Introduction
Population recovery programmes using captive-reared individuals make an important contribution to conservation (Saint-Jailme, Reference Saint-Jalme2002; Seddon et al., Reference Seddon, Armstrong and Maloney2006; Armstrong & Seddon, Reference Armstrong and Seddon2008; Zając et al., Reference Zając, Florek, Zając, Adamski, Bielański and Ćmiel2018). However, before the late 1980s a significant number of such projects failed (IUCN, 1987; Griffith et al., Reference Griffith, Scott, Carpenter and Reed1989; Witkowski et al., Reference Witkowski, Adamski, Kosior and Płonka1997; Adamski & Witkowski, Reference Adamski, Witkowski, Bijok and Prus1999a; Fisher & Lindenmayer, Reference Fisher and Lindenmayer2000; Suding et al., Reference Suding, Gross and Houseman2004; Seddon et al., Reference Seddon, Armstrong and Maloney2006; Moseby et al., Reference Moseby, Hill and Lavery2014) or relied on constant replenishment with captive-reared individuals (Young et al., Reference Young, Hastie and Cooksley2003; Pedrono et al., Reference Pedrono, Smith, Clobert, Massot and Sarrazin2004). The results achieved frequently did not match the initial predictions, for a number of reasons (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith and Toone1996; Fisher & Lindenmayer, Reference Fisher and Lindenmayer2000; Seddon et al., Reference Seddon, Griffiths, Soorae and Armstrong2014), including legal restrictions or organizational difficulties (Kleiman et al., Reference Kleiman, Beck, Dietz, Dietz and Gipps1991; Caughley & Gunn, Reference Caughley and Gunn1996; Fisher & Linderman, Reference Fisher and Lindenmayer2000), or the biological or ecological constraints of the species being restored. Such constraints may relate to the genetic pool and local adaptations (Sarrazin & Barbault, Reference Sarrazin and Barbault1996; Moritz, Reference Moritz1999; Hedrick & Kalinowski, Reference Hedrick and Kalinowski2000; Baums, Reference Baums2008) or to difficulties ensuing from recovery projects being carried out in highly modified ecosystems that had already achieved a resilient alternative state (Bakker & Berendse, Reference Bakker and Berendse1999; Beisner et al., Reference Beisner, Haydon and Cuddington2003; Suding et al., Reference Suding, Gross and Houseman2004; Young et al., Reference Young, Petersen and Clary2005). For animals with highly developed nervous systems, distress or behavioural reactions may affect the outcome of conservation activities, and the implementation of stress-reducing release strategies has been widely discussed (Griffith et al., Reference Griffith, Scott, Carpenter and Reed1989; Wolf et al., Reference Wolf, Garland and Griffith1998; Moseby et al., Reference Moseby, Hill and Lavery2014).
To restore a population and then maintain it in a stable state, reliable estimates of population parameters such as birth and death rates and migration are required (Seddon et al., Reference Seddon, Armstrong and Maloney2006), and the use of population models incorporating such parameters have been recommended (Seddon et al., Reference Seddon, Armstrong and Maloney2006; Converse et al., Reference Converse, Moore and Armstrong2013). However, population parameters are usually difficult to estimate or require long-term study (Beissinger & Westphal, Reference Beissinger and Westphal1998; Brook et al., Reference Brook, O'Grady, Chapman, Burgman, Akçakaya and Frankham2000; Adamski & Witkowski, Reference Adamski and Witkowski2007; Parlato & Armstrong, Reference Parlato and Armstrong2012; Converse et al., Reference Converse, Moore and Armstrong2013). By definition, recovery programmes involve a threatened species or population, the abundance and habitat of which are usually restricted. This may imply a trade-off between the methodological aptness of the research and the effectiveness of conservation measures (IUCN/SSC, 2013; Moseby, Reference Moseby, Hill and Lavery2014).
