Introduction
Micronutrient deficiencies are prevalent across the world, mostly affecting low-income countries in sub-Saharan Africa and Asia. Deficiencies in vitamins and minerals contribute to disease burden and morbidity, thus affecting human potential globally(Reference Stevens, Beal and Mbuya1). Iron, zinc and vitamin B12 are most likely to be deficient among populations in low- and middle-income countries where diets are mainly plant-based and low in animal products(Reference Gramer and Hoffmann2,Reference Bailey, West and Black3) . For instance, in 2018, mean consumption of meat (processed and unprocessed) in sub-Saharan Africa and South Asia was reportedly 36 g/d and 10 g/d, respectively, which were both lower than the global average of 68 g/d(Reference Miller, Reedy and Cudhea4). Notably, the bioavailability of non-haem iron and zinc from plant sources is poor due to antinutritional factors such as phytates(Reference Bailey, West and Black3,Reference Gupta, Brazier and Lowe5) . Regarding edible insects, the primary form of haem iron has a bioavailability similar to that of animal-source iron (myoglobin and haemoglobin), which is approximately 25%(Reference Mwangi, Oonincx and Stouten6). However, the bioavailability of the non-haem iron component of edible insects (mainly ferritin- and holoferritin-bound) is relatively unknown(Reference Mwangi, Oonincx and Stouten6,Reference Mwangi, Oonincx and Hummel7) . Notably, iron bioavailability could vary depending on the insect species and food matrix differences. For instance, chitin has been cited as a potential inhibitor of iron absorption in maize meals containing house crickets(Reference Mwangi, Oonincx and Hummel7) while Hilal et al.(Reference Hilaj, Zimmermann and Galetti8) ruled out the effect of meal worm chitin on absorption of iron from maize porridge.
Conversely, high-income countries reflect high consumption of animal products. For instance, the regional mean consumption of meat in high-income countries (75 g/d) was higher than the global average of 68 g/d in 2018(Reference Miller, Reedy and Cudhea4). Despite the generally high consumption of animal products in Europe, deficiencies in iron, zinc and vitamin B12 are known to occur among vegetarian populations(Reference Mwangi, Oonincx and Stouten6,Reference Mwangi, Oonincx and Hummel7) . Among vegetarians, the less strict vegetarians, such as entovegans (plant and insect-based diet) or even pescatarians (plant- and seafood-based diet), could benefit from consuming edible insects since it is reported that their attitude towards edible insect consumption is generally positive(Reference Zhou, Wang and Zhou9). Deficiencies in iron, zinc and vitamin B12, have been linked with severe consequences such as birth defects, increased susceptibility to infections, reduced growth, decreased work performance and productivity, and death(Reference Stevens, Beal and Mbuya1).
Notably, children under 5 years, women of reproductive age and pregnant women are particularly at high risk owing to increased nutrient needs(Reference Serra-Majem10). Globally, over a half of pre-school age children (6–59 months) and at least two-thirds of non-pregnant women of reproductive age (15–49 years) suffer from some form of micronutrient deficiency including iron, zinc and vitamin B12 (Reference Stevens, Beal and Mbuya1). Therefore, there is a need to find possible interventions to curb micronutrient deficiencies of iron, zinc and vitamin B12.
Globally, the prevalence of zinc deficiency is estimated to be 17·3%, with the highest in Africa (23·9%) and Asia (19·4%)(Reference Bailey, West and Black3). Zinc performs several important catalytic, structural and regulatory functions. It regulates the functioning of many metallo-enzymes(Reference Zastrow and Pecoraro11). In addition, zinc is a component of structural and regulatory proteins and is involved in cellular growth and differentiation, immunity maintenance and antioxidant defence mechanisms(Reference Zastrow and Pecoraro11–Reference Olechnowicz, Tinkov and Skalny13).
Iron performs important roles in the human body such as haem synthesis, oxygen and electron transport, and immune functionality(Reference Ganz14). Anaemia due to iron deficiency affects over 1·2 billion people globally(Reference Camaschella15). In high-income countries, iron deficiency is associated with secondary causative disorders or multiple pathological disorders in people of an older age, while in low-income countries it is associated with poor nutrition and parasitic infestations(Reference Camaschella15). Regarding parasitic infections, hookworms, for instance, cause iron deficiency anaemia by mechanically rupturing blood vessels in the intestines in addition to secreting anticoagulant and antiplatelet agents leading to chronic blood loss, while round worms (Ascaris lumbricoides) are associated with impaired iron absorption in the duodenum and jejenum(Reference Bhat, Vasaikar and Nxasana16,Reference Banu, Khanum and Hossain17) .
Vitamin B12, or cobalamin, is an essential cofactor in metabolic pathways involving the enzymes methionine synthase and methylmalonyl-CoA mutase(Reference Gramer and Hoffmann2). Cobalamin plays an essential role of promoting better immune health and cellular function, for instance DNA synthesis, replication and repair, and production of neurotransmitters(Reference Gramer and Hoffmann2,Reference Mikkelsen and Apostolopoulos18) . In addition, vitamin B12 is required for efficient erythropoiesis(Reference Gramer and Hoffmann2). Vitamin B12 deficiency is linked to megaloblastic anaemia, dysfunction of cellular metabolic pathways and immune dysfunction, contributing to the pathogenesis of many diseases such as cardiovascular, kidney and neurovascular diseases, osteoporosis and cancer progression(Reference Gramer and Hoffmann2,Reference Mikkelsen and Apostolopoulos18) . Vitamin B12 deficiency is estimated to affect 10–50% of women of reproductive age and pregnant women, globally(Reference Gramer and Hoffmann2,Reference Banu, Khanum and Hossain17) . While vitamin B12 is deemed sufficient in high-income countries, deficiencies are known to occur among people of older age due to malabsorption(Reference Lavriša, Hristov and Hribar19) and among strict vegetarians(Reference Wang, Masedunskas and Willett20).
Micronutrient intervention programs at the population level, such as fortification and supplementation, have been suggested to curb micronutrient deficiencies in the past(Reference Stevens, Beal and Mbuya1). In addition, dietary diversity, an important element of diet quality needs to be emphasised. To diversify diets with regard to animal food sources, edible insects could play a pivotal role either as food ingredients or whole insects.
Edible insects are considered nutritious from a macronutrient point of view. They are good sources of both macro and micronutrients. For instance, the protein and fat content of edible insects ranges from about 35–60% and 2·2–43·0% on a dry weight basis, respectively, comprising essential amino acids and fatty acids(Reference Zhou, Wang and Zhou9). Edible insects also contain vitamins and minerals, for instance house crickets (Acheta domesticus) contain 17·5–19·3 mg/kg and 54·3–67·1 mg/kg on a dry weight basis of iron and zinc, respectively(Reference Zhou, Wang and Zhou9,Reference Finke21,Reference Finke22) while freeze-dried A. domesticus reportedly contains 8·58–9·13 µg/100 g of vitamin B12 on a dry matter basis(Reference Khatun, Claes and Smets23). Currently there is scant information about the vitamin/mineral data of edible insects, including quality assessments. Hence, there is a need to gather existing data about the key micronutrients of interest to guide nutritionists and policy-makers in the field of nutrition. Therefore, this review provides an overview of the iron, zinc and vitamin B12 content of raw and processed edible insects and assess their potential to contribute to the recommended dietary intakes of iron, zinc and vitamin B12.
