure 1, increases in mothers’ RSA were related to declines in infants’ RSA and, in turn, decreases in infants’ RSA were related to increases, albeit slight, in mothers’ RSA on average over the course of interaction. This weakening of RSA concordance in relation to infant declines in RSA was more pronounced during the interactive episodes between mother and infant.

Figure 1. Differences in respiratory sinus arrhythmia (RSA) coregulation as a function of maltreatment severity in mother–infant dyads: “above Avg maltreatment” and “below Avg maltreatment” refer to values plus and minus one standard deviation of maltreatment severity, respectively. Minutes: 0.5−1.5 = baseline, 2.0−3.0 = face-to-face play episode, 3.5−4.5 = still-face episode, and 5.0−6.0 = reunion episode.
Table 2. Effects of child maltreatment severity
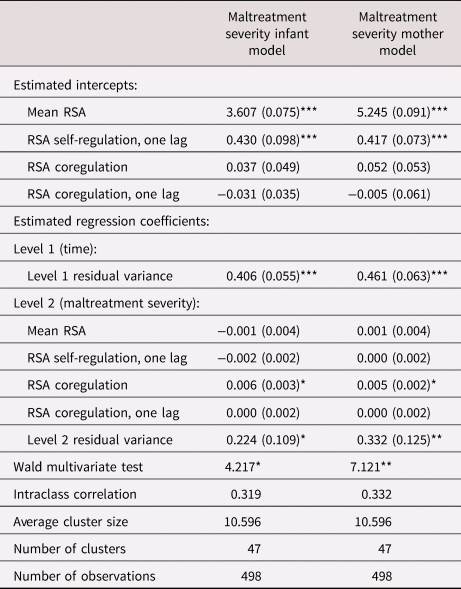
Note: RSA = respiratory sinus arrhythmia. Values in table denote b (SE) unless noted otherwise.
* p < .05, **p < .01, ***p < .001.
Night-to-night sleep variability and mother–infant RSA
We examined whether mothers’ and infants’ mean RSA, self-regulation, and coregulation of RSA were predicted by differences in mothers’ and infants’ night-to-night sleep variability (Table 3). For models including infants’ night-to-night sleep variability, the model intercepts indicated that there were positive effects of one's own mean RSA and self-regulation of RSA on current RSA in both mothers and infants. Once again, the Level 1 effects of coregulation of RSA were not significant, suggesting no RSA concordance in the sample overall in the absence of a predictor. Further, infant night-to-night sleep variability was not a significant predictor of mother or infant self-regulation of RSA. However, there were significant effects of higher levels of infant night-to-night sleep variability on both infant mean RSA levels and mother–infant RSA concordance. With respect to mean levels, higher infant sleep variability was associated with infants’ overall lower mean RSA levels during interaction with mothers, suggesting greater distress (Figure 2). In addition, higher infant night-to-night sleep variability was associated with concordance in lagged RSA coregulation with mothers at a 30-s lag. Specifically, at a one-unit increase in infant night-to-night sleep variability, the parameter for mother-to-infant lagged RSA coregulation was adjusted by b = .037, going from b = −.043 to b = .006. Thus, the coregulation parameter became positive at higher levels of infant night-to-night sleep variability, which could indicate mother and infant were concordant in showing jointly higher or jointly lower RSA; in this case, higher sleep variability was associated with a pattern whereby, during interaction, mothers’ lower RSA in one time unit predicted infants’ lower RSA in the next time unit. Accordingly, over and above the effect of infant sleep variability on infants’ overall lower mean RSA, sleep variability was also associated with mothers’ distress or challenge prompting greater infant distress during the face-to-face interaction. Figure 2 shows an illustration of differences in model-predicted individual mother and infant RSA over time by infant's night-to-night sleep variability. As shown in Figure 2, decreases in mothers’ RSA were actively driving subsequent decreases in infants’ RSA on average over the course of the interaction, demonstrating stronger RSA concordance in mother–infant dyads specifically in relation to interrelated RSA withdrawal.

Figure 2. Differences in respiratory sinus arrhythmia (RSA) coregulation as a function of infants’ night-to-night sleep variability in mother–infant dyads: “above Avg infant night-to-night variability” and “below Avg infant night-to-night sleep variability” refer to values plus and minus one standard deviation of infant night-to-night sleep variability, respectively. Minutes: 0.5−1.5 = baseline, 2.0−3.0 = face-to-face play episode, 3.5−4.5 = still-face episode, and 5.0−6.0 = reunion episode.
Table 3. Effects of mothers’ and infants’ night-to-night sleep variability

Note: RSA = respiratory sinus arrhythmia. Values in table denote b (SE) unless noted otherwise.
