Introduction
Cancer remains a leading global health concern, with the UK reporting 387 820 new diagnoses in 2020, reflecting a nearly 6% increase compared with 2 years prior(1). In 2022, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) reclassified occupational exposure as a firefighter as ‘carcinogenic to humans’ (Group 1)(Reference Demers, DeMarini and Fent2), reflecting a growing body of research demonstrating a significant cancer risk among career firefighters(Reference DeBono, Daniels and Beane Freeman3).
Mesothelioma and bladder cancer have been identified by the IARC as having sufficient evidence to establish a causal link to firefighting. Suggestive evidence also connects firefighting with cancers of the colon, prostate, testis, melanoma and non-Hodgkin lymphoma. Other studies have indicated increased risks of cancers of the airway, thyroid and kidney among firefighters(Reference LeMasters, Genaidy and Succop4). While some studies suggest elevated risks of breast and cervical cancers for female firefighters, the limited sample sizes make it difficult to establish definitive conclusions(Reference Ma, Fleming and Lee5–Reference Stec, Robinson and Wolffe7).
Although UK fire service data support the connection between firefighting and cancer(Reference Stec, Robinson and Wolffe7,Reference Wolffe, Robinson and Dickens8) , the Industrial Injuries Advisory Council (IIAC) currently recognises only mesothelioma as a prescriptive disease for firefighters(9).
The primary driver of the elevated cancer risk among firefighters is their exposure to contaminants contained in fire effluents(10), which include, among others, benzene, polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls (PCB), asbestos, arsenic, formaldehyde and heavy metals (lead, cadmium, chromium, nickel)(Reference Stec, Robinson and Wolffe7,10,Reference Richardson, Watt and Watkins11) . These carcinogens contribute to cancer risk primarily through direct DNA damage but also by promoting chronic inflammation, a recognised hallmark of cancer driven by sustained immune activation, oxidative stress and tissue damage(Reference Ryu and Hong12,Reference Balali-Mood, Naseri and Tahergorabi13) .
While the use of personal protective equipment (PPE) and cleansing methods is promoted by UK fire services and the Fire Brigade’s Union, these measures do not fully eliminate exposure risks(Reference Wolffe, Clinton and Robinson14,Reference Wolffe, Turrell and Robinson15) . Certain contaminants remain in the body for years after their initial uptake(Reference Qu and Zheng16), leaving firefighters who worked before the implementation of recent safety measures potentially at risk from previously absorbed chemicals.
However, chemical exposure is not the only occupational hazard contributing to cancer risk in firefighters. Shift work, classified as ‘probably carcinogenic to humans’ by the IARC, further complicates the risk profile. The disruption of circadian rhythms caused by irregular work schedules has been linked to weight gain, hormonal imbalances, reduced DNA repair efficiency and chronic inflammation – all of which can elevate cancer risk independently of chemical exposures(17). Studies suggest that shift work increases the likelihood of developing hormone-related cancers, such as breast and prostate cancer, as well as other malignancies associated with immune dysfunction(17–Reference Wendeu-Foyet and Menegaux19). This highlights the multifactorial nature of cancer risk among firefighters, where both chemical and non-chemical occupational hazards intersect.
Compounding these risks, changes to the UK firefighters’ pension scheme in 2015 require firefighters to work until a later age to access full benefits. This change carries a genuine risk of increased cancer incidence, due to prolonged exposure to contaminants, coupled with the natural, age-related decline in the body’s antioxidant systems, such as the glutathione cycle(Reference Diaz-Del Cerro, Martinez de Toda and Félix20,Reference Jones, Mody and Carlson21) . This cumulative effect underscores the importance of considering supplementary protective measures.
While contaminant avoidance remains the priority for fire services and their crews, it is vital to explore additional protective strategies, including nutrition, which is non-intrusive and scalable and has wide-reaching implications for health and cancer risk reduction.
Previous papers have provided broad overviews of the possible influences – both positive and negative – of firefighters’ diet and lifestyle on cancer risk(Reference Smith, Matias and Bode22–Reference Sidossis, Lan and Hershey24). The current paper aims to extend this body of work by looking at specific foods and nutrients that may be bioactive against a range of firefighter chemical exposures. It also considers the unique cultural and systemic barriers faced by UK firefighters and explores potential methods for overcoming them.
The role of diet in reducing cancer risk
In addition to occupational exposures, many cancers are linked to lifestyle factors. According to a recent UK government review, four in ten cancer cases are attributable to diet and lifestyle, and up to eight in ten are considered ‘preventable in principle’(Reference Stanton25).
The World Cancer Research Fund (WCRF) lists ten key Cancer Prevention Recommendations that have been shown to reduce risk of cancer(Reference Malcomson, Parra-Soto and Ho26). These are visually summarised in Figure 1(27).

Fig. 1. WCRF cancer prevention recommendations(27). This material has been reproduced from the World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous update project expert report 2018. Available at dietandcancerreport.org.
This falls in line with the recommendations of the UK government(Reference Stanton25), and it comes from examination of thousands of studies and meta-analyses that have explored the links between cancer and dietary and lifestyle factors.
An Italian study followed 5271 participants over 14 years and found that individuals with higher adherence to the WCRF guidelines experienced significantly lower mortality rates. For men, this included reduced risks of death from all causes, digestive system diseases and cancer. For women, high adherence was associated with lower mortality from causes other than cancer(Reference Mirizzi, Aballay and Misciagna28).
The protective effects of nutrition are largely associated with three key factors: the reduction of direct carcinogens (for example, nitrates in processed meats), the avoidance of foods that promote adiposity (for example, saturated fats and sugar-sweetened beverages) and the inclusion of protective dietary components such as dietary fibre and phytochemicals such as carotenoids, dithiothiones, isothiocyanates, flavonoids and phenols. Vegetables and fruits are also a rich source of nutrients linked to a reduced risk of cancer, such as vitamins C and E, selenium and folate. A substantial body of experimental data links many of these compounds with anti-tumorigenic effects in various cells in both animal and in vitro studies(29).
The exposures in Figure 2 show the WCRF summary of conclusions(30). They were graded using a set of pre-defined criteria established by the WCRF which assess the quality and consistency of evidence across studies, the presence of plausible biological mechanisms and the strength of associations(31). This systematic approach ensures that recommendations are based on the strongest available evidence, while areas with limited or conflicting data remain under review to guide future research.
