Inflammatory processes are adaptive physiological mechanisms of organism defense against tissue injury or infection( Reference Medzhitov 1 ). The first description of inflammatory processes in tissue date back to the 19th century by Virchow( Reference Virchow 2 ), who had already linked inflammation to chronic conditions( Reference Heidland, Klassen and Rutkowski 3 ). Over the following centuries, the description of cellular and molecular mechanisms involved in the acute inflammatory response has led to the unraveling of some of the most important characteristics of these adaptive processes( Reference Medzhitov 1 ). Inflammatory response to injury now appears as a highly controlled succession of key stages, the dysregulation of which has been recognised as leading to tissue damage in the long term( Reference Medzhitov 1 ). In particular, chronic low-grade inflammation has been recognised as a key underlying mechanism for several chronic diseases, including obesity, type 2 diabetes, cancer and CVD( Reference Coussens and Werb 4 – Reference Gregor and Hotamisligil 9 ).
Chronic low-grade systemic inflammation appears to be influenced by both non-modifiable and modifiable factors( Reference Medzhitov 1 , Reference Delves and Roitt 10 , Reference Gabay and Kushner 11 ). Among the former are genetic and hormonal factors, which appear to be affected, in part, by ageing( Reference Gruver, Hudson and Sennpowski 12 , Reference Maijo, Clements and Ivory 13 ). In particular, genetically driven modifications appear to be involved in the immune system senescence leading to a higher inflammatory status with ageing( Reference Gruver, Hudson and Sennpowski 12 , Reference Maijo, Clements and Ivory 13 ). In contrast, nutrition is now recognised as one of the key modifiable factors in regulating chronic low-grade systemic inflammation( Reference Calder, Albers and Antoine 14 ). Multiple components of the diet have been identified as having the capacity to intervene in the production or regulation of inflammatory mediators( Reference Hansson 15 , Reference Serhan, Chiang and Van Dyke 16 ). Among the nutritional factors most studied, long-chain fatty acids have been identified as key precursors for pro- or anti-inflammatory mediators( Reference Serhan, Chiang and Van Dyke 16 ). However, investigating the contribution of the whole diet to inflammatory processes requires specific methods such as assigning dietary scores based on culinary practices or dietary recommendations (a priori scores), or by conducting analyses of dietary intakes reported in research studies or surveys (a posteriori scores) (e.g. principal components analysis)( Reference Hu 17 ). Investigating dietary patterns is important since nutrients may have synergistic or antagonistic effects in interaction with the food matrix depending on the combination of foods within meals that compose the diet( Reference Jacobs, Gross and Tapsell 18 ). In a previous paper, we showed that diet characterised by high intakes of antioxidant micronutrients and essential fatty acids were significantly associated with a reduced risk of elevated C-reactive protein (CRP)( Reference Julia, Meunier and Touvier 19 ).
To better characterise the inflammatory properties of the diet, a priori dietary indices, specifically aiming at measuring the dietary inflammatory load have been developed based on the existing literature examining associations between diet and systemic chronic inflammation( Reference Cavicchia, Steck and Hurley 20 , Reference Shivappa, Steck and Hurley 21 ). The dietary inflammatory index (DII) score has been associated to inflammatory biomarkers in cross-sectional studies and repeated measures short-term studies( Reference Cavicchia, Steck and Hurley 20 , Reference Shivappa, Steck and Hurley 22 – Reference Wood, Shivappa and Berthon 25 ), as well as with mortality and several chronic diseases: CVD, cancer and the metabolic syndrome( Reference Wirth, Burch and Shivappa 24 – Reference Zamora-Ros, Shivappa and Steck 46 ). Second, an alternative dietary inflammatory index (ADII) has recently been developed based on the DII by Cavicchia et al.( Reference Cavicchia, Steck and Hurley 20 ) and validated against biomarkers of inflammation( Reference van Woudenbergh, Theofylaktopoulou and Kuijsten 47 ). This ADII differs from the DII by the type and number of items included in the score, and the way energy is taken into account. However, the contribution of the diet to low-grade chronic inflammation in the long term has not been evaluated.