The IUCN recommends that the monitoring that succeeds a population recovery programme should continue for at least as long as the programme's duration (IUCN/SSC, 2013). It is often difficult to act on such guidance, however, as projects may aim to establish wild-captive metapopulations that are more or less dependent on ongoing conservation activities (Pedrono et al., Reference Pedrono, Smith, Clobert, Massot and Sarrazin2004; Converse et al., Reference Converse, Moore and Armstrong2013).
It is important to optimize any restoration. With invertebrates, for example, large numbers of captive-bred individuals can be readily reared (Morton, Reference Morton1983; Witkowski et al., Reference Witkowski, Adamski, Kosior and Płonka1997; Schultz et al., Reference Schultz, Russell and Wynn2008; Thomas et al., Reference Thomas, Taylor and De Leaniz2010; Gum et al., Reference Gum, Lange and Geist2011; Ćmiel et al., Reference Ćmiel, Zając, Lipińska and Zając2018). But the problem lies in the effectiveness of the reintroduction, measured as the population's survivorship or growth rate (Adamski & Witkowski, Reference Adamski and Witkowski2007; Seddon et al., Reference Seddon, Armstrong and Maloney2006; Gum et al., Reference Gum, Lange and Geist2011; Zając et al., Reference Zając, Florek, Zając, Adamski, Bielański and Ćmiel2018). Most restoration programmes concern threatened species, so it is usually assumed that the abundance of the restored population will be below the habitat's carrying capacity (Converse et al., Reference Converse, Moore and Armstrong2013). But this assumption may be flawed for invertebrates. The recovery project of the large blue butterfly Phengaris arion in the UK resulted in an abundance of the species at some sites that was equivalent to the estimated local carrying capacity (Thomas et al., Reference Thomas, Taylor and De Leaniz2010). A similar situation arose during the Apollo butterfly Parnassius apollo recovery project in the Pieniny National Park in southern Poland, albeit on a smaller scale (Adamski & Witkowski, Reference Adamski and Witkowski2007). Effectiveness analysis of the first decade of the project suggested there could be a problem concerning over-supplementation with captive-reared specimens (Adamski & Witkowski, Reference Adamski and Witkowski2007); i.e. that the introduction of too many individuals caused the carrying capacity of the habitat to be exceeded.
Here we analyse the population dynamics of the Apollo butterfly during the 25 years of the recovery project in the Pieniny National Park. This long-term study, in conjunction with the short lifetime of the Apollo, provides a unique opportunity for analysing a multi-generational period. Analysis of the state of a population using classical population growth models may be helpful for assessing the potential harm to a recovery programme of over-supplementation.
Study population
The Apollo (family Papilionidae) is categorized as Least Concern on the IUCN Red list (Nadler et al., Reference Nadler, Bonelli, Dapporto, Karaçetin, Lukhtanov and López Munguira2021). But by the 1950s, the sole remaining population in Poland was in the Pieniny Mountains (Fig. 1a), where it had been known and studied since at least the second half of the 19th century (Siła-Nowicki, Reference Siła-Nowicki1865). By the end of the 1980s, however, it was on the verge of extinction, and it is categorized as Critically Endangered regionally (Witkowski, Reference Witkowski, Głowaciński and Nowacki2004), even though in Europe as a whole it is categorized as Near Threatened (van Swaay et al., Reference van Swaay, Wynhoff, Verovnik, Wiemers, López Munguira and Maes2010). In the mid 20th century, the taxon in the Pieniny Mountains was described as P. apollo frankenbergeri (Slaby, Reference Slabý1955). A combination of several threat factors reduced its abundance in the early 1990s to 20–30 adults per year (Witkowski & Adamski, Reference Witkowski, Adamski, Settele, Margules, Poschol and Henle1996; Adamski & Witkowski, Reference Adamski and Witkowski2007).

Fig. 1 (a) Location of the Pieniny Mountains in Poland, and (b) the spatial structure of the Apollo butterfly Parnassius apollo metapopulation (Adamski & Witkowski, Reference Adamski and Witkowski2007) in the Pieniny National Park, showing the western, central and eastern subpopulations and their patchy structure. The question mark indicates uncertain status.
