INTRODUCTION
Leishmaniases are a group of parasitic diseases endemic in 98 countries worldwide, with over 350 million people living at risk and the annual case incidence ranging from 0.7 to 1.3 million (Alvar et al. Reference Alvar, Vélez, Bern, Herrero, Desjeux, Cano, Jannin and de Boer2012). The disease is prevalent both in the ‘New’ (South and the Central America) and the ‘Old’ World (Southern Europe, Africa, Middle East, Central Asia and Indian subcontinent). Of the 16 categories of neglected tropical diseases (NTDs) assessed for the period from 2005 to 2013, leishmaniasis ranks next only to malaria as the second worst in the age-standardized disability-adjusted life years (DALYs) and second only to dengue fever in the rate of DALY increase, from 5.7 to 5.9 million (Murray et al. Reference Murray, Barber, Foreman, Ozgoren, Abd-Allah, Abera, Aboyans, Abraham, Abubakar, Abu-Raddad, Abu-Rmeileh, Achoki, Ackerman, Ademi, Adou, Adsuar, Afshin, Agardh, Alam, Alasfoor, Albittar, Alegretti, Alemu, Alfonso-Cristancho, Alhabib, Ali, Alla, Allebeck, Almazroa and Alsharif2015). The disease often reaches epidemic proportions in areas of low endemicity due to natural or man-made disasters, including famine, drought, flood, earthquakes and civil wars (Reithinger and Dujardin, Reference Reithinger and Dujardin2007; Bern et al. Reference Bern, Maguire and Alvar2008). The poor socio-political background of the afflicted has largely contributed to the minimal interest shown towards leishmaniases by the policy makers, and even scientists, with resultant lack of good diagnostics and chemotherapeutic agents to enable effective management and control of this infection. Its apparent overlap with the spread of AIDS has highlighted the increasing threat of HIV-Leishmania co-infections, particularly in India and East Africa (Cruz et al. Reference Cruz, Nieto, Moreno, Cañavate, Desjeux and Alvar2006; Bern et al. Reference Bern, Maguire and Alvar2008).
PHENOTYPES AND CAUSATIVE AGENTS
Leishmaniases are considered as a disease complex rather than a single disease. Clinical manifestations occur under three main categories, i.e. cutaneous, mucocutaneous and visceral leishmaniasis (VL) (WHO, 2017). Cutaneous leishmaniasis (CL) is caused by Old World Leishmania species such as, Leishmania major/L. tropica and is marked by the appearance of varying types of skin lesions, which are often innocuous and self-healing. Mucocutaneous leishmaniasis (MCL) is caused by New World Leishmania spp. such as L. braziliensis and is a protracted disease, resulting sometimes in extensive facial disfigurement and tissue destructions in the mouth and nose. Most cases of MCL are not life-threatening per se. However, death could occur due to complications associated with secondary infections. VL caused by L. donovani/L. infantum is far more serious. It is often fatal, if untreated and results from systemic and progressive infection of macrophages in the reticulo-endothelial systems or lymphoid organs, mainly in the spleen, liver and bone marrow. Clinical manifestations of VL include hepatosplenomegaly, fever, anaemia, leucopaenia, hypergammaglobulinaemia and cachexia (Dedet and Pratlong, Reference Dedet, Pratlong, Cook and Zumla2003). The development of leishmaniasis follows a chronic course lasting for months and sometimes years. Interestingly, the traditionally established relationships between the causative species of Leishmania and disease phenotype have become obscure in certain instances and the leishmaniasis situation in Sri Lanka is a case in point (Karunaweera et al. Reference Karunaweera, Pratlong, Siriwardane, Ihalamulla and Dedet2003), with the known visceralizing species L. donovani resulting in essentially dermotropic disease (Fig. 1) (Kariyawasam et al. Reference Kariyawasam, Selvapandiyan, Siriwardana, Dube, Karunanayake, Senanayake, Dey, Gannavaram, Nakhasi and Karunaweera2018). In this context, it is noteworthy that with the accumulation of information over the years has led to the taxonomy of trypanosomatids in general, and Leishmania in particular to be revised, and therefore classical classifications may soon become outdated (Esponosa et al. Reference Esponosa, Serrano, Camargo, Teixeira and Shaw2018).
