Introduction
Rivers are continually undergoing changes that are closely linked to adjacent terrestrial ecosystems. Eutrophication of fluvial ecosystems as a result of anthropogenic activity (e.g. discharge of urban and industrial wastewaters and the use of fertilizers and pesticides in agriculture) leads to important changes in aquatic communities (zooplanktonic, benthic and fish communities) and loss of biodiversity (Gilbert and Avenant-Oldewage, Reference Gilbert and Avenant-Oldewage2017). Environmental conditions can also directly or indirectly affect the presence of parasitic organisms (mainly those parasites with complex life cycles) due to changes in the abundance and distribution of the respective intermediate and definitive hosts (Sures, Reference Sures2004).
Echinorhynchus truttae Schrank, 1788, an acanthocephalan parasite of salmonids, is known to infect a variety of species, including brown trout (Salmo trutta), one of the most important species of freshwater fish in Europe and of high economic value. This parasite is found throughout Europe (including Ireland and the British Islands) and its range extends across Siberia to the Bering Strait (Wayland, Reference Wayland2013). The life cycle of E. truttae requires two hosts. Fish act as final hosts for the adult worms, which inhabit the digestive tract, and amphipods, such as Gammarus, act as the intermediate hosts that harbour the infective form (cystacanth). The life cycle is completed when the infected crustaceans are eaten by an appropriate definitive host (Crompton and Nickol, Reference Crompton and Nickol1985; Kennedy, Reference Kennedy2006).
During a large study of the parasitic fauna of brown trout captured in several rivers in north-western (NW) Spain, remarkable differences in the presence of E. truttae were observed. This work provided data on the prevalence, mean intensity and mean abundance of this acanthocephalan parasite in specimens of S. trutta captured in the adjacent Tambre and Ulla river basins in Galicia and constitutes the first report of this acanthocephalan in Spain.
Materials and methods
This study was carried out in 19 rivers belonging to Tambre (n = 10) and Ulla (n = 9) river basins, located on the Atlantic side of Galicia (NW Spain). The Tambre river is 125 km long and the surrounding basin covers an area of 1530 km2, with an elongation ratio of 0.34. The Ulla river is 132 km long and drains an area of 2803 km2, with an elongation ratio of 0.45 (Río Barja and Rodríguez Lestegás, Reference Río Barja and Rodríguez Lestegás1992) (Fig. 1).
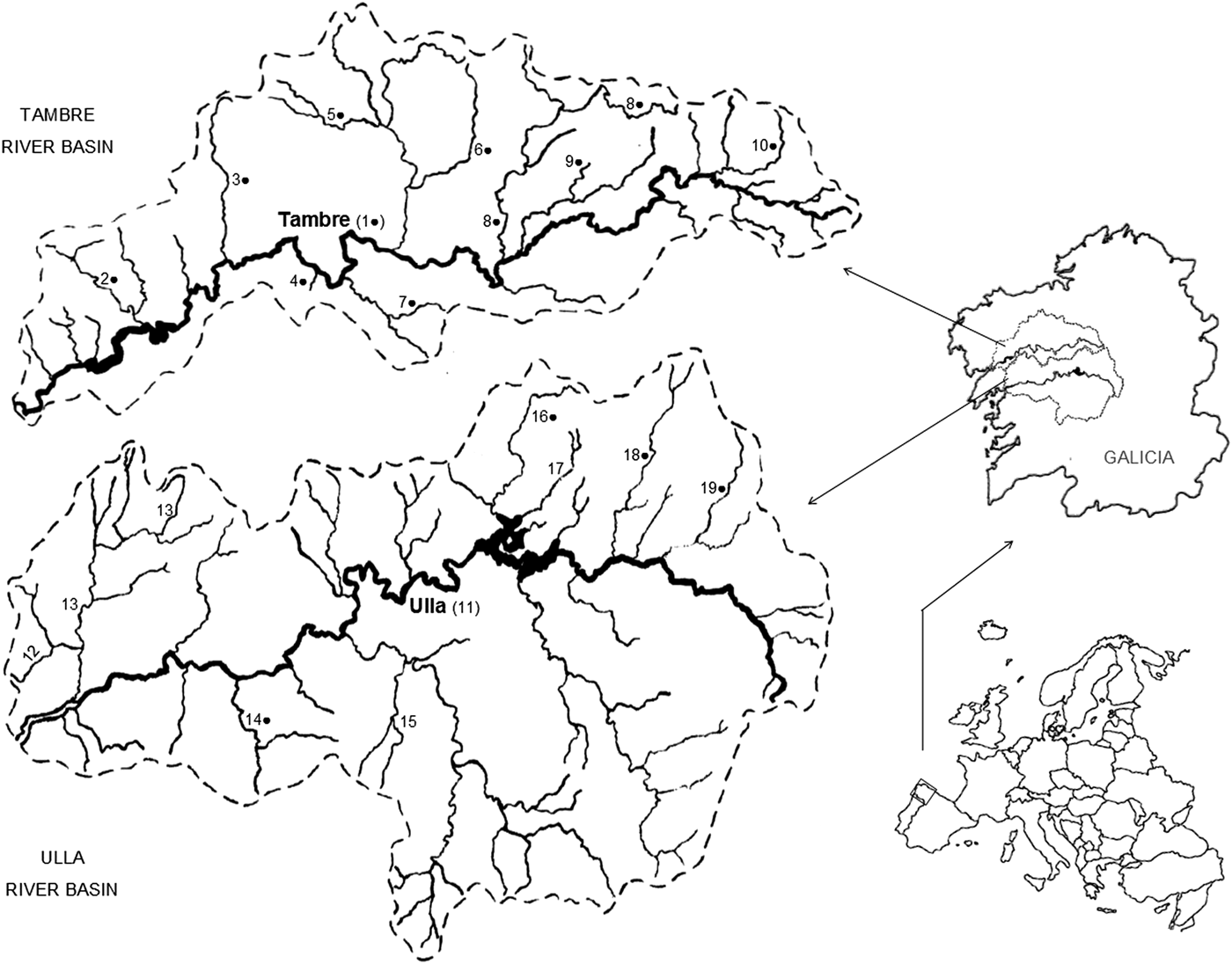