A two-stage recovery project for the subspecies began in 1992. In the first stage, the sites inhabited by the subspecies before its decline were restored by removing the trees and shrubs that had been planted during reforestation programmes or that had grown as a result of succession following scree stabilization and abandonment of management of the mountain meadows. By 1997, these sites had been restored to a suitable state (Witkowski et al., Reference Witkowski, Adamski, Kosior and Płonka1997; Adamski & Witkowski, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016). In the second stage, individuals from the Pieniny National Park's captive breeding programme, which originated from the local population, were reintroduced (Witkowski & Adamski, Reference Witkowski, Adamski, Settele, Margules, Poschol and Henle1996; Adamski & Witkowski, Reference Adamski and Witkowski2007).
During the first stage, the carrying capacity for the Apollo butterfly was estimated from an inventory of host plant abundance (Witkowski et al., Reference Witkowski, Klein and Kosior1992; Adamski & Witkowski, Reference Adamski and Witkowski2007): the anticipated target abundance was 1,200–1,300 adults. The Apollo's abundance was estimated using the capture–mark–recapture method. All reintroduction sites were visited weekly, when the weather was favourable for the species’ activity, and individuals were netted and marked with a unique code on the hindwing, and released (Adamski & Witkowski, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016). Abundance was estimated using Craig's method (Seber, Reference Seber1982). The study population is a mixed type metapopulation (Harrison, Reference Harrison, Gilpin and Hanski1991) comprising western, central and eastern populations and a few smaller, ephemeral subpopulations (Fig. 1b; Adamski & Witkowski, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016).
Methods
Simulation of population recovery based on captive-reared individuals
The model aimed to analyse population growth scenarios in a reinforced population in which the basic model parameters varied in value and stability. The simulation was carried out using Ricker's (Reference Ricker1958) model, a classical discrete population model in which the expected number of individuals in generation t + 1 is a function of the number of individuals in generation t, with the addition of a new variable Nc, the number of captive-reared individuals in generation t:
where r is population growth rate and K is habitat-determined population carrying capacity. Model parameters were estimated from results obtained during the design phase of the project and from data gathered during 1991–2019 using the methods and assumptions described below.
Estimation of model parameters
Carrying capacity and regime shift
The estimated carrying capacity (K) of the study area before the project's inception was based on host plant abundance only (Witkowski et al., Reference Witkowski, Klein and Kosior1992). In the Pieniny National Park, 10,200 stonecrop Sedum maximum L. plants were recorded, 6,600 of which were growing in habitats potentially suitable for the Apollo. Assuming that five stonecrop plants are sufficient for the development of one Apollo imago, the study area's carrying capacity was estimated to be c. 1,320 individuals (Witkowski et al., Reference Witkowski, Klein and Kosior1992). Although this estimate did not take into account caterpillar mortality or variability in stonecrop plant size and its local spatial distribution, it is nevertheless the most appropriate in this case. Information on population abundance available in the literature and unpublished materials (Chrostowski, Reference Chrostowskiundated; Żukowski, Reference Żukowski1959; Witkowski et al., Reference Witkowski, Adamski, Kosior and Płonka1997) is qualitative (e.g. presence/absence at particular sites) or, at most, categorical (e.g. ‘single specimens’, ‘abundant’). We therefore estimated carrying capacity from the abundances attained by the restored population, assuming that the successful reintroduction of a population should enable it to reach the local carrying capacity. However, if over-supplementation has occurred, local habitats may be overexploited, with a subsequent reduction in their carrying capacity (Adamski & Witkowski, Reference Adamski and Witkowski2007). As estimated population abundance fluctuates annually, we used regime shift analysis to distinguish between random fluctuations and directional processes. Hitherto applied mainly in climate analysis, regime shift analysis determines the level around which individual measurements fluctuate randomly, and also detects regime shifts, defined as rapid reorganization of processes from one relatively stable state to another (Rodionov & Overland, Reference Rodionov and Overland2005). Regime shifts in the recovered abundance of the Apollo butterfly were analysed using Rodionov's (Reference Rodionov2004) algorithm implemented in Sequential Regime Shift Detection Software 3.2 (Bering Climate, 2006). The difference between the mean values of neighbouring regimes was assessed using a Student's two-tailed t test with unequal variance, at P = 0.05. The regime means were weighted with Huber's weight function with the parameter = 1. The cut-off length parameter was set at 6.