*p < .05, **p < .01, ***p < .001.
For models including mothers’ night-to-night sleep variability, the model intercepts indicated that there were positive effects of one's own mean RSA and self-regulation of RSA on current RSA in both mothers and infants. However, the Level 1 effects of coregulation of RSA were not significant. In addition, the Level 2 predictor of mothers’ night-to-night sleep variability was not associated with mean RSA, self-regulation, or coregulation of RSA.
Discussion
The purpose of this study was to understand how contextual factors that may reflect adverse caregiving environments, such as child maltreatment and sleep variability, may impact maternal and infant regulatory processes. The findings suggest that maltreatment severity and night-to-night sleep variability are associated with dynamic dyadic mother–infant coregulation of RSA. The findings support prior evidence that RSA concordance was not found in families at risk for child maltreatment overall (Creaven et al., Reference Creaven, Skowron, Hughes, Howard and Loken2014), but that when higher child maltreatment severity was accounted for, it was associated with weakened RSA concordance (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). Thus, the present study replicated prior findings but extended them to infancy, suggesting that disruptions to mother–child RSA coregulation as a function of child maltreatment may begin as early as 6 months of age. The findings also indicate that the risk factor of infant sleep variability was related to positive concordance in the form of mothers’ RSA withdrawal driving infants’ RSA withdrawal, suggesting that infant sleep variability may be an important predictor of interrelated mother and infant distress.
Although extant research in community samples suggests that concordant mother–infant coregulation is essential to support infant biobehavioral regulatory development, our findings show that concordant regulatory processes in mothers and infants with high familial risk may not necessarily signify adaptation. Of note, these findings advance research with respect to the RDoC framework by elucidating important regulatory processes in mother–infant dyads that may be shaped by key health-risk behaviors, such as sleep instability and adverse caregiving, to differentially impact adaptation versus maladaptation. Given that regulatory processes often underlie multiple levels of functioning identified in the RDoC framework, these processes may act as a critical and effective focus for the etiology, prevention, and intervention of later health and developmental outcomes. This study is also situated within a developmental psychopathology perspective (e.g., Cicchetti, Reference Cicchetti2016) such that the developmental stage of the child as well as the nature and severity of familial risk factors are important to consider when understanding parasympathetic coregulation and implications for mother and infant outcomes. Therefore, this study offers preliminary evidence of early behavioral correlates of regulation that may be used in future research to understand both the etiological pathways of later psychopathology and potential targets for early intervention.
Child maltreatment severity disrupts mother–infant RSA coregulation
Consistent with prior research and our first hypothesis, we found that child maltreatment disrupted mother–infant RSA coregulation. Specifically, higher maltreatment severity was associated with weakened concordance in RSA coregulation. These findings align with prior research showing decreases in positive synchrony over time between maltreating mothers and preschool-aged children (Giuliano et al., Reference Giuliano, Skowron and Berkman2015). In addition, they replicate findings regarding weaker concordance or discordance when mother–child RSA coregulation is examined in relation to maltreatment severity (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). For example, mother–child dyads with higher maltreatment severity show greater discordance in RSA in relation to rupture and repair processes in face-to-face interaction, such that RSA trajectories of mother and child diverge over time (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018). Similarly, the present study revealed that a pattern of higher mother RSA coupled with lower infant RSA across the interaction – indicative of greater infant distress and less maternal support in the context of the still-face procedure – was associated with higher child maltreatment severity.
The results also support prior research showing that, on the whole, families at higher risk of maltreatment do not show the overall positive concordance in mother–child RSA that has been shown in some community samples (Creaven et al., Reference Creaven, Skowron, Hughes, Howard and Loken2014; Lunkenheimer et al., Reference Lunkenheimer, Tiberio, Lucas-Thompson, Boker and Timpe2015). This may be due to differences in dyadic RSA processes by maltreatment subtype. An absence of RSA coregulation overall has been associated specifically with neglect (Lunkenheimer et al., Reference Lunkenheimer, Busuito, Brown and Skowron2018), and neglect is the more common form of child maltreatment in the general population (US DHHS, ACF, & ACYF, 2020). Thus, additional research would be useful to tease apart whether null concordance findings are meaningfully related to neglect or are a statistical consequence of collapsing maltreatment subtypes. Although our study was limited with regard to exploration of the influence of subtype, weaker RSA concordance may suggest less coordinated parasympathetic processes between mother and infant overall that may have negative consequences for infant's self-regulatory development. Given that families at risk of maltreatment are typically characterized by intrusive and inconsistent caregiving behaviors, it is possible that mothers were less attuned to their infant during social interaction. Further research is needed that incorporates caregiver behavioral transactions in the study of mother–infant RSA coregulation in the context of child maltreatment to explore this possibility.
