Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disorder primarily affecting synovial joints with the potential for joint damage and systemic or extra-articular involvement(Reference Tanaka1). RA can reduce physical function, quality of life and mental wellbeing(Reference Tański, Szalonka and Tomasiewicz2). It is a significant health burden globally and affects an estimated 0·46% of the world population, or 18 million people(Reference Almutairi, Nossent and Preen3,Reference Black, Cross and Haile4) . The contemporary approach to RA treatment includes pharmacological treatment and is indicated at diagnosis to control inflammation and prevent erosions(Reference Taylor, Fautrel and Piette5,Reference Smolen6) . Conventional synthetic disease-modifying antirheumatic drugs (csDMARD), such as methotrexate, and/or targeted advanced therapies, such as biologic DMARD, are prescribed for RA with the goal of inducing RA remission. This approach can achieve therapeutic remission in up to half of patients(Reference Yu, Jin and Wang7). Many people with RA report ongoing fatigue and pain despite medically optimal RA control(Reference Taylor, Moore and Vasilescu8), highlighting that other approaches are needed to address residual RA symptoms.
There has been longstanding interest in the influence that diet and ingested/excluded foods have on RA disease activity. Elimination diets followed by food reintroductions were popular prior to the evolution of biologic DMARD. The goal of these diets is to reduce disease activity using an elimination protocol, then add foods sequentially to identify specific foods that increase disease activity. By excluding these foods from the diet, it is proposed that RA symptoms could be reduced(Reference McEwen and Morgan9–Reference Brostoff and Gamlin13). The theory that certain foods affect RA is influenced by studies that demonstrate fasting reduces physical symptoms and improves clinical markers of inflammation within 5–10 d(Reference Hafström, Ringertz and Gyllenhammar14). Subsequent research suggests the proposed mechanisms for improvement include fasting-induced reduction in intestinal permeability and beneficial alterations in gut microbiome(Reference Sundqvist, Lindström and Magnusson15–Reference Häupl, Sörensen and Smiljanovic19). These findings support that specific foods may play a role in RA disease activity via their effect on the gut and that further research is needed.
Surveys of the experiences of people with RA found that 24–80% believed that diet played a role in their disease or RA symptoms(Reference Tedeschi, Frits and Cui20–Reference Haugen, Kjeldsen-Kragh and Nordvåg28). Furthermore, 20–67% experienced negative effects from food on symptoms(Reference Felder, De Blecourt and Wüthrich21,Reference New, Terry and Williams22,Reference Lidén, Kristjánsson and Valtysdottir25,Reference Haugen, Kjeldsen-Kragh and Nordvåg28–Reference Tanner, Callahan and Panush30) , while 15–67% reported limiting or avoiding certain foods, or changing their diet(Reference Tedeschi, Frits and Cui20,Reference Grygielska, Kłak and Raciborski24,Reference Goff and Barasi27–Reference Petchiappan, Priyadarshini and Prabhu29,Reference Salminen, Heikkilä and Poussa31) . Overall, 10–33% reported improvements in their condition or reduced joint symptoms following diet modifications(Reference Munshi, Iqbal and Rafique23–Reference Lidén, Kristjánsson and Valtysdottir25,Reference Haugen, Kjeldsen-Kragh and Nordvåg28,Reference Petchiappan, Priyadarshini and Prabhu29,Reference Salminen, Heikkilä and Poussa31) .
To date, position statements from professional organisations do not recommend food exclusions in the management of RA. The British Dietetic Association (BDA) state that although people believe that food intolerances exacerbate inflammation in RA: ‘there is no evidence to support this theory’. The BDA acknowledges that a small subset of individuals with RA may have a genuine intolerance to one or more foods, which can be identified through an exclusion programme conducted under the supervision of a registered dietitian(32). Recent recommendations from the French Society for Rheumatology specifically state that although diet is important, the lack of evidence of benefit from interventional studies means a gluten free diet should not be used in the absence of confirmed Coeliac disease, nor should dairy products be eliminated(Reference Daien, Czernichow and Letarouilly33). The American College of Rheumatology (ACR) 2022 guidelines recommend adherence to a Mediterranean style diet, and recommend against formally defined diets such as vegan, intermittent fasting, elimination, gluten free and paleo diets(Reference England, Smith and Baker34). Professional bodies do, however, recommend limiting intake of added sugars, sodium, highly processed foods, refined carbohydrates, and saturated and trans fats(Reference England, Smith and Baker34,Reference Gwinnutt, Wieczorek and Rodríguez-Carrio35) .
The topic of food intolerance in RA has been the focus of several narrative reviews of elimination diet therapy. However, these reviews are dated and lack a systematic approach(Reference Gamlin and Brostoff36–Reference Darlington and Ramsey39). More recent systematic reviews have briefly addressed the topic; however, these reviews typically include six or fewer studies and generally conclude that the evidence is uncertain due to the moderate-to-high risk-of-bias in the included trials(Reference Philippou, Petersson and Rodomar40–Reference Marquez, Evans and Boltson43). A 2021 systematic review by Philippou et al. evaluated six studies involving elimination and food challenge. The review found evidence of improvements in the number of tender joints (TJ) and inflammatory markers during the elimination phase, while food challenges were associated with increases in inflammatory biomarkers. Responses to elimination diets are individualised and may depend on food allergies or intolerances, potentially offering benefits to some individuals(Reference Philippou, Petersson and Rodomar40).
There is limited cover of the topic of food sensitivity in RA in current reviews, and no focused review since 1997. To address the interest in this topic for both health professionals and those with RA, there is a need for an up-to-date summary, which includes contemporary studies that investigate food intolerance using dietary elimination and food challenges, including assessment of study quality. Given the variability in studies and their quality, a scoping review is appropriate for identifying, synthesising and mapping the breadth of evidence available, and identifying research gaps(Reference Arksey and O’Malley44).
Objectives
This scoping review aims to provide an overview of the research on dietary elimination, reintroduction and food challenge studies, and summarise the methodology, research protocols, outcome measures and gaps in knowledge. The research questions for this review are: (1) ‘what is known about dietary elimination and reintroduction protocols in adults with rheumatoid arthritis?’; (2) ‘how is food intolerance or sensitivity shown to affect people with RA?’
Methods
Protocol and registration
The scoping review protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews and (PRISMA-ScR) guidelines. The protocol was registered prospectively with the Open Science Framework on 30 July 2022 (https://osf.io/dr9m4).
Eligibility criteria/inclusion and exclusion
Inclusion
Study participants were required to be adults ≥ 18 years with RA, confirmed by meeting the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010, the American Rheumatism Association (ARA) 1957 or 1987 classification criteria(Reference Ropes, Bennett and Cobb45–Reference Aletaha, Neogi and Silman47), or diagnosed by a medical specialist. Studies of any design were potentially included, such as case studies, case reports, randomised controlled trials (RCT), uncontrolled trials and experimental or quasi-experimental studies. To be included, studies needed to feature an elimination phase in which one or more foods were excluded for a period of time, with the aim of reducing RA symptoms, followed by a reintroduction or food challenge of individual foods or food groups. Reintroduction was defined by adding a food or food group back one-at-a-time at intervals to determine if they caused adverse symptoms. Food challenges involved exposing a person to a food to which they may react adversely. The effects on symptoms or clinical markers associated with RA were measured or observed, either objectively or subjectively (participant response). Sources included peer-reviewed journals and grey literature, all in English, with no constraints on publication dates. Types of reports included full manuscripts, abstracts, short reports, conference abstracts and letters with case studies.
Exclusion
Studies were excluded if the participant diagnosis was unclear, were aged < 18 years, or if participants had juvenile RA. Studies were also excluded if the food challenge was not done by oral ingestion of a whole food, for example a food extract placed under the tongue, or via rectal patch. Opinion pieces, reviews, surveys and editorials were not included.
Information sources and search
A search strategy was developed using Medical Subject Heading (MeSH) terms, keywords related to diet, food intolerance, food challenge or reintroduction, elimination diet and rheumatoid arthritis, supplemented by additional keywords identified in articles from a pilot search. Search strategies were drafted by the primary researcher (J.M.), with expert advice from an experienced research librarian. The literature search was executed in MEDLINE (EBSCO), Scopus, Cochrane and CINAHL (EBSCO) databases from inception until 16 January 2025. Google Scholar (the first 50 pages were title scanned for potentially relevant papers), ProQuest Dissertations and Theses, as well as open-access theses and dissertations (OATD) were searched. Open-Grey was explored for grey literature. Reference lists from relevant reviews and citations in studies were checked for potentially eligible studies. All searches were conducted by the primary researcher (J.M.). Search strategies for each online database are included in Supplementary Information Table S1.
Selection of sources of evidence
All search records were imported into EndNote (X9, Clarivate Analytics), and duplicates were removed. Titles and/or abstracts were screened by J.M. to exclude irrelevant records. The remaining records were independently screened in Rayyan (Rayyan Systems Inc.) by two reviewers (J.M. and S.G.) against inclusion and exclusion criteria. Full texts were sourced for any article where the abstract was not clear. Where there were discrepancies on study selection, full papers were discussed until a consensus was reached. If there was still uncertainty, papers were discussed with a third reviewer (C.Z.). Where an RA diagnosis was unclear, articles were reviewed by a rheumatologist (R.G.).
Data charting
Data charting templates were developed, one for case studies, and one for trials, both RCT and non-RCT by the primary researcher (J.M.). This was done using standardised JBI data extraction templates plus specific sections to address the research questions and to describe elimination diet protocols, reintroduction protocols and study outcomes.