Fig. 1. Geographical location of the rivers in the Tambre and Ulla basins (Galicia, NW Spain) where brown trout specimens (Salmo trutta) were captured. (1) Tambre, (2) Barcala, (3) Dubra, (4) Oufín, (5) Paradela, (6) Lengüelle, (7) Sionlla, (8) Samo, (9) Gaiteiro, (10) Cabalar, (11) Ulla, (12) Rois, (13) Sar, (14) Liñares, (15) Toxa, (16) Iso, (17) Boente, (18) Furelos, (19) Pambre. ● Echinorhynchus truttae presence.
During the 2015 fishing season (15 March–15 August), a total of 343 specimens of brown trout (S. trutta) were captured by local anglers, who removed the gastrointestinal tracts from the fish and stored them in hermetically sealed plastic bags at −20° C, before sending them to the Laboratory of Parasitology in the Faculty of Pharmacy (University of Santiago de Compostela) for analysis. The anglers also provided data such as the length of the fish and the river where they were caught. Fish age was estimated from length as described by Sánchez-Hernández et al. (Reference Sánchez-Hernández, Servia, Vieira-Lanero, Barca-Bravo and Cobo2012) for specimens captured in the same study area: 19.0–19.1 cm (<2 years); 19.2–25.9 cm (2–3 years); >26.0 cm (>3 years).
The intestines were opened longitudinally and the adult forms of E. truttae were removed, washed with physiological saline solution and conserved in 70% ethanol. The parasites were stained with lactophenol cotton blue and identified following Crompton and Nickol (Reference Crompton and Nickol1985) and Buchmann and Bresciani (Reference Buchmann and Bresciani2001). The intestinal contents were also removed and ground in a mortar with 0.04 M phosphate-buffered saline (PBS) pH 7.2. The homogenates thus obtained were filtered through a set of two sieves (mesh size 150 and 45 µm) before being subjected to diphasic concentration in PBS (0.04 M pH 7.2)/diethyl ether (2:1) by centrifugation at 1250 g , 4 °C, for 15 min. The supernatants were carefully discarded, and the concentration step was repeated until lipid-free sediments were obtained. Aliquots of 10 µL of the sediments were examined under bright field microscopy (×200 magnification) (AX70, Olympus Optical Co., Ltd., Tokyo, Japan). Prevalence rates, mean intensities and mean abundances of adults and eggs were used to describe the parasite infection according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Statistical analyses were performed with Statgraphics® Centurion XVI v.16.2.04 Statistical Software (©1982–2013 StatPoint Technologies, Inc., Warrenton, VA, USA). Differences in the prevalence rates in relation to the river basin and fish age were investigated using Fisher's exact test and the χ 2 test. The mean intensities and mean abundances were compared by Mann–Whitney test. Differences were considered statistically significant at P < 0.05.