Population growth rate
In both classical (Ricker, Reference Ricker1958) and modern approaches the population growth rate (r) is related to the average life-history traits in a given population, such as fecundity and developmental mortality (Caswell, Reference Caswell1978), which are mediated by environmental factors determining the carrying capacity. However, studies of wild population dynamics yield only the net population growth, and it is therefore difficult to separate the influence of carrying capacity from other factors affecting population abundance. We therefore used the maximum growth rate recorded during the recovery process. For the years when the population was supplemented with captive-reared individuals (1992–2001 and 2004), the introduced individuals were included in the calculation in a manner analogous to the calculation of the reintroduction effectiveness coefficient proposed by Adamski & Witkowski (Reference Adamski and Witkowski2007), using the following formula:
where r is population growth rate, Wt is the estimated wild population in year t, Wt −1 is the estimated wild population in year t–1, and Ct −1 is number of captive-reared individuals introduced into the population in year t–1.
Modelled scenarios
Because of the shifting level of population abundance during the restoration process, two variants of the model were run: (1) assuming a constant habitat carrying capacity (K1) throughout the process, and (2) assuming that over-supplementation during 2003–2005 reduced the carrying capacity from (K1) to (K2). In addition, each set of parameters was separately modelled in two versions: (1) with, and (2) without the inclusion of the captive-bred individuals. As a result, six theoretical population growth scenarios were modelled: Scenario 1 (WO K 1): not including captive-reared individuals (wild only); carrying capacity K 1 constant; Scenario 2 (WO + shift): not including captive-reared individuals; carrying capacity reduced from K 1 to K 2; Scenario 3 (WO K 2): not including captive-reared individuals; carrying capacity K 2 constant; Scenario 4 (CI K 1): including captive-reared individuals (captive-reared included); carrying capacity K 1 constant; Scenario 5 (CI + shift): including captive-reared individuals; carrying capacity reduced from K 1 to K 2; Scenario 6 (CI K 2): including captive-reared individuals; carrying capacity K 2 constant.
Results
Estimation of model parameters
The annual estimated population abundances, the numbers of captive-reared individuals released into the wild and the growth rate are listed in Table 1. Based on these data, the estimated growth rate used in the models was r = 0.6. Regime shift analysis showed there were two periods when the estimated population abundance changed: a rapid increase during 1996–1997, and a decrease during 2003–2004 (Fig. 2). Before the decrease, the estimated mean regime was 1,294, afterwards it was 716; accordingly, carrying capacities K 1 and K 2 were set to these values. The difference between the average absolute residual values of the neighbouring regimes was significant (F = 15.9876, df = 5, P < 0.0001).