Our objective was to investigate the association between the DII and the ADII scores and levels of CRP in the long term, in a middle-aged French cohort study. Moreover, as the various non-modifiable factors involved in inflammatory processed are reported to be modified with ageing, age was tested as a potential effect modifier of the relationship.
Methods
Study population
The study population was selected from participants of the SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) 2 study. The initial SU.VI.MAX study was designed in 1994–1996 as a randomised, double-blind, placebo-controlled, primary prevention trial designed to evaluate the effect of an 8-year supplementation with antioxidant vitamins and minerals at nutritional doses on the incidence of CVD and cancer( Reference Hercberg, Galan and Preziosi 48 ). In 2007–2009, participants were offered the opportunity to enroll in an additional observational follow-up study, the SU.VI.MAX2 study – on which our study sample was based( Reference Kesse-Guyot, Fezeu and Jeandel 49 ). Subjects who agreed to enroll underwent a clinical examination and had blood drawn for biological tests. In the SU.VI.MAX2 study, a subsample selected on geographical criteria for operative and logistical aspects (including availability to travel to the evaluation centres of the SU.VI.MAX2 study) was included in the ‘CRP study’.
Both the SU.VI.MAX and the SU.VI.MAX2 studies were approved by the Ethics Committee for Studies with Human Subjects of Paris-Cochin Hospital (no. 706 and no. 2364, respectively) and the Comité National Informatique et Liberté (no. 334641 and no. 907094, respectively). All subjects gave written informed consent to participate in the study. The initial SU.VI.MAX trial was registered at clinicaltrial.gov (no. NCT00272428).
All subjects included in the CRP study were eligible for the present study, whether they were members of the placebo or supplementation group of the initial SU.VI.MAX trial phase. Subjects having less than three dietary records in the first 2 years of the SU.VI.MAX study (1994–1996) and subjects having missing data on covariates were excluded from the analyses.
Data collection
Dietary data assessment
Dietary information was collected every 2 months using 24-h dietary record via computerised questionnaires. A total of six randomly distributed records could be completed per year, covering each day of the week and all seasons. Participants were assisted by an instruction manual, which included validated photographs of >250 generic foods shown in three main portion sizes( Reference Le Moullec, Deheeger and Preziosi 50 ). A French food composition table was used to estimate the daily nutrient intake( Reference Hercberg 51 ). Dietary records were considered as invalid if energy intake was or <418 or >25 104 kJ/d (<100 or >6000 kcal/d). In addition, men reporting <3347 kJ/d (<800 kcal/d) and women reporting <2092 kJ/d (<500 kcal/d) across at least one-third of their dietary records were excluded to account for energy under-reporting. Mean daily nutritional intakes were calculated as the mean of all available 24-h dietary records during the first 2 years of follow-up (mean number of 24 h records/subject=10·1 (sd 3·07)).
Dietary inflammatory indices
Two DII scores were computed in the present analysis, the DII and ADII. The DII is a score initially designed with forty-five dietary parameters determined from a literature review. A first version of the DII was published in 2009( Reference Cavicchia, Steck and Hurley 20 ), and the score was updated with the inclusion of more than 1943 articles in 2014( Reference Shivappa, Steck and Hurley 21 ). The computation of the updated DII has been described in detail previously( Reference Shivappa, Steck and Hurley 21 ). A literature-derived inflammatory effect score is assigned to every micronutrient, macronutrient or food parameter associated with an increase (+1), a decrease (−1) or no effect (0) on six of the following inflammatory biomarkers: IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP, based on a detailed literature review. An overall inflammatory weight is attributed to each of the dietary components, based on the number and design of each study investigating that component. The weights of the various components therefore range between −0·908 and 0·429( Reference Shivappa, Steck and Hurley 21 ). The mean individual intake of every food parameter in the sample is converted to a z-score using standardised values from a world database, then converted to a percentile and centred, in order to correct for the skewness of the initial variables distribution. Finally, the centred percentile score for each parameter is multiplied with its associated inflammatory weight; then these are summed across the parameters, thus providing an individual DII score. In the present study, the DII was based on thirty-six dietary parameters available in the database: total energy intake, protein, carbohydrate, total fat, SFA, cholesterol, vitamin B12, Fe, MUFA, PUFA, n-3 fatty acids, n-6 fatty acids, alcohol, fibre, Mg, niacin, thiamine, riboflavin, vitamin B6, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, anthocyanidins, flavan-3-ol, flavonols, flavanones, flavones, isoflavones, garlic, ginger, pepper (seasoning), onion and tea. Lower (i.e. more negative) DII scores represent more anti-inflammatory diets, whereas higher (i.e. more positive) DII scores represented more pro-inflammatory diets.
