Introduction
The organisms living in and around the Galápagos archipelago are some of the most well-studied life-forms on our planet relative to development and understanding of natural selection as the ultimate driver of organismal evolutionary change and speciation (Lack, Reference Lack1940; Boag and Grant, Reference Boag and Grant1981; Schluter and Grant, Reference Schluter and Grant1984; Gould, 2002; Lamichhaney et al., Reference Lamichhaney, Han, Webster, Andersson, Grant and Grant2018; Zink, Reference Zink2002). Interestingly, Darwin (Reference Darwin1845) wrote only a few pages about his time in the Galapagos where he summarized his scientific collecting work while there and where he superficially noted the geological, climatic, zoological and botanical diversity of the islands. It was only later, after he had returned to England and distributed his collection of biological specimens accumulated during his voyage on the HMS Beagle to various experts at museums that his colleagues in those museums soon let him know that every island of the Galapagos archipelago was inhabited by different species of animals and plants. This finding both stunned and dismayed Darwin because when he was initially collecting specimens on the various islands, he had mixed together his scientific collections from at least 2 of the islands. He stated (Darwin, Reference Darwin1845, pp. 393–394):
I have not yet noticed by far the most remarkable feature in the natural history of this archipelago; it is, that the different islands to a considerable extent are inhabited by a different set of beings. My attention was first called to this fact by the vice governor, Mr. Lawson, declaring that the tortoises differed from the different islands and that he could with certainty tell from which island any one was brought. I did not for some time pay attention to this statement and I had already partly mingled together the collections from two of the islands. I never dreamed that islands, about fifty or sixty miles apart, and most of them in sight of each other, formed of precisely the same rocks, placed under a quite similar climate, rising to a nearly equal height, would have been differently tenanted; but we shall soon see that this is the case.
The Galápagos Islands, rising from the floor of the Pacific Ocean on the equator, are volcanic in origin, being formed from the action of a stationary sub-crustal magmatic plume or hot spot situated under an easterly moving piece of the earth’s crust called the Nazca plate (Holden and Dietz, Reference Holden and Dietz1972; Geist et al., Reference Geist, Snell, Snell, Goddard and Kurz2014a, b). In this archipelago, the current estimate of the maximum age of the easternmost islands is around 3.5 million years (White et al., Reference White, McBirney and Duncan1993; Christie et al., Reference Christie, Duncan, McBirney, Richards, White, Harpp and Fox1992) with an estimated minimum age of around 500,000 years for the islands to the west (Christie et al., Reference Christie, Duncan, McBirney, Richards, White, Harpp and Fox1992; Harpp and Geist, Reference Harpp and Geist2018). The islands are biologically isolated being located on the equator about 950 km west of continental South America. The expedition of biological exploration led by Darwin commenced in the Galápagos on September 15, 1835, while the collecting expeditions (directed and led by Dr Robert C. Dowler, Angelo State University, San Angelo, Texas) that ultimately led to the discovery of the parasites identified and described herein occurred in 1999. Dowler et al. (Reference Dowler, Carroll and Edwards2000) reported on the collecting trips to the Galápagos that occurred in 1995 and 1997 where endemic rodents, that had been considered extinct, were rediscovered. The collecting trip in 1999 was informed by the previous 2 expeditions and specimens of both parasites and their mammalian hosts were preserved as museum specimens (Dowler et al., Reference Dowler, Carroll and Edwards2000).
Knowledge of the approximate ages of individual islands and thus the history of the geological evolution of the emergence of the Galápagos chain into dry-land habitats has a direct impact on our ability to understand the potentially reciprocal biological evolution of the flora and fauna of the islands. As such, one of the best-known examples of natural selection in action comes from studies of the Galápagos finches, which are members of the Tanager family Thraupidae Cabanis, 1847, and the evolution of the 13 species of Darwin’s finches appears to have been from an initial colonization event that occurred around 2–3 million years ago (Sato et al., Reference Sato, O’huigin, Figueroa, Grant, Grant, Tichy and Klein1999; Abzhanov, Reference Abzhanov2010). Interestingly, the divergence time among some of the 7 species of the endemic Galápagos lava lizards of the genus Microlophus Duméril and Bibron, 1837, has been estimated to be as old as 9 million years (Rassmann, Reference Rassmann1997). Since this estimated species divergence time for the species of lizards in the Galápagos is older than the oldest known island, various hypotheses relative to the ages of the islands and arrival times into the Galápagos of animal groups have been proposed, but the dynamic nature of appearance and disappearance of these volcanic islands plays a large part in forming this complex biota (Heads, Reference Heads2014).
If emergent volcanoes existed over the Galápagos magmatic hot spot prior to the emergence and establishment of the current islands, then much of the Galápagos biota could have evolved on these past islands and then transferred or hopped to the new islands when they arose above the surface of the sea, thus explaining species divergence times older than the current islands themselves (Rassmann, Reference Rassmann1997; Heads, Reference Heads2014). The presence of sub-surface seamounts situated southeast of the islands suggests there have been islands forming over the Galápagos magmatic hot spot for at least 14 million years and these now submerged islands may have served as stepping stones or initial landing spots for sweepstakes dispersalists from mainland habitats (Christie et al., Reference Christie, Duncan, McBirney, Richards, White, Harpp and Fox1992; Hoernle et al., Reference Hoernle, van den Bogaard, Werner, Lissinna, Hauff, Alvarado and Garbe-schönberg2002). Initial sweepstakes dispersal via oceanic rafting from the mainland is likely how the Galápagos archipelago first saw the arrival of rice rats, as they are hypothesized to be good dispersers across saltwater (Castañeda-Rico et al., Reference Castañeda-Rico, Johnson, Clement, Dowler, Maldonado and Edwards2019).