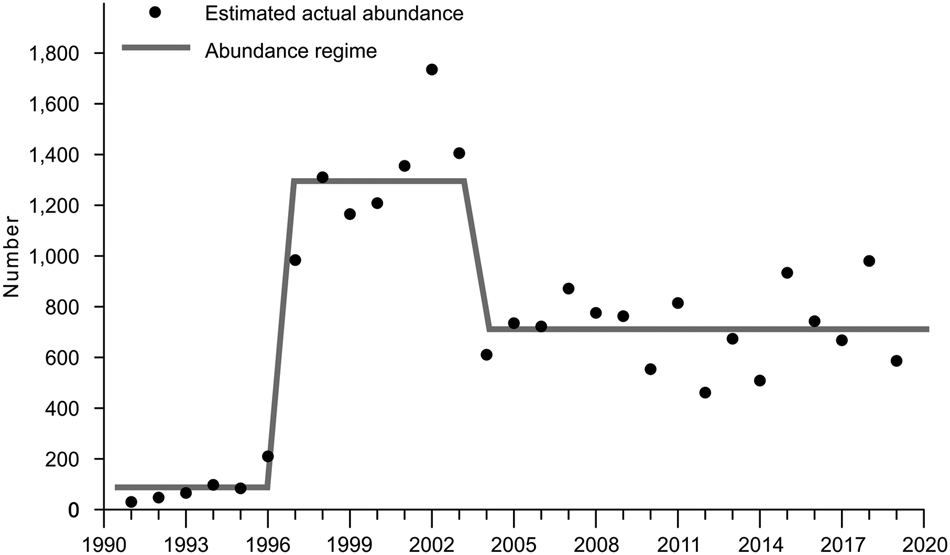
Fig. 2 Population abundance changes and the regime shift of this parameter from 1990 to 2019. The regime line represents the value around which the population abundance fluctuates.
Table 1 Population parameters for the whole metapopulation of the Apollo butterfly Parnassius apollo in the Pieniny Mountains, Poland (Fig. 1) during 1991–2019.

1 Using Craig's method (Seber, Reference Seber1982).
Modelled scenarios
Figures 2–3 illustrate the modelling results. The correlations between the modelled population growth and the population abundance estimates based on the field data (Table 2) varied between R = 0.43 and R = 0.85 (Fig. 3, Table 2). The highest correlation was achieved by the model with the shifted carrying capacity and no supplementation (Scenario 2: WO + shift). For consecutive years, analysis of the differences between the population abundance estimated from field data and that predicted by the models shows that the difference is at the same level for the whole study period only for the WO + shift scenario. For each of the other scenarios there is at least one regime shift in the level of differences (Fig. 4).
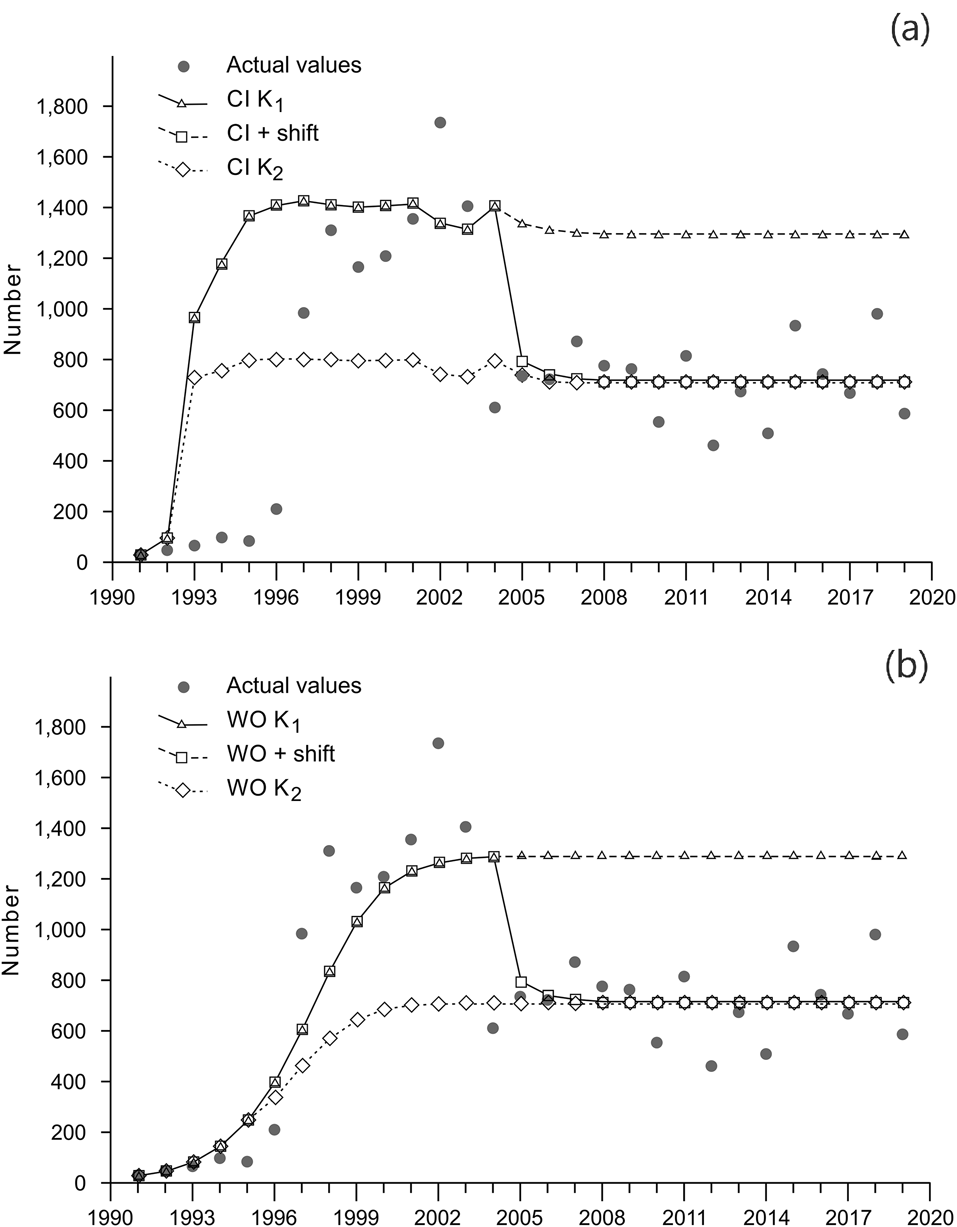
Fig. 3 Changes in the abundance of the restored population and theoretical scenarios (a) including and (b) not including supplementation with captive-reared individuals, from 1990 to 2019. See text for details of the six scenarios.