Materials and methods
Article inclusion and exclusion criteria
Data on the zinc, iron and vitamin B12 contents of edible insects were obtained using the following search strategy: Web of Science and Scopus were first systematically searched using the following keywords: (edible insects) AND (iron content) OR (zinc content) OR (vitamin B12 content) OR (nutritional composition). The search was carried out in May 2024 and limited to the publication years from 2001 to May 2024, since the majority of edible insect research happened after the year 2000. Data from journal articles were included if they fulfilled the following inclusion criteria:
The insects analysed are listed under the world list of recorded edible insects(Reference Jongema24).
Primary studies with original results of analyses for nutrients including iron and/or zinc and/or vitamin B12 for any edible insect species whose scientific names and the extent of processing/preparation prior to analysis are clearly described.
To ensure a standardised comparison of processed edible insect foods, articles in which the insects were fortified or enriched with nutrients and other foods during their processing were excluded. Processed edible insects for which little or no information about the method of processing was provided; for instance, dried insect powders from venders for which processing conditions were not described, were excluded. In addition, analytical findings with the highest sample sizes for each edible insect were used for comparisons with dietary reference values.
Data quality
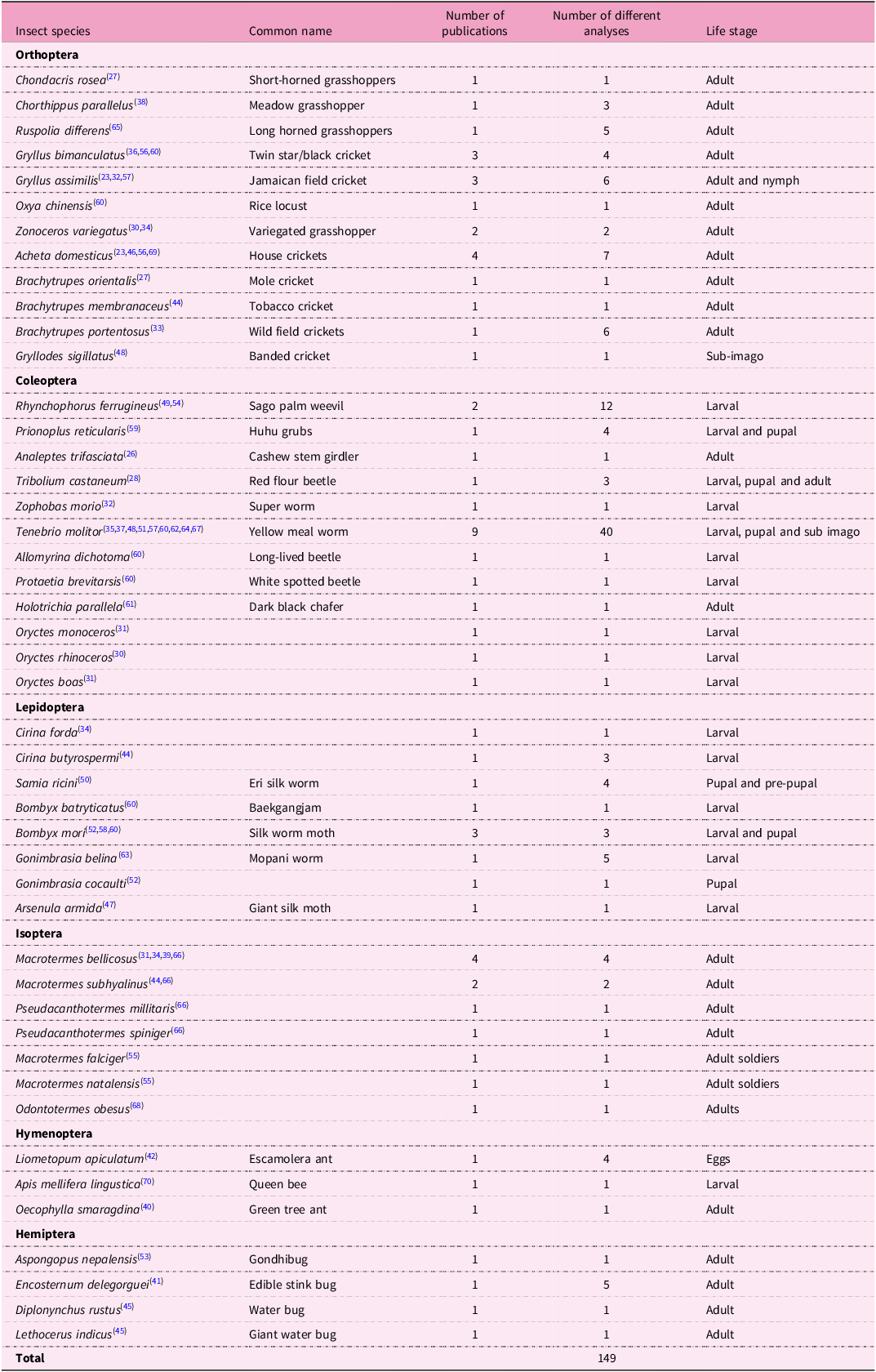
To evaluate the quality of the data, further analyses of the published records were conducted using the EuroFIR quality index. EuroFIR quality guidelines(Reference Salvini, Oseredczuk and Roe25) were used to attribute scores of 1–5 (1 = low quality, 5 = high quality) for seven categories: food description, component identification, sampling plan, number of analytical samples, sample handling, analytical method and analytical method quality control. Each of the seven categories has its own set of criteria(Reference Salvini, Oseredczuk and Roe25) to ensure that an appropriate score is objectively determined. The combination of scores for each of the categories were used to calculate the quality index, whose maximum possible value is 35 (Table 1). Data recorded according to the inclusion criteria has been grouped to indicate the number of analyses considered for each insect species (Table 2).
Table 1. Quality index scores for each of the forty-six data sources used for data extraction

A, food description; B, component identification; C, sampling plan; D, number of analytical samples; E, sample handling; F, analytical method and analytical quality control. All categories score from 1 to 5 except F which is scored from 1 to 10. Highest possible EuroFIR score = 35. 1 = low quality, 2 = better than low quality but less than intermediate, 3 = intermediate, 4 = less than high quality but better than intermediate, 5 = high quality.