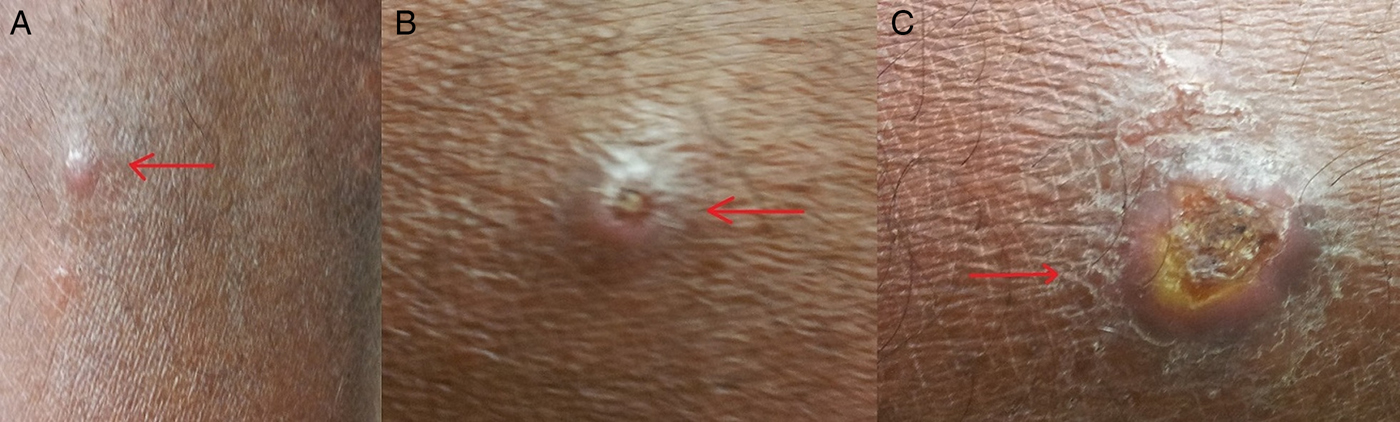
Fig. 1. Skin lesions observed in cutaneous leishmaniasis patients. (a) papule; (b) scaling nodule; (c) ulcer.
There are over 20 species of Leishmania that cause disease in humans (Maroli et al. Reference Maroli, Feliciangeli, Bichaud, Charrel and Gradoni2013; Akhoundi et al. Reference Akhoundi, Kuhls, Cannet, Votýpka, Marty, Delaunay and Sereno2016). Transmission occurs through the bite of an infected female sandfly (Phlebotomus or Lutzomyia spp), which introduces the infective stages of Leishmania to a mammalian host. Sand flies are inconspicuous, fragile and hairy winged dipterans, similar to, but smaller in size than an average sized mosquito. Most Leishmania species are considered as zoonotic parasites with humans acting as accidental hosts (Dedet and Pratlong, Reference Dedet, Pratlong, Cook and Zumla2003). The causative agent of VL in the Indian subcontinent, L. donovani is however widely considered as strictly anthroponotic, although this view remains debateable (Bhattarai et al. Reference Bhattarai, Van Der Auwera, Rijal, Picado, Speybroeck, Khanal, De Doncker, Das, Ostyn, Davies, Coosemans, Berkvens, Boelaert and Dujardin2010; Ready, Reference Ready2014; Akter et al. Reference Akter, Alam, Nakao, Yasin, Kato and Katakura2016).