Fig. 4 Residual analysis of the actual population abundance and the values expected according to the six modelled scenarios, from 1990 to 2019. See text for details of the scenarios.
Table 2 The fit of the six modelled scenarios (see text for details) to the field data.
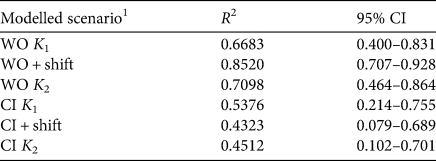
1WO K1, not including captive-reared individuals, constant carrying capacity K1 = 1,294; WO + shift, not including captive-reared individuals, carrying capacity reduced from K 1 = 1,294 to K 2 = 716; WO K 2, not including captive-reared individuals, constant carrying capacity K2 = 716; CI K 1, including captive-reared individuals, constant carrying capacity K1 = 1,294; including captive-reared individuals, carrying capacity reduced from K 1 = 1,294 to K 2 = 716; CI K2, including captive-reared individuals, constant carrying capacity K2 = 716.
2Correlation coefficient; all significant at P < 0.0001.
Discussion
The results suggest that of the six population growth scenarios, the one involving a substantial reduction in habitat carrying capacity best fits the data. This is expected: the population is unlikely to have maintained itself above the habitat's carrying capacity for six consecutive years (1998–2003). Otherwise, given the constant, high carrying capacity of the habitat, it is difficult to explain the population's stabilization at the lower level of abundance after the collapse in 2004.