Table 2. Selected insect species based on availability of as is/fresh basis data or for those that processed moisture content is reported
Data extraction and processing
Data extraction was done to include data points which reported a fresh or as is basis, or for which fresh basis or as is basis could be calculated on the basis of the data available in the journal article. Records for which dry basis data were reported, but moisture content was provided as well, the iron, zinc and vitamin B12 content results were calculated using the following formula (equation 1):
 $$\eqalign{& {\rm{content\;}}\left( {{\rm{{\it x},\;fresh\;or\;as\;is\;basis}}} \right) \cr & = {\rm{content\;({\it x},\;dry\;matter\;basis)\; \times \;(mc\; - \;100)}} \over{{{\rm{100}}}}}$$
$$\eqalign{& {\rm{content\;}}\left( {{\rm{{\it x},\;fresh\;or\;as\;is\;basis}}} \right) \cr & = {\rm{content\;({\it x},\;dry\;matter\;basis)\; \times \;(mc\; - \;100)}} \over{{{\rm{100}}}}}$$
where x is zinc (mg/100 g), iron (mg/100 g) or vitamin B12 (µg/100 g) and mc is moisture content (%). For different data sources, means and standard deviations of iron, zinc and vitamin B12 were calculated (Table 3). Where more than one author reported on the same edible insect, new means and standard deviations were calculated, while for those with only one author, the reported technical or biological mean and standard deviation were used. Logarithmic box plots were made for the iron, zinc and vitamin B12 concentrations of the different insect orders using the chart builder function of JMP Pro software, version 17. Using JMP Pro software, the data were log transformed and extreme values were excluded from the box plots (Supplementary Data) on the basis of unacceptably high values and standard deviations. To compare with dietary reference values, a heatmap was applied (Fig. 3).
Table 3. Iron, zinc and vitamin B12 content (as is/fresh basis) of edible insect species processed using different processing methods
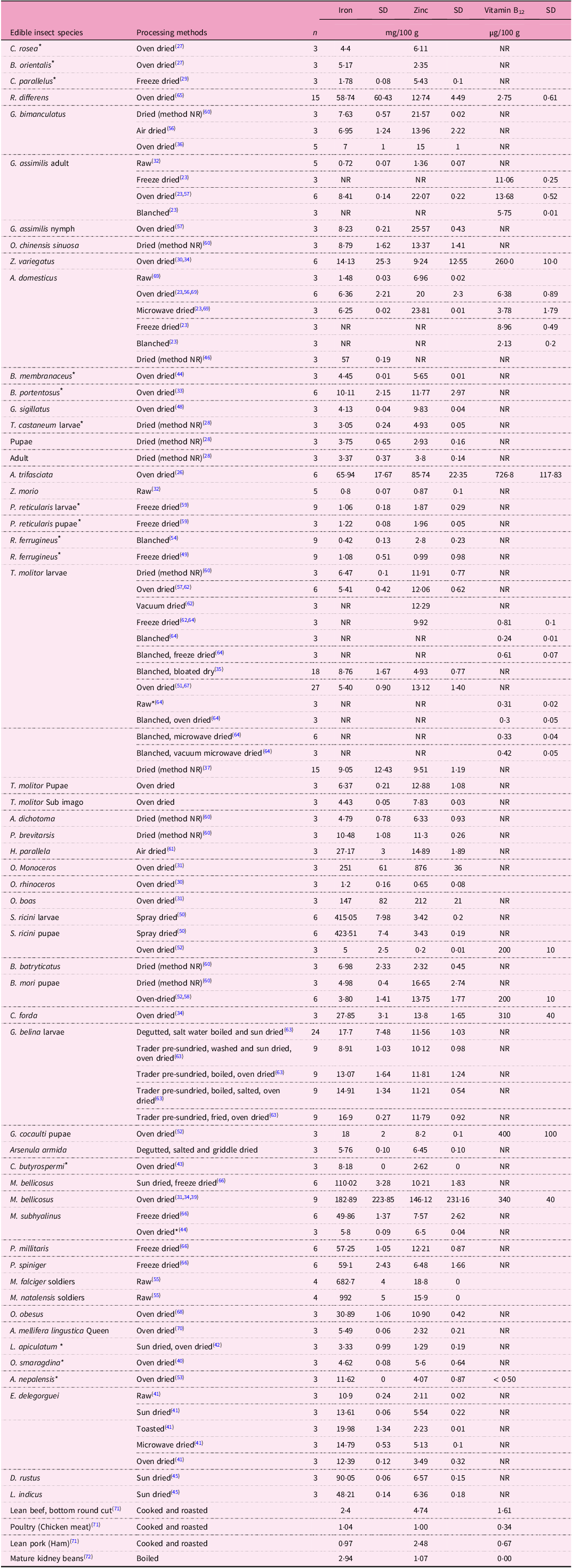
* Underwent processing/preparation but results are reported on fresh weight basis. NR, not reported; SD, standard deviation. Where data was pooled from more than one author, n (sample size) reflects the pooled sample size from the different authors. For C. rosea and B. orientalis the author did not report the standard deviation of the three replicates
Comparison of iron, zinc and vitamin B12 content of processed edible insects with lean beef, lean pork, poultry meat and mature kidney beans
The iron, zinc and vitamin B12 content was compared with lean beef and pork, poultry meat (all cooked and roasted) and boiled mature kidney beans (Table 3).
Kidney beans (Phaseolus vulgaris), also referred to as ‘common bean’ is the most important legume for human consumption, especially in low-income settings. Bean consumption is highest in low- and middle-income countries; for instance, the highest consumption of beans in Africa is estimated at 60 kg/capita/year for western Kenya and Rwanda(Reference Uebersax, Cichy and Gomez73). However, bean consumption is generally low in industrialised nations (e.g. 3 kg/capita/year for the United States)(Reference Uebersax, Cichy and Gomez73). Kidney beans are good sources of both macro and micronutrients. For instance, boiled mature kidney bean seed flour contains carbohydrates (54·49%), protein (24·04%), iron (21·07 ppm) and zinc (17·94 ppm)(Reference Olanipekun, Omenna and Olapade74), but beans are not known to contain vitamin B12.
Poultry (14·7 kg/capita), pork (11·1 kg/capita) and beef (6·4 kg/capita) represent the first, second and third most consumed meat types globally, respectively(Reference Whitton, Bogueva and Marinova75). They are important sources of iron, zinc and vitamin B12 in the human population. Notably, the iron, zinc and vitamin B12 in animal-source foods has good bio-accessibility.
Comparison of iron, zinc and vitamin B12 contents of edible insects with recommended dietary allowances (RDA)
The extracted nutrition data was further compared with recommended dietary allowances (RDA) for different human life stages (Fig. 3). The RDA of iron, zinc and vitamin B12 referred to in this study, were stipulated by the Institute of Medicine (IOM) of the National Academies(76,77) . A solid food was regarded as a ‘source of” a nutrient if the micronutrient value was found greater than 15% of the RDA and regarded as being ‘rich in’ a micronutrient if the micronutrient value doubles that of the requirement for ‘source’(78).