An alternative score (ADII), based on the DII by Cavicchia et al. and following the methodology laid down by van Woudenbergh et al.( Reference van Woudenbergh, Theofylaktopoulou and Kuijsten 47 ) was also computed. In brief, the ADII differs from the original DII, as it does not include those variables for which all subparts are already included in the score (e.g. energy intake, as all macronutrients are already included in the score, total fat, as all fatty acids are included), and it does not take into account polyphenol components (anthocyanidins, flavan-3-ol, flavonols, flavanones, flavones, isoflavones). Moreover, it uses energy-adjusted standardised intakes in the computation of the final score( Reference van Woudenbergh, Theofylaktopoulou and Kuijsten 47 ). Energy-adjusted residuals are used in order to reduce the variation in dietary intake resulting from differences in physical activity, body size and metabolic efficiency( Reference Willett, Howe and Kushi 52 ).
Inflammation measurement
Blood samples were drawn from participants in the SU.VI.MAX2 study (2007–2009), immediately centrifuged and stored frozen at −80°C. CRP concentrations were measured using an immunoturbidimetric assay (reagent: Tina quant C-reactive protein (latex) assay; Roche), with a detection limit of 1 mg/l for CRP. Intra-assay and inter-assay CV were 0·61 and 2·87 %, respectively.
Covariate assessment
Baseline self-administered questionnaires were used to collect data regarding socio-demographic factors (marital status (single/cohabiting), educational level (primary, secondary, superior)), physical activity (subjects were asked to report if they regularly practiced physical activity (yes or no) and if yes, if they practiced the equivalent of >1 h walking/d (yes or no), herein coded as: irregular, <1 h equivalent walking/d, ≥1 h equivalent walking/d) and smoking status (never smoked, former smoker, current smoker)). Data were obtained through self-administered questionnaire at baseline (1994–1996).
Anthropometric measurements were taken at a clinical examination 1 year after inclusion in the SU.VI.MAX study (1995–1997) and considered as baseline data. Weight was measured to the nearest 0·1 kg with subjects wearing only light clothing and without shoes. Height was measured to the nearest cm using a wall-mounted stadiometer in the same conditions. BMI was calculated as the weight (kg) divided by the square of height (m), and obesity was defined as BMI≥30 kg/m2.
Data from a total of 3476 subjects were included in the CRP study. Of these, 861 were excluded for insufficient number of dietary records, 502 for missing data on anthropometric measurements, forty-three for missing data on socio-demographic variables and thirty-nine for missing data on tobacco use or physical activity. Finally, fifty-one subjects were further excluded for CRP values >10 mg/l, leaving a final sample of 1980 subjects for analyses. Compared with the 8112 subjects having three available dietary records in the SU.VI.MAX study, subjects included in the study were more likely to be male, older, non-smokers and with higher physical activity.