Despite the importance of the endemic fauna and flora of the Galápagos archipelago to the development of the theory of speciation and subsequently the theoretical aspects of evolution (Darwin, Reference Darwin1859; Lack, Reference Lack1940, Reference Lack1947; Grant and Grant, Reference Grant and Grant1992; Grant, Reference Grant1986), relatively little work has been done on the evolutionary biology and phylogeny of parasites of vertebrates of these islands. Most published studies related to parasite diversity there are biased towards the avifauna and their ectoparasites with a significant blank in the research literature regarding helminth diversity of avian hosts, as shown in Table 1. Up to the current time, we are not aware of any publications discussing, describing, or even mentioning the diversity of helminth parasites from either the autochthonous land mammals or from any of the introduced synanthropic rodents which currently include only species of Rattus and Mus. Even so, some work on the parasites of vertebrates has been accomplished and Bataille et al. (Reference Bataille, Levin, Sari and Parker2018) published a summary of all known ecto- and endoparasites of vertebrates of the Galápagos biota and provided a discussion of their likely mode of arrival in the island chain. Their review shows that species of the phylum Apicomplexa make up the majority of the documented endoparasites of the Galápagos endemic avifauna (Table 1). Gettinger et al. (Reference Gettinger, Martins-Hatano and Gardner2011) published on mites of the family Laelapidae and described a new species from Aegialomys galapagoensis (Waterhouse, 1839). From birds, Jiménez-Uzcátegui et al. (Reference Jiménez-Uzcátegui, Sarzosa, Encalada, Rodríguez-Hidalgo, Celi-Erazo, Sevilla and Huyvaert2015).reported several helminths from the Waved Albatross, Phoebastria irrorata (Salvin, 1883) collected from the island of Española including unidentified species of Nemata (genus Contracaecum Railliet and Henry, 1912), a species of cestode assignable to Tetrabothrius Rudolphi, 1819, and a species of trematode in the genus Cardiocephaloides Sudarikov, 1959. From the islands of Isabela and Fernandina, an unidentified species of Contracaecum and an unidentified trematode of the family Heterophyidae were reported from the flightless cormorant, Nannopterum harrisi (Rothschild, 1898) (see Carrera-Jativa et al., Reference Carrera-Jativa, Rodriguez-Hidalgo, Sevilla and Jiménez-Uzcátegui2014) and species of Contracaecum and the trematode Renicola sp. were reported from the Galápagos brown pelican, Pelecanus occidentalis Linnaeus, 1766, from several islands (Table 1) (Parker et al., Reference Parker, Whiteman and Miller2006). Finally, an unidentified trematode was reported from the Galápagos rail, Laterallus spilonota (Gould, 1841) (see Bataille et al., Reference Bataille, Levin, Sari and Parker2018). All of these helminth parasites are known to have narrow host-ranges (sensu Agosta, Reference Agosta2006, Reference Agosta2022) occurring only in birds and as will be seen herein, there have been no documented cases of ecological fitting (Janzen, Reference Janzen1980; Agosta, Reference Agosta2006) involving birds and rodents now occurring on the islands.
Table 1. Recorded endoparasites found present in Galápagos endemic vertebrates, including birds, mammals and reptiles

Curiously, up to the current time, the only reported endoparasite from Darwin’s finches is the coccidian Isospora geospizae (McQuistion and Wilson, Reference McQuistion and Wilson1989), although there was also an anecdotal account by Grant who said: ‘… virtually nothing is known about parasites and disease beyond the discovery of parasitic worms in a cactus finch (Salvin 1877), the occasional observation of worms in the feces of ground finches (D. Schluter, pers. comm.), …’ (Grant, Reference Grant1986, p. 65).
Similar to the avifauna of the area, Galápagos reptiles have also been found infected with species of Apicomplexa (Couch et al., Reference Couch, Stone, Duszynski, Snell and Snell1996), and it is interesting that a significant literature has developed around the diversity of pinworms (Nemata: Oxyurida) and other nematodes of the Galápagos tortoise species group, see Petter (Reference Petter1966); Petter and Douglas (Reference Petter and Douglas1976); Bouamer and Morand (Reference Bouamer and Morand2006), Walton AC (1942), and Fournié et al. (Reference Fournié, Goodman, Cruz, Cedeño, Vélez, Patiño, Millins, Gibbons, Fox and Cunningham2015) and references therein.
McIntosh (Reference McIntosh1939) described Infidum luckeri McIntosh, Reference McIntosh1939, a digenetic trematode recovered from the gall bladder of a specimen of the Jubo snake, Phylodryas hoodensis (Van Denburgh, 1912) that died in the US National Zoo (snake specimen No. 7485, parasite specimen – former United States National Parasite Collection (USNPC) Helm. Coll. No. 43409) and was collected most likely either from the island of Española or from Gardner Island, near the island of Española by members of the 1938 Presidential Cruise; these are the only 2 islands from which this species of snake is known (Thomas, Reference Thomas1997).
Parasites of mammals
To our knowledge, the Galapagos sea lion, Zalophus wollebaeki Sivertsen, 1953, is the only mammal in the archipelago reported to be infected with endoparasites prior to the current study; here, individual sea lions were reported to host the eye fluke, Philophthalmus zalophi (Dailey et al., Reference Dailey, Ellin and Parás2005) (Digenea: Philophthalmidae) collected from the islands of Santa Cruz and San Cristobál. In addition, from these sea lions, ascaridoid nematode eggs, other unidentified juvenile nematodes, coccidian oocysts and some cestode eggs, identified as belonging to the order Pseudophyllidea were reported (Dailey et al., Reference Dailey, Ellin and Parás2005; Walden et al., Reference Walden, Grijalva, Páez-Rosas and Hernandez2018). Mites and lice recovered while examining sea lions for the trematode study are also deposited in the former USNPC, but no identifications were attempted (Dailey et al., Reference Dailey, Ellin and Parás2005).