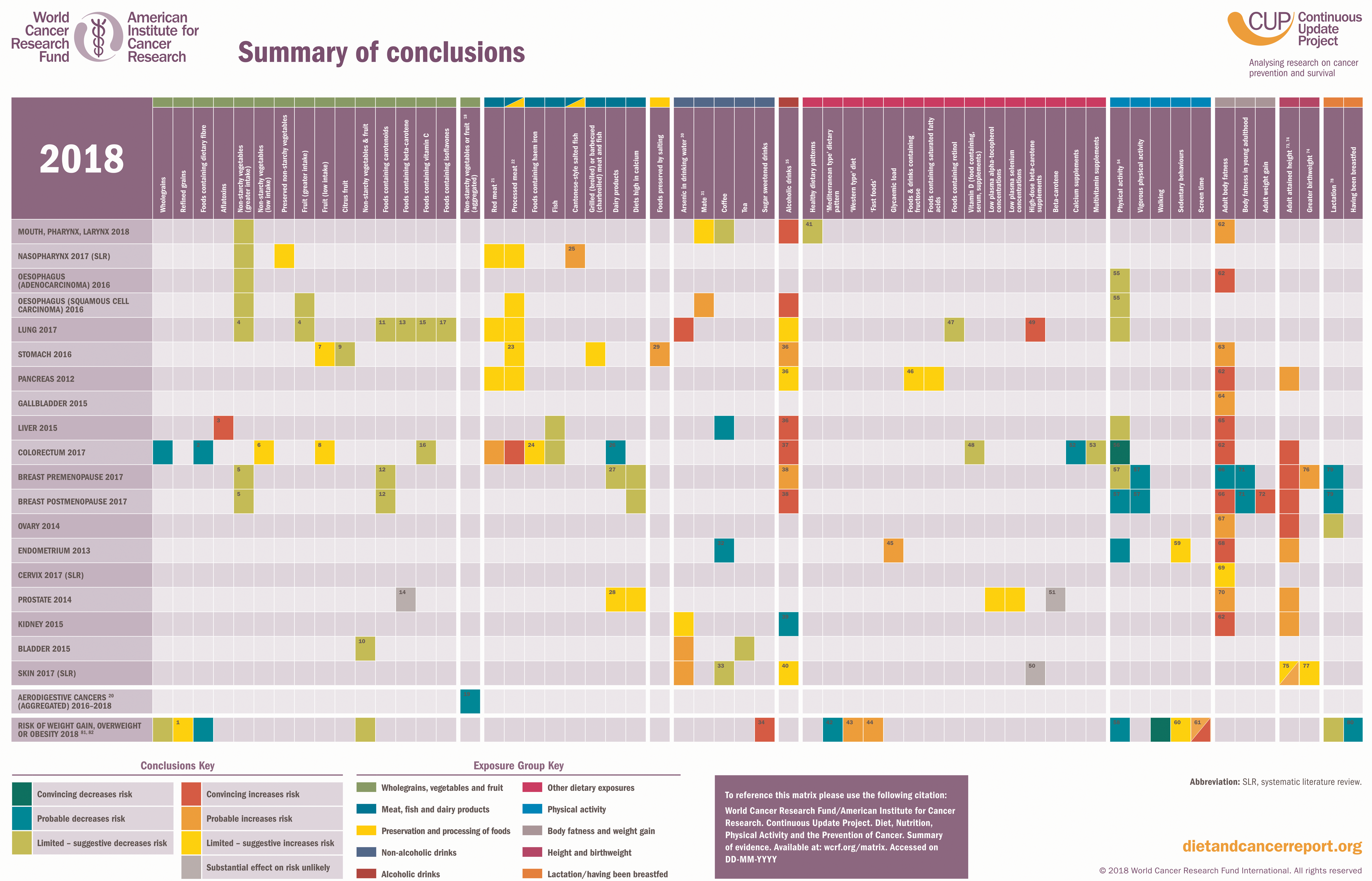
Fig. 2. WCRF summary of conclusions(30). Figure 2 shows the WCRF 2018 summary matrix, which details associations where the exposure was graded as having a ‘strong convincing’/ ‘strong probable’ or ‘limited suggestive’ likelihood causality for its impact on cancer risk. This material has been reproduced from the World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous update project expert report 2018. Available at: https://www.wcrf.org/research-policy/library/matrix-for-all-cancers/.
The 2018 Third Expert Report emphasised the importance of viewing the recommendations as a comprehensive lifestyle approach conducive to cancer prevention. The report suggested that adopting a holistic perspective would yield greater benefits than concentrating on individual foods or isolated risk factors.
Looking at cancers currently linked with firefighting, higher intake of vegetables and fruits and fibre-containing foods have been shown to reduce the risk of bladder cancer(Reference Yao, Yan and Ye32), prostate cancer(Reference Berenguer, Pereira and Câmara33), colorectal cancer(Reference Aune, Chan and Lau34), lymphoma(Reference Zhang, Hunter and Rosner35), oesophageal cancer(36), thyroid cancer(Reference Jung, Kim and Tae37) and breast cancer(Reference De Cicco, Catani and Gasperi38).
Dairy products have been associated with a reduced risk of colorectal cancer(Reference Aune, Chan and Lau34) and breast cancer(Reference Knekt, Järvinen and Seppänen39,Reference Dong, Zhang and He40) . However, it should be noted that suggestive links between higher levels of calcium and increased risk of prostate cancer have also been found(Reference Pernar, Ebot and Wilson41).
Fish and omega-3 has shown inverse correlation with colorectal cancer(Reference Aglago, Huybrechts and Murphy42). Coffee has shown promising evidence of reduced risk for several cancers, including melanoma(Reference Dong, Wei and Yang43), whereas tea (in particular green tea) has been linked with protective effects from bladder cancer(Reference Al-Zalabani, Wesselius and Yi-Wen Yu44). By contrast, red and processed meats are linked with higher risk of colorectal cancer(Reference Chan, Lau and Aune45) and oesophageal cancer(36).
Alcohol intake is positively associated with multiple cancers, including oesophagus, liver, breast, colorectal, mouth, throat and skin(Reference Pöschl and Seitz46). Conversely, some studies suggest that moderate alcohol intake is associated with a reduced risk of kidney cancer(Reference Al-Bayati, Hasan and Pruthi47), non-Hodgkin lymphoma(Reference Tramacere, Pelucchi and Bonifazi48) and thyroid cancer(Reference Hong, Myung and Kim49). Thus, its role in cancer prevention remains complex and should be approached cautiously.
Linked with dietary intake, adult body fatness is correlated with at least thirteen different cancers(Reference Lauby-Secretan, Scoccianti and Loomis50), including liver, colon, kidney, oesophageal, breast, thyroid and malignant melanoma cancers, and is the highest single risk factor shown in the WCRF summary of evidence.
Physical exercise is identified by the WCRF as a key protective element against cancer risk, with its functions extending beyond modifying body composition. Friedenreich et al. (2010) concluded, from an analysis of data from fifteen European countries, that up to 19% of cancer cases could have been prevented through sufficient levels of physical activity(Reference Friedenreich, Neilson and Lynch51).
The Mediterranean diet
The basic parameters of the WCRF dietary advice fall closely in line with the Mediterranean Diet (MD)(Reference Mirizzi, Aballay and Misciagna28) – a concept first coined in the 1960s by Ancel Keys, which remains one of the most well-known and well-researched diets in the world. While several diets exist which follow similar guidelines to the WCRF report, there is consensus that the ‘Mediterranean diet reigns supreme’ considering all the evidence and pros and cons of each(Reference Mineo52).
While there remains no single fixed definition of the MD pattern (with the lack of homogeneity making meta-analyses difficult)(Reference Schwingshackl, Schwedhelm and Galbete53), it is characterised by high consumption of vegetables, fruits, legumes, whole grains, nuts and extra virgin olive oil, with moderate intake of fish, dairy products and red wine, and low consumption of red meat, processed foods and sweets. Many definitions, including that of the United Nations Educational, Scientific and Cultural Organization (UNESCO), also include a holistic view of the Mediterranean lifestyle, incorporating social connections and reinforcing cultural traditions through shared meals, respect for the land and preservation of biodiversity(Reference Mentella, Scaldaferri and Ricci54) (see Figure 3 for a representation of this).