While the insect serving size is a significant factor in this regard, a consensus on this matter was not found in the literature. Maya et al.(Reference Maya, Wilderspin and Costa79) examined the impact of exposing families to insect-based or plant-based dinner menus on dietary patterns, meat intake and protein intake over a 6-week period; however, standard insect serving sizes were not defined. Furthermore, the typical serving sizes of edible insects could vary depending on seasonal availability and different cultural settings. For instance, Yhoung-Aree et al.(Reference Yhoung-Aree, Puwastein and Attig80) estimated an average edible insect consumption level of 15 g per person per day for school age children (6–13 years) for a range of edible insects consumed in Thailand, while Acuña et al.(Reference Acuña, Caso and Aliphat81) specified the typical portion size per person as the amount of insects equivalent to a 220 g capacity chilli container in Mexico. Following a cross-sectional study among insect eating communities in central and southwestern Uganda, Kasozi et al.(Reference Kasozi, Namazi and Basemera82) reported the typical amount of grasshoppers eaten by an adult per day as 345·86 g and 310·69 g in peri-urban and rural areas, respectively.
Accordingly, the iron, zinc and vitamin B12 contents utilised for the comparisons in this study were based on the weight per 100 g of edible portion of the edible insect, which is consistent with the nutrition labelling regulation in the European Union(Reference Drewnowski, Maillot and Darmon83), and also compares favourably with 100 g of beef, which is the reference used in food composition tables. For example, the protein content of 100 g of mealworm larvae (13·68–22·32% on fresh weight basis)(Reference Nowak, Persijn and Rittenschober84) is very similar to the protein content of beef (16·62–21·80% on fresh weight basis)(Reference Hwang and Joo85,Reference Jung, Hwang and Joo86) . In light of the aforementioned considerations, this article estimates a serving of beef to be equivalent to a serving of meal worms (and by extension, other insect species) based on protein consumption.
Results
Data search and quality
With the ‘English’ filter activated, the search yielded 515 and 62 results for Web of Science and Scopus, respectively. Book chapters, proceeding papers, editorial material and reviews (n = 211) were excluded. Articles were screened on the basis of title and abstract to eliminate those not relevant to the topic, such as oil extraction, antioxidant properties and microbial properties (n = 212), and duplicates (n = 5). Further screening was done by carrying out a full text search to obtain the remaining forty-six records used for data extraction (Fig. 1).
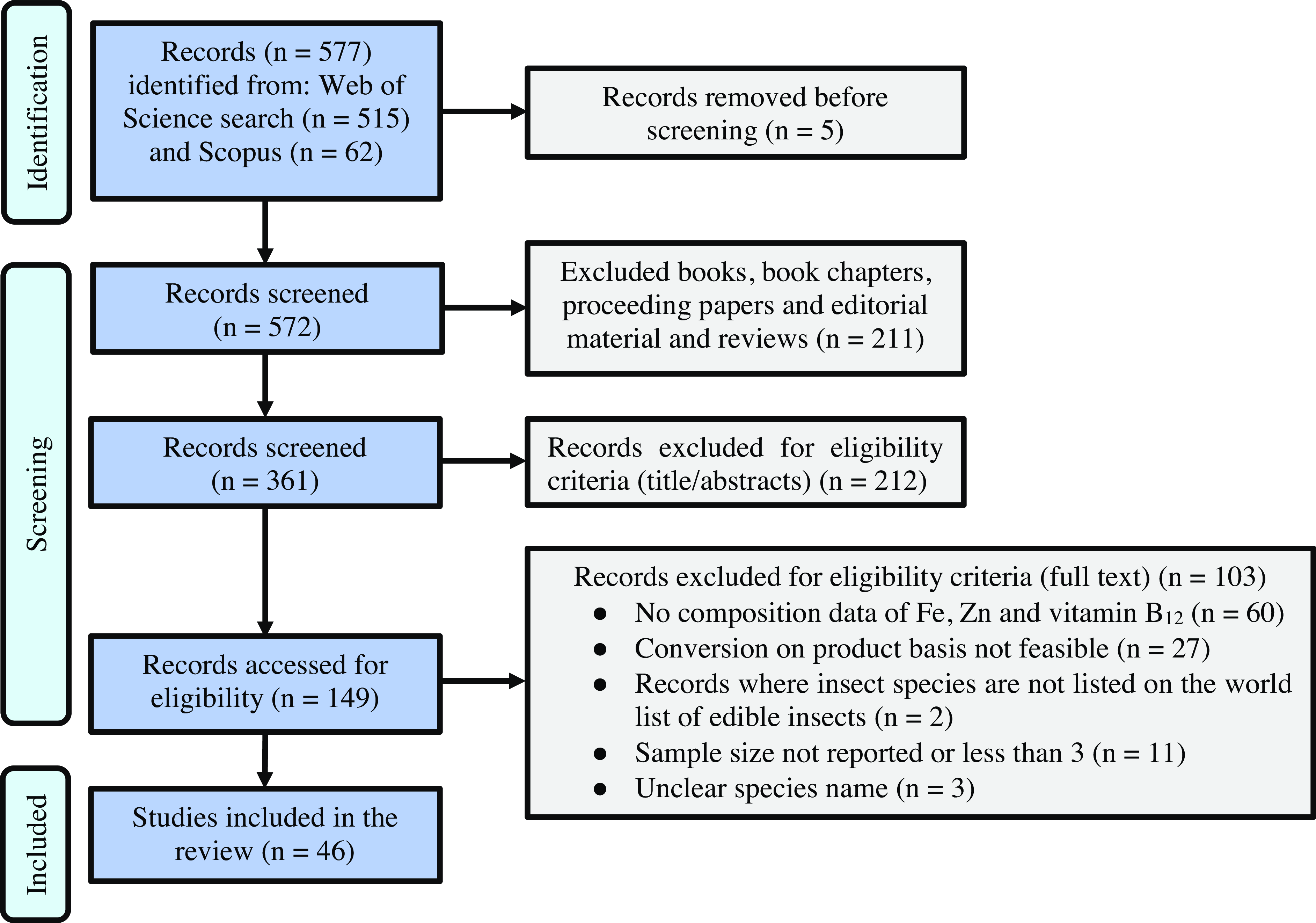
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines flow chart for literature search and study selection.
The overall EuroFIR quality index scores ranged from 15 to 30 for all the records assessed (Table 1). Generally, most articles (89·1%) were of low-to-moderate quality (EuroFIR index score 7–25) while only 10·9% were of high quality (EuroFIR index score 26–35).
Number of analyses and edible insect developmental stages used for data extraction
Table 2 presents the edible insect species for which as is and fresh-weight data are available. The forty-six articles included contain forty-six species of edible insects from six(Reference Mwangi, Oonincx and Stouten6) insect orders, which include the following: orthoptera(Reference Zastrow and Pecoraro11), coleoptera(Reference Zastrow and Pecoraro11), lepidoptera(Reference Hilaj, Zimmermann and Galetti8), isoptera(Reference Mwangi, Oonincx and Hummel7), hymenoptera(Reference Bailey, West and Black3) and hemiptera(Reference Miller, Reedy and Cudhea4). The highest number of insects were adults(Reference Mokwunye, Igbinadolor and Mokwunye26) followed by the larval stage(Reference Mikkelsen and Apostolopoulos18) and pupae(Reference Gupta, Brazier and Lowe5), while sub-imago and eggs comprised only two and one of the insect species, respectively. Data on zinc and/or iron and/or vitamin B12 content were available for only one developmental stage for the majority of edible insects studied. The edible insects for which data on more than one developmental stage were available include Samia ricini (larvae and pupae), Bombyx mori (larvae and pupae), Prionoplus reticularis (larvae and pupae), Tribolium castaneum (larvae, pupae and adults), Tenebrio molitor (larvae, pupae and sub-imago) and Gryllus assimilis (adult and nymph). The most represented insect species was T. molitor (nine publications).
