Ultra-processed foods (UPF) are, according to the NOVA classification, ‘formulations of ingredients, mostly for industrial use only, derived from a series of industrial processes’(Reference Monteiro, Cannon and Levy1). Examples of UPF are breakfast cereals, savoury snacks, reconstituted meat products, frankfurters, pre-packaged frozen dishes, soft and/or sweetened drinks, distilled alcoholic beverages and supplements.
UPF represents an important and growing part of the world’s food supply. Recent studies have reported that these foods account for a significant percentage of about 50–60 % of the energy content in the usual diet of the average US, Canadian or British consumer(Reference Martínez Steele, Baraldi and Louzada2–Reference Rauber, da Costa Louzada and Steele4). The increase in the volume of industrially processed products in the global food supply has coincided with an increasing prevalence of obesity and non-communicable diseases in many countries(Reference Lawrence and Baker5), suggesting a possible association between UPF consumption and obesity risk, but studies on the potential health effects of UPF are limited.
Some cross-sectional studies have reported a significant association between UPF consumption, obesity(Reference Louzada, Baraldi and Steele6–Reference Nardocci, Leclerc and Louzada9) and the metabolic syndrome(Reference Martínez Steele, Juul and Neri10), while others have shown no association(Reference Adams and White11,Reference Nasreddine, Tamim and Itani12) . In addition, results from a large French prospective cohort study, the NutriNet-Santé study, found that high UPF consumption led to a significant increase in the risk of CVD(Reference Srour, Fezeu and Kesse-Guyot13), diabetes(Reference Srour, Fezeu and Kesse-Guyot14), depressive symptoms(Reference Adjibade, Julia and Allès15) and cancer(Reference Fiolet, Srour and Sellem16). To date, despite great interest in the subject in both scientific and lay communities, there is a lack of consensus in terms of evaluation and impact of UPF on health and no systematic reviews, and meta-analyses were conducted so far on adults. Our study aimed to assess the relationship between UPF consumption as defined by NOVA and health status by conducting a comprehensive systematic review with meta-analysis of all the cross-sectional and cohort studies published so far.
Methods
Search strategy and selection of studies
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines(Reference Moher, Liberati and Tetzlaff17). The protocol was registered at www.crd.york.ac./PROSPERO/uk as CRD42020165495. Two authors (G. P. and M. D.) independently performed systematic literature searches in MEDLINE, Embase, Scopus, Web of Science and Google Scholar databases, from inception to June 2020. Further studies were searched by checking the references of the identified articles. The keywords ‘ultra processed’ or ‘ultraprocessed’ or ‘ultra-processed’ or ‘NOVA’, and ‘food’ or ‘foods’ were used in combination as medical subject heading (MeSH) terms and text words. No language limitations were applied. Missing data or additional information were requested from the corresponding authors of the articles.
Two investigators (G. P. and M. D.) independently assessed articles potentially relevant for eligibility. Observational studies that reported a measure of association (risk estimates with CI or standard errors or sufficient data to calculate them) between the UPF consumption – defined by the NOVA Food Classification System(Reference Monteiro18,Reference Monteiro, Cannon and Levy19) and evaluated by dietary recalls, food records or questionnaires – and health indicators were considered eligible for inclusion. The decision to include the studies was based on the study title, abstract and full-text screening. The inclusion and exclusion criteria are summarised in online Supplementary Table S1, following the PECOS (Population, Exposure, Comparison, Outcome, Study) design format. Eligible studies were included if they met the inclusion criteria for the study population (clinically healthy subjects aged ≥18 years), exposure (high UPF consumption), reference group (low UPF consumption), outcome (any health indicator), study design (cross-sectional and prospective cohort studies) and statistics (sufficient data to allow calculation of differences between subjects consuming high UPF levels and those consuming low UPF levels). Case–control studies were excluded to minimise bias in recall and selection. Review articles, letters to the editor, comments, case reports and randomised controlled trials were also excluded. Discrepancies were resolved through consensus and discussion with a third investigator (M. P. M.) if consensus could not be reached.
Data extraction
Data extraction was carried out in duplicate by two investigators (M. D. and G. P.) using a standardised form. Disagreements were resolved by consensus or by a third investigator (M. P. M.) if consensus could not be reached. The following data were extracted from the original articles: main author, year of publication, country of study population, cohort, number of participants evaluated and events, length of follow-up (years), age of the population at baseline, sex, definition of outcome of interest, method used to assess UPF intake, comparison, measures of effect size and CI, and details of adjustment for confounding factors in the multivariate model. If the results were reported separately for men and women, they were included in the analysis as separate cohorts.