Data items
The data charting templates were iteratively refined (J.M. and C.Z.) to ensure all relevant data were extracted including:
-
Publication information (author, year, country and record type)
-
Aim/purpose of study
-
Study population (sample size, demographics, RA diagnosis confirmation, type of RA, years with disease and participant withdrawals)
-
Methodology/methods (study design, blinding and duration)
-
Intervention design (intervention description, comparator/s, study setting, pre-elimination protocol, food sensitivity testing, elimination diet composition and protocol, reintroduction or challenge protocol)
-
Outcome measures used (clinical, laboratory and biometric measures and times, response measures to the elimination, and food challenge response measures)
-
Outcomes (response to elimination diet, short- and long-term outcomes from excluding offending foods, responders and non-responder numbers, foods or food groups identified as provoking an RA response, numbers of participants who reacted to each food or food group, and comparison of food sensitivity test results to food challenge responses)
Critical appraisal of individual sources of evidence
The Mixed Methods Appraisal Tool (MMAT), developed for quality appraisal of common types of empirical studies in systematic reviews(Reference Hong, Fàbregues and Bartlett48), was used to evaluate the methodological quality of the trials. Case studies were evaluated using the Joanna Briggs Institute (JBI) critical appraisal checklist for case reports(Reference Moola, Munn, Tufanaru and Aromataris49).
Synthesis of results
Study characteristics, size, country and distribution dates are displayed as a bubble chart. A flowchart is used to show the overall numbers of participants at intake, the interventions, dropouts and numbers completing studies. Study protocols and outcome measures are summarised in narrative format, and in detail in the supplementary tables. A bar chart is used to show the cumulative numbers of foods identified as affecting RA symptoms in individuals from both trials and case studies.
Results
Selection of sources of evidence
After duplicates were removed, 1414 unique citations were identified. Based on the title and/or abstract, 1211 were irrelevant or did not meet inclusion criteria. Snowball and reference searches identified twenty-eight additional records. After screening the 231 potentially eligible records, of which 187 were full texts, 183 were excluded (Fig. 1). This left forty-eight records for inclusion (Supplementary Information Table S2).
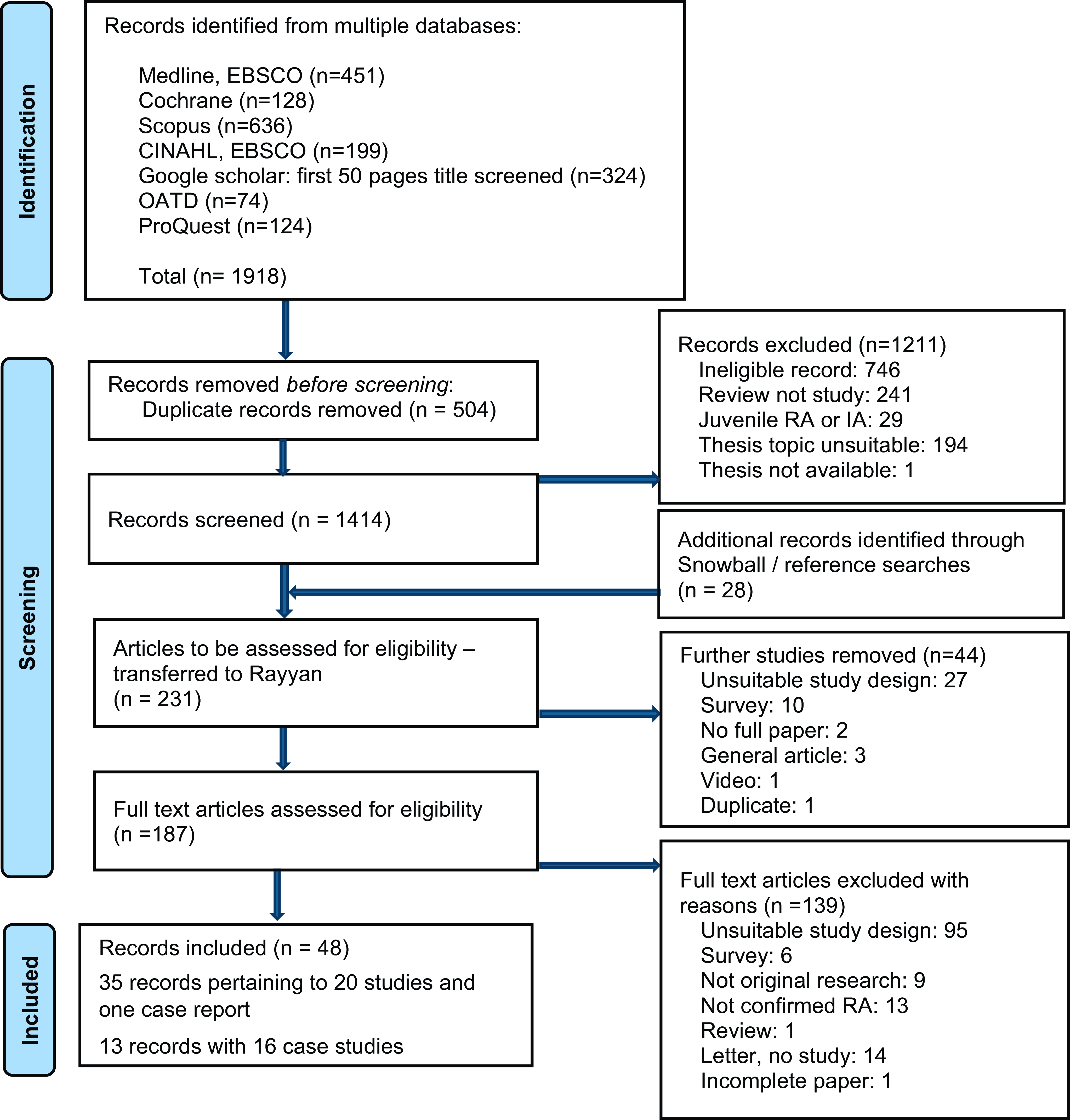
Fig. 1 PRISMA flow chart of search and screening.
Characteristics of included studies
The included 48 records were published between 1949 and 2024. The articles comprised conference abstracts (n = 4), full manuscripts (n = 33), short reports (n = 3), case studies in letters to journals (n = 4), theses (n = 2) and case studies included as part of a paper (n = 2). Thirty-three articles and the two theses described twenty trials and one case report, and thirteen articles described sixteen case reports (Fig. 2). Studies were from twleve countries: USA (n = 9), England (n = 8), Italy (n = 3), Turkey (n = 2), India (n = 1), Spain (n = 1), Australia (n = 1), the Netherlands (n = 2), Norway (n = 1), Switzerland (n = 1), Sweden (n = 2) and Israel (n = 1). Study sizes ranged from 4–94 participants with RA, 85% of whom were female. Three of twenty trials included participants with other types of inflammatory arthritis, with data reported separately for those with RA(Reference Carini, Brostoff and Wraith50–Reference Ratner, Schneeyour and Eshel52).
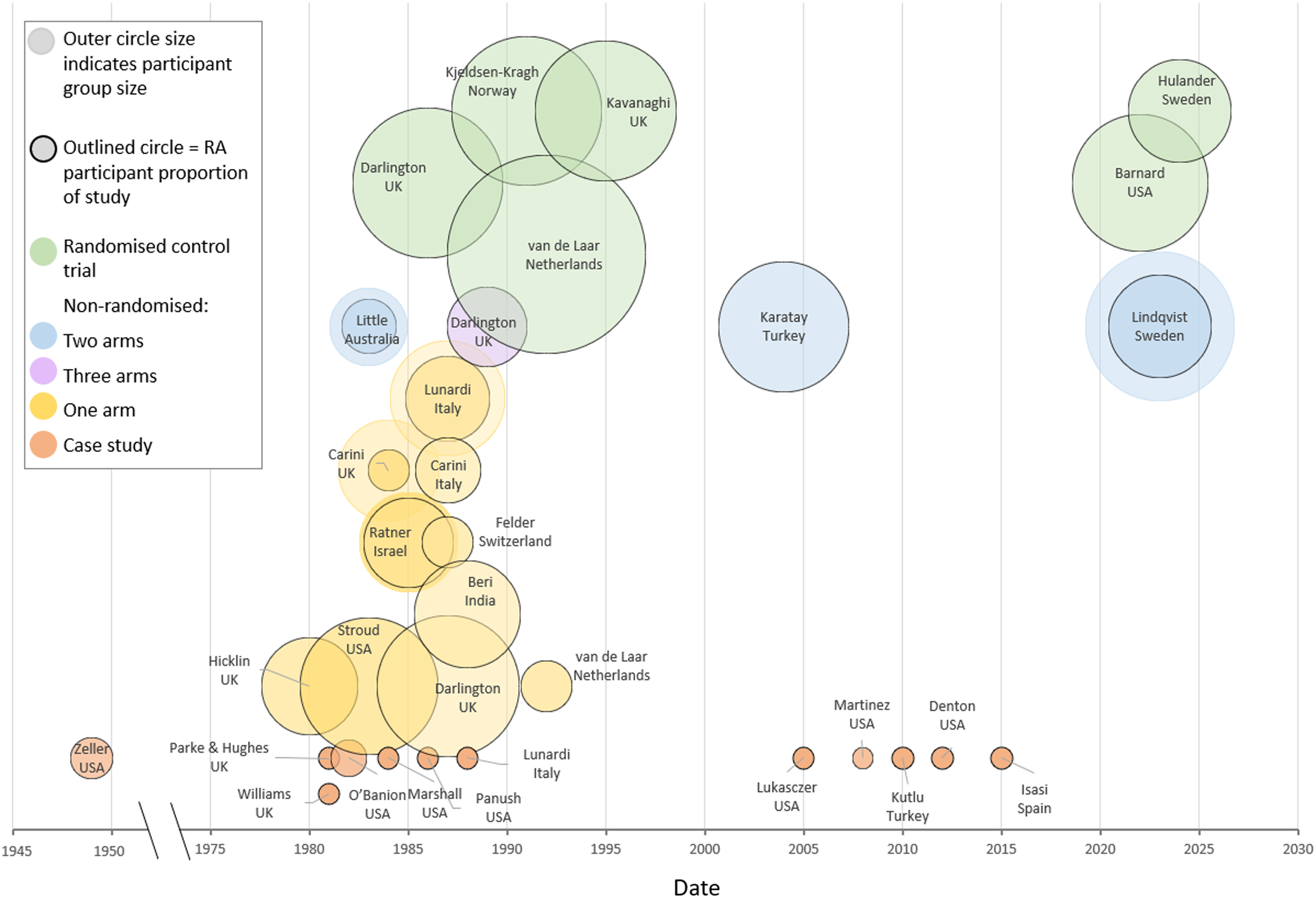
Fig. 2 Characteristics of records describing participants with RA undergoing a dietary intervention, by study size, country, study type and date.