Endemic rodents known from the Galápagos Archipelago include species in the genera Nesoryzomys Heller 1904, Aegialomys Weksler et al., 2006, and Megaoryzomys (Lenglet and Coppois, Reference Lenglet and Coppois1979). Species of Nesoryzomys and Aegialomys are placed in the tribe Oryzomyini (Rodentia: Cricetidae) (see Lenglet and Coppois, Reference Lenglet and Coppois1979; Salazar-Bravo et al., Reference Salazar Bravo, Pardiñas, Zeballos and Teta2016; Ronez et al., Reference Ronez, Brito, Hutterer, Martin and Pardiñas2021). During historical times, 6 species of endemic rodents are known to either have occurred on or currently inhabit various islands of the Galápagos (Tables 2 and 3). Two species of endemic Galápagos rice rats, including Nesoryzomys indefessus (Thomas, 1899) and N. darwini Osgood, 1929, have recently (IUCN, 2019) been declared extinct by the International Union for the Conservation of Nature. Presumably viable populations of 4 other species are still extant, but all are under extreme pressure of anthropogenically mediated imminent obliteration. These species include: Nesoryzomys narboroughi Heller, 1904, N. fernandinae Hutterer and Hirsch, 1979, N. swarthi Orr, 1938, and Aegialomys galapagoensis (Waterhouse, 1839) (see also Prado and Percequillo (Reference Prado and Percequillo2018) and [Tables 2 and 3]). Interestingly, Percequillo et al. (Reference Percequillo, Do Prado, Abreu, Dalapicolla, Pavan, de Almeida Chiquito, Brennand, Steppan, Lemmon, Lemmon and Wilkinson2021) show that a divergence time between the taxa that gave rise to the genera Nesoryzomys and Aegialomys was during Pleistocene time and based on this multi-locus phylogenetic analysis, it appears that precursors of the species of these 2 genera entered into the Galapagos simultaneously and did not evolve from a common ancestor in the islands.
Table 2. Status of all known Galápagos rodents recorded in the literature

* Megaoryzomys curioi has only been identified from remnant skeletal material (Ronez et al., Reference Ronez, Brito, Hutterer, Martin and Pardiñas2021).
Table 3. Distribution and status of rodents recorded on each island of the Galápagos archipelago

Invasive rodents that have successfully colonized various islands in the Galapagos include the black rat, Rattus rattus (Linnaeus, 1758), Norway rat, Rattus norvegicus (Berkenhout, 1769) and house mouse, Mus musculus Linnaeus, 1758. All 3 species arrived on the islands by accompanying humans, with R. rattus founding successful invading populations at least 3 times, with the first occurring between the 17th and 18th centuries (Harper and Carrion, Reference Harper, Carrion, Veitch, Clout and Towns2011; Phillips et al., Reference Phillips, Wiedenfeld and Snell2012) (see Table 3).
The current report provides information derived from a survey where both endemic and invasive rodents in the Galápagos were collected and preserved as museum specimens while giving a description and comparisons of a new species of cestode of the genus Raillietina.
This is the first report of species of Raillietina Fuhrman, 1920, (Cyclophyllidea: Davaineidae) from endemic rodents in the Galápagos. The only other species of Raillietina reported from vertebrates on the islands is R. echinobothrida Mégnin, 1880, from domestic chickens on both San Cristobal and Santa Cruz islands (Gottdenker et al., Reference Gottdenker, Walsh, Vargas, Merkel, Jiménez, Miller, Dailey and Parker2005); this species is known to use both beetles and ants as intermediate hosts (Panich et al., Reference Panich, Tejangkura and Chontananarth2021) and is not known from any endemic Galápagos vertebrates. Interestingly, 2 species of ants of the genus Pheidole Westwood, 1839, were demonstrated to be the intermediate host for R. loeweni (Cestoda) from the black-tailed jackrabbit, Lepus californicus Gray, 1837, in Kansas (Bartel, Reference Bartel1965) and at least 1 species of this genus of ant appears endemic to the Galápagos (Herrera et al., Reference Herrera, Tocora, Fiorentino, Causton, Dekoninck and Hendrickx2024).
Materials and methods
All rodents were captured using ShermanTM and TomahawkTM live traps baited with a mixture of dried rolled oats and peanuts. After capture, specimens were euthanized using chloroform, examined for arthropod (ecto-) and helminth (endo-) parasites, prepared as museum specimens, and transported back to museums in the USA. The pleural and peritoneal cavities were opened and examined for gross evidence of parasites and the intestines were removed, opened and the contents were searched for parasites. All parasites found were fixed in 10% formalin, transported, and stored in a solution of 10% formalin until study. At time of study, specimens of nematodes and cestodes were in placed in 70% ethanol and stored in this solution until staining or clearing. For morphological examination of nematodes, all specimens were transferred to 70% ethanol, rinsed several times in fresh 70% ethanol, cleared for 24 h in lactophenol and mounted in lactophenol on a standard microscope slide under a no. 1 coverslip with a small piece of museum-quality tag-paper under 1 edge of the cover slip to keep the cover slip from squashing the specimen over time. Specimens so prepared were then studied with a Zeiss AxiophotTM digital microscope. All cestodes preserved in the field and transferred to the Manter Laboratory were rinsed several times in 70% ethanol times, stained in Semichon’s Acetic Carmine, destained in 70% acid alcohol, neutralized in 70% ethanol with a few drops of ammonium hydroxide, dehydrated to 100% ethanol in a series of ethanol baths ranging from 70%–85%–95%–100% ethanol (with 2 rinses in 100% with an interval of 20 min), cleared in terpineol, rinsed quickly in xylene and mounted on a microscope slide under a No. 1 cover slip in gum Damar. Larval cestodes found in the livers of Rattus spp. were stained in Semichon’s Acetic Carmine and cleared in lactophenol. To study the hooks of the larval Taeniids, the rostellum was removed and hooks were spread in lactophenol with pressure of a pencil eraser under a 15 mm square coverslip on a standard microscope slide. For the new species of cestodes reported herein, holotype and paratype specimens were deposited in the Parasite Collection of the Harold W. Manter Laboratory of Parasitology, the University of Nebraska-Lincoln (HWML). All helminths recovered and studied are also deposited in the HWML Parasite collections. HWML numbers are given in results.