CLINICAL MANAGEMENT OF LEISHMANIASIS
Confirmation of diagnosis of leishmaniasis is made through laboratory means. Visualization of parasites in the clinical samples from symptomatic patients constitutes the time-honoured gold standard for definitive diagnosis of leishmaniasis (de Vries et al. Reference de Vries, Reedijk and Schallig2015). The routine procedures for this include microscopic examinations of Giemsa-stained smears of lesion aspirates (in the case of CL and MCL; Fig. 2) or splenic or bone marrow aspirates (in the case of VL) for the presence of amastigotes and/or cultivation of the samples in suitable media for their differentiation into and/or replication as promastigotes. These century-old practices have been gradually replaced by less cumbersome and more sensitive and specific methods, i.e. serodiagnosis for the presence of Leishmania-specific antibodies or circulating antigens and by PCR amplification of Leishmania-specific DNAs (Singh and Sundar, Reference Singh and Sundar2015; Akhoundi et al. Reference Akhoundi, Downing, Votýpka, Kuhls, Lukeš, Cannet, Ravel, Marty, Delaunay, Kasbari, Granouillac, Gradoni and Sereno2017).

Fig. 2. Microscopic appearance of a Giemsa-stained smear made from tissue aspirates from a skin lesion of a cutaneous leishmaniasis patient [under oil immersion (×1000 magnification)].
The mainstay of treatment for leishmaniasis is chemotherapy, but none of the drugs in use had been specifically designed and developed for treating this disease, i.e. antimonials (meglumine antimoniate or glucantime®, sodium stibogluconate or Pentostam®), miltefosine, pentamidine, amphotericin B, ketoconazole and paromomycin (Singh et al. Reference Singh, Singh, Chakravarty and Sundar2016a). The antimonials remain as the first line of treatment for VL in most endemic settings for the past so many decades, although the mode of action of these compounds remains largely unknown. As reviewed by Hendrickx et al. (Reference Hendrickx, Guerin, Caljon, Croft and Maes2018) and also Uliana et al. (Reference Uliana, Trinconi and Coelho2018), the chemotherapeutic agents widely available for treatment of leishmaniasis are toxic with prolonged use resulting in significant side-effects and even death due to renal and/or cardiac complications. Better alternatives are urgently needed with drug repurposing used as a promising strategy for finding new agents for oral or topical administration with anti-Leishmania activity (Trinconi et al. Reference Trinconi, Reimao, Bonano, Espada, Miguel, Yokoyama-Yasunaka and Uliana2018). Amphotericin B-liposome (AmBisome®), a superior but expensive drug, is limited in use in endemic areas of poverty. Appearance and spread of drug resistance is also a major cause of concern, as extensively reviewed in this special issue (Sundar and Singh, Reference Sundar and Singh2018). Strategies for boosting hosts’ immune responses to potentiate chemotherapy are considered as important future tools to meet such challenges and are reviewed by Taslimi et al. (Reference Taslimi, Zahedifard and Rafati2018) in this special issue.
Chemotherapy of CL faces the dilemma of its necessity, due to the dogma based on its tendency for self-resolution. However, treatment hastens healing, thereby minimizes the scar formation, prevents spread, progression in to more complicated disease forms, such as MCL and helps to avoid poor responsiveness of protracted disease (Marsden, Reference Marsden1986; Cannella et al. Reference Cannella, Nguyen, Piggott, Lee, Vinetz and Mehta2011).
Prevention of spread, though believed as a hindrance for the potential development of herd immunity, would be important from a public health standpoint at least in countries with CL due to potentially visceralizing L. donovani infections (Kariyawasam et al. Reference Kariyawasam, Selvapandiyan, Siriwardana, Dube, Karunanayake, Senanayake, Dey, Gannavaram, Nakhasi and Karunaweera2018). Alternative approaches for treating CL are now available by using physical means, e.g. thermotherapy that uses radio-frequency generated heat (RFHT) that is cost-effective and safe (Refai et al. Reference Refai, Weerasingha, Sumanasena, Madarasingha, Karunaweera, Fernandopulle, De Silva and Satoskar2017). The outcome of numerous clinical trials using RFHT for the treatment of CL caused by varying Leishmania spp. has been carefully reviewed in this special issue (David, Reference David2018).