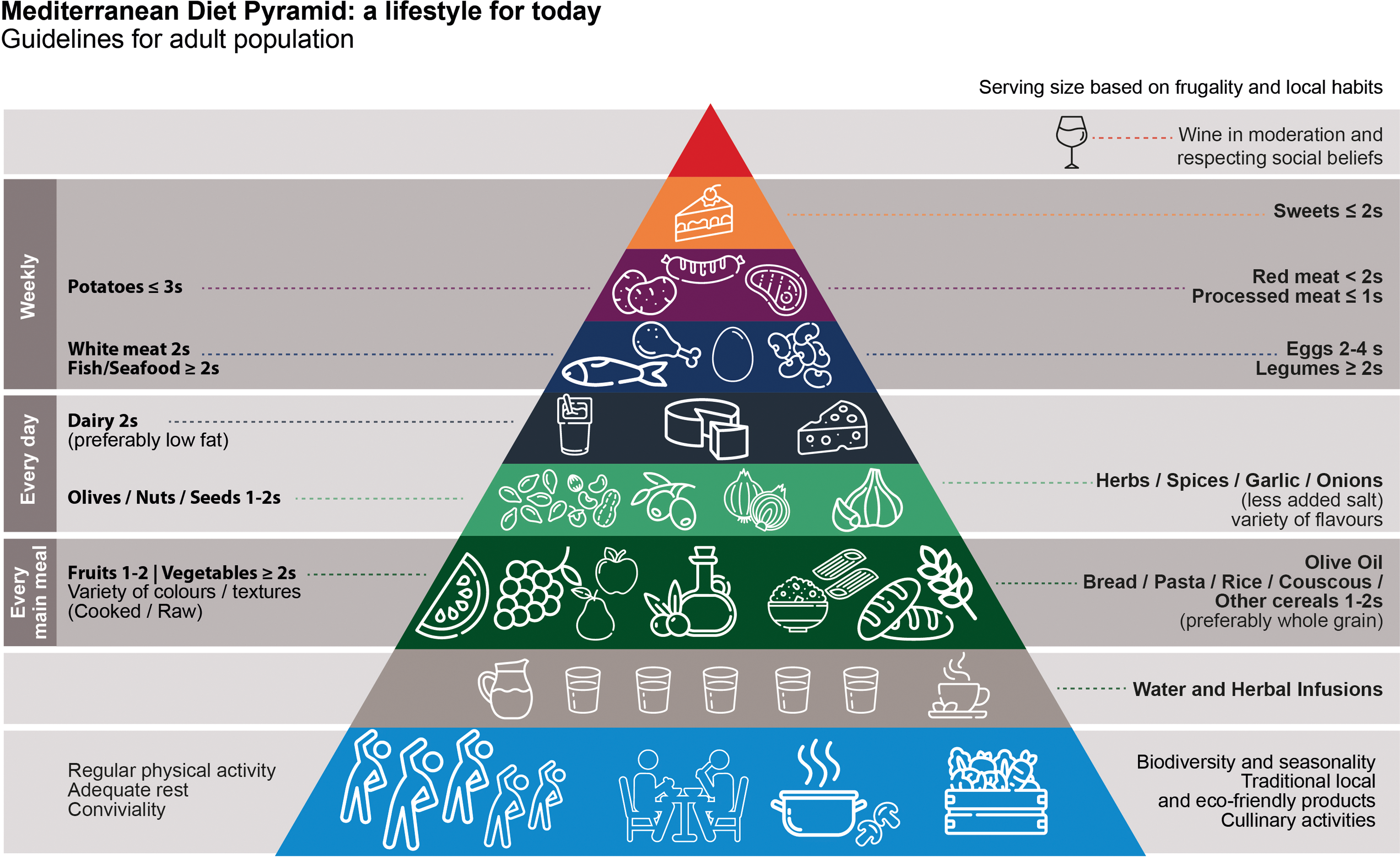
Fig. 3. The Mediterranean diet pyramid. Adapted from Mentella et al.(Reference Mentella, Scaldaferri and Ricci54).
The Mediterranean diet (MD) is viewed as the most evidence-supported dietary option for those at an increased risk of cancer development or currently diagnosed with cancer(Reference Nagy, Petrosky and Beckler55). A recent meta-analysis of 117 studies, encompassing over 3 million participants, found that high adherence to the MD was consistently linked with lower cancer mortality(Reference Morze, Danielewicz and Przybyłowicz56).
While numerous studies and meta-analyses have demonstrated an inverse relationship between adherence to the MD and cancer risk, some research has reported variability in the strength of associations across different cancer types(Reference Fan, Hu and Xie57). These variations are often attributed to differences in study design, definitions of the MD, and genetic or environmental factors, highlighting the inherent complexity of dietary research(Reference Fan, Hu and Xie57,Reference Augimeri and Bonofiglio58) . Nevertheless, the weight of evidence strongly supports the MD as a dietary pattern associated with reduced cancer risk, as highlighted by findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. This prospective cohort study, the largest ever conducted on the connection between diet and disease, included over half a million participants. Using a scoring system for adherence to the MD (where nine is the highest possible conformity and zero is the lowest) the study identified that a two-point increase in the MD score correlated with a 12% reduction in overall cancer incidence. This inverse association was considerably stronger than that predicted on the basis of the associations of the individual components of this diet, and points to the value of the holistic nature of dietary patterns(Reference Benetou, Trichopoulou and Orfanos59).
While reasons for its efficacy are complex, it essentially comprises substances that are high in levels of anti-proliferative, anti-inflammatory, anti-angiogenic, anti-metastatic, anti-oxidant and pro-apoptotic factors(Reference Augimeri and Bonofiglio58) (Figure 4), which have been shown to be potential leading factors behind cancer development in both in vivo and in vitro studies(Reference Dayi and Oniz60). Its rich fruit and vegetable content provides carotenoids, vitamins C and E, folates and flavonoids with antioxidant properties. The diet’s high fibre supports gut health by promoting beneficial bacteria that produce anti-inflammatory short-chain fatty acids (SCFA) such as butyrate, reducing systemic inflammation and oxidative stress(Reference Augimeri and Bonofiglio58,Reference Mahmod, Haif and Kamal61) .
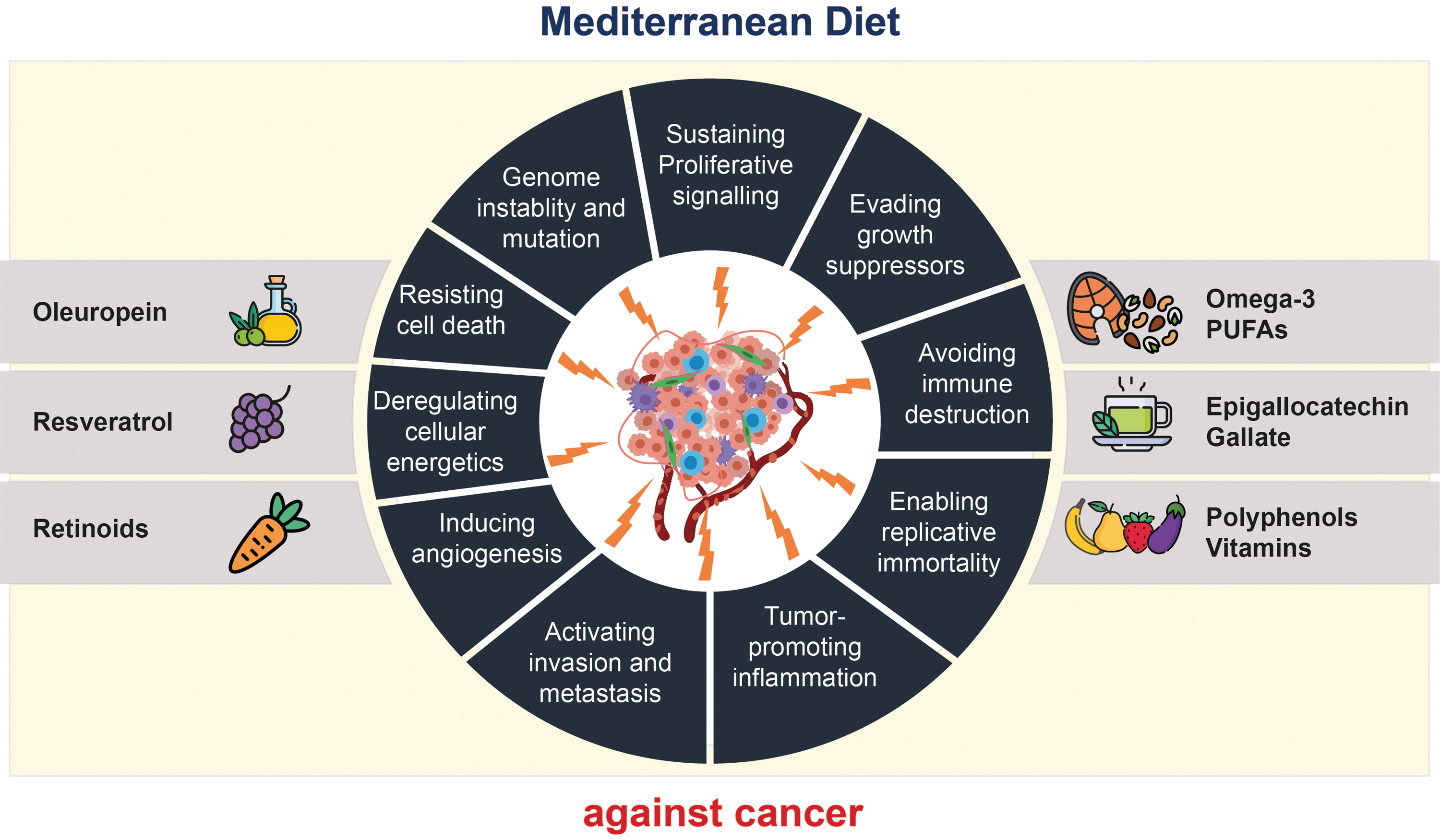
Fig. 4. Mechanisms by which the MD pattern may impact hallmarks of cancer. Foods of the MD that exert anti-tumoural activities by targeting the various hallmarks of cancer include olive oil, red wine, fruits, legumes and vegetables, which contain bioactive molecules such as oleuropein, resveratrol, retinoids, vitamins, epigallocatechin-gallate and omega-3 polyunsaturated fatty acids (PUFA). Adapted from Augimeri and Bonofiglio(Reference Augimeri and Bonofiglio58).