Results
Endemic species studied in this paper included individuals of Nesoryzomys swarthi obtained from near La Bomba, Santiago Island (0°11.21ʹS; 90°42.04ʹW) while individuals of both Nesoryzomys narboroughi and N. fernandinae were collected at Cabo Douglas on Fernandina Island (1°18.24ʹS; 91°39.14ʹW). Specimens of Aegialomys galapagoensis were obtained from suitable habitats on Santa Fe Island (0°48.21ʹS; 90°2.45ʹW). Invasive species studied included Rattus rattus collected on Volcan Wolf (0°3.96ʹN; 91°24.18ʹW) and Cerro Azul (0°55ʹ42.0954ʹʹN; 91°23ʹ36.9ʹʹW) of Isabela Island while specimens of Rattus norvegicus were collected on Rábida Island (0°24ʹ17.3874ʹʹ;90°42ʹ28.0ʹʹ).
Twelve individuals of each species of endemic rodents were collected and processed as museum specimens. Additionally, 22 individuals of R. rattus and 7 individuals of R. norvegicus were collected, processed, and examined for ecto- and endoparasites. See Table 4 for data on prevalence and numbers for individual species of nematode and cestode parasites recovered. Tapeworms identified as Hymenolepis diminuta Rudolphi, 1819, (Cestoda: Hymenolepididae) were found in both R. rattus and R. norvegicus. No tapeworms of the genus Hymenolepis were found in endemic rodents.
Table 4. Prevalence of endoparasites in rodent species collected by Dr Robert Dowler in 1999

During this work, a new species of the cestode genus Raillietina, was found to occur in the small intestines of 5 specimens of N. swarthi collected at La Bomba, on Isla Santiago. Importantly, none of the Rattus that were examined were found to harbour specimens of Raillietina, although, as noted, these rodents did harbour the almost ubiquitous Hymenolepis diminuta.
Following is the description of a new species of Raillietina. Measurements are given in micrometres (µM) unless otherwise indicated and N is the number of individual characters measured. Whenever possible, in all specimens, measurements of each character were averaged from measurements of characters taken from 5 different segments anteriad of the last mature segment. Measurements of characters in mature segments were taken from the last mature segment, defined as the segment immediately anterior to the observed segment in which eggs begin to appear in the developing uterus. Mean and standard deviation are given in parentheses. For measurements of egg characteristics, N represents the number of individual characters measured in the eggs (see Table 5).
Table 5. Measurements for Raillietina dowleri n. sp. found in Nesoryzomys swarthi on the island of Santiago, Galápagos, Ecuador. Measurements are in micrometers

Description
Raillietina dowleri n. sp
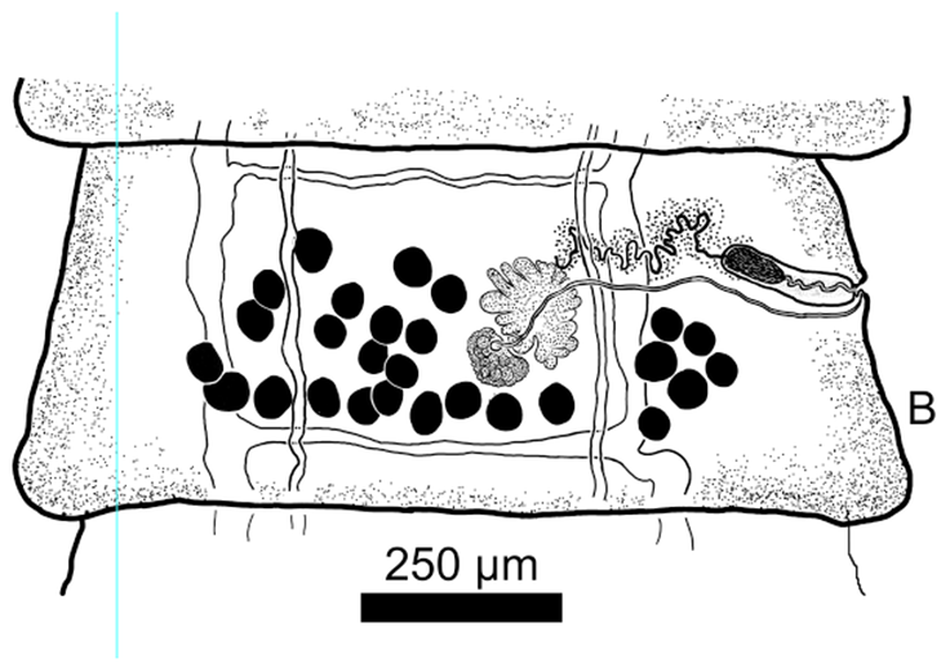
For the following description, 7 full tapeworm specimens were studied. Scolex (Figures 1A; 3A–3C), N = 7, 289–384 (344 ± 36) in maximum width. Suckers, N = 8, 91117 (108 ± 12) long by 64–95 (88 ± 12) wide. Dorsal and ventral osmoregulatory canals join within Scolex at base of rostellum. Rostellum present and armed with approximately 140 claw hammer-shaped hooks, N = 8, 14–16 (15 ± 0.8) long (Figure 3B). Suckers armed with 2 types of hooks or spines showing both thicker falcate shaped hooks with recurved spines (Figure 3C) and thin, claw-shaped hooks (Figure 3D). Neck (Figure 1A), N = 7, 743–1580 (1122 ± 281) long by 212–251 (250 ± 36) in maximum width. Strobila, N = 7, 49–133 mM (94.6 ± 31.9 mM) long, with 250–489 (377 ± 93) segments; maximum width 1137–1569 (1337 ± 139) attained late in gravid segments (Figures 1B, 1C). Strobilae craspedote with intersegmental boundaries well-defined in both mature and gravid segments. Mature segments (Figure 1B) wider than long, gravid segments with developed egg capsules longer than wide (Figures 2A, 2B); strobila attenuated anteriad, with increase in relative length beginning in mature segments; length/width ratio of mature and gravid segments 0.20–0.34 (N = 7) and 0.29–1.69 (N = 7), respectively. Cirrus sac elongate, fusiform, N = 7, 106–179 (137 ± 27) in maximum length by 33–46 (42 ± 5) in maximum width. Cirrus unarmed. Testes, mostly round in overall shape, N = 7, 29–38 (38 ± 6) long by 29–37 (33 ± 3) wide, situated with most testes