It is, however, surprising that the scenarios not involving supplementation with captive-reared individuals were generally a better fit to the field data than those including such supplementation (Table 2, Figs 3–4). This difference is particularly conspicuous with regard to 1992–1997. The model involving captive-reared individuals indicates that the carrying capacity should have been reached within 2 years, but it took twice as long; the recovery schedule could have been responsible for this. The estimated pre-restoration carrying capacity was based only on host plant abundance, whereas one reason for the Apollo population's critical status was the loss of dry grasslands associated with limestone scree (Witkowski et al., Reference Witkowski, Adamski, Settele, Margules, Poschol and Henle1996, Reference Witkowski, Adamski, Kosior and Płonka1997; Adamski & Witkowski, Reference Adamski and Witkowski2002, Reference Adamski and Witkowski2007). In the first phase of the recovery project, captive-reared individuals were introduced in parallel with habitat restoration. The planned habitat conditions at all the sites covered by the project had been achieved by 1997 (Witkowski et al., Reference Witkowski, Adamski, Kosior and Płonka1997; Adamski & Witkowski, Reference Adamski, Witkowski, Bijok and Prus1999a; Adamski, Reference Adamski2016), the year when the population reached a level similar to the carrying capacity estimated on the basis of host plant abundance. It is possible either that host plant resources only become fully available after other habitat elements have reached an appropriate condition, or that host plant abundance is not the factor limiting population abundance. The Apollo is sensitive to disturbance of the non-forest habitat structure, and hence even a slight increase in the number of shrubs in open grassland could make this habitat unattractive or even unsuitable (Fownes & Roland, Reference Fownes and Roland2002; Nakonieczny et al., Reference Nakonieczny, Kędziorski and Michalczyk2007a; Matter et al., Reference Matter, Doyle, Illerbrun, Wheeler and Roland2011). Experience from butterfly conservation practice indicates that habitat-oriented activities are crucial for effectiveness (New, Reference New1991; Thomas, Reference Thomas, Spellerbeg, Goldsmith and Morris1991; Pullin & Knight, Reference Pullin and Knight2001; Schultz and Crone, Reference Schultz and Crone2005; Adamski & Witkowski, Reference Adamski and Witkowski2007). The introduction of captive-reared individuals to sites where habitat is not yet in a suitable condition will not be effective. The Apollo recovery project initially assumed that because of the host plant's rarity, butterfly abundance was the most appropriate measure of habitat quality (Witkowski et al., Reference Witkowski, Płonka and Budzik1993). The host plant inventories (Witkowski et al., Reference Witkowski, Klein and Kosior1992) could have been misinterpreted because the assumptions were oversimplified, but the breakdown of the population after it had reached or exceeded the estimated carrying capacity suggests that estimation was reasonably reliable.
Another question relates to the mechanisms potentially responsible for the reduced carrying capacity. Earlier research suggested that overestimation of food plant abundance led to its excessive exploitation because too many captive-reared individuals were introduced to particular sites (Adamski & Witkowski, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016). This is corroborated indirectly by the fact that introduction effectiveness indices were lower at sites where many individuals were introduced (Adamski & Witkowski, Reference Adamski and Witkowski2007). Moreover, the subpopulations in the eastern part of the metapopulation (Fig. 1b), not supplemented since the decrease in 2004, appeared to be more stable (Adamski, Reference Adamski2016): the shorter distances between subpopulations could have led to a higher migration ratio (Adamski, Reference Adamski2016). That this might be related to the host plant was supported by the observation that a large proportion of fresh stonecrop shoots were consumed by young Apollo caterpillars early in the season (Olejniczak, Reference Olejniczak2011; P. Olejniczak, pers. comm., 2008). The stonecrop plants, whose young shoots had been completely eaten, developed new shoots from the rhizomes. Between consumption of the first shoots and the regrowth, the development of the Apollo's larvae was presumably limited.
These arguments and observations suggest that the reintroduction of too many individuals is not only ineffective but can also lead to long-term negative consequences, such as a reduced carrying capacity. This does not imply, however, that captive breeding has no role in butterfly recovery projects. It has been demonstrated that, in the absence of the maintenance or restoration of appropriate habitat quality, the introduction of captive-reared individuals is ineffective (New, Reference New1991; Thomas, Reference Thomas, Spellerbeg, Goldsmith and Morris1991; Pullin & Knight, Reference Pullin and Knight2001; Schultz & Crone, Reference Schultz and Crone2005; Adamski & Witkowski, Reference Adamski and Witkowski2007). Nonetheless, in addition to obtaining large numbers of individuals for introduction into the wild, there are several conservation advantages of captive breeding. In this particular case, captive breeding combined with field studies enabled two threats to be addressed.