Statistics
For descriptive purposes, nutrient intakes across DII and ADII scores were computed using the residual approach. CRP was categorised as ≤3 and >3 mg/l (considered as ‘elevated CRP’) and values >10 mg/l were excluded( Reference Pearson, Mensah and Alexander 53 ). Logistic regression models were applied to estimate OR (95 % CI) of CRP>3 mg/l across tertiles of DII scores. P trend across tertiles of DII scores were computed using DII tertiles as continuous variables. Crude associations were investigated, as well as several covariate adjustments: (1) sex and initial allocation in the supplementation/placebo group of the SU.VI.MAX trial (although initial supplementation allocation group had no effect on probability of CRP>3 mg/l, for consistency the variable was included in the adjustment procedure); (2) model 1+baseline age, educational level (primary/secondary/university), baseline smoking status (never smoker/former smoker/current smoker), baseline physical activity (irregular/<1 h equivalent walking/d/≥1 h equivalent walking/d), energy intake and number of available dietary records; (3) model 2 + BMI at baseline. Number of available dietary records was included in the adjustment procedure in order to take into account the accuracy of the collected data. Interactions were tested between DII scores and sex, age (cut-off points at the median age, 50 years old), initial allocation to the supplementation or placebo group in the SU.VI.MAX trial period, overweight status at baseline, energy and alcohol intakes (cut-off points at the median intake). Stratified analyses were conducted in case of significant interactions (P<0·20). Type I error was increased in interaction tests in order to improve power( Reference Marshall 54 ). In sensitivity analyses, we excluded subjects who had provided less than six dietary records (thus augmenting the precision of the dietary data). Additional sensitivity analyses excluded obese subjects at baseline and subjects with a cancer or CVD diagnosis during the first 2 years of follow-up.
All analyses were carried using SAS software (version 9.3; SAS Institute Inc.). All tests were two-sided and a P value<0·05 was considered significant.
Results
In the included population, DII and ADII were strongly correlated, as the Spearman’s correlation between DII and ADII was 0·72 (κ coefficient between tertiles 0·44).
Overall, subjects in the highest tertile of DII scores (i.e. with a pro-inflammatory diet) were more likely to be women, younger, less educated, current smokers and to practice physical activity irregularly (Table 1). Similar results were observed for ADII scores (see online Supplementary Table S1), except for sex, as subjects in the highest tertile of ADII were more likely men. They also were more likely to have lower total energy intakes, lower energy intakes from carbohydrates, lower intakes of PUFA (both n-3 and n-6 fatty acids) and lower intakes of vitamin and fibre. Conversely, they were more likely to have higher energy intakes from lipids and proteins, higher intakes of alcohol and SFA (Table 2). Again, similar results were found with ADII scores (see online Supplementary Table S2), except for energy intakes and macronutrient intakes. For ADII, as expected, energy intakes across tertiles were similar. Carbohydrates and lipid intakes were similar across tertiles, and protein intakes increased with increasing scores, whereas they decreased with increasing scores for DII. Finally, overall, the differences in nutrient intakes between tertile 1 and tertile 3 were higher for ADII compared with DII.
Table 1 Characteristics of the Supplémentation en Vitamines et Minéraux Antioxydants population included in the study (n 1980) according to tertiles of dietary inflammatory index (DII) score (Numbers and percentages; mean values and standard deviations)

CRP, C-reactive protein.
* P values obtained using χ 2 tests or ANOVA tests, as required.
Table 2 Dietary intakes of the Supplémentation en Vitamines et Minéraux Antioxydants population included in the study according to tertiles of dietary inflammatory index (DII) scores (n 1980)Footnote † (Mean values and standard deviations)

* P values obtained using ANOVA tests.
† Nutrient intakes expressed as energy-adjusted residuals.
At the 12-year follow-up examination, a total of 266 subjects had elevated CRP measurements. Long-term associations between DII and ADII and low-grade inflammation are tabulated in Table 3. Overall associations between DII, ADII and long-term inflammation were not statistically significant. No interaction was detected between the DII and age (P=0·36) or between DII or ADII and sex, group allocation in the SU.VI.MAX trial period, overweight status at baseline, energy or alcohol intakes. In contrast, a qualitative interaction was found between ADII and age (P=0·16), and stratified analyses according to age showed that the ADII was prospectively associated with CRP only among younger participants of the SU.VI.MAX study (age at inclusion <50 years: tertile 3 v. tertile 1: OR 1·79 (95 % CI 1·04, 3·07)) SU.VI.MAX study participants (Table 4). The restriction to subjects with at least six dietary records led to similar results (n 1748), though the relationships were no longer significant. The exclusion of obese subjects at baseline or subjects diagnosed with CVD or cancer during the first 2 years of follow-up did not modify results.