occurring in segment antiporal and only a few poral relative to the ovary (Figures 1B, 1C). Number of testes per mature proglottid N = 3, 22–29 (25 ± 4). Seminal receptacle, N = 2, 117–148 (133 ± 22) long by 18–23 (21 ± 4) in maximum width, extending porad, mostly anterior to ovary. Ovary (lobate, with small or large lobes), N = 7, 107–258 (168 ± 60) in maximum width by 73–201 (121 ± 48) in maximum length. Vitelline gland with relatively smooth margins, N = 7, 37–61 (45 ± 8) wide by 45–74 (60 ± 10) in maximum length, situated dorsal and posterior to ovary. Genital ducts always passing between excretory canals (Figures 1B, 1C). Eggs subspherical with thin outer shell, N = 4, 22–26 (24 ± 2) long by 18–22 (20 ± 2) wide. Egg capsules (Figures 2A, 2B) N = 2, 21–25 (22 ± 2), 4–8 eggs per capsule.

Figure 1. (A) Anterior end (Scolex and neck) of Raillietina dowleri n. sp., (B) Mature segment of Raillietina dowleri n. sp., ventral view. (C) Photographic image of testes to the left, vitelline gland center compact gland, and ovary with oöcapt and ovarian lobes evident. To the right of image c can be seen the ventral osmoregulatory duct running from top to bottom of image. (D) Expanded view of drawing of vitelline gland, ovary, vagina distal and of the cirrus sac, testes (black), and convoluted seminal duct (vas deferens). All ducts can be seen to pass between the dorsal and ventral osmoregulatory canals.

Figure 2. (A) Photograph of a gravid proglottid of Raillietina dowleri n. sp. showing the distribution of egg capsules and eggs. (B) Drawing of a gravid proglottid of Raillietina dowleri n. sp. showing the distribution of the egg capsules and the disposition of the cirrus sac being pushed anteriad by the developing egg capsules.

Figure 3. (A) Photograph of the anterior part of the Scolex of Raillietina dowleri n. sp., showing the hooks around the rostellum. (B) Expanded image of 2 of the hooks dissected out of the rostellum of the specimen shown in FIGURE 3A. (C) Image of the hooks lining the anterior part of the suckers, and (D) The small claw shaped hooks lining the posterior parts of the suckers of Raillietina dowleri n. sp.
Taxonomic summary
Symbiotype host (see Frey et al., Reference Frey, Duszynski, Gannon, Yates and Gardner1992): Santiago Galápagos Mouse, Nesoryzomys swarthi Orr, 1938 (Rodentia: Cricetidae). Symbiotype Number: NMNH:USNM570194).
Type Locality: La Bomba, Santiago: Galápagos, Ecuador, 0°11.21–s; 90°42.04–W.
Collection date: 7 July 1999.
Site of infection: Small intestine, duodenum.
Prevalence: Five of twelve specimens of Nesoryzomys swarthi examined (33%).
Specimens deposited: Holotype: HWML217626, Field Collection Number: ASK5508; Paratypes: HWML217627, HWML217628, HWML217629, HWML217630, HWML217631, HWML217632, HWML217633, HWML217648; Additional specimens examined: HWML217634, HWML217635, HWML217636.
Etymology: This species was named after Robert C. Dowler, Professor of Biology, Emeritus, Angelo State University, San Angelo, Texas in honour of his long-term commitment to research in mammalogy, mammalian biodiversity, museum collections and mammalian parasitology. Without his dedication to this project and his leadership in collecting under rigorous field conditions, the occurrence and diversity of these species of parasites in the Galápagos would still remain unknown.
Comparisons
The cestode genus Raillietina (Order Cyclophyllidea: Family Davaineidae) contains more than 200 described species with a cosmopolitan distribution in birds and mammals (Schmidt, Reference Schmidt1986). However, because it is unlikely that any species of oryzomyine rodents have made it across the Pacific Ocean to the Indomalayan or Australasian zoogeographic regions and there is no evidence of this occurring, we restrict our comparisons to those species of Raillietina occurring in mammals of the Neotropical and southern Nearctic regions (see Table 6). In addition (as noted earlier), invasive rodents of the genera Rattus and Mus were collected from either the same localities or near the same areas as from where individuals of the endemic species of rodents were collected and no evidence of this new cestode species was discovered in any of the invasive murids.