Assessment of methodological quality
Two investigators (G. P. and M. D.) independently assessed the methodological quality of each included study using the National Institutes of Health study quality assessment tool for observational cohort and cross-sectional studies(20). This tool has fourteen items in total, with an overall rating based on weaknesses in critical domains (see online Supplementary Table S2). The critical domains were the following: research question, exposure assessed prior to outcome measurement, exposure measurements and evaluation, outcome measurements, and statistical analysis. The final results lead to an overall methodological evaluation of good, fair or poor. Disagreements were resolved by consensus or by a third investigator (M. P. M.) if consensus could not be reached.
Statistical analysis
All data were analysed using Review Manager (RevMan; version 5.3 for Macintosh). A random-effects model (DerSimonian and Laird method) was applied to combine multivariable-adjusted risk ratios (RR) or OR of the highest v. the lowest category of UPF consumption. The pooled results were reported as RR and were presented with 95 % CI with two-sided P values. Meta-analysis was conducted if ≥2 studies were available for an outcome. Outcomes expressed in β-coefficient or prevalence ratio have been excluded from the meta-analysis. The statistical heterogeneity between studies was estimated using the χ 2 Cochran’s Q-test with the I 2 statistic, which provides an estimate of the amount of variance between studies due to the heterogeneity rather than sampling error(Reference Higgins, Thompson and Deeks21). Where I 2 exceeded 50 %, heterogeneity was considered substantial and subgroup analyses were performed to explore the source of the heterogeneity(Reference Higgins and Green22). The robustness of the results was established by eliminating each study one by one from the meta-analysis and recalculating the summary estimate (the ‘leave one out’ approach). If ≥5 studies were available, the possibility of publication bias was explored by visual inspection of funnel plot of the effect size against standard error. A P <0·05 was considered statistically significant.
Results
Search results
The selection process is shown in Fig. 1, according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. The search produced 2619 articles. After title and abstract screening, sixty-three articles were selected for the evaluation of the full text. At the end of the selection process, twenty-three articles were included in the qualitative analysis and nineteen in the quantitative analysis.

Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram for search strategy. RCT, randomised controlled trials.
Selected cross-sectional studies (n 10 studies) examined the association between the UPF consumption and the following health outcomes: overweight/obesity (n 5), high waist circumference or abdominal obesity (n 5), BMI gain (n 3), hypertension (n 3), low HDL-cholesterol (n 3), the metabolic syndrome (n 3), hypertriacylglycerolaemia (n 3), hyperglycaemia (n 3), waist circumference gain (n 2), irritable bowel syndrome (n 1), and C-reactive protein levels (n 1). Selected prospective cohort studies (n 13) examined the association between the UPF consumption and all-cause mortality (n 5), CVD risk/mortality (n 3), overweight/obesity (n 2), depression (n 2), IHD/cerebrovascular risk/mortality (n 2), waist circumference gain (n 1), hypertension (n 1), frailty (n 1), CHD (n 1), overall cancer risk/mortality (n 1), breast cancer (n 1), prostate cancer (n 1) and colorectal cancer (n 1).
Cross-sectional studies
Table 1 presents the main characteristics of the ten cross-sectional studies included in the systematic review. The overall analysis comprised 113 753 participants. Three studies were conducted in Brazil(Reference Louzada, Baraldi and Steele6,Reference Silva, Giatti and de Figueiredo8,Reference Lopes, Araújo and Levy23) , two in Canada(Reference Nardocci, Leclerc and Louzada9,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) , two in the USA(Reference Juul, Martinez-Steele and Parekh7,Reference Martínez Steele, Juul and Neri10) , one in France(Reference Schnabel, Buscail and Sabate25), one in the UK(Reference Rauber, Steele and Louzada26) and one in Lebanon(Reference Nasreddine, Tamim and Itani12). The evaluation of UPF consumption was conducted through 24-h dietary recall in five studies(Reference Juul, Martinez-Steele and Parekh7,Reference Nardocci, Leclerc and Louzada9,Reference Martínez Steele, Juul and Neri10,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24,Reference Schnabel, Buscail and Sabate25) , 24-h food records in one study(Reference Louzada, Baraldi and Steele6), 4-d food records in one study(Reference Rauber, Steele and Louzada26) and FFQ in the remaining three studies(Reference Silva, Giatti and de Figueiredo8,Reference Nasreddine, Tamim and Itani12,Reference Lopes, Araújo and Levy23) . Exposure was assessed through the total energy contribution from UPF. The methodological quality score was fair in six studies(Reference Louzada, Baraldi and Steele6–Reference Silva, Giatti and de Figueiredo8,Reference Martínez Steele, Juul and Neri10,Reference Schnabel, Buscail and Sabate25,Reference Rauber, Steele and Louzada26) and poor in four studies(Reference Nardocci, Leclerc and Louzada9,Reference Nasreddine, Tamim and Itani12,Reference Lopes, Araújo and Levy23,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) .