Results
Macroscopic and microscopic analysis of the intestinal contents of 343 brown trout revealed the presence of adults and/or eggs of E. truttae in 53 of the 123 trout from Tambre river basin (43.1%). By contrast, parasitic forms of this acanthocephalan parasite were only observed in eight of the 220 fish from the Ulla basin (3.6%), showing significant differences between the prevalences obtained in two adjacent watersheds (P < 0.001; odds ratio 19.0). The mean intensity and mean abundance of adult forms also differed significantly between the two river basins studied (P < 0.01) (Table 1). Furthermore, E. truttae was found in trout captured in all sampled rivers in the Tambre basin (n = 10), whereas the parasite was only detected in trout from four of the nine rivers sampled in the Ulla basin (Fig. 1).
Table 1. Prevalence, mean intensity and mean abundance of Echinorhynchus truttae in brown trout (Salmo trutta) captured in two adjacent river basins in Galicia (NW Spain) in relation to the length and estimated age of the fish specimens

N, number of trout; P, prevalence (%); MI, mean intensity (adults/infected trout); MA, mean abundance (adults/examined trout); SD, standard deviation.
a Estimated according to the length of the fish following Sánchez-Hernández et al. Reference Sánchez-Hernández, Servia, Vieira-Lanero, Barca-Bravo and Cobo2012.
b Minimun legal size = 19.0 cm.
Regarding the age of the specimens, both the prevalence and mean abundance of E. truttae adults in trout captured from Tambre river basin were significantly higher in fish older than 3 years that in younger trout (<2 and 2–3 years) (P < 0.05) (Table 1).
Discussion
Since the last quarter of the 20th century, when Cordero del Campillo and Álvarez Pellitero (Reference Cordero del Campillo and Álvarez Pellitero1976) mentioned the presence of acanthocephalan species in brown trout (S. trutta) captured in Galician rivers, no data have been made available on these parasites in the geographical area considered in the present study. Álvarez Pellitero (Reference Álvarez Pellitero1979) carried out a wide study on 1205 trout from several rivers in León, a region close to Galicia. However, this researcher did not detect any acanthocephalan parasites in the trout. To our knowledge, the present study is the first study providing data on the prevalence, mean intensity and mean abundance of E. truttae in brown trout in Spain.
The prevalences of infection by E. truttae, determined after macroscopic and microscopic examination of the intestines of 123 and 220 trout from Tambre and Ulla basins, were 43.1 and 3.6%, respectively. These values are within the range described for the same host in different areas of Europe. Thus, the prevalence of infection ranged between 5.5 and 93.3% for ten of 21 locations studied in Central Scotland (Dorucu et al. Reference Dorucu, Crompton, Huntingford and Walters1995), while the prevalence rates of 1.9, 17.4 and 47.1% were reported for trout captured in three streams in northern Italy (Dezfuli et al. Reference Dezfuli, Giari, Biaggi and Poulin2001). Recently, in Turkey, Amin et al. (Reference Amin, Heckmann, Evans and Tepe2016) reported a prevalence rate of 84.5% for Echinorhynchus baeri in S. trutta. By contrast, acanthocephalan species were not detected in 140 trout captured in four Irish lakes (Byrne et al. Reference Byrne, Grey, Holland and Poole2000) or in 484 fish captured in ten hydrographic drainage basins in the Mediterranean island of Corsica (Quilchini et al. Reference Quilchini, Foata, Mouillot, Mattei and Marchand2010).
The effect of the fish size on both the abundance and richness of helminth parasites is well documented (Guégan and Hugueny, Reference Guégan and Hugueny1994). Higher prevalence and abundance in older trout can be attributed to a higher infection risk than younger specimens because older fish are exposed to infective prey for a longer time period and, also, larger fish ingest greater amounts of prey. Dezfuli et al. (Reference Dezfuli, Giari, Biaggi and Poulin2001) observed that the number of E. truttae adults per fish was significantly correlated with fish length in one of the three streams studied in northern Italy. Similarly, in our study, the percentage of positive samples and the number of E. truttae adults per fish were higher in trout of length >26 cm than in smaller specimens. This correlation was observed in trout captured in the Tambre basin, but it was not detected in specimens from the Ulla basin, possibly due to the small number of positive samples obtained from this river basin.