Table 6. List of species of Raillietina from rodents and primates in the Caribbean, central, and South America. Measurements of characters from original papers are given

Differential diagnosis
Raillietina dowleri n. sp. can be recognized as distinct from R. demerariensis Daniels, 1895, described from the red howler monkey, Alouatta seniculus (Linnaeus, 1766) in South America based on the width of the strobila; R. dowleri n. sp. has a much larger strobilar width with a mean width of 1, 337 µM, whereas the maximum width of the strobila of R. demeriensis does not exceed 640 µM (Stunkard, Reference Stunkard1953). In addition, R. dowleri n. sp. can be recognized as distinct from R. alouattae Baylis, 1947, described from the Guyanan red howler monkey Alouatta macconelli (Linnaeus, 1766) also from South America, by possessing many fewer testes: R. dowleri n. sp. has from 22–29 testes in each mature proglottid whereas R. alouattae sports 110–130 in each mature proglottid.
Raillietina dowleri n. sp. can be recognized as distinct from R. trinitatae Cameron and Reesel, 1951, described from the Paca, Cuniculus paca (Linnaeus, 1766), from the island of Trinidad in the Caribbean, in having much larger eggs: Eggs of R. dowleri n. sp., are 22–26 µM by 18–22 µM while gravid proglottids of R. trinitatae have eggs that average only about 10 µM in width. In addition, R. dowleri possesses from 4 to 8 eggs per egg capsule and only 21–25 egg capsules per gravid proglottid compared to 50–70 egg capsules with 8–12 eggs per capsule in R. trinitatae. The rostellar hooks of R. dowleri are claw-hammer shaped (Figures 3B-3D) while those of R. trinitatae are a single fork shape [see Fig. 6 in Cameron and Reesel (1951)].
Raillietina dowleri n. sp. can be recognized as distinct from R. guaricana (César and Luz, Reference César and Luz1993) described from Sooretamys angouya (Fisher, 1814) [syn. Oryzomys ratticeps (Hensel, 1872)] in Brazil, in having many fewer hooks on the rostellum with from 120 to 140 rostellar hooks occurring in R. dowleri vs only 66–78 in R. guaricana; in having a much smaller strobila, both in length and maximum width, smaller size of suckers, and by the much smaller size of the egg capsules which range from 21 to 25 µM in R. dowleri compared to 92–121 µM in R. guaricana (see César and Luz, Reference César and Luz1993).
From R. halli (Vigueras, Reference Vigueras1943) collected from Capromys pilorides (Say, 1822) in Cuba in the early 1940’s, R. dowleri n. sp. can be recognized as distinct by having fewer hooks on the rostellum, with R. halli possessing from 200 to 220 hooks, while R. dowleri has only from 120 to 140 hooks on the rostellum, while each gravid proglottid of R. halli contains from 40 to 60 egg capsules, compared to 21–25 per proglottid as found in R. dowleri [see Vigueras (Reference Vigueras1943) for a complete description of this species].
Raillietina dowleri n. sp. can be recognized as distinct from R. celebensis (Baer and Sandars, Reference Baer and Sandars1956) originally described from Rattus norvegicus by having a much shorter strobila, shorter hooks on the rostellum, and many fewer egg capsules per gravid proglottid. For additional information on R. celebensis, see Baer and Sandars (Reference Baer and Sandars1956), and the re-description by de Oliveira et al. (Reference de Oliveira, Simões, Luque, Iñiguez and Júnior2017).
Raillietina dowleri n. sp. can be recognized as distinct from R. oligocapsulata (Sato et al., Reference Sato, O’huigin, Figueroa, Grant, Grant, Tichy and Klein1999) described from the tapeti or forest cottontail rabbit [cf. Sylvilagus brasiliensis (Linnaeus, 1758)] based on the number of hooks on the rostellum (124–140 in R. dowleri vs 170, in R. oligocapsulata) number of eggs per egg capsule, having 4–8 eggs/capsule whereas R. oligocapsulata has 15–20 eggs/capsule (see description by Sato et al., Reference Sato, Okamoto and Basáñez1998).
Finally, R. dowleri n. sp. can be recognized as distinct from R. multitesticulata Perkins, 1950, described from the Colombian red howler monkey (Alouatta seniculus) collected near Kongarooma in the former British Guiana based on number of testes with R. dowleri sporting from 22 to 29 testes in each mature proglottid whereas R. multitesticulata has 115–120 testes in each proglottid (Perkins, 1950).
Summary of additional species of parasites recovered from rodents collected
Phylum Nemata
Physalopteridae
Physaloptera calnuensis (Sutton, Reference Sutton1989)
Locality, deposition and host records: Santa Fe: Galápagos, Ecuador, 0°48.21ʹS 90°2.45ʹW, 16 July 1999, 2 males (HWML17007) from Aegialomys galapagoensis; Volcan Wolf, Isabela: Galápagos, Ecuador, 0°3.96ʹN 91°24.18ʹW, 7 September 1999, 2 males and 3 females (HWML17053) from Rattus rattus.
Remarks: Sutton’s type host for P. calnuensis was Calomys laucha (Fischer, 1814) from the stomach (Sutton, Reference Sutton1989). Physaloptera calnuensis, originally described from Calomys laucha from Argentina may have transferred to the Galápagos with the original endemic rodents. The existence of this nematode in Rattus in the islands may indicate ecological fitting from endemic rodents to the muroid invaders.
Prevalence: Physaloptera calnuensis occurs in 1 of 12 specimens of A. galapagoensis examined (8·83%) and from 1 of 22 specimens of R· rattus examined (4·55%).