By visualizing these regulatory processes, we were also able to illustrate oscillations in infant RSA and stability of mother RSA across the dyadic interaction related to differences in maltreatment severity. Specifically, weaker RSA concordance in relation to infant declines in RSA was more pronounced during the episodes of social interaction during the still-face procedure. Normative patterns of infant RSA suggest that higher RSA is associated with greater social engagement and maternal sensitivity, and lower RSA is associated with adaptive regulation in the context of stress (Bazhenova et al., Reference Bazhenova, Plonskaia and Porges2001; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996). Accordingly, our findings suggest that mother–infant dyads with higher maltreatment severity show the opposite of the typical pattern: infants may have had greater difficulty regulating themselves during social engagement with their mothers, as compared to episodes of disengagement. It is possible that the caregiving behaviors of mothers with higher maltreatment severity were less positive or supportive during the face-to-face play and reunion episodes. Therefore, interactive episodes may have been more physiologically taxing for infants, but they became more regulated during the still-face stressor when mothers were asked to disengage. Although task-specific differences were not assessed in the current study, these patterns may lead to problems in the development of infants’ self-regulated behavior or could be reflective of how infants adapt to less available or consistent caregiving in the context of families with higher maltreatment risk. These findings corroborate research with other mother–infant samples exposed to adversity, such that some infants become distressed when they resume interaction with less sensitive mothers (Conradt & Ablow, Reference Conradt and Ablow2010).
With regard to parenting, RSA patterns in mothers are less clear (Giuliano et al., Reference Giuliano, Skowron and Berkman2015). Sensitive and responsive caregiving has been implicated in both higher RSA (Skowron et al., Reference Skowron, Loke, Gatzke-Kopp, Cipriano-Essel, Woehrle, Van Epps and Ammerman2011; Weisman, Zagoory-Sharon, & Feldman, Reference Weisman, Zagoory-Sharon and Feldman2012) and lower RSA (Mills-Koonce et al., Reference Mills-Koonce, Propper, Gariepy, Barnett, Moore, Calkins and Cox2009; Moore et al., Reference Moore, Hill-Soderlund, Propper, Calkins, Mills-Koonce and Cox2009) during interactive tasks. However, in mothers with higher maltreating behaviors, higher RSA has been observed, reflecting greater social disengagement with their infants (Skowron et al., Reference Skowron, Cipriano-Essel, Benjamin, Pincus and Van Ryzin2013). Given our finding that mothers with higher levels of maltreatment severity had slightly elevated RSA over time, it suggests that they could have been less engaged throughout the interaction. Despite the regulatory patterns shown in mothers and infants from the present study, these findings should be interpreted within broader caregiving contexts and in relation to infant outcomes to better understand whether these parasympathetic processes undergird adaptive versus maladaptive outcomes.
Infant sleep variability and mother–infant RSA coregulation
Adverse caregiving environments also influence infant sleep behavior and promote sleep problems. According to the transactional model of infant sleep (Sadeh et al., Reference Sadeh, Tikotzky and Scher2010), caregiving behaviors are the most immediate and direct path to infant sleep problems: inconsistent routines or negative nighttime interactions between mothers and infants are linked to infants’ inability to develop their own self-regulation skills as well as fragmented sleep (Sadeh et al., Reference Sadeh, Tikotzky and Scher2010). Poor maternal bedtime strategies in relation to infant sleep difficulties and subsequent self-regulatory capacities have been found in families with high versus low adversity (Cronin, Halligan, & Murray, Reference Cronin, Halligan and Murray2010). Therefore, greater infant sleep variability may be implicated with more inconsistent caregiving.
Although mothers’ sleep duration during a 24-hr period was, on average, consistent with the recommended amount of sleep for adults (7 hr or more), infants’ average sleep duration was just under the recommended amount of sleep for 4- to 12-month-old infants (12–16 hr) according to the American Academy of Sleep Medicine and the Sleep Research Society (Paruthi et al., Reference Paruthi, Brooks, D'Ambrosio, Hall, Kotagal, Lloyd and Wise2016; Watson et al., Reference Watson, Badr, Belensky, Bliwise, Buxton, Buysse and Tasali2015). There was also considerable variability in sleep duration across the consecutive days of sleep assessment for both mothers (range = 4.72–10.11 hr per 24-hr period) and infants (8.54–13.40 hr per 24-hr period) in the present study. We found that higher infant night-to-night sleep variability was associated with infants’ overall lower mean RSA levels during interaction with their mothers. Given that sleep is an important regulated activity that is critical to early development, infants with more variable sleep may also have more difficulties with physiological aspects of regulation. We also found that higher infant sleep variability was associated with positive concordance in RSA, specifically in terms of mothers’ greater RSA withdrawal driving infants’ greater RSA withdrawal over time (i.e., coordinated declines in RSA) during face-to-face interactions. One possible interpretation is that an adverse caregiving environment indicated by higher infant sleep variability also contributes to maternal distress, elevating infant distress in challenging interactions. In other words, higher-risk mothers may not adequately support infant regulation in various forms, including consistent sleep and social support under conditions of challenge. Given that impairments in attachment relationships have been associated with infant sleep problems (Scher, Reference Scher2001), it is also possible that infants with higher sleep variability show more overall stress during interaction with their mothers in the context of adverse environments, resulting in more pronounced RSA declines over time.