Trial interventions
Six studies were RCT, involving a total of 317 participants with RA. Of these, 209 completed the studies, with durations from 3 weeks to 13 months. Four compared interventions (elimination, fast or a small number of foods followed by food reintroductions), with a control (no diet change)(Reference Kavanaghi, Workman and Nash53–Reference Darlington, Ramsey and Mansfield56). Two of these had crossover arms(Reference Barnard, Levin and Crosby55,Reference Darlington, Ramsey and Mansfield56) . Another RCT compared two meal replacements, one allergen-free and the other containing two allergens(Reference van de Laar and van der Korst57). The most recent study used a randomised control crossover design, in which twenty-five participants with RA were each challenged with three different protein food meals(Reference Hulander, Bärebring and Winkvist58).
Two studies divided participants into two or three non-randomised groups. In one study, 15 participants with RA were allocated into three groups of five, undergoing unblinded, blinded and placebo food challenges with foods previously identified as affecting their RA symptoms(Reference Darlington, Jump and Ramsey59). Another study enrolled twenty people with RA who had one or more positive skin prick tests to food allergens (SPTP group), and twenty with RA who tested negative to all skin prick tests (SPTN group). The SPTP group underwent a challenge with all the foods that tested positive concurrently, while the SPTN group was challenged with rice and corn(Reference Karatay60).
Two studies compared laboratory measures following oral food challenges between individuals with RA and healthy controls. In one study, seven participants with RA consumed foods previously identified as causing joint symptoms, while seven healthy controls consumed the same foods(Reference Little, Stewart and Fennessy61). The other study involved twenty-two women with RA and twenty-two matched women without RA. Both groups were challenged with a red meat meal and postprandial serum metabolites were assessed(Reference Lindqvist, Gjertsson and Hulander62).
Eight single arm uncontrolled studies included a total of 208 participants, 190 of whom had RA (and eighteen with another inflammatory arthritis). Of these, 144 undertook food reintroductions, fifteen dropped out and thirty-one completed only an elimination diet phase(Reference Felder, De Blecourt and Wüthrich21,Reference Ratner, Schneeyour and Eshel52,Reference Beri, Malaviya and Shandilya63–Reference Hicklin, McEwen and Morgan67) .
Three studies included a single arm undergoing an intervention (n = 61, forty-one with RA), and included comparator groups for laboratory tests only. These comparator groups consisted of healthy controls (n = 70), those with: RA (n = 62); other types of arthritis (n = 20); and allergies (n = 45)(Reference Carini, Brostoff and Wraith50,Reference Carini, Fratazzi and Aiuti68,Reference Kjeldsen-Kragh, Hvatum and Haugen69) . Figure 3 presents the cumulative numbers of participant types, interventions and the flow of participants to study endpoints, showing dropouts, across the twenty trials.
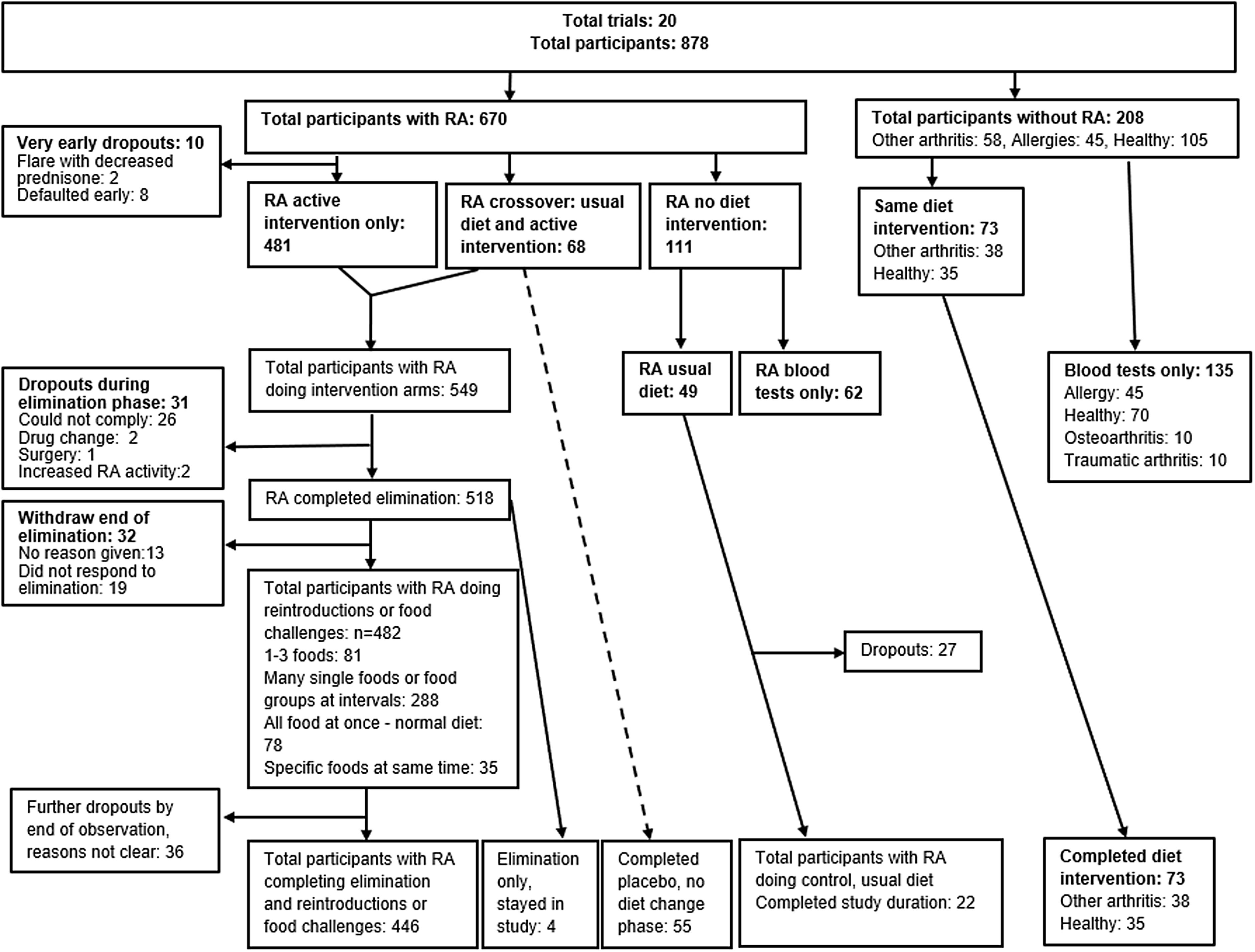
Fig. 3 Sample numbers, interventions and dropouts.
Critical appraisal
We conducted a quality appraisal using the appropriate MMAT questions for RCT and non-randomised trials. Where questions could not be answered with certainty, usually due to the brevity of information in the records, boxes were marked with both ‘unclear’ and ‘yes/no’ with a reason (Table 1). The JBI critical appraisal tool for case series was used to appraise the quality of each case study (Table 2).
Table 1. The mixed methods appraisal tool evaluation of the methodological quality of the trials

CT; cannot tell, Blank cell; not applicable.
a Not enough information to be sure of adherence.
b Limited information on participants other than an RA diagnosis
c Inadequate reporting, limited outcome data reported
d Details missing such as specific time frames for measures.
e Confounders considered, related data measures not recorded
f Adherence assumed owing to results, however, inadequate reporting to be certain.
Table 2. Joanna Briggs Institute Critical appraisal for case reports
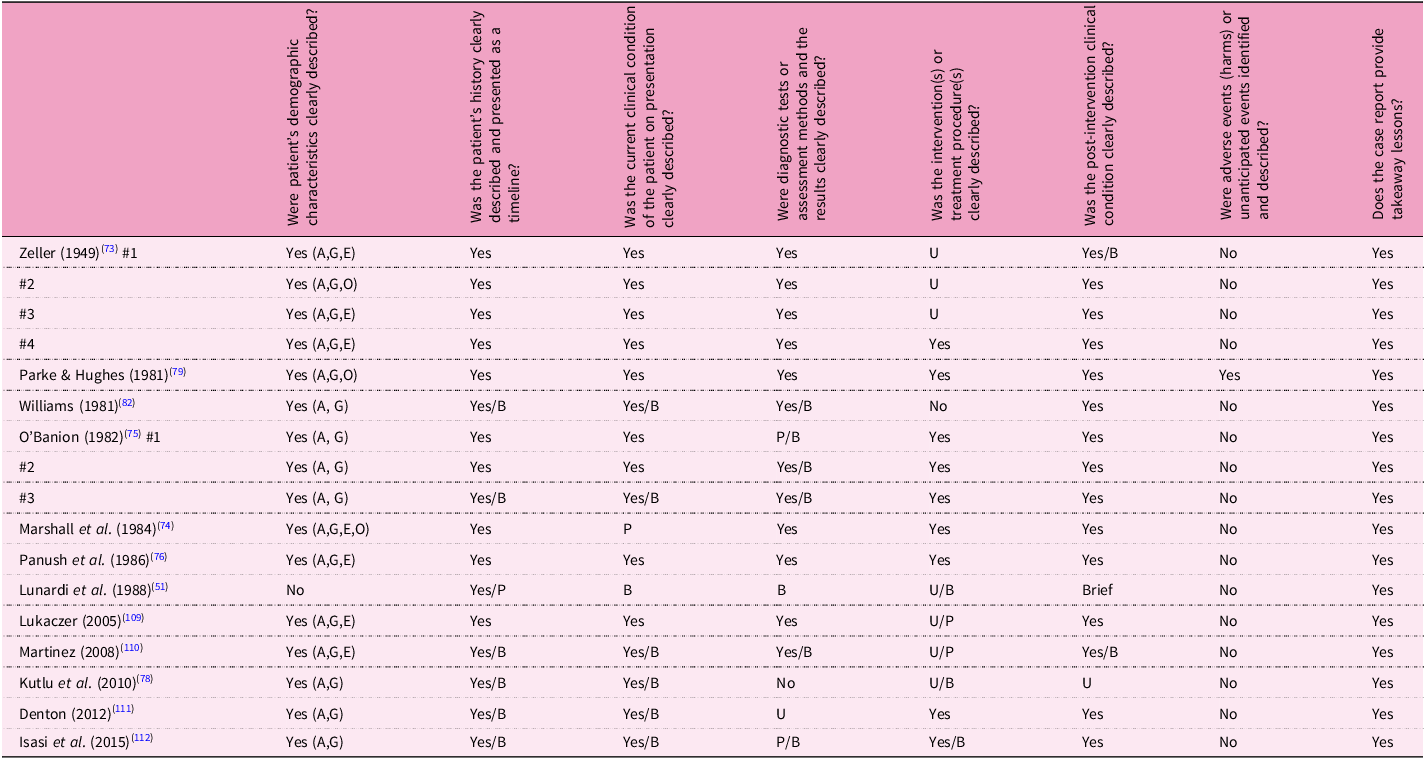
A, age; B, brief; E, ethnicity; G, gender; O, occupation; P, partial; U, unclear.