Spiruridae
Mastophorus muris (Gmelin, 1790)
Locality, deposition, and host records: Cabo Douglas, Fernandina: Galápagos, Ecuador, 1°18.24ʹS 91°39.14ʹW, 7 November 1999, 6 females/4 juveniles (HWML17049, HWML17052, HWML17050, HWML17047, HWML17046, HWML17048) from Nesoryzomys fernandinae; Santa Fe: Galápagos, Ecuador, 0°48.21ʹS 90°2.45ʹW, 16 July 1999, 2 females (HWML17013) from Aegialomys galapagoensis; East of Eden, Santa Cruz: Galápagos, Ecuador, 0°33ʹ40.2114ʹʹ–90°31ʹ40.8ʹʹ, 15 July 1999, 3 females (HWML17016, HWML17017) from Rattus rattus; South of Cerro Bruho, San Cristobal: Galápagos, Ecuador, 0°47ʹ6ʹʹ–89°28ʹ5.8794ʹʹ, 24 July, 1999, 6 females/7 males (HWML17012) from Rattus rattus; West of Punta Pitt, San Cristobal: Galápagos, Ecuador, 0°42ʹ43.1994ʹʹ–89°15ʹ11.8794ʹʹ, 25 July 1999, 3 specimens (HWML17014) from Rattus rattus.
Remarks: Gmelin’s type host for M. muris was Myodes glareolus Gmelin 1780, (see: Quentin, Reference Quentin1971). It appears that this species of nematode now occurs in endemic mammals after host-switching from invasive Rattus or Mus.
Prevalence: We found these nematodes in 6 of 12 N. fernandinae examined (50%); 1 of 12 A. galapagoensis examined (8·33%); 4 of 22 R. rattus examined (18·18%).
Protospirura numidica Seurat, Reference Crook and Grundmann1914
Locality, deposition and host records: North of Cerro Bruho, San Cristobal: Galápagos, Ecuador, 0°44ʹ44.988ʹʹ–89°26ʹ22.92ʹʹ, 26 July 1999, 3 females (HWML118823) from Rattus rattus.
Remarks: Seurat’s type host for P. numidica was Felis ocreata Bate, 1905, from the stomach of the cat (Crook and Grundmann, Reference Crook and Grundmann1964).
Prvalence: 1 of 22 R. rattus examined (4·55%).
Phylum Platyhelminthes
Hymenolepididae
Hymenolepis diminuta (Rudolphi, 1819)
Locality, deposition and host records: Volcan Wolf, Isabela: Galápagos, Ecuador, 0°3.96ʹN 91°24.18ʹW, September 7, 1999, 1 specimen (HWML217637) from Rattus rattus; La Bomba, Santiago: Galápagos, Ecuador, 0°11ʹ12.5874ʹʹ–90°42ʹ2.5194ʹʹ, 7 July 1999, 4 individuals (HWML217638, HWML217639, HWML217640) from Rattus rattus; North of Cerro Bruho, San Cristobal: Galápagos, Ecuador, 0°44ʹ44.988ʹʹ–89°26ʹ22.92ʹʹ, 26 July 1999, 1 individual (HWML217641) from Rattus rattus.
Remarks: Rudolphi’s listed type hosts for H. diminuta include Rattus rattus and Mus musculus and this cestode is a common parasite of the small intestine of rodents (Oldham, Reference Oldham1931; Gardner and Schmidt Reference Schmidt1986; Dursahinhan et al., Reference Dursahinhan, Botero-Cañola and Gardner2023). In addition to the discovery of these cestodes in invasive rats in the Galápagos, it is interesting to note that this cestode is found in rodents (especially species of the genus Rattus) world-wide, probably having been distributed globally by humans with their synanthropic species of Rattus. Thus, the presence of these cestodes in Rattus on Santiago Island can probably be attributed to natural infections in the invasive rats; however, it is interesting to note that no instances of H. diminuta are known from the endemic species of rodents that were sampled.
Prvalence: 5 of 22 R. rattus examined (22·73%).
Taeniidae
Taenia taeniaeformis (Batsch, 1786)
Locality, deposition and host records: Volcan Wolf, Isabela: Galápagos, Ecuador, 0°3.96ʹN 91°24.18ʹW, 7 September 1999, 2 individuals (HWML217642, HWML217643) from Rattus rattus; Caleta Iguana, Cerro Azul, Isabela: Galápagos, Ecuador, 0°55ʹ42.0954ʹʹ–91°23ʹ36.96ʹʹ, 13 July 1999, 4 individuals (HWML217647) from Rattus rattus; South of Cerro Bruho, San Cristobal: Galápagos, Ecuador, 0°42ʹ43.1994ʹʹ–89°15ʹ11.8794ʹʹ, 24 July 1999, 1 individual (HWML217646) from Rattus rattus; West of Punta Pitt, San Cristobal: Galápagos, Ecuador, 0°42ʹ43.1994ʹʹ–89°15ʹ11.8794ʹʹ, 25 July 1999, 1 individual (HWML217644) from Rattus rattus; North of Cerro Bruho, San Cristobal: Galápagos, Ecuador, 0°44ʹ44.988ʹʹ–89°26ʹ22.92ʹʹ, 26 July 1999, 1 individual (HWML217645) from Rattus rattus.
Remarks: Batsch’s type host for T. taeniaeformis was Felis sp. This cestode has a worldwide distribution with adults in cats and rodents serving as intermediate hosts.
Prevalence: 8 of 22 R. rattus examined (36·36%). These findings indicate that feral cats on the islands are consuming R. rattus and these rodents are living in a commensal relationship with cats.
Discussion
Both classical and evolutionary parasitology has been understudied in the Galápagos even though it is such an important geographic location for the development of the theory of evolution. The present paper starts to alleviate this dearth of information on parasites, at least in mammals, by outlining occurrence and prevalence of endoparasites in both endemic and invasive species of rodents. However, since few specimens were collected, examined and necropsied, and only metazoan parasites were preserved (see also Gettinger et al., Reference Gettinger, Martins-Hatano and Gardner2011) the true parasite diversity within the Galápagos rodent fauna is still not well-known and remains understudied.
Additional collecting and analysis of parasites from both introduced and endemic mammals and birds would shed light on their transmission dynamics as shown by levels of network connections and would enable a local and robust network analysis (e.g. Dursahinhan et al., Reference Dursahinhan, Botero-Cañola and Gardner2023) using both occurrence data at the species level as well as levels of connectedness that would be shown in a molecular phylogeographic analysis.