Table 1. Characteristics of cross-sectional studies evaluating ultra-processed food (UPF) consumption and different health outcomes
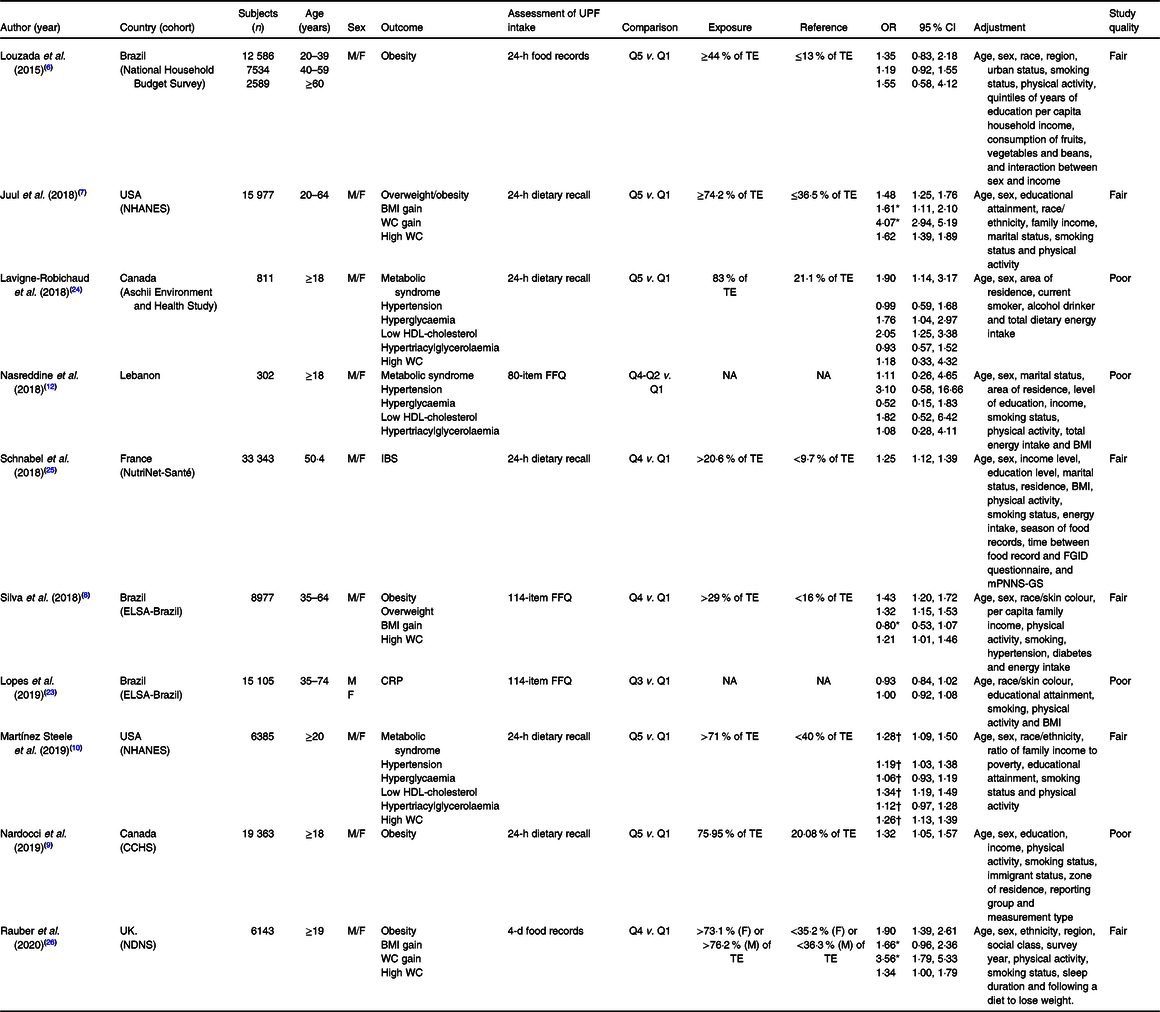
CCHS, Canadian Community Health Survey; CRP, C-reactive protein; E, energy; ELSA, Brazilian Longitudinal Study of Adult Health; F, female; FGID, four functional gastrointestinal disorders; IBS, irritable bowel syndrome; NHANES, National Health and Nutrition Examination Survey; M, male; mPNNS-GS, Modified Programme National Nutrition Santé Guideline Score; NDNS, UK National Diet and Nutrition Survey; TE, total energy; WC, waist circumference.