Study characteristics, protocols and methodology
Elimination diet protocols and outcome measures
Elimination diets were described in sixteen studies, employing four main types: a water or juice fast (n = 2); a low allergen food replacement (n = 3); a highly restricted whole food diet (n = 10); a single food group exclusion (n = 1). Two studies used an overnight fast prior to a single food challenge, with no prior dietary exclusions(Reference Hulander, Bärebring and Winkvist58,Reference Lindqvist, Gjertsson and Hulander62) . The elimination diet was not described in two studies(Reference Felder, De Blecourt and Wüthrich21,Reference Little, Stewart and Fennessy61) .
The fasting protocols included water-only for 4–9 d(Reference Stroud, Hahn and Arnett64), and a juice-only fast lasting 7–10 d(Reference Kjeldsen-Kragh, Borchgrevink and Laerum54). Three studies employed a low allergen food replacement plus a limited selection of solid foods for 4 weeks or until clinical remission was achieved(Reference Kavanaghi, Workman and Nash53,Reference van de Laar and van der Korst57,Reference van de Laar, Aalbers and Bruins65) . Ten studies used a very limited range of whole foods for durations of 5–14 d (n = 7), 2–4 weeks (n = 2) and until observed improvement (n = 1). Seven of these studies prescribed omnivorous diets, including one or two types of animal protein, typically lamb or fish, a limited range of vegetables and fruit, plus mineral or spring water(Reference McEwen and Morgan9,Reference Pachor, Andri and Nicolis10,Reference Carini, Brostoff and Wraith50,Reference Darlington, Ramsey and Mansfield56,Reference Darlington, Jump and Ramsey59,Reference Darlington and Ramsey66–Reference Carini, Fratazzi and Aiuti68,Reference Lunardi, Pachor and Nicolis70,Reference Rayman and Callaghan71) . Three studies were exclusively plant-based, allowing fruit, vegetables, refined oil and sugar in a study from India(Reference Beri, Malaviya and Shandilya63), while a Turkish study included cooked vegetables, legumes, fruit compote and rye bread(Reference Karatay60). The most recent study prescribed a gluten-free diet with a limited selection of plant foods(Reference Barnard, Levin and Crosby55). One study eliminated a single food group, dairy, for 3–4 months(Reference Ratner, Schneeyour and Eshel52).
Outcome data comparing the end of the elimination period with baseline was detailed in four studies, and included changes in clinical and laboratory variables associated with disease activity in RA, including improvements in joint tenderness, joint pain, grip strength (GS), articular index (AI), morning stiffness (MS), erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP)(Reference Kavanaghi, Workman and Nash53,Reference Darlington, Ramsey and Mansfield56,Reference van de Laar and van der Korst57,Reference Stroud, Hahn and Arnett64) . In addition, five studies briefly mentioned outcomes, such as noting improvement in a certain number of participants(Reference Ratner, Schneeyour and Eshel52,Reference Beri, Malaviya and Shandilya63,Reference van de Laar, Aalbers and Bruins65,Reference Hicklin, McEwen and Morgan67,Reference Kjeldsen-Kragh, Sumar and Bodman-Smith72) . Another study documented laboratory and clinical markers at the end of the elimination period, treating these as baseline figures without providing pre-elimination measures(Reference Karatay60).
Among seven studies reporting clinical data and participant response numbers to the elimination diet on a 4–9 d water fast, twenty-five of thirty-one participants showed a 25% or greater improvement in RA symptoms, and an improved ESR (p < 0·001)(Reference Stroud, Hahn and Arnett64). A 1-week few-foods elimination diet consisting of fish, pears and carrots reported that seven variables in the forty-seven participants decreased significantly, including pain by day and night, duration of morning stiffness (DMS), and painful joint count (PJC), (p < 0·01)(Reference Darlington, Ramsey and Mansfield56). In an RCT, on a 4-week low allergen food replacement diet, one with added lactalbumin and azo dye, both groups improved significantly (p < 0·05), with nine of seventy-eight showing a greater than 20% improvement, and no significant change in CRP or ESR(Reference van de Laar and van der Korst57). In a follow up study with six of nine participants who responded, four achieved total or partial remission on the same allergen free diet(Reference van de Laar, Aalbers and Bruins65). In a 4-week elemental diet supplemented with chicken, rice and carrots, in twenty-four participants, GS and the Ritchie articular index (RAI) improved (p = 0·006), with no significant change in ESR or CRP(Reference Kavanaghi, Workman and Nash53). A 2-week few foods vegan diet (primarily fruits and vegetables) led to ten of fourteen experiencing a 25–54% improvement, with a 33% reduction in ESR, however, four had no benefit(Reference Beri, Malaviya and Shandilya63). One study, which eliminated dairy products and beef, reported that in six of nineteen participants with RA, joint swelling reduced in 1 week, becoming asymptomatic in 3–4 weeks. The other thirteen had no noticeable changes(Reference Ratner, Schneeyour and Eshel52).
Food reintroduction and challenge protocols
Prior to the elimination phase, DMARD and non-steroidal anti-inflammatory drugs (NSAID) were withdrawn in four studies, with the rationale to reduce the masking of reactions to food reintroductions(Reference Darlington, Ramsey and Mansfield56,Reference Little, Stewart and Fennessy61,Reference Beri, Malaviya and Shandilya63,Reference Stroud, Hahn and Arnett64) . Another study excluded participants on antihistamines, glucocorticoids and NSAID that could affect skin prick test results(Reference Karatay60). Two further studies excluded those on medications that reduced interleukin (IL)-6, lipid lowering medications and glucocorticoids(Reference Hulander, Bärebring and Winkvist58,Reference Lindqvist, Gjertsson and Hulander62) .
Reintroductions of foods were spaced in the following ways: one food every 2–3 d (n = 6)(Reference Kavanaghi, Workman and Nash53–Reference Barnard, Levin and Crosby55,Reference Stroud, Hahn and Arnett64,Reference van de Laar, Aalbers and Bruins65,Reference Zeller73,Reference Marshall, Stroud and Kroker74) ; every 5–7 d (n = 3)(Reference Carini, Brostoff and Wraith50,Reference Hicklin, McEwen and Morgan67,Reference Carini, Fratazzi and Aiuti68,Reference Lunardi, Pachor and Nicolis70) ; and one food group every 2 weeks (n = 1)(Reference Beri, Malaviya and Shandilya63). One study and one case series introduced three new foods per day, spaced 5 h apart, and foods from the same biological family were separated by 4 d, to avoid complicating responses due to cross-reactivity(Reference Darlington, Ramsey and Mansfield56,Reference O’Banion75) . The sequence of reintroductions, prioritising less reactive foods first, was considered in two studies(Reference Darlington, Ramsey and Mansfield56,Reference O’Banion75) , with one of these reintroducing potentially more reactive foods at a rate of one per 2 d, rather than three foods per day(Reference Darlington, Ramsey and Mansfield56). One study tested foods in both organic and non-organic forms, the latter containing additives including artificial colouring, flavour enhancers, preservatives and pesticide residues(Reference Marshall, Stroud and Kroker74). Another study group reintroduced all foods identified by positive skin prick tests simultaneously(Reference Karatay60). In one case series and one RCT, participants were challenged with their normal pre-elimination diet(Reference van de Laar and van der Korst57,Reference O’Banion75) . If there was uncertainty about a negative food reaction, two studies and one case series reported repeating the food challenges more than once(Reference Kjeldsen-Kragh, Borchgrevink and Laerum54,Reference Carini, Fratazzi and Aiuti68,Reference O’Banion75) .
The protocol following a reaction to food was specified in three studies: participants would return to the baseline diet or consume only safe foods until symptoms subsided(Reference Beri, Malaviya and Shandilya63,Reference van de Laar, Aalbers and Bruins65,Reference Marshall, Stroud and Kroker74) . In one study, laxatives were used to expediate gut emptying for more severe reactions(Reference Marshall, Stroud and Kroker74).
The duration over which foods were reintroduced spanned from 4 weeks to 4 months recorded across six studies: 4 weeks(Reference Kavanaghi, Workman and Nash53); 5 weeks(Reference Darlington, Ramsey and Mansfield56); 6 weeks(Reference Darlington and Ramsey66); 9 weeks(Reference Barnard, Levin and Crosby55); 10 weeks(Reference Beri, Malaviya and Shandilya63); 4 months(Reference van de Laar, Aalbers and Bruins65).
While most studies tested many different foods, five studies were designed to challenge 1–3 specific foods to assess the effect on RA symptoms or metabolites, and measure responses in a clinical setting. Three studies challenged one food per person, with 6 h to 3 d of observation(Reference Felder, De Blecourt and Wüthrich21,Reference Little, Stewart and Fennessy61,Reference Lindqvist, Gjertsson and Hulander62) , and two challenged three foods per person, with challenges separated by approximately a week(Reference Hulander, Bärebring and Winkvist58,Reference Darlington, Jump and Ramsey59) .