Key components such as fish, rich in omega-3 fatty acids, combat inflammation, angiogenesis, metastasis and cell proliferation while lowering the reliance on red and processed meats. Whole grains add fibre, protecting the gut by minimising carcinogen interaction and endotoxin absorption, thereby reducing colon cell damage(Reference Nagy, Petrosky and Beckler55). Although not yet formally assessed in the WCRF’s graded evidence, nuts – an integral part of the Mediterranean diet – have been linked in other research to reduced risk of several cancers relevant to firefighters, including breast, colorectal and prostate cancer, through mechanisms including anti-inflammatory and antioxidant effects via their content of healthy fats, polyphenols and micronutrients(Reference Bolling, Aune and Noh62).
Hence, the main elements for which the MD is known have been shown to have positive associations for reducing cancer incidence (with the potential exception of red wine, given its complex relationship with cancer risk(Reference Rehm, Gmel and Gmel63)), providing a basis for its use to mitigate work place cancer risks. Moreover, the principles of the MD can be adapted to fit UK dietary preferences and budgets, using alternatives such as rapeseed oil instead of olive oil or tinned fish for fresh fish. Beyond practicality, the MD framework can also accommodate seasonal and environmental considerations, with local substitutions helping maintain dietary integrity in non-Mediterranean climates. The New Nordic Diet, for instance, was developed on similar principles using regionally available produce and offers a useful model for how dietary traditions can be both nutritionally effective and ecologically adapted(Reference Krznarić, Karas and Ljubas Kelečić64).
It is worth noting that the original studies that observed the health benefits of the MD focused on its reduction of coronary heart disease risk(Reference Keys65). Studies since then have linked it with multiple other disease outcomes(Reference Dinu, Pagliai and Casini66).
Owing to the MD’s correlation with reduced obesity and associated poor health outcomes(Reference D’Innocenzo, Biagi and Lanari67), American fire services have recently begun to promote this dietary pattern among firefighters, prompted by the high levels of obesity among US fire service workers(Reference Poston, Haddock and Jahnke68). This led to the US Department of Homeland Security awarding a Harvard team of scientists a $1·5-million, 3-year competitive research grant entitled ‘Feeding America’s Bravest: MedDiet-Based Interventions to Change Firefighters’ Eating Habits and Improve Cardiovascular Risk Profiles’ which focused on changing the American Firefighters’ eating plans to align with the MD(Reference Korre, Sotos-Prieto and Kales69).
Current dietary habits and cancer risk in firefighters
While several studies have examined the diet of American firefighters, similar research on UK firefighters is lacking. In the absence of such data, it is reasonable to hypothesise that the diet of UK firefighters follows similar patterns to those of the general population, suggesting dietary factors that may influence cancer risk for UK firefighters.
Conformity to the MD style of eating in the UK is extremely low. In 2007, the UK Department of Health developed the Eatwell Plate (now the Eatwell Guide) to provide nutritional guidelines. This guide outlines recommended portions of dietary intake in a structure very similar to the MD. An assessment by Sheelbeek et al.(Reference Scheelbeek, Green and Papier70) found that only 1 in 1000 adhere to the nine key government guidelines. According to the National Diet and Nutrition Survey published in 2018(71), less than one-third of UK adults consume the recommended amount of fruit and vegetables. Sugar intake is more than double the recommended limit across all age groups, oily fish consumption is half the guideline, and while females meet recommendations for red and processed meat, males exceed them. Despite a 30 g daily fibre guideline, the UK average is just 18 g.
Comparisons with other European countries, as demonstrated by data from the EPIC study(Reference Slimani, Fahey and Welch72), reveal the extent to which British dietary habits differ from those followed on the continent, as shown in Figure 5.
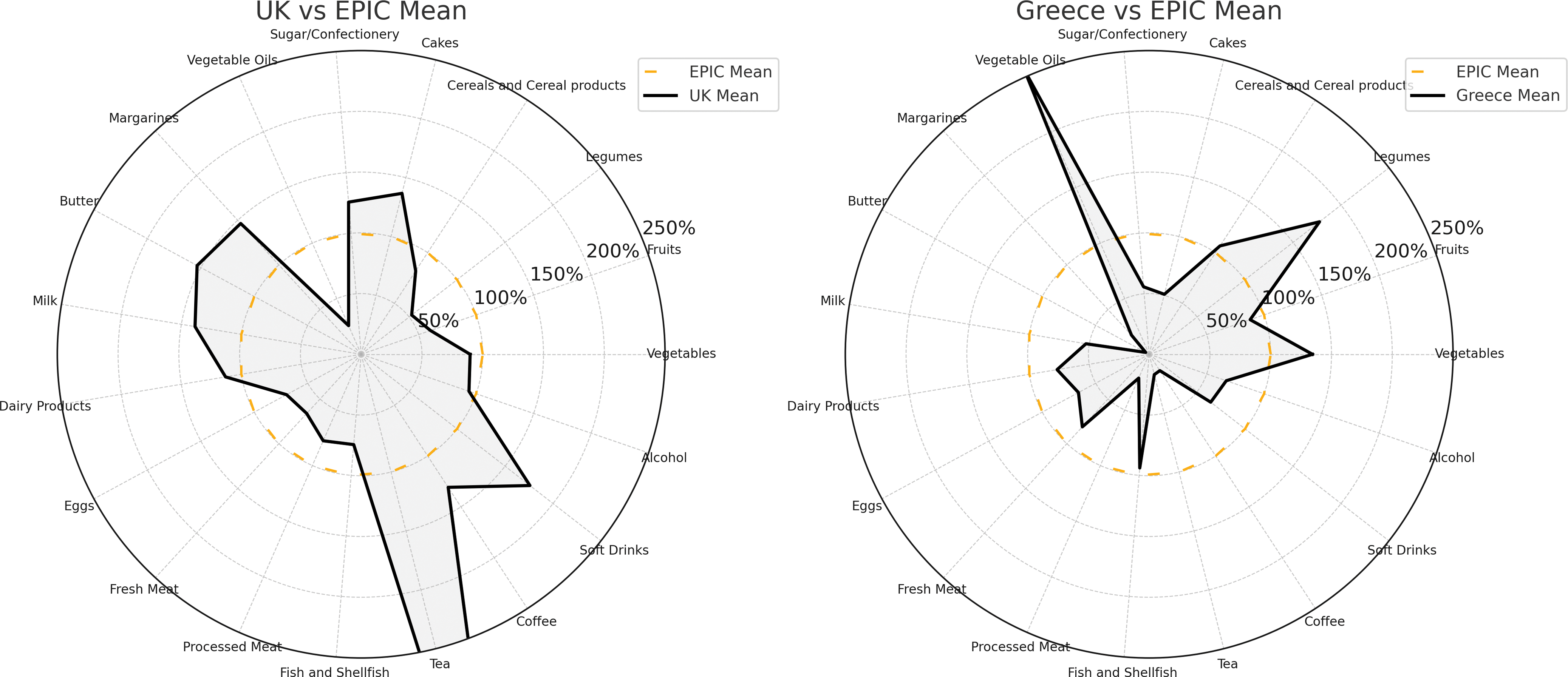
Fig. 5. Comparison of average food consumption in Greece and the UK relative to all-EPIC-country averages. Points inside the dotted circle indicate below-EPIC-average consumption; points outside indicate above-average consumption. Greece demonstrates high intake of vegetable oils, legumes and vegetables, whereas the UK shows high consumption of sugars, cakes, soft drinks and butters/margarines. Adapted from Slimani et al.(Reference Slimani, Fahey and Welch72).