* Values are expressed as β-coefficients.
† Values are expressed as prevalence ratios.
Meta-analytic pooling under a random-effects model indicated a significant association between the highest UPF consumption and increased risk of overweight/obesity in five studies(Reference Louzada, Baraldi and Steele6–Reference Nardocci, Leclerc and Louzada9,Reference Rauber, Steele and Louzada26) with a total population of 73 169 subjects (OR 1·39, 95 % CI 1·29, 1·50; P < 0·00001), without any evidence of statistical heterogeneity between studies (I 2 = 0 %; P = 0·47) (Fig. 2). Similarly, a statistically significant association was found between highest UPF consumption and increased risk of high waist circumference or abdominal obesity in four studies(Reference Juul, Martinez-Steele and Parekh7,Reference Silva, Giatti and de Figueiredo8,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24,Reference Rauber, Steele and Louzada26) with a population of 31 908 (OR 1·39, 95 % CI 1·16, 1·67; P = 0·0003), without any statistical heterogeneity between studies (I 2 = 49 %; P = 0·12). In addition, highest UPF intake was associated with increased risk of the metabolic syndrome (OR 1·79, 95 % CI 1·10, 2·90; P = 0·02) and reduced HDL-cholesterol levels (OR 2·02, 95 % CI 1·27, 3·21; P = 0·003), with no evidence of statistical heterogeneity between studies (I 2 = 0 %; P = 0·49 and I 2 = 0 %; P = 0·86, respectively) in two studies(Reference Nasreddine, Tamim and Itani12,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) involving a limited population of 1113 subjects. On the other hand, no statistically significant associations emerged between highest consumption of UPF and hypertension(Reference Nasreddine, Tamim and Itani12,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) (OR 1·31, 95 % CI 0·50, 3·43; P = 0·58), hyperglycaemia(Reference Nasreddine, Tamim and Itani12,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) (OR 1·10, 95 % CI 0·34, 3·52; P = 0·87) or hypertriacylglycerolaemia(Reference Nasreddine, Tamim and Itani12,Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) (OR 0·95, 95 % CI 0·60, 1·50; P = 0·82).
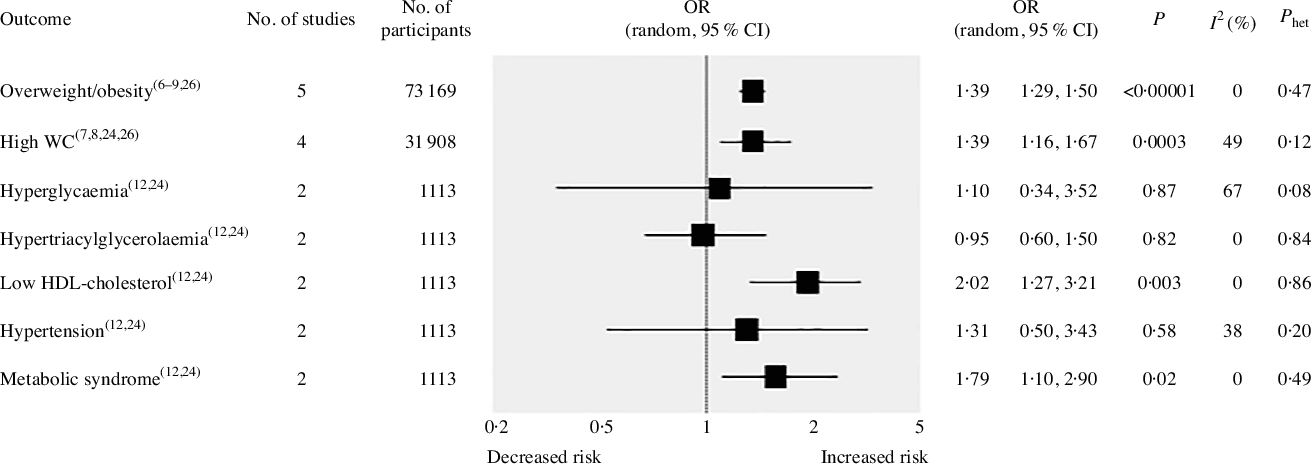
Fig. 2. Forest plot of cross-sectional studies investigating the association between ultra-processed foods consumption and different health outcomes. P value is for Z test of no overall association between exposure and outcome; P het is for test of no differences in association measure among studies; I 2 estimates from heterogeneity rather than sampling error. WC, waist circumference.