Blinded food challenges
Blinded food challenges were conducted in five studies with a total of thirty-four participants who were rechallenged with previously implicated foods. These foods had been identified through exclusion reintroduction diets(Reference Carini, Brostoff and Wraith50,Reference Darlington, Jump and Ramsey59,Reference Carini, Fratazzi and Aiuti68,Reference Panush, Stroud and Webster76) , or via allergy testing and symptom diaries(Reference van de Laar, Aalbers and Bruins65). The foods were disguised within an allergen free carrier (Pepti 2000) with added rice, caramel and beet sugar(Reference van de Laar, Aalbers and Bruins65); lentil soup (n = 2)(Reference Darlington, Jump and Ramsey59,Reference Carini, Fratazzi and Aiuti68) ; or lyophilised (freeze dried) foods encapsulated in opaque capsules to mimic a serving size (n = 2)(Reference Carini, Fratazzi and Aiuti68,Reference Panush, Stroud and Webster76) . In addition, one study conducted a double-blind test on four participants, comparing tap water with mineral water, to confirm sensitivity(Reference Kavanaghi, Workman and Nash53).
Three studies included clinical data in the results of blinded challenges. In Darlington’s 1989 study, five participants underwent double-blind food challenges using lentil soup laced with the challenge food. The results showed deteriorations in 24-h pain, PJC, GS, DMS, walk time and haemoglobin levels (p = 0·000036)(Reference Darlington, Jump and Ramsey59). In a closely monitored study with four participants, disease activity was scored after each food challenge or placebo using an allergen-free carrier. Assessment measures included DMS, TJ, swollen joints (SJ), RI, Thompson score, GS, 100-ft walk time, global assessment, fatigue score, CRP and ESR. All four participants showed a significant difference in disease activity between the food challenges and placebo, as determined by a change-of-point test. In three participants, specific foods were clearly identified as affecting disease activity(Reference van de Laar, Aalbers and Bruins65,Reference van de Laar77) . A separate case study using lyophilised milk, or a placebo in capsules, resulted in reproducible and predictable RA joint symptoms within 6–12 h, as well as an increase in ESR (p < 0·05)(Reference Panush, Stroud and Webster76).
Measures for reactions to food challenges
Following a food challenge or reintroduction, reactivity was assessed by an exacerbation of RA symptoms reported by the participant, and/or measured changes by a clinician. Participant-reported exacerbations in symptoms such as joint pain, swelling and stiffness were used in seven studies(Reference Kavanaghi, Workman and Nash53–Reference Darlington, Ramsey and Mansfield56,Reference Darlington and Ramsey66,Reference Hicklin, McEwen and Morgan67,Reference Lunardi, Pachor and Nicolis70) . Across all studies, clinical reactivity assessments included joint diameter (n = 3), joint pain score, tender joint count (TJC), dolorimeter pain index (n = 4), RAI (n = 4), swollen joint count (SJC) (n = 2), GS (n = 4), visual analogue scale (VAS)-pain (n = 2), 24-h pain (n = 1), timed walk (n = 2), participant symptom diary (n = 3), global assessment (n = 3), VAS-fatigue (n = 1) and health assessment questionnaires (HAQ) (n = 1). Laboratory measures used include ESR (n = 3) and CRP (n = 2). Leucocyte counts were measured in one older case series(Reference Zeller73) and pulse rate in another(Reference O’Banion75) (Supplementary Information Tables S3 and S4). The time to onset and resolution of symptoms was documented in six studies(Reference Carini, Brostoff and Wraith50,Reference Ratner, Schneeyour and Eshel52,Reference Little, Stewart and Fennessy61,Reference Hicklin, McEwen and Morgan67,Reference Carini, Fratazzi and Aiuti68,Reference Marshall, Stroud and Kroker74) and seven case studies(Reference Zeller73,Reference O’Banion75,Reference Panush, Stroud and Webster76,Reference Kutlu, Öztürk and Taşkapan78,Reference Parke and Hughes79) . Four studies recorded changes in non-joint symptoms such as rhinitis, headaches, gastrointestinal changes and skin rashes(Reference Carini, Brostoff and Wraith50,Reference Stroud, Hahn and Arnett64,Reference Carini, Fratazzi and Aiuti68,Reference Lunardi, Pachor and Nicolis70) .
In response to specific food challenges previously identified as triggers for joint inflammation, one study measured serotonin (5-HT), and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), in platelet rich plasma before and up to 6 h after food consumption in seven individuals with RA, compared with seven healthy controls(Reference Little, Stewart and Fennessy61). In those with RA, there was a significant and sustained decrease in 5-HT and an associated increase in 5-HIAA, as well as increased joint pain and swelling. By contrast, healthy controls exhibited a temporary decrease of 5-HT at 75 min post challenge(Reference Little, Stewart and Fennessy61). Another study measured serum immune complexes immunoglobulin (Ig)G anti-IgE after food challenges in ten individuals with both allergies and RA. These immune complexes were detectable in three participants after consuming aggravating foods but were absent after consuming non-reactive foods(Reference Carini, Fratazzi and Aiuti68). In two case studies, multiple clinical and laboratory measures were assessed in response to dairy challenges(Reference Panush, Stroud and Webster76,Reference Parke and Hughes79) .
Two recent studies investigated the effects of single food challenges in participants with RA who did not report any obvious food intolerances. One study measured serum metabolites in twenty-two women with RA and twenty-two matched healthy controls after consuming a red meat meal. The findings revealed a higher concentration of phenylalanine in both fasting and postprandial samples of women with RA(Reference Lindqvist, Gjertsson and Hulander62). The other study examined blood lipids and IL-6 levels following three different protein meals – red meat, salmon or soy. No significant differences in IL-6 or triglycerides were observed, although the salmon meal resulted in increased very-low-density lipoprotein (VLDL) particles(Reference Hulander, Bärebring and Winkvist58).
Outcomes from food reintroductions
Nine studies documented foods identified as exacerbating symptoms. A total of 173 participants with RA across these studies (ranging from four to forty-eight per study) completed reintroductions, with 138 identifying foods(Reference Carini, Brostoff and Wraith50,Reference Kavanaghi, Workman and Nash53,Reference Darlington, Jump and Ramsey59,Reference Beri, Malaviya and Shandilya63,Reference van de Laar, Aalbers and Bruins65,Reference Hicklin, McEwen and Morgan67,Reference Carini, Fratazzi and Aiuti68,Reference Marshall, Stroud and Kroker74,Reference Kjeldsen-Kragh, Haugen and Borchgrevink80) . The number of foods reintroduced ranged from five food groups in one study(Reference Beri, Malaviya and Shandilya63) to approximately sixty foods in two studies(Reference Darlington, Jump and Ramsey59,Reference Marshall, Stroud and Kroker74) . Six studies reported that the number of foods reacted to per person ranged from one to ten foods, and only one study reported more, showing 27% of participants reacting to over ten foods(Reference Darlington, Jump and Ramsey59). The average reactions per person were reported as 2·5(Reference Hicklin, McEwen and Morgan67), 4·5(Reference Kavanaghi, Workman and Nash53) and 8·8(Reference Stroud, Hahn and Arnett64,Reference Marshall, Stroud and Kroker74) . An additional study documented a single food per participant identified by elimination and reintroduction, and used a food challenge protocol for retesting(Reference Little, Stewart and Fennessy61). Case studies highlighted reactions from one(Reference Panush, Stroud and Webster76,Reference Parke and Hughes79,Reference Isasi, Tejerina and Morán81,Reference Williams82) to fourteen foods(Reference O’Banion75) (Supplementary Information Tables S3 and S4).
The time to onset of symptoms following food challenges was recorded in six studies. In five studies the onset ranged from 1 to 48 h(Reference Carini, Brostoff and Wraith50,Reference Little, Stewart and Fennessy61,Reference Carini, Fratazzi and Aiuti68,Reference Marshall, Stroud and Kroker74) , however, one study reported reactions could occur anywhere from 2 h to 2 weeks(Reference Hicklin, McEwen and Morgan67). In seven case studies the reported onset ranged from 45 min to 24 h, with symptoms subsiding within 24 h to 10 d(Reference Zeller73,Reference O’Banion75,Reference Panush, Stroud and Webster76,Reference Kutlu, Öztürk and Taşkapan78,Reference Parke and Hughes79) . The study that excluded dairy and beef reported that, among nineteen people with RA, joint swelling subsided in six individuals within 1 week, and they became asymptomatic 3–4 weeks after starting the diet. These six responders were reintroduced to dairy food at 3 months, which resulted in joint swelling and pain within 2–3 weeks, requiring anti-inflammatory drugs. Three of the six participants repeated the dairy provocation at 6 months and experienced the same result(Reference Ratner, Schneeyour and Eshel52).
Where food sensitivity tests such as the radioallergosorbent test (RAST) for antibodies to foods, enzyme-linked immunosorbent assays (ELISA) for food antibodies, assays for food antibody and food immunoglobulin (Ig) complexes, and skin prick tests to foods were done, none demonstrated significant associations with the foods identified using a reintroduction protocol(Reference Carini, Brostoff and Wraith50,Reference Ratner, Schneeyour and Eshel52,Reference Darlington, Jump and Ramsey59,Reference van de Laar, Aalbers and Bruins65,Reference Kjeldsen-Kragh, Hvatum and Haugen69) .
A compilation of specific foods identified across ten studies and sixteen case reports involving 161 participants is depicted in Fig. 4, showing the cumulative number of individuals reacting to each food. The foods most frequently reported to increase RA symptoms were wheat, corn, milk/dairy, eggs, beef and pork.

Fig. 4 Cumulative numbers of people reported as reacting to foods.
Outcomes measured following exclusion of identified foods
In thirteen of eighteen studies, foods that were found to increase RA symptoms were excluded by the end of the study period, with the outcome measures reported to varying degrees. Six of these studies reported a range of clinical and laboratory measures, three utilised a grading system for the severity of symptoms, and four had inadequate reporting.