The diet of trout is mainly determined by habitat, season, prey availability and ontogeny (Knutsen et al. Reference Knutsen, Knutsen, Gjosaeter and Jonsson2001; Lehane et al. Reference Lehane, Walsh, Giller and O'Halloran2001; Lagarrigue et al. Reference Lagarrigue, Céréghino, Lim, Reyes-Marchánt, Chappaz, Lavandier and Belaud2002). As the fish grow, their diet changes both qualitatively and quantitatively and they feed on larger macroinvertebrates as well as on other more energetically valuable prey (Oscoz et al. Reference Oscoz, Escala and Campos2000) and, therefore, having higher risk of acquiring parasitic infection through the trophic chain. Prey selection by trout can also play a role in the pattern of E. truttae infection. However, Sánchez Hernández (Reference Sánchez Hernández2009) concluded, after the study of stomach contents of brown trout captured from rivers in the Tambre and Ulla basins, that the feeding of the trout of this geographical area is determined by the most abundant and widely distributed prey through the river.
The parasitization of S. trutta by the acanthocephalan parasite E. truttae implies ingestion of gammarid amphipods harbouring the cystacanth form. This infective form can modify the crustacean behaviour so that it reacts differently to light, becoming more positively phototropic, active and swimming closer to the water surface. The crustaceans thus become more conspicuous to fish and more vulnerable to predation by the definitive host (Fielding et al. Reference Fielding, MacNeil, Dick, Elwood, Riddell and Dunnam2003; MacNeil et al. Reference MacNeil, Fielding, Hume, Dick, Elwood, Hatcher and Dunnam2003; Lagrue et al. Reference Lagrue, Güvenatam and Bollache2013). Unfortunately, we do not have any data on the parasite status of the gammarid amphipod. However, the different prevalence rates of E. truttae suggest that the presence/abundance of the crustacean that acts as the intermediate host may be different in the two river basins. This is supported by information provided by the Environmental Observation Network of Environmental Laboratory (ROAGA-LMAG, Xunta de Galicia) (http://siam.xunta.gal/roaga-lma-descricion) on benthic macroinvertebrate fauna inhabiting several water bodies in the Tambre and Ulla river basins, indicating a higher abundance of gammarids in the Tambre basin (data not shown). Moreover, differences in the prevalence rates of helminth parasites in fish from close water bodies were observed by other authors. Hartvigsen and Kennedy (Reference Hartvigsen and Kennedy1993) carried out studies on composition and richness of helminth communities in S. trutta from ten water reservoirs situated close to each other in a well-defined region of south-west England, and observed that local factors promoting distinctiveness have a greater influence than regional factors which induce similarity.
According to previous reports, the amphipods involved in the biological cycle of E. truttae are species of the genus Gammarus, specifically Gammarus pulex (Crompton and Nickol, Reference Crompton and Nickol1985; Kennedy, Reference Kennedy2006). Although we did not find any reference to the presence of this species in freshwater environments in the Iberian Peninsula, at least ten other species of the genus Gammarus have been described (García and Jaume, Reference García and Jaume2008). The Gammaridae family is the most common group of crustaceans in freshwater environments in Galicia, with Echinogammarus lusitanicus being the most abundant species, although Echinogammarus beriyoni is also present in the middle and low stretches of the rivers (González González and Cobo Gradín, Reference González González and Cobo Gradín2006). As occurs in Ireland with the indigenous Gammarus duebeni (MacNeil et al. Reference MacNeil, Dick and Elwood2000; Prenter et al. Reference Prenter, MacNeil, Dick, Riddell and Dunn2004), our findings suggest that native Galician gammarid species may act as intermediate hosts of E. truttae, although this remains to be confirmed.
Finally, the remarkable difference in the prevalence of E. truttae in brown trout captured in Tambre and Ulla basins may be a consequence of various conditions such as lack of oxygen due to organic pollution and low pH (Meijering, Reference Meijering1991), temperature and salinity changes (Foucreau et al. Reference Foucreau, Cottin, Piscart and Hervant2014; Vereshchagina et al. Reference Vereshchagina, Lubyaga, Shatilina, Bedulina, Gurkov, Axenov-Gribanov, Baduev, Kondrateva, Gubanov, Zadereev, Sokolova and Timofeyev2016), recognized as important factors which influence and alter natural distribution of gammarid amphipods, intermediate hosts for this acanthocephalan parasite.
Acknowledgements
The authors thank anonymous local anglers for their collaboration and the Environmental Observation Network of Environmental Laboratory (ROAGA-LMAG, Xunta de Galicia) for providing information about benthic macroinvertebrate communities.
Financial support
The study was funded by the Autonomous Government of Galicia (grants GPC2014/069 and ED431C 2017/31).
Conflict of interest
None.