Table 3 Logistic associations between tertiles and continuous dietary inflammatory index (DII) scores with long-term elevated C-reactive protein in the Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) study (n 1980)(Odds ratios and 95 % confidence intervals)

ADII, alternate dietary inflammatory index.
* Model 1 adjusted on sex and initial allocation in the supplementation/placebo group of the SU.VI.MAX trial.
† Model 2: model 1+baseline age, educational level (primary/secondary/university), baseline smoking status (never smoker/former smoker/current smoker), baseline physical activity (irregular/<1 h equivalent walking/d/≥1 h equivalent walking/d), energy intake and number of dietary records available.
‡ Model 3: model 2+BMI at inclusion.
Table 4 Multivariate stratified logistic associations between dietary inflammatory index (DII) scores and long-term elevated C-reactive protein in the Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) study (n 1980)Footnote * (Odds ratios and 95 % confidence intervals)
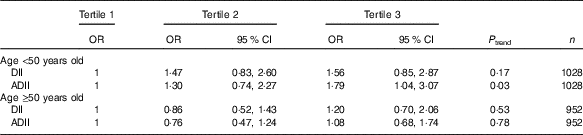
ADII, alternate dietary inflammatory index.
* Model adjusted on sex and initial allocation in the supplementation/placebo group of the SU.VI.MAX trial, baseline age, educational level (primary/secondary/university), baseline smoking status (never smoker/former smoker/current smoker), baseline physical activity (irregular/<1 h equivalent walking/d/≥1 h equivalent walking/d), energy intake and number of dietary records available, BMI at inclusion.
Discussion
In this study on adults issued from the French general population, the inflammatory potential of the diet, measured through the a priori score ADII, which uses energy-adjusted intakes, was prospectively associated with long-term inflammation measured using CRP only in the younger subjects at inclusion (35–50 years old). To the best of our knowledge, this is the first study to investigate the relationship between dietary inflammatory scores (DIS) with different computational methods and low-grade inflammation with a long-term longitudinal follow-up.
Short-term longitudinal studies and cross-sectional studies investigating the association between DIS and low-grade biological inflammation have shown somewhat mixed results. In a sample of 600 subjects from the Seasonal Variation of Blood Cholesterol (SEASONS) study (mean age=48 years old), an anti-inflammatory diet estimated by the DII was associated with a lower risk of having elevated CRP (high-sensitivity CRP (hs-CRP)>3 mg/l)( Reference Cavicchia, Steck and Hurley 20 ); similar results were found in the same population when updating the original DII score, with pro-inflammatory diets being associated with higher odds of having elevated CRP (OR of 3rd tertile v. 1st tertile of DII score=1·47 (95 % CI 1·03, 2·12)( Reference Shivappa, Steck and Hurley 22 ). In the Buffalo Cardio-Metabolic Occupational Police Stress study, a similar association between DII score and elevated hs-CRP was found( Reference Wirth, Burch and Shivappa 24 ). In other studies, the association between DII scores and hs-CRP were not statistically significant( Reference Shivappa, Hebert and Rietzschel 23 , Reference Wood, Shivappa and Berthon 25 , Reference Zamora-Ros, Shivappa and Steck 46 , Reference Alkerwi, Shivappa and Crichton 55 ), although the DII was found to be associated with other biological markers of inflammation (namely, IL-6 and IL-4)( Reference Shivappa, Hebert and Rietzschel 23 , Reference Wood, Shivappa and Berthon 25 ). Overall, these results suggest cross-sectional to short-term associations between DII scores and biological inflammatory biomarkers. However, further studies are needed to better characterise relationships with different types of biomarkers involved.
DII and ADII take into account a wide range of nutrients, some of which also have been identified as being associated with overall quality of the diet. As such, DII and ADII share some components with existing a priori diet quality indices. For instance, the Mediterranean diet score, which shares with DII and ADII some components both directly (ethanol intake or MUFA intakes) or indirectly (through the use of fruit and vegetables or fish and seafood consumption components) has repeatedly been associated with biological inflammatory components in cross-sectional studies( Reference Carter, Roberts and Salter 56 , Reference Chrysohoou, Panagiotakos and Pitsavos 57 ). In the Multi-Ethnic Study of Atherosclerosis, an a priori defined comprehensive healthy dietary pattern including forty-seven food groups (positive foods groups including fruit, vegetables and whole grains, negative food groups including meat, fats and snacking products) was found to be associated with various biomarkers of inflammation, including CRP( Reference Nettleton, Schulze and Jiang 58 ).