Instrumental techniques used to obtain iron, zinc and vitamin B 12 results
The main instrumental techniques used for iron and zinc analyses were atomic absorption spectrometry (AAS) (either flame or oven AAS), inductively coupled plasma-optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS) and titrimetric methods. The main techniques used for vitamin B12 analyses included immunoassays and spectrophotometric techniques, sometimes coupled with a chromatographic technique.
Effect of processing on the zinc, iron and vitamin B12 contents of selected insect species
Data extraction was done to include data points which reported on an as is or fresh weight basis, or for which fresh or as is basis could be calculated. Table 3 indicates the iron, zinc and vitamin B12 contents of the edible insects processed using different methods, on an as is or fresh basis.
The iron content (mg/100 g) varied from 0·4 for blanched Rhynchophorus ferrugineus to 992·0 mg/100 g for Macrotermes natalensis soldiers. When it comes to zinc content, oven-dried S. ricini pupae has an amount of 0·2 mg/100 g, while oven-dried Oryctes monoceros contain a substantial 876·0 mg/100 g. There was scant data on vitamin B12 content. Blanched T. molitor had a vitamin B12 content of 0·2 µg/100 g, whereas oven-dried Analeptes trifasciata contained 726·8 µg/100 g.
An unfairly high value of vitamin B12 (138·19 ± 195·06 mg/100 g) for Z. variegatus was obtained from the average of values reported by Anaduaka et al.(Reference Anaduaka, Uchendu and Osuji30) (276·12 ± 21·64 mg/100 g) and Atowa et al.(Reference Atowa, Okoro and Umego34) (0·26 ± 0·01 mg/100 g). Both authors(Reference Anaduaka, Uchendu and Osuji30,Reference Atowa, Okoro and Umego34) used a spectrophotometric technique for vitamin B12 quantification with variations in extraction procedures and wavelengths at which the quantifications were carried out (530 and 325 nm) (Table 3). The high value of vitamin B12 reported by Anaduaka et al. could be caused by errors during data processing and reporting, hence it was omitted from Table 3.
Similarly, the average of vitamin B12 values reported for raw A. domesticus by Bawa et al.(Reference Bawa, Songsermpong and Kaewtapee69) (0·50 ± 0·84 mg/100 g, as is basis) was omitted from Table 3 because the unacceptably high standard deviation could be caused by a reporting error for one of the values and hence cannot be exclusively explained by the differences in the substrates used.
Supplementary Fig. 1 shows the results of the effects of processing on iron, zinc and vitamin B12 contents of edible insects. Results indicate that oven drying was mostly used. Data on oven-dried insects (Fig. 2) indicates wide variations in iron and zinc content among different insect orders, as indicated by the box plots. For the majority of the treatments, the vitamin B12 content of the edible insect species was not studied.
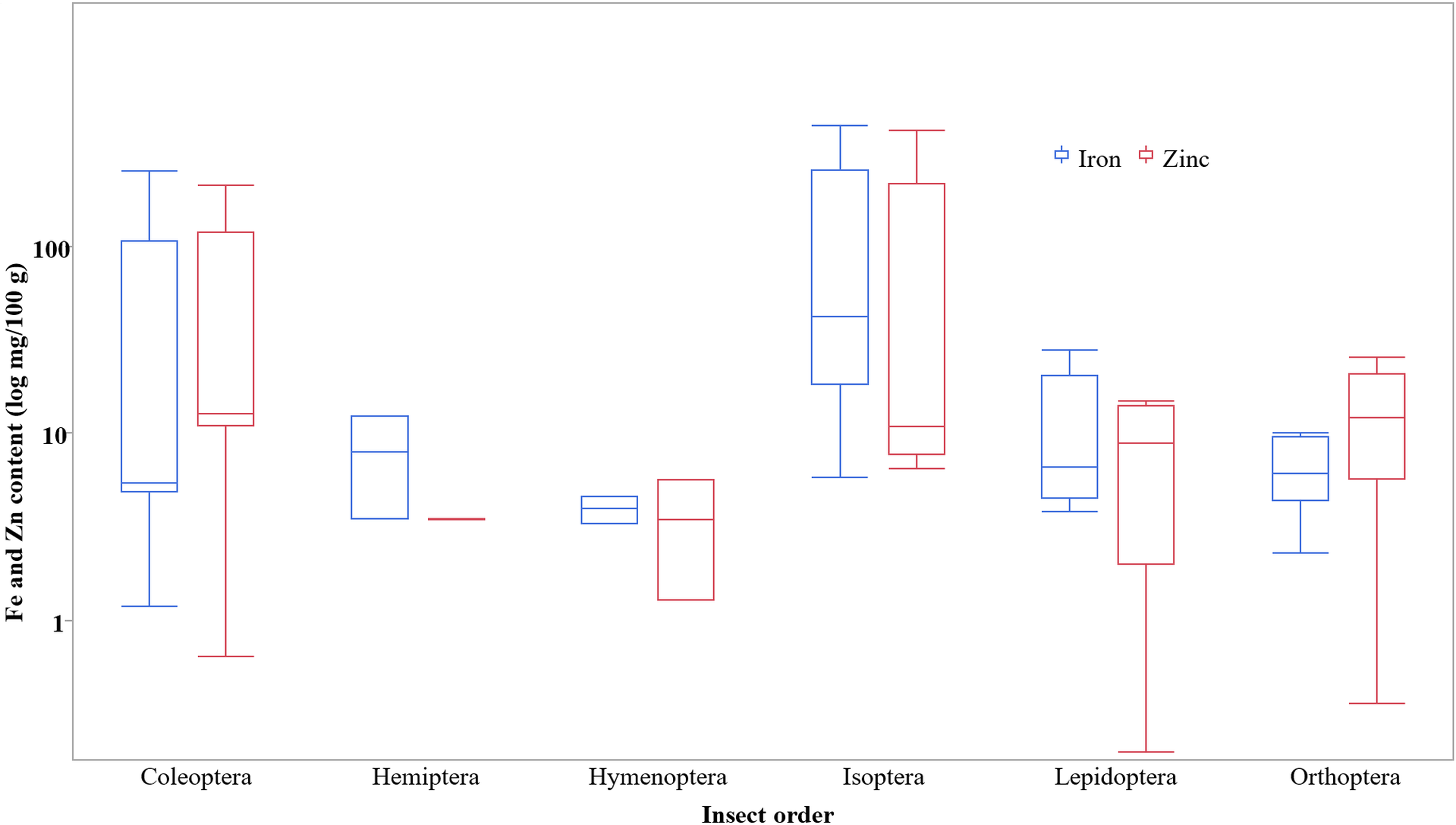
Fig. 2. Box plot of iron and zinc content of oven dried edible insects (fresh and as is basis).