Recent body mass index (BMI) figures for UK firefighters show a mean value of 26·7(Reference Stevenson, Turner and Siddall73). While BMI does not always accurately reflect body composition – especially in muscular individuals – it is nonetheless a useful population-level indicator. Robust UK data on firefighter body composition are limited, highlighting the need for further targeted research. In the meantime, studies among firefighters in the USA have shown that elevated BMI in this group often reflects true excess adiposity rather than increased muscle mass(Reference Poston, Haddock and Jahnke68). While global studies of firefighter BMI are limited(Reference Soares, Smith and Grossi Porto74), data from the USA(Reference Moffatt, Stewart and Jack75), Turkey(Reference Demiralp and Özel76), Iran(Reference Montazerifar, Karajibani and Hosseini77) and Germany(Reference Strauß, Foshag and Przybylek78) all follow a similar trend towards excess body weight, reflecting a working population potentially vulnerable to cancer associated with greater body fatness, as well as work exposure to carcinogens.
Preliminary research into the gut microbiome of firefighters, although currently limited to USA-based studies, suggests significant disruptions likely linked to occupational exposures and dietary patterns. For example, a recent pilot study observed reduced microbial diversity and an increased presence of pathogenic bacteria among firefighters, potentially contributing to immune dysregulation and chronic inflammation(Reference Yoo, McSkimming and Rajan79). Another study linked occupational hazards, such as toxin exposure, to changes in gut health that might exacerbate vulnerability to disease(Reference Houser, Smith and Rhodes80). Although these findings are preliminary, they underscore the interplay between occupational risk factors and dietary inadequacies, warranting further investigation in UK contexts.
Firefighter specific diets part 1 – the potential for future development
Although the MD has demonstrated promising results in reducing cancer risk among the general population, it is crucial to consider the unique carcinogen exposure that firefighters face. While current human trials are limited, various mechanistic and early-stage studies have explored the chemo-preventive potential of specific foods and nutrients against carcinogens present in fire effluents, offering hope for the development of more tailored nutritional guidelines in the future. The following examples highlight emerging research in this area. These studies were selected to illustrate the breadth of potential nutrient-mediated detoxification or mitigation strategies but are not exhaustive. As many are derived from animal models or in vitro research, they should be viewed as hypothesis-generating and interpreted within the broader hierarchy of nutritional evidence. As discussed later in the manuscript, such findings remain exploratory and are not yet directly prescriptive.
For instance, sulforaphane, a compound found in cruciferous vegetables, and in particularly high concentration in broccoli sprouts, has garnered attention for its potential anticancer properties(Reference Nestle81). Trials in mice have shown it to reduce carcinogen induced stress when the lungs were subjected to benzo[a]pyrene (BaP) (one of the most prevalent PAH to which firefighters are exposed)(Reference Kalpana Deepa Priya, Gayathri and Sakthisekaran82).
A human trial conducted in China used a broccoli sprout beverage to examine the potential detoxification of benzene in people subject to high levels of airborne pollution.
Urinary analysis of subjects found it elicited dose-dependent benzene detoxification(Reference Chen, Johnson and Egner83).
Fish oils also show promise in cancer prevention. In mice, they were found to block the formation of hepatic DNA adducts by detoxifying multiple PAHs, including BaP, benz(a)anthracene and chrysene(Reference Zhou, Zhu and Phillips84). The chemo-protective potential of fish oil has also been demonstrated in in vitro studies using human lung cells treated with BaP(Reference Barhoumi, Mouneimne and Chapkin85), while omega-3s found in oily fish have been shown to inhibit free radical increases in rats exposed to high levels of formaldehyde(Reference Zararsiz, Meydan and Sarsilmaz86). However, caution is warranted due to the bioaccumulation of heavy metals in fish, which may compound the heavy-metal burden in firefighters. To balance benefits and risks, intake of fish products should not exceed recommended levels. Smaller or shorter-lived fish species such as salmon, sardines and mackerel offer safer options owing to their lower potential for heavy metal bioaccumulation(Reference Mozaffarian and Rimm87).
Beyond nutrients like omega-3s and sulforaphane, plant-based compounds and micronutrients such as garlic, selenium and folate have demonstrated protective effects against specific fire contaminants through detoxification and cancer-preventive mechanisms. Tea (in particular, green tea and its constituents) has been found in animal studies to reduce the potential toxic risks of Asbestos(Reference Luo, Liu and Wang88), BaP(Reference Luo, Liu and Wang88–Reference Kumar, Sharma and Sehgal90), PCB 126(Reference Newsome, Petriello and Han91) and nickel(Reference Chen, Su and Wu92). Similarly, retinoids – derivatives of vitamin A, found naturally in various plant and animal sources – have been linked with inhibiting the cytotoxic effects of BaP in both in vitro and in vivo studies(Reference Huang, Ohnishi and Jiang93–Reference McCarthy, Lindamood and Hill95). Garlic, a food rich in bioactive compounds, has demonstrated protective effects against chromium-induced carcinogenicity in laboratory studies, attributed to its antioxidant properties and ability to activate detoxification pathways(Reference Das, Dhundasi and Das96,Reference Kalayarasan, Sriram and Sureshkumar97) . Selenium and folate have also demonstrated protective roles against arsenic exposure. Selenium intake has been associated with reduced incidence of arsenic-related premalignant skin lesions in populations exposed to arsenic-contaminated drinking-water(Reference Chen, Hall and Graziano98), while folate supplementation was shown to enhance arsenic methylation and urinary excretion in a randomised controlled trial, potentially lowering health risks associated with arsenic exposure(Reference Gamble, Liu and Ahsan99). Although these studies focus on arsenic exposure from drinking-water, their findings suggest protective mechanisms that may benefit firefighters. Soy-derived genistein has shown the ability to mitigate DNA damage in breast cells exposed to 7,12-dimethylbenzo[a]anthracene (DMBA) in an in vitro analysis(Reference Leung, Yung and Poon100). Zinc, found in several animal and vegetable foods, has similarly been linked to protective effects against benzene(Reference Ibrahim, Saleh and Farrag101), as well as cadmium accumulation in rats(Reference Waalkes102).
Fruit and vegetables, and phytochemicals found within them, have been associated in laboratory studies with protective effects against carcinogen-induced damage. For instance, spinach, peaches and quercetin have been shown to reduce genotoxicity caused by BaP exposure in mouse bone marrow models, highlighting their potential to mitigate DNA damage(Reference Edenharder, Krieg and Köttgen103). In an in vivo study, a green vegetable cocktail effectively reduced the toxic impact of benzene in rats(Reference Berroukche, Boufadi and Soltani104), while human case–control studies have similarly noted that higher fruit and vegetable intake is associated with lower oxidative stress markers in individuals exposed to high benzene levels(Reference Costa, Ozcagli and Gangemi105).