The clinical measures reported included the Disease Activity Score, GS, VAS-pain, Global Assessment, and assessments of joint pain and swelling, as well as the number of joints affected. Laboratory measures included ESR and CRP(Reference Kjeldsen-Kragh, Borchgrevink and Laerum54–Reference Darlington, Ramsey and Mansfield56,Reference Karatay60,Reference Stroud, Hahn and Arnett64,Reference Marshall, Stroud and Kroker74) . A small study (n = 4) used biopsies of the synovial membrane of a large joint and the proximal small intestine to count mast cell numbers before and after a 6-week period of partial disease remission on an allergy-free diet(Reference van de Laar, Aalbers and Bruins65). One study with eleven published papers explored many clinical parameters and included comparisons between responders and non-responders in the intervention arm. Of significance were differences in calprotectin levels(Reference Kjeldsen-Kragh, Mellbye and Haugen83), anti-Proteus mirabilis IgG titres(Reference Kjeldsen-Kragh, Rashid and Dybwad84) and changes in faecal flora(Reference Peltonen, Kjeldsen-Kragh and Haugen85). Three other studies utilised a grading system that rated disease severity, comparing the end of the study period with the baseline. Severity of symptoms was graded from 0 (none), to 3 or 4(Reference Carini, Brostoff and Wraith50,Reference Lunardi, Bambara and Biasi51,Reference Carini, Fratazzi and Aiuti68) .
Four studies (including two conference abstracts) reported the numbers of participants who improved, with no clinical data(Reference Kavanaghi, Workman and Nash53,Reference Darlington, Jump and Ramsey59,Reference Beri, Malaviya and Shandilya63,Reference Hicklin, McEwen and Morgan67) .
Study outcomes at completion
In four single-blind RCTs, overall outcomes were compared between the elimination reintroduction protocol and the usual diet control, and two reported the number of responders.
Darlington’s study included two groups: group one (n = 25) followed a restricted omnivore diet for 1 week followed by individual food re-introductions over 5 weeks. Group two (n = 24) consumed their usual diet plus placebo pills for 6 weeks before crossing over to the intervention. At the conclusion of the 6-week intervention thirty-three out of forty-seven participants who completed the study reported feeling ‘better’ or ‘much better’, with reductions in pain, the number of PJC or SJC, and ESR compared with baseline and the control group (p < 0·05). Eleven participants reported no change or worsening of symptoms(Reference Darlington, Ramsey and Mansfield56).
Kjeldsen-Kragh’s study began with a 1-week juice fast, followed by individual food reintroductions every 2 d, ultimately transitioning to an individualised vegetarian diet by 9 months. At the end of the 13-month study, twelve out of twenty-seven participants in the diet group and two out of twenty-six in the control group reported significant clinical improvement. By the study’s conclusion, seventeen participants remained in each group. The intervention group showed significant improvements in ESR and CRP (p < 0·0001), and reductions in pain, DMS, global assessment, TJ, SJ and RAI compared with the control group (p < 0·02). However, radiographic scores in both groups deteriorated slightly(Reference Kjeldsen-Kragh, Borchgrevink and Laerum54).
The third and most recent study conducted 2022, was an RCT crossover trial with twenty-two participants in each group. The intervention involved a gluten-free, vegan, few-foods diet with reintroductions every 2 d over 16 weeks. At the end of the study (n = 32), there was significant improvement in DAS28, Modified Health Assessment Questionnaire (MHAQ) and the number of SJ compared with baseline and the control group (p < 0·05)(Reference Barnard, Levin and Crosby55).
Another RCT (n = 47) included a diet group (n = 24) that underwent a 4-week elimination diet, followed by 8 weeks of single food reintroductions. Of the twenty-four participants, eleven identified foods that exacerbated symptoms. However, by the 24-week endpoint, the study reported no significant changes from baseline (without providing figures) and observed a high dropout rate (eleven out of twenty-four). The authors noted that participants appeared to have abandoned the diet(Reference Kavanaghi, Workman and Nash53).
A two-arm study (n = 20 in each group) compared participants with RA who reacted positively to skin prick tests (SPTP) with those who reacted negatively (SPTN). Both groups consumed a low allergenic, few foods vegan diet for 12 d. The SPTP group then reintroduced foods that had elicited positive skin prick reactions, while the SPTN group added corn and rice for 12 more days before re-eliminating these foods for another 12 d. Upon food reintroduction, thirteen out of eighteen participants in the SPTP group and three out of seventeen in the SPTN group experienced an increase in disease activity. However, after re-elimination, only one out of thirteen participants in the SPTP group showed a reduction in disease activity(Reference Karatay60,Reference Karatay, Erdem and Kiziltunc86) .
Among the single-arm trials, one study took place in a controlled, low-allergy, live-in environment. Participants began with a water fast, followed by individual food reintroductions every 2 d over an unspecified period. Results from twenty-six participants showed significant improvements in GS, joint tenderness index, joint swelling index and PIP circumference (p < 0·001), with a non-significant reduction in ESR(Reference Marshall, Stroud and Kroker74).
Three additional single-arm studies used a few-foods omnivore diet with food reintroductions spaced 5–7 d apart over an unspecified timeframe. These studies employed a grading system to assess disease severity from baseline to the end of the study period. Two of the studies used a scale ranging from 0 (lowest severity) to 3 (highest severity), and results for fourteen participants with RA, assessed by a physician, showed severity reductions of 1–3 points per person(Reference Carini, Brostoff and Wraith50,Reference Carini, Fratazzi and Aiuti68) . In the other study, participants kept daily symptom diaries, rating severity from 0 to 4. At study completion, the seropositive RA group (n = 9) improved from a mean score of 3·25 ± 0·41 to 1·75 ± 1·00, while the seronegative group (n = 8) improved from 2·22 ± 0·98 to 1·57 ± 1·11 (p < 0·005). Four individual participants showed Waaler Rose test results becoming negative, while the Reuma tests also decreased(Reference Lunardi, Bambara and Biasi51).
Two conference abstracts reported on studies using a few-foods omnivore diet followed by food reintroductions. One study (n = 22) reported improvements in twenty participants(Reference Hicklin, McEwen and Morgan67), while another study (n = 15) stated that participants experienced ‘significant improvements with diet therapy’(Reference Darlington, Jump and Ramsey59).
Microbiome and gut relevant outcomes
Measures related to microbiome changes and gut permeability were reported in only three studies.
In a 13-month study, by Kjeldsen Kragh (1991), faecal flora changes were analysed using gas–liquid chromatography (GLC) of bacterial cellular fatty acids from direct stool samples. The twenty-seven participants began with a juice fast, reintroducing foods at 2 d intervals to transition to an individually adjusted vegan and then lacto-vegetarian diet. Significant alterations in intestinal flora were observed between each dietary phase and compared with the baseline omnivore diet. Participants were categorised into a high improvement index (HI) group (> 20% improvement in five core variables compared with baseline) and a low improvement index (LI) group. The study found a strong association between intestinal flora composition and disease activity, with a highly significant difference between the HI and LI groups at 1 month and 13 months (p < 0·001)(Reference Peltonen, Kjeldsen-Kragh and Haugen85). At baseline, all participants with RA mean plasma calprotectin levels measured eight times higher than the upper limit of the normal range for healthy individuals. Over the course of the study, a significant decrease in calprotectin was observed in the HI group (p < 0·03) but not in the LI group(Reference Kjeldsen-Kragh, Mellbye and Haugen83).
Another study involved three participants who had identified specific foods that aggravated their symptoms. Biopsies were taken from the proximal small intestine both before dietary exclusion and after 6 weeks of allergen elimination, during which disease activity had decreased. In two of the three participants, there was a marked reduction in mast cells during periods of reduced disease activity(Reference van de Laar, Aalbers and Bruins65,Reference van de Laar77) .
A third study examined the effects of sodium cromoglycate (SCG), a mast cell stabiliser, in participants who had identified foods that increased arthralgia symptoms. Participants were administered 500 mg of SCG or a placebo 3·5 h before a food challenge consisting of either 5 oz of milk or two eggs. All twelve participants who received SCG were protected from increased symptoms, while none in the placebo group experienced symptom relief(Reference Carini, Brostoff and Wraith50).
Follow-up after study completion
Six studies reported follow-up assessments of participants after study completion, with intervals ranging from 3 months(Reference Darlington, Jump and Ramsey59,Reference Beri, Malaviya and Shandilya63) , to 8 months(Reference van de Laar77), 12 months(Reference Kjeldsen-Kragh, Haugen and Borchgrevink80), and 1–4 years(Reference Carini, Brostoff and Wraith50,Reference Carini, Fratazzi and Aiuti68) .
Carini et al. followed up all participants in two studies, from 1 to 4 years. In one study, all ten participants maintained dietary exclusions and experienced reduced disease activity(Reference Carini, Fratazzi and Aiuti68). In the other study, twenty out of twenty-four participants with arthralgia maintained dietary exclusions; however, it was unclear whether any of these individuals were among the four RA participants(Reference Carini, Brostoff and Wraith50).
The study from India which initially reported ten out of fourteen participants who identified foods that increased RA symptoms, found that at the 10-month follow-up, three out of ten participants had adhered to the diet and remained off medication(Reference Beri, Malaviya and Shandilya63).
Van de Laar reported that of four participants who had identified foods increasing RA symptoms during the study period, two maintained dietary exclusions and experienced prolonged improvement, one for 8 months(Reference van de Laar, Aalbers and Bruins65).
Only one study (Kjeldsen-Kragh et al.) conducted a formal follow-up that included the same range of clinical data collected during the intervention. One year after the study ended, forty-five of the original fifty-three participants were assessed, twenty-three out of twenty-six from the control group, and twenty-two out of twenty-seven from the diet group, including ten of the twelve responders. Eight clinical variables were measured and compared with baseline, along with an assessment of foods participants continued to avoid. The responders were found to have maintained their clinical improvement(Reference Kjeldsen-Kragh, Haugen and Borchgrevink80).
Case studies also reported long-term control of disease activity, with follow-up periods ranging from 5 months to 4 years (Supplementary Table S4).
Detailed characteristics of included studies, including study design, participants, methods, elimination and reintroduction protocols, and outcome measures used for twenty trials are included in Supplementary Table S3. Supplementary Table S4 provides an overview of the case studies, detailing the elimination reintroduction protocols as well as the individual outcomes.