However, unless well-trained (in field methods) mammalogists/parasitologists are involved with field collections, endoparasites (helminths and protozoa) as well as ectoparasites are almost never actually collected nor are they considered as important components of the ecological communities of rodents or other mammals. Or they are collected as an afterthought, with little effort being made to preserve specimens of parasite of high quality that can be used for both morphology and molecular investigations into the future.
The intrinsic value of parasites cycling in natural ecosystems is a difficult parameter to estimate, mostly because the majority of biologists think of parasites as unattractive, unappealing and unnecessary inhabitants of their favourite animal groups or species. In fact, the first thing that many field biologists do when they begin to prepare a specimen for a museum study skin is to discard the intestinal tracts of any specimens collected (Gardner, pers. obs.). This occurs now on a regular basis despite the continued and relatively recent calls for training and the fact that there are available published papers that outline methods and provide examples of the importance of collecting parasites from their associated vertebrate hosts [see: (Gardner, Reference Gardner, Wilson, Cole, Nichols, Rudran and Foster1996) (mammals); (Gardner and Jiménez-Ruiz, Reference Gardner, Jiménez-Ruiz, Kunz and Parsons2009) (bats); (Gardner et al., Reference Gardner, Fisher, Barry, McDiarmid, Foster, Guyer and Gibbons2012) (herps); (Galbreath et al., Reference Galbreath, Hoberg, Cook, Armién, Bell, Campbell, Dunnum, Dursahinhan, Eckerlin, Gardner and Greiman2019) (mammals)]. This appalling destruction of a significant portion of the biodiversity of potentially endangered or rare species in an area that is being surveyed for preservation or conservation purposes is significant as parasites have been shown to have not only intrinsic value to natural ecosystems, but extrinsically, these organisms can serve as indicators of ecological health (Marcogliese, Reference Marcogliese2005) as well as probes for current as well as ancient biodiversity (Gardner and Campbell, Reference Gardner and Campbell1992).
Following the Document, Assess, Monitor, Act protocol (Brooks et al., Reference Brooks, Hoberg, Gardner, Boeger, Galbreath, Herczeg, Mejía-Madrid, Racz and Dursahinhan2014) we call for more parasite surveys on the mammalian fauna of the Neotropics followed by subsequent phylogenetic studies to be completed on these cestodes (Raillietina spp.) on mainland South America and the Galápagos Islands before the habitats are forever obliterated by the continued encroachment by humans and their machines into residual natural areas. A phylogenetic/phylogeographic analysis including all known species of Raillietina using both morphology and molecules would give deeper insight into whether Raillietina dowleri from Nesoryzomys swarthi is derived from a direct ancestral invasion of the islands of its rodent hosts or the presence of these cestodes in individuals of N. swarthi is the result of ecological fitting in the archipelago that occurred after the establishment of rice-rats in the islands. Parent et al. (Reference Parent, Caccone and Petren2008) point out that most of the terrestrial fauna diversified in parallel to the geological formation of the islands, so it is to be expected that there is more diversity of these tapeworms and their associated hosts than has yet been recorded. At this point, with no information on the helminth-parasite fauna of the passerines of the Galápagos and only limited collections that were made of the rodents, the level of parasite biodiversity of the rodent fauna of the islands is still relatively unknown.
Data availability statement
All specimens of helminths collected and analysed herein are freely available for study at the Harold W. Manter Laboratory of Parasitology: Contact information is provided at the permanent web site of the Manter Lab. See: https://hwml.unl.edu.
Acknowledgements
Work by undergraduates and staff in the Manter Laboratory was funded by the National Science Foundation grant numbers: REU supplements to DEB-9631295, and DEB-0097019, DBI-1458139, and DBI-1756397 to Scott L. Gardner. A hearty thank you is extended to Dr Gábor Rácz, Collection Manager at the Manter Laboratory, for assistance throughout this project. We also thank Clark R. Gardner and Grant S. Gardner (who kept this project going with enthusiasm in a middle school science fair poster), and Jay Johnson (for collecting literature on ants in the Galápagos). We also acknowledge our friend and colleague, Donald Gettinger who provided technical and moral support during these years of work on this paper. Finally, we acknowledge the field work and the honest support of Dr Robert C. Dowler, Emeritus Professor, Angelo State University, and field team of Joseph Flanagan, Nick Dexter, Sharelle Hart, and Marcia Revelez, for collection of the parasites described and discussed herein and for making this project possible. Thank you also to Dr Miguel Pinto, Principal Coordinator of CDRS Collections and Curator of the Vertebrate Collection, Fundación Charles Darwin, Galapagos for assistance with information on extinct and extant and invasive mammal species in the islands.
Author contributions
After specimens were received in the Manter Laboratory, S.L.G. conceived of the analyses, guided all aspects of the work, and wrote most of the manuscript. As an undergraduate, M.C.F. conducted the early work in the HWML on sorting, clearing, and mounting specimens received from Dr Dowler in the 1990’s. In the last few years, E.K.C. finished mounting and clearing of specimens and helped finish the spreadsheets of all data used in this analysis. Expertise in microscopy and data analysis was provided by A.T.D.
Financial support
The research was supported in the Manter Laboratory by the National Science Foundation DEB-9496263, the NSF REU programme, and grants from the NSF: DBI-1458139, and DBI-1756397 to S.L.G. Fieldwork in the Galápagos Islands was supported by National Geographic Society grant 6517-99 and the Angelo State University Research Enhancement program to R.C. Dowler.
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
Collection of specimens was approved through permits issued by Galapagos National Park. Field research methods were approved by Angelo State University and were in accordance with guidelines established for research on mammals by the American Society of Mammalogists; see summary in Sikes (Reference Sikes2016).