Body composition and dietary habits have also been studied for their impact on contaminant effects. A ‘Western diet’, high in saturated fats, was linked to accelerated BaP-induced colon cancer development in rat models(Reference Harris, Pulliam and Okoro106). Excess body fat, often associated with such dietary patterns, has been shown to slow benzene removal from the body, increasing susceptibility to its effects(Reference Sato, Nakajima and Fujiwara107). In addition, two separate in vivo studies highlighted that diets high in fat and low in minerals significantly increased lead absorption in in vitro and animal models(Reference Barltrop and Eng Khoo108) and in rats(Reference Barltrop and Khoo109).
The amino acids glycine and cysteine (in the form of N-acetylcysteine) have been shown to improve glutathione deficiency and mitigate oxidative stress in studies involving aged mice and a limited sample of older adults(Reference Kumar, Liu and Suliburk110). Glutathione plays a central role in detoxification and cellular defence, and its decline with age may contribute to increased cancer susceptibility(Reference Diaz-Del Cerro, Martinez de Toda and Félix20,Reference Jones, Mody and Carlson21) . This may be particularly relevant to older firefighters with long-term contaminant exposure. These findings suggest potential dietary strategies for supporting antioxidant capacity in this at-risk group. Alpha-lipoic acid has also been found to attenuate the age-related decreases in glutathione synthesis in in vitro studies, and in vivo studies on rats(Reference Suh, Shenvi and Dixon111).
Also of note in the potential reduction of cancer risk is the option of manipulating diet through controlled energy intake(Reference Key, Bradbury and Perez-Cornago112). In mice treated with 3-methylcholanthrene (a PAH and known firefighter risk factor), tumour development and tumour incidence were greatly reduced under caloric restriction(Reference Konno, Hishinuma and Hashimoto113). Fasting-mimicking diets, tested on mice, were shown to reduce risk factors/biomarkers for cancer, as well as diabetes and cardiovascular disease, without significant adverse effect(Reference Brandhorst, Choi and Wei114).
A recent study has supported the feasibility of time restricted eating (TRE) for firefighters in America. The Healthy Heroes randomised controlled trial (RCT) separated a body of firefighters into two groups. The control group and the study group were both set a fixed dietary intake matching the MD format. The TRE group restricted eating to a 10-hour window and reported improved quality of life and showed improved cardiometabolic health markers with no adverse effects(Reference Manoogian, Zadourian and Lo115).
These studies provide a sample of the extensive research that illustrates how nutrition can potentially reduce the cancer risk associated with exposure to fire effluents and are summarised in Table 1.
Table 1. Examples of nutrients and dietary factors with potential effects on fire contaminants
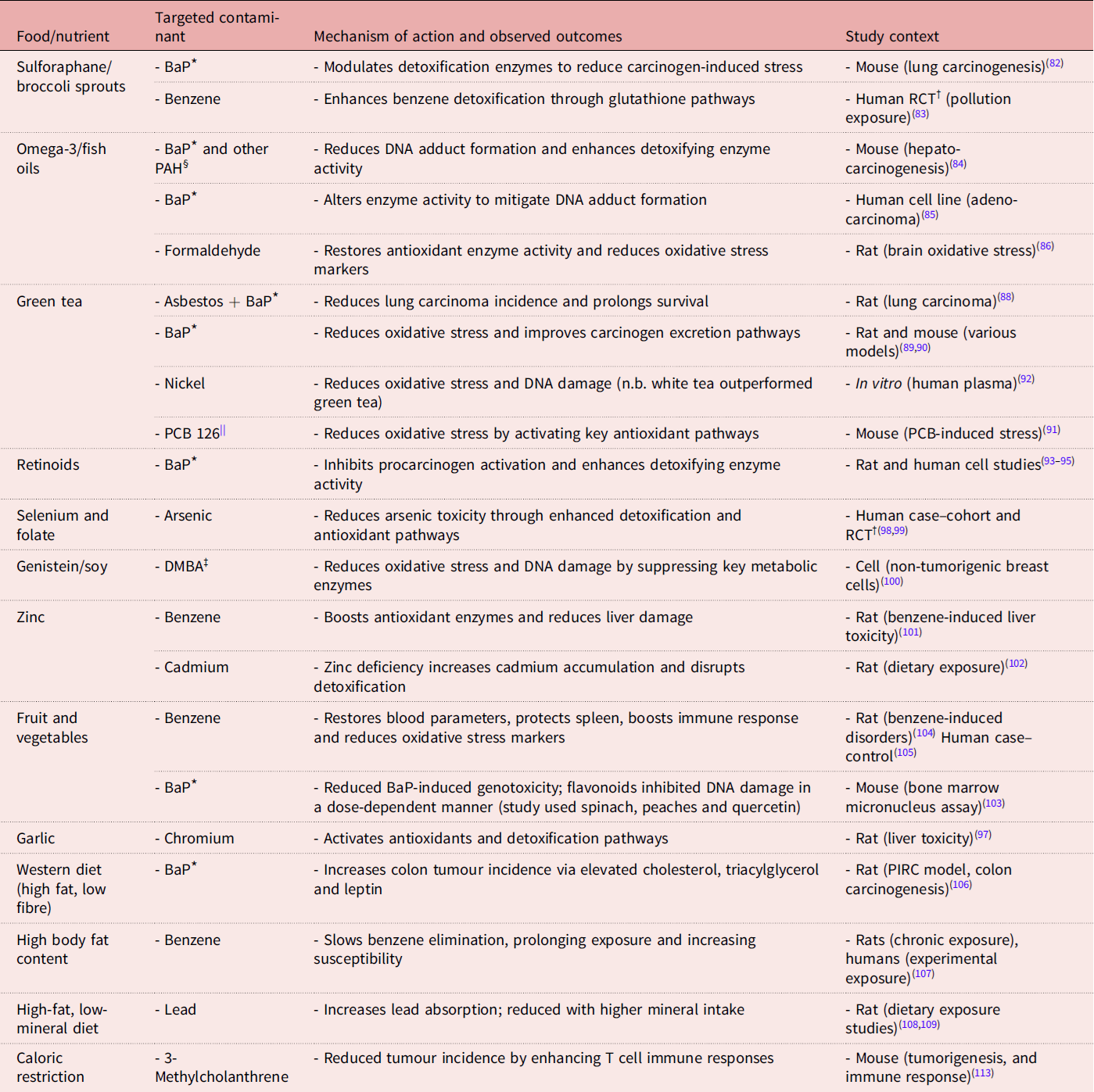
Note: This table provides selected examples of studies exploring nutrient-mediated detoxification or mitigation of fire contaminant toxicity. It is not exhaustive but aims to demonstrate the breadth of current research. The ‘Food/nutrient’ column includes a mix of whole foods, bioactive compounds and micronutrients, reflecting the diverse forms in which protective effects have been studied.
* BaP, Benzo[a]pyrene.
† RCT, randomised controlled trial.
‡ DMBA, 7,12-dimethyl-benz[a]anthracene.
§ PAH, polycyclic aromatic hydrocarbons.
|| PCB, polychlorinated biphenyl.
Firefighter-specific diets part 2: challenges in future development
While the studies discussed in part 1 highlight the potential of specific nutrients and dietary patterns in mitigating the cancer risks associated with exposure to fire effluents, no definitive conclusions can be drawn about the practical feasibility or safety of the proposed protective mechanisms. None of these mechanisms has undergone the rigorous evaluation needed for recognition by the WCRF’s Global Cancer Update Project(29). While chemical interactions are demonstrated in laboratory settings, and human studies suggest correlations between certain nutrients and cancer outcomes, a comprehensive range of analytical methods is necessary before causality can be confidently established.
In vivo and in vitro studies contribute valuable insights for future exploration, demonstrating biological plausibility for human study. However, these studies alone cannot provide proof of efficacy in human subjects. In vitro studies fail to assess nutrient bioaccessibility or replicate the complex interactions of real diets, while animal studies, despite controlled conditions, use carcinogen and nutrient levels unrepresentative of human exposure. This makes it hard to assess how reflective, or safe, any results would be in real life.