The first was inbreeding in the wild population, which was disrupted by introducing captive-bred individuals. In the early years of the captive breeding programme, Apollo butterflies from the larger population in the Slovak part of the Pieniny Mountains were incorporated. This decision was controversial, even though both populations were the same subspecies, P. apollo frankenbergeri, and there were reliable reports that until at least the early 1950s butterflies occasionally migrated between the two populations. After the introduction of the Slovak butterflies into the captive breeding programme, symptoms of genetic erosion (a high level of developmental mortality, Witkowski et al., Reference Witkowski, Płonka and Budzik1993; wing deformations, and significant numbers of individuals incapable of stretching their wings upon emergence, Adamski & Witkowski, Reference Adamski and Witkowski1999b) decreased. In addition, the average individual fluctuating asymmetry (Adamski & Witkowski, Reference Adamski and Witkowski2002) was significantly reduced (Adamski & Witkowski, Reference Adamski, Witkowski, Bijok and Prus1999a, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016).
The second threat was that as a result of long-term isolation, the Apollo butterflies in the Pieniny National Park no longer migrated between subpopulations (Adamski & Witkowski, Reference Adamski, Witkowski, Bijok and Prus1999a, Reference Adamski and Witkowski2007). This changed after the introduction of individuals from Slovakia into the captive-breeding programme: individuals from the Slovak and Polish–Slovak breeding lines were significantly more likely to perform both long- and short-distance migrations (Adamski & Witkowski, Reference Adamski and Witkowski2007; Adamski, Reference Adamski2016). An additional benefit of captive breeding is that studies carried out in captivity have provided new information on Apollo butterfly biology and ecology, which may be of use in the conservation of this species (Adamski et al., Reference Adamski, Witkowski, Bijok and Prus1999; Adamski, Reference Adamski2004; Nakonieczny & Kędziorski, Reference Nakonieczny and Kędziorski2005; Nakonieczny et al., Reference Nakonieczny, Kędziorski and Michalczyk2007a,Reference Nakonieczny, Michalczyk and Kędziorskib; Łozowski et al., Reference Łozowski, Kędziorski, Nakonieczny and Łaszczyca2014).
Our modelling confirms that if a butterfly population is to be restored, habitat-oriented measures are crucial for achieving long-term stability. On the other hand, the introduction of captive-reared individuals to habitats where the conditions are inappropriate is ineffective. Over-supplementation with introduced individuals can be counterproductive, as the habitat carrying capacity can be permanently reduced. Long-term studies of restored populations are crucial, as they facilitate the analysis of the time-lagged results of conservation measures such as reintroduction.
Acknowledgements
This study was financed from the statutory funds of the Institute of Nature Conservation, Polish Academy of Sciences. We thank the Management of the Pieniny National Park for facilitating data collection and analysis, and members of the Park's staff, particularly Bogusław Kozik and Jacek Berezicki, for their assistance in gathering field data.
Author contributions
Study concept and design: PA, AMĆ; fieldwork: PA; model construction: AMĆ; data analysis: PA; writing: PA, AMĆ.
Conflicts of interest
None.
Ethical standards
The research was conducted in accordance with the ethical standards applicable in the EU and those of Oryx. All research related to establishing the captive breeding programme and releasing individuals was with permission of the Polish Ministry of the Environment (Permit No. MOŚ/II/4185/91). The exchange of material with the Slovak part of the Pieniny Mountains was in compliance with Permit No. 4772/96/95 from the Polish Ministry of the Environment and Permit No. 00045/95 from the Slovak Ministry of the Environment. Habitat restoration was part of the Annual Conservation Measure Plans for the Pieniny National Park (Roczne Plany Zadań Ochronnych dla Pienińskiego Parku Narodowego), approved by the Polish Ministry of the Environment. No animals were collected or harmed during this research.