Prospective cohort studies
Table 2 presents the main characteristics of the thirteen prospective cohort studies included in the systematic review. The overall analysis comprised 183 491 participants followed over a period ranging from 3·5 to 19 years. Two cohorts were based in Spain(Reference Mendonça, Pimenta and Gea27–Reference Sandoval-Insausti, Blanco-Rojo and Graciani32), one in France(Reference Srour, Fezeu and Kesse-Guyot13,Reference Adjibade, Julia and Allès15,Reference Fiolet, Srour and Sellem16,Reference Schnabel, Kesse-Guyot and Allès33) , one in Brazil(Reference Canhada, Luft and Giatti34), one in Italy(Reference Bonaccio, Di Castelnuovo and Costanzo35) and one in the USA(Reference Kim, Hu and Rebholz36). The evaluation of UPF consumption was conducted through 24-h dietary recalls, FFQ and dietary history. Exposure is extremely variable and ranges from the contribution of the total energy of UPF to servings per d or daily intake. The methodological quality score was good in all studies but one(Reference Sandoval-Insausti, Blanco-Rojo and Graciani32).
Table 2. Characteristics of prospective cohort studies evaluating ultra-processed food (UPF) consumption and different health outcomes
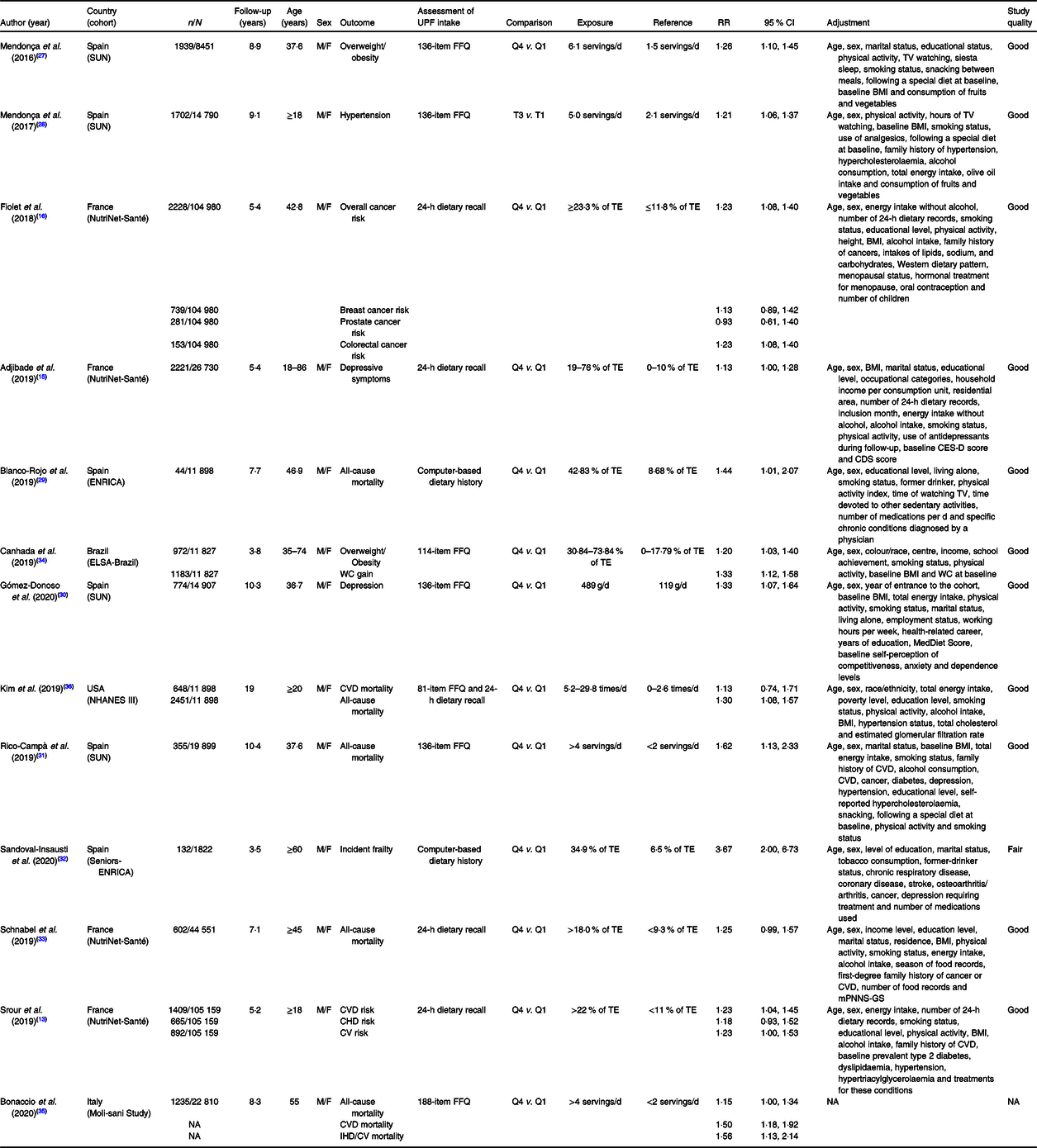
CDS, Cognitive Difficulties Scale; CES-D, Center for Epidemiologic Studies Depression Scale; CV, cerebrovascular; ELSA, Brazilian Longitudinal Study of Adult Health; ENRICA, Study on Nutrition and Cardiovascular risk factors in Spain; F, female; F-up, follow-up; M, male; mPNNS-GS, Modified Programme National Nutrition Santé Guideline Score; NA, not available; NHANES, National Health and Nutrition Examination Survey; RR, risk ratio; TV, television; SUN, University of Navarra Follow-Up Project; WC, waist circumference.