RCT in humans are the gold standard for identifying factors involved in the development and prevention of disease, but they are challenging in nutrition and cancer research due to confounding factors, the length of time required between intervention and cancer outcomes, non-adherence and the complexity of dietary interactions(31,Reference Mayne, Playdon and Rock116) .
These trials often use supplements rather than whole dietary changes, but the efficacy of supplements is uncertain because they lack the synergistic mix of nutrients found in whole foods(Reference Bouayed and Bohn117). For example, a Portuguese RCT on antioxidants in firefighters showed no significant improvements in immune response, concluding that supplements were less effective than natural dietary components in supporting immunocompetence and reducing inflammation(Reference Santos, Rama and da Silva118).
Beyond the potential inefficacy of supplement-based interventions, safety concerns also exist. The WCRF advises against using supplements to prevent cancer(27). This reflects the 1996 CARET study, which investigated the use of high-dose β-carotene and vitamin A supplements to prevent lung cancer in smokers. The trial was halted when early analysis revealed not only a lack of benefit but also an increased risk of harm, with 28% more lung cancers and 17% more deaths in the active intervention group. The implications of this trial for future studies and study design have been widely accepted: safety and efficacy should be demonstrated before recommending vitamin supplements to any population(Reference Omenn, Goodman and Thornquist119).
In 2014, Fardet and Rock criticised the analyses of single nutrients or food groups as a reductionist approach not adequate in studies on the preventive effects of nutrition in chronic diseases such as cancer(Reference Fardet and Rock120). By contrast, dietary patterns may take into account synergistic and antagonistic interactions between the components of a food matrix, thus yielding a holistic net effect of diet(Reference Tapsell, Neale and Satija121). For this reason, the MD should remain the key aim for firefighter nutritional protection, due to the synergistic effect of its components(Reference Augimeri and Bonofiglio58).
Therefore, the MD remains a practical strategy for firefighters to reduce cancer risk. Yet, cultural and occupational barriers, including food habits and job demands, must be addressed to enable sustainable dietary improvements that extend beyond theoretical recommendations.
Challenges and solutions for changing firefighter diets
Understanding the obstacles that hinder the implementation of tailored dietary strategies is crucial. Research into UK firefighters’ understanding of cancer risk is lacking; however, UK population data suggest a widespread lack of awareness on this subject. In 2023, a Cancer Research UK survey found that only 21% of the UK population understood the link between cancer and diet, and fewer still recognised the risks posed by physical inactivity (17%) and obesity (10%)(Reference Whitelock122).
The lack of knowledge on cancer risk is not limited to the UK. Surveys of American firefighters, published in 2024, showed that most, including those affected by cancer, had received no education on diet’s role in cancer prevention(Reference McClanahan, Sanchez and Gant123).
However, even among populations who are given direct dietary advice for health conditions, information alone rarely drives significant change(Reference Madigan, Graham and Sturgiss124).
This is partly influenced by the UK’s food culture and environment, which is commonly described as obesogenic(Reference Omoleke125). Drawing on WHO data and broader public health sources, Omoleke – author of the cited review – explains how the widespread availability of ultra-processed foods contributes to this environment and can shape dietary habits. Vending machines, online food delivery services and convenience outlets serve as prime examples of such easy-access sources of energy-dense, nutrient-poor foods. As discussed by Roberto et al. in a Lancet series on obesity(Reference Roberto, Swinburn and Hawkes126), although individuals have some control over their diet, the modern food environment has introduced an influx of hyper-palatable foods high in sugar, fat and salt that appear to surpass the rewarding properties of non-processed foods. Emerging evidence suggests these foods have effects on the reward pathways in the brain, making it harder for people to regulate their food intake and maintain a healthy diet(Reference Schulte, Avena and Gearhardt127).
Such foods are often cheaper than their healthier counterparts, with the UK Fenland study noting that the higher cost of adherence to the MD was seen as a barrier by many. However, the study noted that this cost difference could potentially be mitigated not only by the natural reduction in unhealthy items such as red and processed meats, inherent to the MD, but also by prioritising lower-cost healthy components, such as pulses, legumes and canned fish, over more expensive items within the diet(Reference Tong, Imamura and Monsivais128). While such substitutions might lower costs, they require substantial changes in purchasing habits and taste preferences and, in some cases, time and cooking skills – which may well be particularly challenging for individuals accustomed to the rewarding and convenient nature of highly processed foods.
Firefighters face unique challenges that heighten the difficulties discussed, such as work-related stress, trauma exposure and disrupted sleep(Reference Sidossis, Lan and Hershey24,Reference Dyal, Smith and DeJoy129) . These job-related factors intensify barriers to maintaining healthy dietary and lifestyle habits, contributing to a cycle of poor nutrition, mental health issues and overall well-being.
A recent UK paper explored sleep, mental health and contaminant exposure, finding high levels of sleep problems among UK firefighters (61%). Those who reported sleep problems were over 4 times more likely to report any mental health disorder, 2·9 times more likely to report anxiety and 2·3 times more likely to report depression than those who did not report sleep issues. The study found a direct correlation between such disorders and likelihood of contaminant exposure. While causality cannot be confirmed, the authors suggest that contaminant exposure may contribute to mental health difficulties through direct biological effects – such as neurotoxicity or endocrine disruption – or indirectly via concern over health risks and contaminant-linked illness. These pathways may also disrupt sleep, further undermining psychological well-being(Reference Wolffe, Robinson and Clinton130).
Evidence shows that poor mental health, poor sleep and poor dietary choices are interconnected, with declines in one often exacerbating issues in the other two(Reference Bremner, Moazzami and Wittbrodt131,Reference Hill, Conner and Clancy132) .
An American observational study of firefighters, examining job-related stresses and health habits, found a direct link between the two. Headrick(Reference Headrick133) reported that emotional demands during the shift positively correlated with unhealthy eating and alcohol use during the time off duty. Such off-duty habits led to fatigue before the next shift, which exacerbated the perception of emotional demands during the shift, leading to further unhealthy behaviour(Reference Headrick133).
A Swedish study into shift workers noted a high incidence of metabolic disorders and diseases, linked to the quality of the diet and irregular timing of eating. However, other factors that affect metabolism are likely to play a part with the association to metabolic disorders, including psychosocial stress, disrupted circadian rhythms, sleep debt, physical inactivity and insufficient time for rest and revitalisation(Reference Lowden, Moreno and Holmbäck134).
Biochemical factors at least partly underpin the association. Sleep curtailment is associated with decreased leptin levels and elevated ghrelin levels, leading to increased hunger and appetite(Reference Spiegel, Tasali and Penev135).
Inadequate sleep may especially increase consumption of unhealthy foods, as demonstrated by an Australian study on firefighter eating habits which found that night shifts are associated with a higher intake of foods rich in sugar, fat and energy. Fatigue was identified as a key contributor to this pattern(Reference Bonnell, Huggins and Huggins136)
A study examining Spanish firefighters showed similar results, identifying night shift work and the associated sleep difficulties as the cause of a higher intake of saturated fats and a higher injury incidence(Reference López-Bermudo and Gómez-Landero137).
These studies demonstrate that there are multiple factors within firefighters’ work culture which can lead to an accumulation of unhealthy behaviours and make poor dietary choices more likely. However, supportive environments can mitigate the effects of work stress on diet. The aforementioned study by Headrick(Reference Headrick133) shows that partner support for healthy eating at home and a perception of healthy eating among colleagues help to moderate unhealthy behaviours, demonstrating the importance of a positive food culture at work. Such change relies heavily on group norms. Without peer participation, individual intentions are less likely to translate to long-term dietary changes in firefighters(Reference Headrick133).
The value of group norms in shaping dietary behaviour is further illustrated by findings from the PHLAME (Promoting Healthy Lifestyles: Alternative Models’ Effects) intervention. This team-centred programme demonstrated that improving dietary norms among coworkers, alongside increasing knowledge of the benefits of fruits and vegetables, significantly enhanced intake among firefighters(Reference Ranby, MacKinnon and Fairchild138).