Discussion
Summary of evidence
This scoping review examined current published literature on dietary elimination, reintroduction, and food challenge protocols in adults with RA. The twenty studies and seventeen case reports included a typical RA patient population but displayed considerable methodological heterogeneity, including a variety of protocols for both elimination diets and food challenges. Of the 878 total participants across all trials, 670 had RA, 518 undertook an elimination diet, and 446 completed a reintroduction or food challenge protocol. The overall dropout rate in the studies involving elimination and reintroduction was 18% (range 0–46%). Only four studies have been conducted since 2000, with the remainder from 1980 to 1995. The decline in interest in this type of dietary therapy may be related to the enhanced effectiveness of modern drug therapies. DMARD have significantly evolved over the last 35 years, with new biological response modifiers (biologics) in the late 1990s offering a broader range of options for effective control of RA activity(Reference Upchurch and Kay87). Most studies were low-to-medium quality, and many had inadequate data reporting. This may be attributed to the fact that reporting standards were not established until 1996 with the CONsolidated Standards of Reporting Trials (CONSORT) Statement(Reference Altman and Simera88). The geographical distribution and historical range of the studies, along with feedback from people with RA in multiple surveys, indicates a broad and sustained interest in this type of dietary intervention in RA.
The elimination strategies were highly varied, as was duration of dietary exclusion prior to food reintroductions and challenges. Only nine studies recorded responses to the elimination diet phase, with five detailing changes in laboratory and clinical measures. The lack of comparative data on the efficacy of different exclusion protocols before food reintroduction is a notable gap in the research.
Although fasting has been confirmed as being effective in disease activity measures in RA(Reference Häupl, Sörensen and Smiljanovic19), its short-term feasibility and the need for supervision limits its practicality, and its impact on subsequent food reintroductions remains unexplored. Alternatives such as the ‘few foods diet’ or an elemental diet offer more tolerable and practical approaches. The common feature for all elimination diets is they only include foods considered least likely to produce a non-immediate hypersensitivity or immune response. Foods removed are typically dairy, nuts and seeds, soy, cereal grains, especially gluten, eggs, some meats, coffee, sugar and additives. Only one study included a gluten grain, rye, as it was a staple in Turkey where the study was conducted(Reference Karatay, Erdem and Kiziltunc86).
Recent studies specifically excluding arthritogenic food groups in RA are scarce. The ITIS diet pilot study, conducted with twenty people demonstrated a 50% reduction in pain in 7/20 and fatigue in 9/20 participants within 2 weeks. The ITIS diet excludes certain food groups associated with increased inflammation, including sugar, alcohol, refined grains, gluten, nightshades, dairy and red meat. It also enhances dietary quality by incorporating foods associated with being anti-inflammatory, such as fermented foods as well as polyphenol, fibre and omega-3 rich foods(Reference Coras, Martino and Gauglitz89,Reference Coras, Martino and Gauglitz90) . Other diets that remove foods commonly associated with symptom aggravation in RA, include vegan diets, which eliminate dairy, eggs and red meat, and vegetarian diets which remove red meat, along with other animal protein. A systematic review of vegan and vegetarian diets found that a pooled analysis showed a small but significant reduction in pain compared with control diets, but no significant improvement in disease activity or physical functioning(Reference Søndergaard, Jensen and Thomsen91). One RCT explored the effects of a gluten free (GF) vegan diet compared with a well-balanced control diet in patients with RA. After 9 months, participants on the GF vegan diet recorded significant improvements in all measured variables including CRP levels, with 9/22 meeting the ACR20 response criteria, compared with 1/25 in the control group. IgG antigliadin and anti-β lactoglobulin levels decreased significantly in responders, leading the authors to suggest the benefits may be due to a diminished immune response to exogenous food antigens(Reference Hafstrom92).
Although these diets indicate the exclusion of certain foods groups may contribute to symptom improvement, a key confounder in elimination diet studies is the concurrent improvement in dietary quality. For example, most elimination diets promote the consumption of unprocessed, additive-free, whole foods and increased fibre intake, which are independently associated with anti-inflammatory benefits. Adding dietary fibre without other dietary changes has been shown to produce a favourable change in the Th1/Th17 ratio, decrease markers of bone erosion, and significantly reduce serum calprotectin and zonulin, intestinal markers of inflammation and gut barrier function in subjects with RA(Reference Häger, Bang and Hagen93). Improving dietary quality alone has been shown to improve some RA disease measures, as observed in diet studies on the Mediterranean diet and other whole-food-based diets(Reference Philippou, Petersson and Rodomar40). A systematic review assessing the effects of anti-inflammatory diets on inflammation and pain in RA, found that Mediterranean diets, ketogenic diets, vegan and vegetarian diets all resulted in significantly lower pain compared with standard diets (p = 0·0002)(Reference Schönenberger, Schüpfer and Gloy94). In this scoping review none of the RCT controlled for the confounding effect of dietary quality by comparing two diets of similar quality, one with food exclusions and one without.
This confounding factor has been addressed in only one recent study. Guagnano et al. (2021) compared two versions of a Mediterranean diet. The version excluding dairy, gluten, and meat, showed significant improvements in quality-of-life (as measured by the 36-Item Short Form health survey), VAS-Pain scores and high-sensitivity CRP after 3 months, as compared with the standard Mediterranean diet. Despite a high dropout rate of 30%, this outcome suggests that specific food exclusions may influence RA activity when diet quality is controlled for(Reference Guagnano, D’Angelo and Caniglia95). Given these findings, this confounder should be considered in the design of future RCT exploring the effects of food exclusions in RA.
Food challenge protocols varied widely in this scoping review, with reintroduction intervals of three foods per day, one food every 2, 3, 7 d, or two weeks. Most trials introduced a single food or a food group separately, such as dairy products, while two of the twenty reintroduced multiple foods together(Reference van de Laar and van der Korst57,Reference Karatay, Erdem and Kiziltunc86) . Four studies measured the effects of single foods on various serum markers in a food challenge protocol after an overnight fast(Reference Felder, De Blecourt and Wüthrich21,Reference Hulander, Bärebring and Winkvist58,Reference Little, Stewart and Fennessy61,Reference Lindqvist, Gjertsson and Hulander62) . The methods for assessing food reactivity also varied from subjective approaches, where participants observed an increase in pain or inflammation, to objective approaches, such as a clinician measuring joint diameter, joint pain or tenderness counts. Reactivity was reported as taking as short as 45 min to as long as 2 weeks after food consumption, however, most studies and case reports reported that onset of symptoms began between 1 and 48 h and lasted from 18 h to 10 d. The variability in response times suggests that spacing reintroductions 2 or more days apart may enhance the accuracy of identifying trigger foods.
The unblinded nature of many food reintroductions and reliance on self-reported symptoms are problematic, introducing the risk of subjective bias or nocebo effects. This was demonstrated in one study where participants, who believed they reacted to a specific food, showed no changes in pain, swelling or clinical markers when observed for 36 h after its consumption(Reference Felder, De Blecourt and Wüthrich21). However, in a study with monitored open food reintroductions in twenty-two people, significant differences (p < 0·001) in GS, dolorimeter pain index and arthrorcircameter of PIP joints measures were documented after reactive compared with non-reactive foods(Reference Marshall, Stroud and Kroker74). In another study, where seven participants with RA consumed a food previously confirmed as reactive, in addition to joint diameter increases, platelet serotonin release was significantly increased for 5 h post consumption(Reference Little, Stewart and Fennessy61). Van de Laar (1991) conducted double-blind food challenges with fresh foods and measured twelve variables: DMS; VAS-Fatigue; TJC (77); SJC (74); RAI (78); GS; 100 ft walk time; Thompson score; Physician’s Global Assessment; weight; ESR; CRP, in response to food challenges. Change of point tests showed disease activity parameters changed significantly between reactive foods and placebo challenges in three of four participants(Reference van de Laar77). These data suggest a role for objective measurement of reactivity to foods and blinding of food challenges in study design.
Studies documenting foods that increased RA symptoms in individuals show variability in both the types and numbers of reactive foods identified. This variability may reflect the heterogeneity in reintroduction protocols, as well as individual food sensitivities. Some studies specifically included participants with RA with pre-existing allergies or food intolerances, hypothesising that they may be more sensitive to certain foods(Reference Carini, Brostoff and Wraith50,Reference Carini, Fratazzi and Aiuti68,Reference Lunardi, Pachor and Nicolis70) , whereas other studies explicitly excluded this group(Reference Hulander, Bärebring and Winkvist58,Reference Lindqvist, Gjertsson and Hulander62) . Darlington (1986) observed that 90% of participants with a family history of atopy had a good response to the elimination reintroduction protocol, compared with 70·6% of those without such a family history(Reference Darlington, Ramsey and Mansfield56). Foods that were identified in studies as increasing RA symptoms, when added together, showed a cumulative pattern, and those that elicited a greater number of reactions were wheat, corn, dairy products, eggs, beef and pork. While these results require confirmation in contemporary studies, they may inform the design of elimination and reintroduction protocols. Studies included in this scoping review indicate that food sensitivity tests such as RAST, ELISA, IgG and skin prick tests do not accurately predict foods that trigger RA symptoms.
The precise mechanisms by which certain foods increase RA activity (and high-specificity measurements for these) should be considered in future studies. In this scoping review four studies measured specific markers post-challenges: platelet serotonin release(Reference Little, Stewart and Fennessy61); IgE anti-IgE(Reference Carini, Fratazzi and Aiuti68); amino acid metabolites(Reference Lindqvist, Gjertsson and Hulander62); IL-6 and blood lipids(Reference Hulander, Bärebring and Winkvist58). In another study, histological studies, including mast cell infiltration from synovial and proximal small intestine biopsies, were compared during partial remission to the baseline period with active disease for those on a diet that excluded aggravating foods(Reference van de Laar, Aalbers and Bruins65). Further research is required to determine whether repeat studies are warranted, and which biomarkers may elucidate mechanisms, for example, changes in gut microbiome, intestinal permeability, immune modulation and inflammation.