With more relevance for the present results, the investigation of dietary patterns specifically related to intakes in pro- and anti-inflammatory dietary components (with the use of reduced rank regression methods) in the SU.VI.MAX study showed that a diet with high intakes of linoleic acid and antioxidant vitamins (energy-adjusted residuals of β-carotene, vitamins C and E) was negatively associated with long-term elevated CRP( Reference Julia, Meunier and Touvier 19 ). These results underline the difficulty of identifying and quantifying the specific contribution of dietary components to low-grade inflammation and to disentangle their effect from those of the overall nutritional quality of the diet.
The findings of our study also imply that the association between DIS and long-term inflammation depends on the computation algorithm. Indeed, the ADII was only associated with long-term chronic inflammation. Noteworthy, the original ADII developed by van Woudenbergh et al.( Reference van Woudenbergh, Theofylaktopoulou and Kuijsten 47 ) was associated with a summary score for low-grade inflammation including CRP, IL-6, IL-8, TNF-α, serum amyloid A and soluble intercellular adhesion molecule-1. Differences between the DII and the ADII reside in: (1) the use of energy-adjusted components using the residual method, (2) the exclusion of certain items of the score, which are already comprised in other components of the score (i.e. total fat and types of fatty acids, and energy and macronutrients) and (3) the exclusion of polyphenol components( Reference van Woudenbergh, Theofylaktopoulou and Kuijsten 47 ). Control for energy intake in the development of dietary indices – whether focusing on specific components of the diet or overall diet quality indices – is of major importance, as energy intake is a confounder in the relationship between diet and health outcomes( Reference Waijers, Feskens and Ocke 59 ). Energy intakes act as confounders in multiple ways: first, subjects with higher overall food consumption tend to have higher intakes in all micronutrients – therefore higher dietary scores – but also energy intakes; second, energy can act in itself as a pro-inflammatory factor, through the increase in adipose tissue. This second confounding is somewhat taken into account in the DII computation, as energy appears as a parameter of the score. Moreover, the balance between the various types of fatty acids (long-chain n-6 and n-3 fatty acids in particular) rather than their total amount is thought to have a major impact on inflammatory processes( Reference Simopoulos 60 ). Therefore, the use of both total fat and the various fatty acids in the DII might add a confounding factor to the relationship between diet and long-term inflammation. These computational differences led to a higher discrimination of subjects according to nutrient intakes for ADII compared with DII.
Our results suggest that the inflammatory potential of the diet is associated with long-term CRP only in subjects under 50 years old at baseline. Several hypothesises may be proposed to explain these findings. Ageing is associated with a wide range of modifications in the immune system, both in the adaptive and the innate immunity. These changes include the decline in T-cell functions linked to thymic atrophy, modifications in the cellularity of bone marrow associated with systemic growth hormone decrease or decline in capacity to produce inflammatory cytokines by ageing macrophages( Reference Gruver, Hudson and Sennpowski 12 ). Hormonal replacement therapy in menopausal women has also been shown to increase CRP( Reference Vitale, Gebara and Mercuro 61 ). Low-grade systemic inflammation has been reported to be a characteristic of ageing processes, so-called ‘inflammaging’( Reference Chung, Cesari and Anton 62 , Reference Franceschi and Campisi 63 ). Inflammaging has been hypothesised to be related to increased production and/or inadequate elimination of damaged cells and molecules with ageing( Reference Franceschi and Campisi 63 ), or to alterations in the balance between pro- and anti-inflammatory mediators and cytokines( Reference Maijo, Clements and Ivory 13 ). Though nutrition may mediate the regulation of inflammatory cytokines, many of the mechanisms involved in the immune system senescence and inflammaging are non-modifiable and genetically driven( Reference Maijo, Clements and Ivory 13 ). In this context, it may be inferred that the inflammatory potential of the diet could be of particular importance during life periods in which non-modifiable ageing processes have not yet become the main determinant of the inflammatory state of an individual. As hormone replacement therapy may be considered as a confounder for the relationship, we ran a sensitivity analysis adjusting for this confounder, and results were not modified.