Herein lies one of the key factors to firefighter diet: the work dietary culture.
In the UK, firefighters typically eat communally through a messing system (often referred to simply as ‘the mess’) – a long-standing tradition where each crew co-operatively funds, prepares and eats shared meals. This shared eating format aligns with the social aspect of the MD. Field research in the USA has shown that this approach, free from management influence, fosters a co-operative work environment essential for group performance(Reference Kniffin, Wansink and Devine139). Both qualitative and quantitative analyses from the study highlighted a direct correlation between the level of fire station commensality and work-group performance.
However, the mess culture presents both challenges and opportunities for improving firefighter diets.
In an examination of American firefighter diets, Sotos-Prieto identified fire service food culture and a lack of nutritional education within stations as being key barriers to change(Reference Sotos-Prieto, Jin and Rainey140). Even when firefighters recognise the importance of a healthy eating pattern, they reported that their own food choices were often influenced by colleagues(Reference Joe, Hatsu and Tefft141).
The main barriers to change include a number of cultural, social, economic and logistical factors.
In most fire crews, one team member voluntarily manages shared meal planning and grocery shopping. Choices are normally shaped by practical needs: meals must be affordable, hearty and able to handle disruptions from fire calls without losing their appeal. For this reason, where food education is lacking, less healthy, preservative rich options high in protein, saturated fats and refined carbohydrates are staple choices of the UK mess rota.
Attempts to modernise messing have found that small-scale interventions have little success(Reference Bucher Della Torre, Wild and Dorribo142,Reference Brown, Poston and Jahnke143) , concluding that ‘intensive and culturally tailored prevention interventions targeting nutritional behaviours are needed at the individual, group, and organisational levels’(Reference Bucher Della Torre, Wild and Dorribo142).
By adopting these approaches, successful interventions have been done in the USA and in the UK, harnessing the mess culture as a tool to drive positive mindset around healthy eating.
Hershey et al. found that the Harvard-led intervention to encourage the MD among fire crews brought about significant improvements in participants’ MD adherence(Reference Hershey, Chang and Sotos-Prieto144). This came from an on-station intervention which used multiple angles to promote healthy eating. Education was provided via an online platform, which contained brochures on MD recommendations, a firefighter-specific Mediterranean Diet Pyramid, grocery shopping tips, sample recipes, video interviews with exemplary firefighters practicing the MD, and access to additional resources, such as chef-led cooking demonstrations in fire station kitchens, access to supermarket discounts, free samples of MD foods, email announcements and reminders, and family and peer education and support.
Analyses in the UK and in the USA have shown that, when the MD is presented to firefighters, it is viewed more positively than other potential dietary schemes(Reference Lessons and Bhakta145,Reference Yang, Farioli and Korre146) .
Two separate London-based interventions in fire stations, which aimed to promote the MD to stations, found that, when teaching was given regarding how to make low-cost, low-burden meals in line with MD principles, participant feedback was overwhelmingly positive even from low-intensity formats of input(Reference Lessons and Bhakta145,Reference Lessons and Bhakta147) .
As a final tie-in with diet and lifestyle, it should be highlighted that physical activity is also a key component of the MD pyramid(Reference Mentella, Scaldaferri and Ricci54). Beyond its direct effects on body composition and internal functioning, UK research on firefighters shows that physical activity reinforces the feedback loop between positive lifestyle habits and healthy eating(Reference Siddall, Turner and Stevenson148). Furthermore, studies highlight its critical role in reducing cancer risk among firefighters, particularly through improving cardiorespiratory fitness and reducing inflammation, underscoring the importance of its integration into occupational health strategies(Reference Smith, Matias and Bode22).
Discussion
Cancer is a significant occupational health risk for UK firefighters due to chronic exposure to carcinogens such as benzene, asbestos and PAH in fire effluents. While protective measures have reduced risks, exposure persists, compounded by factors including disrupted sleep and stress, which impair the body’s detoxification and repair mechanisms. This underscores the need for supplementary strategies, such as dietary interventions, to mitigate risks.
The Mediterranean diet offers a promising approach, supported by evidence linking it to reduced cancer incidence and mortality through its anti-inflammatory and antioxidant properties. Despite these benefits, UK firefighters face barriers to adopting healthier diets. Elevated BMI within this group, coupled with broader UK population data and the known dietary trends of shift workers under stress, suggests low adherence to healthy dietary patterns within this workforce. However, the communal culture of the fire service provides an opportunity for promoting healthier eating and lifestyle through targeted education and peer support. Successful interventions in the USA and UK show that integrating MD principles into communal dining can encourage long-term dietary improvements, but only when supported by targeted education, peer involvement and culturally tailored strategies, as outlined in this review. These methodologies are essential to ensure that such interventions are practical, sustainable and effective, while preserving teamwork and camaraderie.
This review serves as a dual call to action. First, it highlights the need for more firefighter-specific nutritional research, particularly randomised controlled trials, to identify and validate effective strategies for reducing cancer risk. An example of the type of targeted research required is the ongoing trial in Tucson, USA, which investigates sulforaphane’s detoxification potential in firefighters undergoing live fire training(Reference Savani149). Results, expected in late 2027, could offer valuable insights into practical interventions tailored to this population. This trial exemplifies the critical shift toward targeted research – a direction this review strongly advocates.
Second, beyond research, this review calls for fire service officials to prioritise investments in dietary education, training and support. These efforts must be collaborative, leveraging the communal bonds fostered through crew commensality to promote sustainable change. Ethical considerations, such as the affordability of healthy foods and the responsibility placed on firefighters to adopt dietary changes in the absence of systemic support, must be addressed to ensure equitable and sustainable interventions.
In addition, organisational and environmental factors should be considered when designing interventions, such as kitchen resources for preparing healthy meals, the nutritional quality of staple ingredients kept on station and the constraints of fixed shift schedules. For example, initiatives to provide basic whole foods or form partnerships with local suppliers could help fire stations prepare healthy meals more easily and affordably.
The findings of this review extend beyond firefighting, offering valuable insights for other high-risk professions such as mining, construction and industrial work, where occupational carcinogen exposure and barriers to healthy eating are also prevalent. Future research could refine these insights to address specific challenges faced by these groups.
While this review highlights promising dietary strategies, it also underscores the complexities of developing a universal diet that addresses the diverse needs of this population. Factors such as genetic variability, differing exposure levels to fire contaminants, and the unpredictable nature of firefighting operations – where the frequency and intensity of fire encounters can vary greatly between stations and individuals – mean that no single dietary approach will suit everyone perfectly. Nevertheless, foundational dietary patterns like the MD provide a scientifically supported starting point for population-wide recommendations. Looking ahead, advancements in research and technology – such as genetic testing, real-time biomarker tracking and personalised health analytics – may enable further refinement of dietary strategies. Bridging the current research gaps will be critical to achieving this goal, while existing knowledge can already guide meaningful dietary improvements.
Conclusion
Addressing the cancer risks faced by UK firefighters demands a holistic approach that integrates protective measures with sustainable lifestyle changes. Efforts must prioritise whole foods, community involvement and physical activity to create a positive cycle of health that aligns with the unique occupational realities of firefighters. Such an approach positions the Mediterranean diet not merely as an ideal but as a practical, sustainable strategy to reduce cancer risks and safeguard the long-term health of those who risk their lives for public safety.
Acknowledgements
The authors are grateful for the knowledge and insights shared by Anna Stec, Sarah Lewis, Sarah Kefyalew, Jeff Burgess and Stefanos Kales.
Authorship
Ben Jones: conducted the research, performed the literature review and drafted the manuscript. Shelly Coe: reviewed the manuscript critically, provided intellectual guidance and supervised the preparation of the final version for submission.
Financial support
None.
Competing interests
The author(s) declare none.









