The size of quaternary 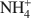 cations defines their packing configurations in the interlayers and the basal spacings of organoclays, and hence strongly influences the sorptive properties of organoclays. A series of organoclays (TAA-SACs) was prepared from a smectite (SAC) fully exchanged with symmetrical tetraalkylammonium (TAA) cations of progressively increasing sizes with the carbon number of single alkyl group from 1 to 6. X-ray diffraction analysis indicated the packing configurations of monolayer, monolayer-to-bilayer transition, bilayer, and bilayer-to-trilayer transition in the interlayers of SAC. Calculations of the interionic distances between TAA ions support such packing configurations. Sorption of benzene by TAA-SACs displayed a high-low-high uptake trend and progressively weaker sorptive interactions as the size of TAA ions increased. Both the siloxane surfaces and TAA ions contributed to the overall sorption, with their relative contributions dependent on the TAA interionic distances and the basal spacings of TAA-SACs. High benzene sorption by small TAA-SAC (tetramethylammonium(TMA)-SAC) was attributed to the strong interactions between the siloxane surfaces and benzene molecules. With large TAAs, high sorption was due to the effective solute partitioning. Compared to benzene sorption, TCE sorption by small TAA-SACs (TMA-SAC and tetraethylammonium(TEA)-SAC) was less effective and displayed an abnormal trend, due largely to the lack of the siloxane surface-TCE interactions and to the stronger hydration of TMA as compared to TEA ions. The results provide strong evidence to support the use of either small or large quaternary
cations defines their packing configurations in the interlayers and the basal spacings of organoclays, and hence strongly influences the sorptive properties of organoclays. A series of organoclays (TAA-SACs) was prepared from a smectite (SAC) fully exchanged with symmetrical tetraalkylammonium (TAA) cations of progressively increasing sizes with the carbon number of single alkyl group from 1 to 6. X-ray diffraction analysis indicated the packing configurations of monolayer, monolayer-to-bilayer transition, bilayer, and bilayer-to-trilayer transition in the interlayers of SAC. Calculations of the interionic distances between TAA ions support such packing configurations. Sorption of benzene by TAA-SACs displayed a high-low-high uptake trend and progressively weaker sorptive interactions as the size of TAA ions increased. Both the siloxane surfaces and TAA ions contributed to the overall sorption, with their relative contributions dependent on the TAA interionic distances and the basal spacings of TAA-SACs. High benzene sorption by small TAA-SAC (tetramethylammonium(TMA)-SAC) was attributed to the strong interactions between the siloxane surfaces and benzene molecules. With large TAAs, high sorption was due to the effective solute partitioning. Compared to benzene sorption, TCE sorption by small TAA-SACs (TMA-SAC and tetraethylammonium(TEA)-SAC) was less effective and displayed an abnormal trend, due largely to the lack of the siloxane surface-TCE interactions and to the stronger hydration of TMA as compared to TEA ions. The results provide strong evidence to support the use of either small or large quaternary 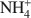 cations in preparation of organoclays as effective sorbents for removing organic contaminants from water.
cations in preparation of organoclays as effective sorbents for removing organic contaminants from water.