Individual sleep variability has also been associated with infant temperamental style. Our findings regarding higher infant night-to-night sleep variability in relation to RSA synchrony may reflect an infant with a more irritable temperament or biological reactivity. These infants may be both more variable in sleep behavior and more reactive to their mother's stress during interaction. For example, negative behavioral and emotional states such as fussiness are negatively correlated with the proportion of sleep during a 24-hr period (Hayes, McCoy, Fukumitzu, Wellman, & DiPietro, Reference Hayes, McCoy, Fukumitzu, Wellman and DiPietro2011). Accordingly, infants with difficult temperament styles often show poor physiological regulation. In contrast, infant adaptation has been associated with higher RSA and more organized sleep patterns (Feldman, Rosenthal, & Eidelman, Reference Feldman, Rosenthal and Eidelman2014). In addition, higher RSA is related to positive social interactions and decreased risk of later psychopathology (Beauchaine, Reference Beauchaine2001; Patriquin et al., Reference Patriquin, Lorenzi, Scarpa, Calkins and Bell2015). However, given the limited number of studies on the associations between sleep problems and dyadic parasympathetic processes in mothers and infants, more research is needed. Although mother and infant sleep is often bidirectional, mother night-to-night sleep variability was not related to mother–infant coregulation of RSA. These results may be due to differential contextual factors or individual differences among mothers in relation to the dynamic coupling of physiology in dyads with higher familial risk.
Limitations, future directions, and implications
The current study is not without limitations. Although our methodological approach was sophisticated in the measurement of sleep variability and the use of time series modeling of observed physiological regulatory processes among mothers and infants in a naturalistic setting, modeling nonlinear patterns of RSA trajectories in parent and infant and incorporating the influence of concurrent behaviors and task condition changes could be informative next steps to give more context to these findings. In addition, a larger sample could have helped strengthen analytic power and allowed us to account for more sociodemographic factors, such as family structure and race and ethnicity, as well as additional maternal and infant characteristics, such as caregiver sensitivity and intrusiveness, sleep routines and hygiene, and/or infant temperament and sex. Future research could also incorporate broader contextual factors, such as experiences of prejudice, racism, and structural inequities, that may increase stressors in families and disparately affect the health outcomes of marginalized mothers and infants. Furthermore, we were unable to examine differences in regulatory processes by experiences of abuse or neglect, so future work could investigate whether maternal–infant coregulation of RSA differs as a function of maltreatment subtype. With respect to generalizability, the present findings may be characteristic of lower-income, ethnically diverse families at risk for child maltreatment and may not extend to lower-risk community samples or specific clinically referred populations.
Conclusion
Infancy is a sensitive period of development whereby parasympathetic processes are more susceptible to adverse conditions and largely shaped by interactions with caregivers. Changes in self- and coregulation among mother–infant dyads may explain how social and environmental experiences become “biologically embedded” to compromise health and development. Mother-infant interactions characterized by high familial risk have been shown to lead to disturbances in regulatory capacities and in vagal reactivity. In line with the RDoC framework, this work has important implications related to early identification of transdiagnostic markers of risk that may better classify later psychopathology. RSA, in particular, may be a key regulatory process associated with both maltreatment risk and sleep instability, which may affect long-term health and developmental outcomes. Interventions that aim to increase positive and consistent caregiving may help strengthen mothers’ physiological regulatory capacities, which may in turn strengthen infants’ self-regulation. Self- and coregulatory processes may therefore be important targets of future interventions to promote adaptation and optimal outcomes among vulnerable mother–infant dyads.
Acknowledgments
The authors are indebted to their community partners for their time and are extremely grateful to the families who shared their experiences with us. Thanks also go to Tiffany Koppels and Sara Chaparro Rucobo, without whom the data could not have been collected.
Funding Statement
The research reported here was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number K01HD098331 and the Society for Research in Child Development Victoria S. Levin Award for Early Career Success in Young Children's Mental Health Research awarded to S. M. Brown. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Society for Research in Child Development.
Conflicts of Interest
None