Comparison of iron, zinc and vitamin B 12 content of edible insects with lean beef, lean pork, poultry meat and mature kidney beans
Most edible insect species have a higher content of iron (g/100 g, as is basis) than lean beef (2·4 mg/100 g) and kidney beans (2·94 mg/100 g), except raw A. domesticus, G. assimilis and Zophobas morio; oven-dried O. rhinoceros; freeze-dried Chorthippus parallelus and P. reticularis; and blanched R. ferrugineus (Table 3). Similarly, most edible insects have a higher iron content than lean pork (0·97 mg/100 g), except raw G. assimilis and Z. morio, and blanched R. ferrugineus. Most of the studied edible insects have a higher zinc content than either poultry (1·00 mg/100 g) or mature kidney beans (1·07 mg/100 g), except oven-dried S. ricini, and O. rhinoceros, and raw Z. morio. There were thirteen species in either raw, toasted or oven-dried form that had lower zinc contents than either lean beef (4·74 mg/100 g) or pork (2·48 mg/100 g). Regarding vitamin B12, A. domesticus (raw), R. differens (oven-dried) and G. assimilis (freeze-dried, oven-dried and blanched), O. rhinoceros (oven-dried), Gonimbrasia cocaulti (oven-dried), B. Mori (oven-dried) and S. ricini (spray-dried) had higher contents of vitamin B12 than lean beef, lean pork, poultry and mature kidney beans, while all edible insects were superior to boiled mature kidney beans with regard to vitamin B12 content. Notably, most heat treatments involving a blanching step reflected a lower vitamin B12 content in edible insect species than in pork, beef, poultry and kidney beans. Results also indicate that there were few records of vitamin B12 content for the edible insect species studied.
Comparison of iron, zinc and vitamin B 12 contents of edible insects with recommended dietary allowances (RDA)
Regarding roasted lean beef, results indicated that while it can be regarded as a ‘source of’ iron for infants, adolescents and lactating women, and ‘rich in’ iron for male adults and the elderly, it is ‘poor in’ iron for female adults and pregnant women (Fig. 3). Generally, roasted lean beef was found to be ‘rich in’ zinc and vitamin B12 for all human life stages. The content of iron (0·89 mg/100 g), zinc (0·44 mg/ 100 g) and vitamin B12 (0·0 µg/100 g) of boiled mature kidney beans indicated that they are ‘poor in’ iron, zinc and vitamin B12 for all human life stages.
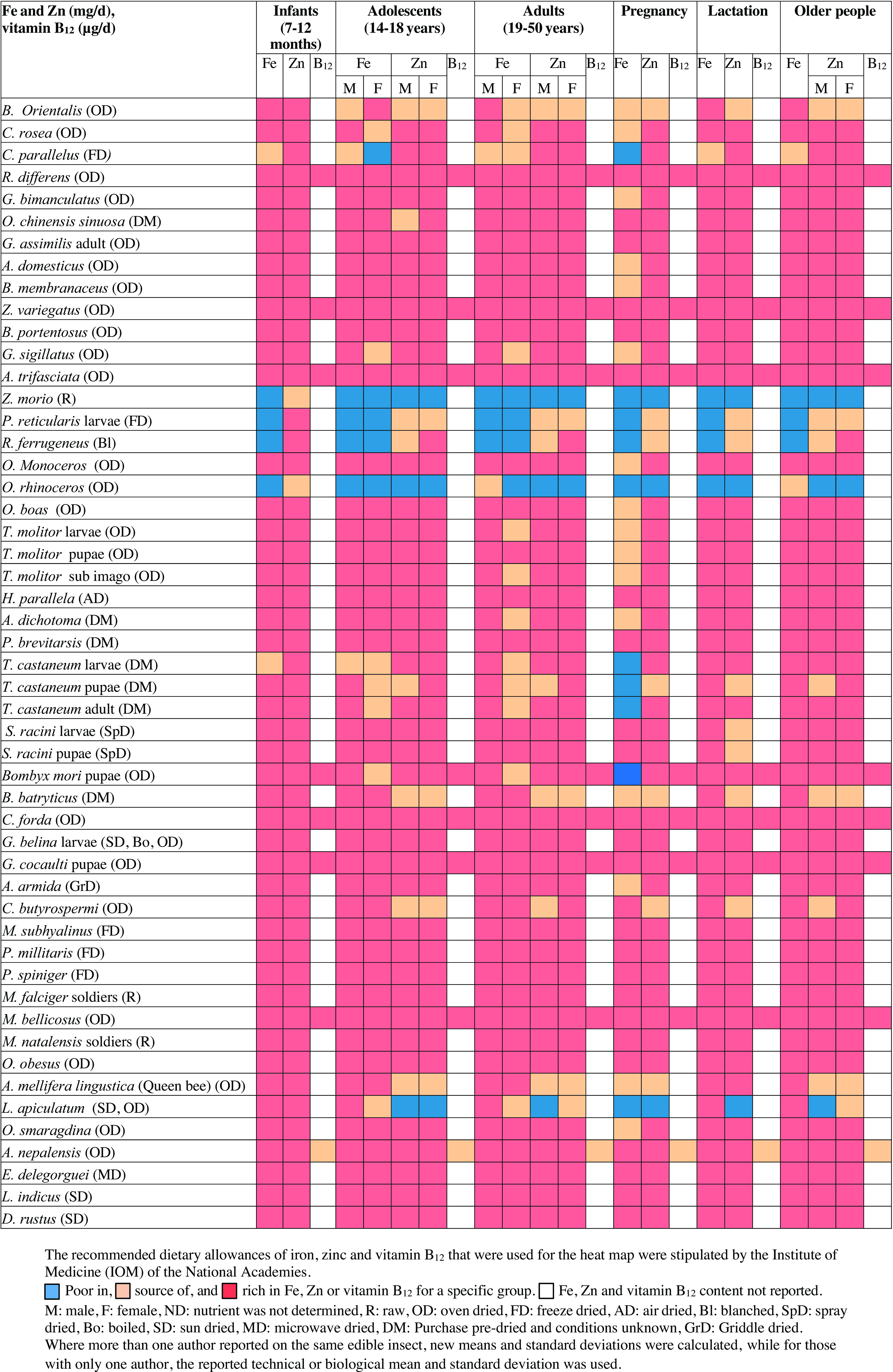
Fig. 3. Heatmap representing the adequacy of iron, zinc and vitamin B12 content from edible insect species for different human life stages.
With regard to iron, most of the studied insects can be considered ‘rich in’ or ‘sources of’ iron for different human life stages, except for C. parallelus (female adolescents and pregnant women); G. assimilis, Z. morio, P. reticularis and R. ferrugineus (all human life stages); O. rhinoceros (infants, adolescents, female adults, pregnant women and lactating women); and T. castaneum and Liometopum apiculatum (pregnant women).
Most insect species are rich in zinc. However, the zinc content of G. assimilis is poor for male adults and elderly, pregnant and lactating women. Z. morio and O. rhinoceros are poor sources of zinc for all human life stages except infants. Liometopum apiculatum is a poor source of zinc for adolescents, male adults and elderly, pregnant and lactating women.