The relationship between the gut microbiome, intestinal permeability, diet and RA has gained increasing attention in recent years. Research shows that microbiota in individuals with early RA differs from that of healthy controls, with reductions in Bifidobacterium and Bacteroides, and an increase in Prevotella species(Reference Horta-Baas, Romero-Figueroa and Montiel-Jarquín96). In addition increased levels of Collinsella and Eggerthella, and reduced microbiome diversity have been observed, correlating with disease duration and auto-antibody levels(Reference Chen, Wright and Davis97). Altered gut barrier function and increased intestinal permeability has also been demonstrated in RA. Biomarkers such as elevated levels of soluble CD14 (sCD14) and lipopolysaccharide-binding protein (LPS-BP), indicate bacterial translocation from the colon to the bloodstream, with higher levels correlating positively with RA disease activity. These biomarkers decrease in response to DMARD treatment(Reference Audo, Sanchez and Rivière18). Other recent studies found faecal and serum zonulin measures in sixty-one people with RA were above reference ranges in 98% of cases, and serum and faecal calprotectin were above reverence ranges in 38%(Reference Heidt, Kämmerer and Fobker98). Colonic tissue biopsies from subjects with RA show altered tight junction proteins, along with increased serum biomarkers of intestinal permeability, which is associated with systemic inflammation(Reference Audo, Sanchez and Rivière18).
The permeability of the intestinal epithelium is tightly regulated by the mucosal immune system and the integrity of intercellular tight junctions. Certain dietary components may directly affect tight junction function, increasing intestinal permeability. Studies using Caco-2 cell lines in vitro show that alcohol, specific fatty acids, gliadin, and proteins from milk and cheese can increase permeability(Reference De Santis, Cavalcanti and Mastronardi99). In addition, food additives commonly used in the food industry, such as emulsifiers, organic solvents, gluten, and microbial transglutaminase, may exacerbate tight junction leakage(Reference Lerner and Matthias100).
Dietary components may also directly impact gut mucosa negatively in RA. In a rectal patch study involving twenty-seven individuals with RA, increased mucosal reactivity was observed in response to cow’s milk in 22% of participants and to gluten in 33%, while reactivity was detected in one of eighteen healthy controls(Reference Lidén, Kristjánsson and Valtysdottir25). Another study using jejunal perfusions of dietary antigens – including prolamins from wheat and oats, casein, α- and β-lactalbumin, ovalbumin, and protein extracts from soy, pork and codfish – found localised immune activation in fourteen participants with RA. This was evidenced by moderately or highly increased levels of jejunal IgG, IgA and IgM activities to nearly all test antigens, when compared with healthy controls(Reference Hvatum101).
Increased intestinal permeability allows microbial and immunogenic antigens to translocate into the subepithelial space, potentially triggering immune responses. Recent fasting studies highlight the profound effects of food withdrawal. A 7 d fasting regimen combined with bowel cleansing resulted in rapid improvements in disease activity (DAS28 and SDAI), as well as significant reductions in IL-6 and zonulin, a key marker of mucosal barrier dysfunction(Reference Häupl, Sörensen and Smiljanovic19).
Microbiome and metabolome in dietary response
Several dietary studies have explored the impact of dietary changes on the gut microbiome in individuals with RA. The diets both exclude foods thought to aggravate RA symptoms, and include foods shown to promote gut health and reduce inflammation. The ITIS study aimed to improve diet quality by incorporating foods with the potential to decrease inflammation, such as phytonutrient-rich, fibre-rich and omega-3-rich foods, along with fermented foods, while excluding potentially inflammatory foods such as red meat, alcohol, gluten, dairy and nightshades. The study found that responders – defined as those experiencing a ≥ 50% reduction in pain (n = 7) – differed from non-responders in both their baseline microbiome composition and their response to dietary changes. Microbiome alpha diversity at baseline was significantly higher in responders. Following dietary intervention, plasma metabolome beta diversity significantly differed between responders and non-responders, suggesting that specific metabolites may influence pain levels in RA(Reference Coras, Martino and Gauglitz102). A previous study examined faecal microbiome changes in response to a 3-month dietary intervention in forty-three adults with RA, who were randomised to their habitual diet, or an uncooked vegan diet rich in lactobacilli, which increased fibre intake 2–4 times. Significant alterations in gut microflora were observed in the intervention group, that were associated with a reduction in subjective symptoms of RA(Reference Peltonen, Nenonen and Helve103). Further analysis indicated that the reduction in disease activity correlated with a higher intake of fermented drinks and fibre, as demonstrated through stepwise regression modeling(Reference Nenonen, Helve and Rauma104). More recently a plant-based dietary intervention in seventy-seven participants with RA showed preliminary evidence of improved intestinal barrier integrity, as measured by faecal albumin levels(Reference Wagenaar, Pereira and Redanz105).
The extent to which the exclusion of specific food groups influences gut microbiome composition and inflammatory marker changes in RA remains unclear. It is possible that improvements in overall dietary quality or increased fibre intake alone contribute to these observed benefits. A questionnaire-based study found that individuals with RA who adhered more closely to Mediterranean diet (MD) principles, exhibited a healthier gut microbiome composition, with an almost complete absence of Prevotella copri, a gut bacterium associated with RA pathogenesis. These individuals also had significantly lower CRP and reduced disease activity, compared with those with low adherence to the MD diet(Reference Diamanti, Panebianco and Salerno106). A study examining the effects of a prebiotic rich fibre supplement (15 g/d) in participants with RA (n = 29), reported an increase in systemic serum anti-inflammatory short chain fatty acids, and a reduction in pro-arthritic cytokines IL-18 and IL-33, both of which are implicated in RA disease progression(Reference Dürholz, Hofmann and Iljazovic107). Future research is needed to distinguish the effects of dietary eliminations from those of overall dietary quality in relation to gut microbiome changes and intestinal permeability.
The studies included in this scoping review contained only three that investigated changes related to gut permeability or dysbiosis in RA, with key findings highlighting differences in biomarker outcomes in responders compared with non-responders. Responders to dietary interventions exhibited distinct microbiome changes compared with non-responders(Reference Peltonen, Kjeldsen-Kragh and Haugen85). Small intestinal biopsies from two responders showed marked reductions in mucosal mast cells(Reference van de Laar, Aalbers and Bruins65). Increased mast-cell numbers are observed in the intestinal mucosa of individuals with altered gut barrier function(Reference Albert-Bayo, Paracuellos and González-Castro108). An increase in serum serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) is observed in response to foods identified to aggravate joint symptoms in seven individuals(Reference Little, Stewart and Fennessy61), potentially linking mast cell aggravation to dietary triggers(Reference Albert-Bayo, Paracuellos and González-Castro108).
Future directions
In designing future studies, it would be beneficial to reduce confounders in RCT. For example, ensuring identical dietary quality with the only variable being specific food exclusions would help isolate the effects of dietary modifications. In current studies that incorporate food exclusions, authors could consider implementing a structured reintroduction phase in responders to evaluate whether certain foods exacerbate RA symptoms. Observations from this scoping review suggest that meal-sized portions should not be reintroduced less than 2 d apart. Blinded food challenges would be appropriate for confirming sensitivity. If specific foods are confirmed to aggravate RA symptoms, further investigations into the underlying mechanisms would be valuable.
The growing body of research linking gut dysbiosis, intestinal permeability and RA, along with evidence of microbiome and biomarker alterations following dietary changes, suggests that future studies should incorporate these measures. For example, future research could assess microbiome and metabolome changes and compare responders with non-responders. Intestinal permeability and inflammation biomarkers, such as serum lipopolysaccharide-binding protein, and stool and serum zonulin and calprotectin, could provide insights into participants responses to dietary interventions. Conducting dietary analyses to correlate specific nutrient changes with biomarker variations would further contribute to understanding the relationship between diet and RA.
Long-term participant follow-up measures were reported in only one study. As effective dietary changes will need to be maintained, the assessment of long-term sustainability of dietary interventions and their impact on disease progression should be a key priority in future studies.
Research is also needed to establish clear guidelines for an evidence-based protocol for an elimination reintroduction diet, as well as establish to what extent this type of diet is useful as an adjunct to medical treatment for this population. Food intolerance patterns appear to be very individual, and guidelines on the optimal approach require further research.
Strengths and limitations of the scoping review process
This is the first scoping review solely focused on food challenge studies in RA. Every effort was made in the search to identify relevant texts on this topic, with a considerably larger number of records included compared with previous reviews. The format of a scoping review allows a wide range of records; however, this inclusivity means the quality of studies was not assessed against the more stringent criteria used for systematic reviews of RCT. The main limitations stem from the varied quality of study designs, heterogeneity in methodologies and outcomes, incomplete reporting, and the inclusion of some studies only available as conference abstracts. In addition, case studies, which represent the lowest evidence level, tend to report only successful dietary interventions, potentially biasing the findings.
Conclusions
On the basis of the studies included in this scoping review, subgroups of people with RA reporting both subjective and objective effects of certain foods on RA, and improvements in disease activity following food exclusions are consistently found. These findings suggest that some people with RA may benefit from an elimination and food challenge protocol to identify arthritogenic foods. However, the need for further research is clear, both to replicate the findings of studies using laboratory markers and to elucidate the mechanisms by which foods or additives affect RA. For health and nutrition professionals working with clients with RA who suspect foods may contribute to their disease activity, there are no current accepted protocols in use. Clinical studies are needed to standardise elimination and reintroduction protocols for patients with RA, that are practical for implementation in clinical practice. If foods are found by this method, long term participant follow-up to monitor disease activity and progression, as well as patient tolerance of a restricted diet is an important consideration.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954422425000083
Acknowledgements
The authors acknowledge the contribution of Sylvia Goedeke as a reviewer for the screening of abstracts and papers for inclusion in this review.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare none.
Authorship
J.M. and C.Z. conceptualised the review. J.M. conducted the literature search, extracted data and drafted the manuscript. C.Z., G.M. and R.G. contributed to critical revision and edits of the manuscript. C.Z. reviewed the data extraction and screening of papers. All authors approved the final version.