LEISHMANIASIS IN THE INDIAN SUBCONTINENT
The Indian subcontinent accounts for nearly 70% of the world's anthroponotic VL cases, amounting to several hundred thousand annual cases (Alvar et al. Reference Alvar, Vélez, Bern, Herrero, Desjeux, Cano, Jannin and de Boer2012). VL is however substantially under-reported, with reported coverage varying not only between countries but even between districts within a given country (Bern et al. Reference Bern, Maguire and Alvar2008; Alvar et al. Reference Alvar, Vélez, Bern, Herrero, Desjeux, Cano, Jannin and de Boer2012). India has the world's highest national VL incidence, Nepal and Bangladesh being the next. Taken together, the population at risk of acquiring VL is ~200 million in these three countries. VL data with regard to other south Asian countries such as Bhutan and Sri Lanka, however, are sparse with the general belief of it being sporadic and scattered upheld in most part (Alvar et al. Reference Alvar, Vélez, Bern, Herrero, Desjeux, Cano, Jannin and de Boer2012).
Clinical features of VL is marked by splenomegaly as the most noticeable symptom and is commonly accompanied by other non-specific signs such as anaemia, weight loss and hepatomegaly. Post-kala azar dermal leishmaniasis (PKDL) is also an intriguing clinical scenario described in India and occurs in 5–10% of VL cases following apparent cure of VL (and sometimes with no history of preceding VL). PKDL patients present with macular papular skin lesions and are speculated to act as reservoirs for VL transmission (due to the parasite-rich nature of the skin lesions) (Ready, Reference Ready2014), although such significance of PKDL remains debatable (Le Rutte et al. Reference Le Rutte, Coffeng, Bontje, Hasker, Ruiz Postigo, Argaw, Boelaert and De Vlas2016; Hirve et al. Reference Hirve, Kroeger, Matlashewski, Mondal, Banjara, Das, Be-Nazir, Arana and Olliaro2017).
Leishmaniasis is a relatively newly established disease in Sri Lanka with the first autochthonous case of CL reported in 1992 (Athukorale et al. Reference Athukorale, Seneviratne, Ihalamulla and Premaratne1992). There has been a steady increase in the numbers and distribution of CL across the country during the past two decades (http://www.epid.gov.lk/web/images/pdf/wer/2015/vol_42_no_24-english.pdf). The causative agent of CL in Sri Lanka is L. donovani, of type MON-37 (Karunaweera et al. Reference Karunaweera, Pratlong, Siriwardane, Ihalamulla and Dedet2003), which is indeed a cause for concern, and is transmitted by Phlebotomus argentipes, the same vector found elsewhere in the south Asian region (Gajapathy et al. Reference Gajapathy, Peiris, Goodacre, Silva, Jude and Surendran2013). Leishmania donovani is an established agent of human VL in other south Asian countries, though dermotropism, as abundantly seen in Sri Lanka, has only been occasionally observed elsewhere (Elamin et al. Reference Elamin, Guizani, Guerbouj, Gramiccia, El Hassan, Di Muccio, Taha and Mukhtar2008; Kumar et al. Reference Kumar, Srinivasan, Anish, Nandakumar and Jambulingam2015). This has led to investigations on the role for both the parasite (Siriwardana et al. Reference Siriwardana, Noyes, Beeching, Chance, Karunaweera and Bates2007; Zhang et al. Reference Zhang, Ramasamy, McCall, Haydock, Ranasinghe, Abeygunasekara, Sirimanna, Wickremasinghe, Myler and Matlashewski2014) and host genetics in determining the disease phenotype (Samaranayake et al. Reference Samaranayake, Fernando and Dissanayake2010). However, the debate continues and the theories on CL-inducing L. donovani being ‘essentially’ dermotropic still remain inconclusive.