Among the studied species for which vitamin B12 content was available, Ruspolia differens, G. assimilis, Z. variegatus, A. trifasciata, O. rhinoceros and B. mori were ‘rich in’ vitamin B12 for all human life stages, while Aspongopus nepalensis can be considered a ‘source of” vitamin B12 for all human life stages. While T. molitor, Cirina forda and Macrotermes bellicosus were ‘rich in’ vitamin B12 for infants, they can be considered poor in vitamin B12 content for other human life stages, as shown in Fig. 3.
Discussion
Generally, edible insects could fulfil the dietary reference values of iron, zinc and vitamin B12 for the majority of the human population across different life stages. However, laboratory analytical gaps and a dearth of micronutrient data have been identified.
Data quality
Decent sampling and sample preparation procedures are necessary for accurate and reliable analytical results. Sampling and sample preparation gaps could have contributed to the inaccuracy and lack of precision of the results obtained. In this study, for instance, it was not clear whether the sampling sites reflected consumption, different seasons and important outlets, while some authors did not report about appropriate stabilisation treatments for the samples. In addition, regarding food description, a few authors(Reference Grdeń and Sołowiej46,Reference Park, Kang and Choi60) who purchased ready-made insect powder lacked details about sample treatment, such as the extent of drying and the drying method for some edible insects such as A. domesticus, Protaetia brevitarsis and B. mori.
Furthermore, analytical method and analytical method quality control generally scored poorly using the EuroFIR index. This could be partly attributed to the lack of already validated official analytical methods adapted to edible insect matrices. While instrumental techniques used for analysis of iron, zinc and vitamin B12 of edible insects in this study were generally consistent with those recommended by the EuroFIR guidelines for assessment of methods of analysis (EuroFIR GAMA)(Reference Castanheira, Saraiva and Rego87), the lack of method consistency and lack of validated official analytical methods for edible insect matrices remain a challenge. An analytical method refers not only to an instrumental technique used, but also to steps such as sample pre-treatments and reporting of results. The lack of validated official analytical methods for edible insects is reflected by unreasonable variations in some of the values for iron, zinc and vitamin B12 of the studied insect species. For instance, Atowa et al.’s method of analysis was based on AOAC (88) standard procedures, even if there is no indication that the procedure was developed and validated for edible insect matrices. Worryingly, Atowa did not mention the exact AOAC method number, which also makes it difficult to know which food matrix the method had been validated for. Therefore, despite the use of recommended instrumental techniques, there is need to develop and validate official analytical methods for edible insect matrices in reference to AOAC guidelines for standard method performance requirements(88).
Relatedly, method quality control procedures were not considered. There were no records of analytical method validity for all the records used for data extraction. One consideration for method validity, which was lacking for all records, is the incorporation of appropriate standard reference materials in the method of analysis(Reference Castanheira, Saraiva and Rego87,Reference Nielsen89,Reference Ambrus90) . Notably, standard reference materials based on edible insects do not yet exist. A few articles(Reference Kavle, Carne and Bekhit59,Reference Addeo, Roncarati and Secci70) used cod muscle and/or fish protein as standard reference materials, which might not be appropriate for edible insect matrices. Kinyuru et al.(Reference Kinyuru, Konyole and Roos66) used in-house control materials, which are not certified reference materials despite being of edible insect matrices. This article therefore proposes future research into the development of edible insect matrix-based certified reference materials to guarantee the accuracy of analytical results obtained.
Furthermore, the lack of any forms of laboratory and/or method certification(Reference Salvini, Oseredczuk and Roe25) for most records reduces confidence in the accuracy of the recorded data for zinc, iron and vitamin B12 contents of edible insects. Only a few studies(Reference Grdeń and Sołowiej46,Reference Jankauskienė, Aleknavičius and Kiseliovienė67,Reference Ranjith, Kalleshwaraswamy and Deshmukh68) reported to have some form of laboratory/method accreditation. In terms of data precision, individual data records were good except for a few records where precision was uncertain since standard deviations were not recorded, for instance the iron and zinc content of Chondacris rosea and Brachytrupes orientalis (Reference Chakravorty, Ghosh and Jung27).
Factors affecting the iron, zinc and vitamin B 12 composition of the studied insect species
The observed wide variations in the iron, zinc and vitamin B12 contents of edible insects could be explained by a number of factors such as insect species, developmental stage, geographic and climatic conditions, insect’s food intake, processing methods and methods of analysis applied (see supra). It is however not yet known which of the factors have the biggest influence on nutritional composition of edible insects.
Species differences
The differences in iron, zinc and vitamin B12 contents of edible insects are species specific. Different species of edible insects, even within the same insect order, exhibit differences in iron, zinc and vitamin B12 content. This could indicate that observed differences are species specific and may not necessarily be a reflection of taxonomic distance(Reference Mwangi, Oonincx and Stouten6).
Developmental stages
A few studies have reported on iron, zinc and vitamin B12 contents of more than one insect developmental stage because most of the times each insect is eaten at a certain life stage(Reference Mwangi, Oonincx and Stouten6). Nevertheless, more than one stage of some insects can be consumed, for instance, the adult and nymph stages of A. domesticus (Reference Sun-Waterhouse, Waterhouse and You91). Hence, this study also highlights the lack of comprehensive data for iron, zinc and vitamin B12 content of all the possible consumable life stages. The variations in iron and zinc content within species could be explained by differences in developmental stages in some edible insects.
Ecosystem and insect habitat
Ecosystem factors such as season of harvesting and geographical origin of the harvested insect can lead to variations in iron, zinc and vitamin B12 contents of edible insects in case of wild harvesting. The long-horned grasshopper (R. differens) for instance, is generally harvested from the wild during two annual swarming seasons(Reference Mmari, Kinyuru and Laswai92,Reference van Huis93) . Results published by Ssepuuya et al.(Reference Ssepuuya, Smets and Nakimbugwe94) indicate that R. differens harvested in Uganda had significant variations in their iron, zinc and vitamin B12 contents across the two swarming seasons and different districts. Similar findings were recorded by Kababu et al.(Reference Kababu, Mweresa and Subramanian65) for R. differens harvested in Uganda. This could be attributed to differences in food sources in different geographical locations, as well as seasonal changes.
Edible insect diet
The chemical composition of edible insects is generally influenced by their diet composition. For instance, A. domesticus fed on five differently formulated commercial feed-based diets expressed significant differences in the iron, zinc and vitamin B12 contents(Reference Bawa, Songsermpong and Kaewtapee69). Such differences can be explained by differences in substrate micronutrient content and possible regulation to maintain insect body homeostasis and prevent toxicity(Reference Mwangi, Oonincx and Stouten6). It was demonstrated that the zinc content of the body of Gryllus assimilis is regulated by changes in assimilation and elimination rate depending on the dietary zinc content(Reference Bednarska, Opyd and Zurawicz95). Therefore, the iron, zinc and vitamin B12 content closely depends on the diet of edible insects.