The results of the pooled analysis for all included studies are shown in Fig. 3. The highest consumption of UPF was found to be associated with an increased risk of all-cause mortality in five studies(Reference Blanco-Rojo, Sandoval-Insausti and López-Garcia29,Reference Rico-Campà, Martínez-González and Alvarez-Alvarez31,Reference Schnabel, Kesse-Guyot and Allès33,Reference Bonaccio, Di Castelnuovo and Costanzo35,Reference Kim, Hu and Rebholz36) involving 111 056 subjects and 4687 deaths (RR 1·25, 95 % CI 1·14, 1·37; P < 0·00001), with no statistical heterogeneity between studies (I 2 = 2 %; P = 0·40). In addition, highest UPF intake showed a significant association with increased risk of CVD incidence and/or mortality in three studies(Reference Srour, Fezeu and Kesse-Guyot13,Reference Bonaccio, Di Castelnuovo and Costanzo35,Reference Kim, Hu and Rebholz36) with 2501 cases (RR 1·29, 95 % CI 1·12, 1·48; P = 0·0003; I 2 = 7 %, P = 0·34), cerebrovascular disease incidence and/or mortality in two studies(Reference Srour, Fezeu and Kesse-Guyot13,Reference Bonaccio, Di Castelnuovo and Costanzo35) with 1150 cases (RR 1·34, 95 % CI 1·07, 1·68; P = 0·01; I 2 = 32 %, P = 0·22) and depression in two studies(Reference Adjibade, Julia and Allès15,Reference Gómez-Donoso, Sánchez-Villegas and Martínez-González30) with 2995 cases (RR 1·20, 95 % CI 1·03, 1·40; P = 0·02; I 2 = 42 %, P = 0·19). The statistically significant association was also found for overweight/obesity in two studies(Reference Mendonça, Pimenta and Gea27,Reference Canhada, Luft and Giatti34) with 2911 cases, (RR 1·23, 95 % CI 1·11, 1·36; P < 0·0001) and no evidence of heterogeneity between studies (I 2 = 0 %, P = 0·64).
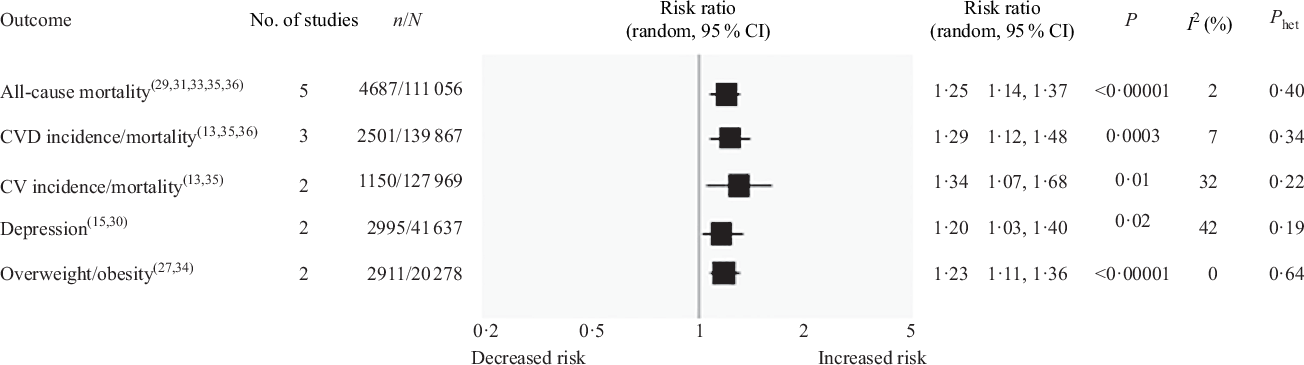
Fig. 3. Forest plot of prospective cohort studies investigating the association between ultra-processed foods consumption and different health outcomes. P value is for Z test of no overall association between exposure and outcome; P het is for test of no differences in association measure among studies; I 2 estimates from heterogeneity rather than sampling error. CV, cerebrovascular.