ELIMINATION OF LEISHMANIASIS FROM THE INDIAN SUBCONTINENT
A memorandum of understanding was signed in May 2005 during the World Health Assembly between the World Health Organization and the government representatives of India, Nepal and Bangladesh that committed themselves to work in mutual cooperation to achieve the elimination of VL from these countries by 2015 (World Health Organization., 2005). The objective of the VL elimination initiative as laid down at the outset was to reduce the annual incidence of VL below one per 10 000 population at district, subdistrict or upazilla level in India, Nepal and Bangladesh, respectively (Picado et al. Reference Picado, Dash, Bhattacharya and Boelaert2012). The strategies adopted were based on case detection and management together with vector control. Though there was a substantial progress in selected areas with conspicuous reduction of the VL burden in the region, certain gaps acted as road blocks and prevented the expected progress towards the achievement of targeted outcome. Such deficiencies included the low coverage of health services at community level, parasite resistance to antimonial compounds, limited availability and high cost of alternative therapeutic agents and the lack of effectiveness of vector control measures (Bhandari et al. Reference Bhandari, Angdembe, Rijal and Boelaert2011; Chowdhury et al. Reference Chowdhury, Mondal, Chowdhury, Faria, Alvar, Nabi, Boelaert and Dash2014; Muniaraj, Reference Muniaraj2014). The VL elimination framework was subsequently updated (World Health Organization, 2012a). In the new framework, there were five key strategies described, viz. early diagnosis and complete case management, effective disease and vector surveillance, social mobilization and building partnerships, and clinical and operational research, to achieve the elimination goal. A more recent initiative led the WHO to define a road map for prevention, control, elimination and eradication of 17 NTDs, including VL, as a step towards achieving the Sustainable Development Goals (World Health Organization, 2012b). These efforts included the extension of support to enable better access to drugs and related interventions, and monitor progress towards VL elimination by 2020 with all stake holder involvement (Hirve et al. Reference Hirve, Kroeger, Matlashewski, Mondal, Banjara, Das, Be-Nazir, Arana and Olliaro2017).
There are obvious advantages of moving towards elimination of leishmaniasis from the South Asian region. However, the deficiencies would need to be carefully addressed in order to ensure a more successful outcome at least by year 2020. In addition to the obvious factors that may have contributed to the sustained parasite transmission (Hirve et al. Reference Hirve, Kroeger, Matlashewski, Mondal, Banjara, Das, Be-Nazir, Arana and Olliaro2017), the presence of phenotypic/genotypic variants of L. donovani in the region with the potential to act as reservoirs of infection has obvious implications on the ongoing plans for the elimination of VL from the South-Asian region; a factor that has been largely overlooked thus far.
Though vector control is an essential component in the overall strategy towards disease elimination, there have been obvious lack of innovations in that field going back to many decades (Picado et al. Reference Picado, Dash, Bhattacharya and Boelaert2012). On the other hand, there have been some notable progress in the fields of diagnostic, therapeutic and vaccine development (Singh and Sundar, Reference Singh and Sundar2015; Srivastava et al. Reference Srivastava, Shankar, Mishra and Singh2016; Sundar and Singh, Reference Sundar and Singh2016), but many hurdles persist (Singh et al. Reference Singh, Hasker, Boelaert and Sundar2016b). For regional elimination of L. donovani-induced leishmaniasis to become a reality, effective deployment of existing and new tools will be essential. A strong political as well as active community participation would be imperative and so would inter country cooperation and partnerships. Furthermore, appropriate diagnostic and treatment services as well as effective epidemiological surveillance also would need to be ensured in order to achieve a more successful outcome of the renewed efforts in the regional drive towards the elimination of L. donovani-induced leishmaniasis.
ACKNOWLEDGEMENT
Assistance provided by Ms. Udeshika Kariyawasam in formating of text and images is greatly appreciated.
FINANCIAL SUPPORT
Financial support for NDK is through the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI099602 and U01AI136033. The content of this editorial is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
None.