Strengths of our study include its long-term design with over 12 years of follow-up, the collection of extensively accurate data from repeated dietary records and the investigation of multiple inflammatory dietary scores. Moreover, we were able to investigate DIS, taking in to account multiple confounders, with the use of several sensitivity analyses.
Some limitations need to be addressed. First, we did not have access to hs-CRP. This limited our capacity to investigate low-grade inflammation, as 61 % of our sample was under the detection limit for CRP, impeding us to use linear regression methods. However, the lower limit of our CRP measurements (i.e. 1 mg/l) still allowed us to investigate low-grade inflammation using an internationally validated cut-off point( Reference Pearson, Mensah and Alexander 53 ). Second, we had only one measurement of CRP at the end of the follow-up and no baseline measurement. Therefore, we were not able to exclude subjects with elevated CRP at baseline or conduct repeated measures analysis. However, the results from our sensitivity analyses, excluding subjects susceptible of having elevated CRP (obese subjects at baseline or subjects diagnosed with cancer or CVD during the first 2 years of follow-up) showed consistent results, therefore suggesting that this bias may be of limited importance. Third, we did not have access to other inflammatory biomarkers or to repeated measurements of CRP. Though the inclusion of multiple biomarkers appears preferable to evaluate inflammatory status, CRP has been shown to be a good marker of low-grade inflammation, acting as an independent inflammatory predictor for multiple chronic diseases( Reference Coussens and Werb 4 , Reference Libby 6 , Reference Mantovani, Allavena and Sica 7 , Reference Pradhan, Manson and Rifai 64 ). However, generalisation of our results to all inflammatory biomarkers or inflammatory status should be taken with caution. Other studies are needed to confirm the results of our study, and expand to other inflammatory biomarkers. Studies with repeated measurements are also needed to better understand the concurrent modifications of diet and inflammation. Finally, subjects in our study were volunteers recruited for a long-term cohort study (initially a randomised trial followed by an additional observational phase) on nutrition. Compared with subjects with at least three dietary records at baseline, subjects included in our study were more likely older, non-smokers and with higher physical activity levels, which could have strengthened this selection bias. The risk profile of our population is therefore very likely different and non-representative from the general population, with lower risks, which could have led to an underestimation of the associations.
Overall, our results suggest a differential effect of the inflammatory potential of the diet on inflammation according to age. Such results should be taken into account when analysing the prospective association between diet and inflammatory-related diseases.
Acknowledgements
The authors thank Paul Flanzy, Mohand Ait Oufella, Yasmina Chelghoum, Than Duong Van (computer scientists), Cédric Agaesse (dietitian), Nathalie Arnault, Veronique Gourlet, Fabien Szabo, Laurent Bourhis, Stephen Besseau (statisticians) and Rachida Mehroug (logistics assistant) for their technical contribution to the study.
K. E. A. was supported by a doctoral fellowship by the University of Paris 13, Sorbonne Paris Cité. Drs N. S., J. R. H. and M. D. W. were supported by the United States National Institute for Diabetes, Digestive and Kidney Diseases (grant no. R44DK103377). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
C. J. wrote the statistical analysis plan, analysed the data, and drafted and revised the paper. E. K.-G. participated in statistical analysis plan, analysed the data and critically revised the paper for important intellectual content. M. T. and K. E. A. analysed the data and critically revised the paper for important intellectual content. S. H. and E. K.-G. designed data collection tools, implemented the study, monitored data collection for the whole study and critically revised the draft paper for important intellectual content. J. R. H., M. D. W. and N. S. aided in the interpretation of data related to the dietary inflammatory index, as well as provided these calculations, and critically revised the paper for important intellectual content. All authors have read and approved the final manuscript.
J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. N. S. and M. W. are employees of CHI. None of the other authors declares any conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114517000034