Sensitivity analysis and publication bias
A leave-one-out sensitivity analysis was performed by iteratively removing one study at a time to confirm that our results were not determined by a single study. There were few changes in the quantitative measurements of OR, RR and the 95 % CI, without any study affecting the results for almost all of the outcomes investigated. The only exceptions were found in cross-sectional study analyses for the metabolic syndrome, low HDL-cholesterol levels and hyperglycaemia. For the metabolic syndrome and low HDL-cholesterol levels, the removal of the study by Lavigne-Robichaud et al.(Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez24) changed the relative effect from significant (in the main analysis) to non-significant in the sensitivity analysis (OR 1·11, 95 % CI 0·26, 4·65; P = 0·89 and OR 1·82, 95 % CI 0·52, 6·42; P = 0·35). Conversely, for hyperglycaemia, the removal of the study by Nasreddine et al.(Reference Nasreddine, Tamim and Itani12) changed the relative effect from non-significant in the main analysis to significant in the sensitivity analysis (OR 1·76, 95 % CI 1·04, 2·97; P = 0·03).
The publication bias was evaluated for all-cause mortality (online Supplementary Fig. S1). The shape of the funnel plot did not show any evident asymmetry, suggesting the absence of possible publication biases.
Discussion
The present study is the first systematic review with meta-analysis that evaluated all available observational studies that assessed the association between UPF consumption and health status. By comparing the highest v. the lowest UPF consumption, the pooled analysis of cross-sectional studies, carried out for each result in a limited number of studies, showed a possible increase in the risk of overweight/obesity, high waist circumference, reduced levels of HDL-cholesterol and the metabolic syndrome. Similarly, for prospective cohort studies, the increased UPF consumption was associated with an increased risk of all-cause mortality in five studies, CVD in three studies, cerebrovascular disease and depression in only two studies, and confirmed the significant association found for overweight/obesity, but only in two studies. These results, although reporting interesting and useful data to formulate a hypothesis, must be carefully interpreted due to the low number of subjects and studies investigated.
In recent years, the global food system has undergone a profound transformation in terms of technology and food processing. The food profile of the world’s countries has changed significantly in favour of the consumption of highly processed industrial products for reasons of economic convenience, industrial competition and attractiveness to the consumer(Reference Monteiro, Moubarac and Cannon37). For all these reasons, the availability and consumption of UPF have increased significantly in all countries, regardless of economic level(Reference Moodie, Stuckler and Monteiro38). These foods are defined based on a classification system called NOVA, which classifies foods into four groups according to the industrial processing used in their production(Reference Monteiro, Cannon and Levy1). The NOVA classification is a simple classification based on the food technology and does not provide any indications on the nutritional content of the food. According to this classification, UPF are defined as products ‘created mostly or entirely from substances extracted from food or derived from food constituents with little or no intact food’. Since Monteiro coined the term UPF, there have been an increasing number of studies that have associated UPF consumption with negative health outcomes in adult subjects(Reference Moubarac, Batal and Louzada3,Reference Martínez Steele, Popkin and Swinburn39) , including cardiometabolic risk factors(Reference Silva Meneguelli, Viana Hinkelmann and Hermsdorff40), CVD(Reference Bonaccio, Di Castelnuovo and Costanzo35), cancer(Reference Fiolet, Srour and Sellem16) and many other outcomes(Reference Adjibade, Julia and Allès15,Reference Schnabel, Buscail and Sabate25,Reference Sandoval-Insausti, Blanco-Rojo and Graciani32) .
In the present study, we have thoroughly evaluated all the observational studies that investigated the possible association between UPF consumption and health status, and we made a quantitative assessment of the association through the meta-analytical procedure. The number of studies included was limited, especially for individual outcomes, and does not allow us to be sure that the results obtained are completely reliable but is sufficient to hypothesise the nature of the association that must then be tested in intervention studies for validation. The analysis of the ten cross-sectional studies showed an increased risk of overweight/obesity, high waist circumference, reduced HDL-cholesterol levels and the metabolic syndrome but not of the other outcomes such as hypertension, hyperglycaemia or hypertriacylglycerolaemia in adults who consume high levels of UPF compared with those who consume less. The analysis of prospective cohort studies confirmed the significant increase in the risk of overweight/obesity and documented a 29 % increase in the risk of CVD incidence and/or mortality, a 34 % increase in the risk of cerebrovascular disease and a 20 % increase in the risk of depression.
The explanations for the possible harmful effects of UPF on health are different and may lie in the fact that these foods are, de facto, indicators of poor food quality, containing high amounts of free or added sugars, fats, low levels of fibre and high energy density(Reference Gibney41). These characteristics can reasonably explain the negative effect of these products on cardiovascular and cardiometabolic risk factors, as well as the risk of overweight/obesity. However, beyond the nutritional composition, UPF could also explain their harmful effects through other mechanisms, such as the presence of compounds that are formed during the processing of the food, and therefore more present in UPF. For example, both acrylamide – a contaminant present in heat-treated processed food products – and acrolein – a compound formed during fat heating – have been associated with an increased risk of CVD(Reference Zhang, Huang and Zhuang42,Reference DeJarnett, Conklin and Riggs43) . In addition, bisphenol A – an industrial chemical used in some UPF plastic packaging – has been found associated with an increased risk of cardiometabolic disorders(Reference Rancière, Lyons and Loh44). Although bisphenol A is banned for use in food packaging in many countries, it has now been replaced by other components such as bisphenol S, which also has endocrine-disrupting properties, and is suspected to be absorbed more orally than bisphenol A(Reference Buckley, Kim and Wong45). Recent studies have confirmed that UPF consumption is associated with increased exposure to endocrine-disrupting chemicals and phthalates used in industrial plastic packaging(Reference Buckley, Kim and Wong45,Reference Serrano, Braun and Trasande46) . Another possible explanation for the harmful effects of UPF on health status is related to their organoleptic characteristics, which have led to an increase in the eating rate and delayed satiety signalling, leading to higher overall food intake. Hall et al. recently(Reference Hall, Ayuketah and Brychta47) conducted a randomised controlled trial on twenty weight-stable adults who were randomised to receive either ultra-processed or unprocessed ad libitum diets for 2 weeks. At the end of the study, a significant increase in body weight was reported along with an overall increase in energy intake only after the UPF-rich diet. In addition, it was also hypothesised that UPF can adversely affect health by modifying the gut microbiome in such a way that it disturbs the energy balance and promotes the selection of microbes that promote inflammation-related diseases such as CVD and metabolic diseases and even depression(Reference Foster and McVey Neufeld48,Reference Zinöcker and Lindseth49) .
The present study has several limitations that should be addressed. First, the included studies evaluated UPF consumption through self-reported tools (FFQ, food records and 24-h recalls), which are generally accepted, but which are susceptible to recall bias, and which are not specifically designed to collect UPF data as described by the NOVA classification. This may result in an over- or underestimation of the UPF intake level. Indeed, the application of the National Institutes of Health study quality assessment tool suggested that the methodological quality of all the cross-sectional studies included was fair or poor, mainly due to the lack of details on the validity and reliability of the questionnaires used to assess UPF consumption. Secondly, the overall analyses for each different outcome were carried out in a limited number of studies, thus reducing the statistical power of the analysis. Third, only a limited number of studies included total energy intake as a confounding variable in the multivariable models, thus introducing a possible limitation in the interpretation of the results. However, it should be noted that total energy intake can also be part of the causal pathway of UPF intake; therefore, this aspect is not necessarily a study limitation. In addition, it is well known that unhealthy eating habits (i.e. high consumption of UPF) are commonly associated with other unhealthy lifestyle behaviours, such as sedentary habits, which in turn are associated with adverse health outcomes. Thus, the results of the present meta-analysis should be interpreted with caution, since not all the included studies considered unhealthy lifestyle behaviours as confounding factors in the multivariable models. On the other hand, the study presents also some strengths such as a rigorous search and selection strategy that identified all available cross-sectional and prospective cohort studies examining the relationship between UPF consumption and health status, and the fact that all but one of the included cohort studies had good methodological quality, with an adequate follow-up, and high participation rates.
In conclusion, we reported for the first time in a systematic review with meta-analysis the possible association between high UPF consumption, worse cardiometabolic risk profile (reported mainly by an increased risk of overweight/obesity, elevated waist circumference, reduced HDL-cholesterol levels and increased risk of the metabolic syndrome), and greater risk of all-cause mortality, CVD, cerebrovascular disease and depression. The available literature still has several limitations and the methods used to classify these foods need careful review, so reducing the applicability and transferability of these results to the general population. However, these findings have important public health implications, especially for food policymakers who should discourage the consumption of UPF and promote fresh and minimally processed foods to improve health status.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. B., L. I., F. S. designed the research; G. P. and M. D. conducted the systematic literature search, performed the quality assessment and the data extraction; G. P. performed the statistical analysis; M. D., G. P., M. P. M. wrote the paper; M. B., L. I., F. S. critically reviewed the manuscript and had primary responsibility for final content; all authors contributed to writing and reviewing the manuscript and read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520002688