Processing
Processing conditions could lead to changes in iron, zinc and vitamin B12 contents of edible insects. In cultures where insects are a traditional delicacy, they are either consumed raw or processed(Reference Melgar-Lalanne, Hernández-Álvarez and Salinas-Castro96). For instance, raw edible termite body parts or whole raw/alive termites can be eaten as they emerge from the mound holes in many parts of sub-Saharan Africa(Reference Netshifhefhe, Kunjeku and Duncan97,Reference van Huis98) while R. differens (wings and legs removed) can be eaten raw in Tanzania(Reference Melgar-Lalanne, Hernández-Álvarez and Salinas-Castro96). Both termites and R. differens can also be processed using different techniques such as steaming, roasting, smoking, frying, stewing and curing for better sensory quality and improved shelf life(Reference Melgar-Lalanne, Hernández-Álvarez and Salinas-Castro96). Novel technologies aimed at utilising edible insects as ingredients in a non-recognisable form such as powders/flours have been developed to increase consumer interest in developed countries, such as those in Europe(Reference Hartmann and Siegrist99). Such novel technologies include freeze drying, oven drying, fluidised bed drying and microwave drying(Reference Melgar-Lalanne, Hernández-Álvarez and Salinas-Castro96). The iron, zinc and vitamin B12 contents of edible insects (on as is basis) processed using different techniques are presented in Table 3. Processing methods could alter the iron and zinc content of edible insects to varying extents either negatively or positively(Reference Dandadzi, Musundire and Muriithi41).
In addition, the extent of the effect of a processing method on iron and zinc contents of edible insects depends on the edible insect species. This could be attributed to species-specific differences in matrices mineral release. Comparisons between processing methods were difficult for many of the processing methods in this study because of existing data gaps, especially for vitamin B12 content. Therefore, the data were disaggregated by insect orders, which also created a problem of very large variations due to different individual insect matrices as indicated by logarithmic scale box plots (Supplementary Fig. 1).
Oven drying is the most commonly used processing method, with data gaps still evident for other processing methods used, such as freeze drying, toasting and microwave drying. For instance, microwave-dried processing was not used for Z. variegatus which makes it difficult to compare the effect of the microwave drying processing on iron, zinc and vitamin B12 levels with available data for other processing methods. Therefore, more analyses are needed to provide more data on the effect of processing methods on iron, zinc and vitamin B12 contents of different edible insect species.
Comparison of zinc, iron and vitamin B 12 contents of edible insects with lean beef, lean pork, poultry meat and mature kidney beans
Most of the edible insects studied (for which data were available) have more zinc, iron and vitamin B12 than lean pork and beef, poultry and mature kidney beans (Table 3) despite the existence of wide variations. Consistent with our findings, it was reported by Payne et al.(Reference Payne, Scarborough and Rayner100) that the median iron content of crickets (5·46 mg/100 g) and honeybees (18·50 mg/100 g) is 180% and 850% greater than that of raw beef (1·95 mg/100 g), which has the highest iron content out of three commonly consumed meat types (beef, poultry and pork)(Reference Payne, Scarborough and Rayner100). Notably, this review reflects even higher quantities of iron in the different meat types due to roasting, whereas Payne et al.’s comparison was with raw beef.
Similarly, Locusta migratoria and A. domesticus reportedly contain higher amounts of iron and similar amounts of zinc, compared with beef, pork and poultry(Reference Mwangi, Oonincx and Stouten6). Relatedly, it was reported that edible grasshoppers (Z. variegatus) contain up to 10 and 1·2 times more iron and zinc, respectively, than bean seeds (P. vulgaris)(Reference Halliru, Muhammad and Ahmed101). However, several factors (discussed earlier) could explain why up to thirteen edible insects were inferior to beef and pork in terms of zinc content. Therefore, on the basis of the overview, insect species having high zinc and iron contents can be selected as a source of these micronutrients and could be a substitute for commonly consumed meat types, even for non-strict vegetarian population groups such as pescaterians and ento-vegetarians who are likely to be open to eating edible insects.
Among the edible insects for which data is available, the vitamin B12 content of R. differens, A. domesticus and G. assimilis is higher than that of roasted beef for instance. Similarly, the vitamin B12 content values reported for T. molitor larvae (1·08 µg/100 g), L. migratoria adults (0·84 µg/100 g), G. assimilis adults (2·88 µg/100 g) and Shelfordella lateralis adults (13·21 µg/100 g dry basis) are either comparable to or higher than pork meat (1 µg/100 g)(Reference Schmidt, Call and Macheiner102). All edible insects were superior to kidney beans in terms of vitamin B12 content irrespective of species, since kidney beans do not contain any vitamin B12. For Z. variegatus, Anaduaka et al. reported an unreasonably high value of 276·12 ± 21·64 mg/100 g. The latter could be attributed to serious errors during laboratory analysis or data processing, and ultimately a lack of official analytical methods for edible insects (see supra). There is also likely a negative effect of processing on the observed low levels of vitamin B12 for some insect species; for instance, most pre-blanched insects in our study could have lost some of the vitamin B12 due to leaching.
Comparison of zinc, iron and vitamin B 12 contents of edible insects with dietary reference values
Generally, the edible insects included in this study can be considered as ‘rich in’ or ‘sources of’(78) iron and zinc for different human life stages irrespective of the processing methods used (Fig. 3). Some edible insect species could be considered poor/inadequate in iron and zinc for different human life stages. These include raw, freeze-dried or blanched insect species, oven-dried O. rhinoceros and sun-dried/oven-dried L. apiculatum. The raw and blanched insects could have had a high moisture content, which creates a dilution effect on iron and zinc. In addition, during blanching there is a likelihood of iron and zinc loss due to leaching into the blanch water. Drying methods have also been reported to have varying effects on the iron and zinc contents depending on the edible insect species.
For most edible insects, data on vitamin B12 contents are not available, making comparisons with dietary reference values difficult. Furthermore, while it is possible that vitamin B12 content varies by species(Reference Mwangi, Oonincx and Stouten6), processing methods(Reference Oonincx and Finke103), geographical region of sourcing(Reference Ssepuuya, Smets and Nakimbugwe94) and diet(Reference Bawa, Songsermpong and Kaewtapee69) certain variations obtained were unreasonably high. Some of the unfairly high variations could possibly be explained by methodological differences and inter-laboratory errors for means and standard deviations calculated from results of more than one author.
Bioaccessibility and bioavailability of iron, zinc and vitamin B 12 of edible insects
The current study did not report about the bioaccessibility and bioavailability of iron, zinc and vitamin B12 from edible insects. Notably, inhibitors such as phytates for insects fed on a plant-based diet(Reference Mwangi, Oonincx and Hummel7), different processing methods(Reference Manditsera, Luning and Fogliano104) and the form of iron (haem v. non-haem) could influence the bioaccessibility and bioavailability of iron and zinc.
Regarding the form of iron, edible insects contain both haem and non-haem iron. The primary form of haem-iron in edible insects is found in cytochromes and its bioavailability is reportedly similar to that of vertebrate iron (myoglobin and haemoglobin)(Reference Mwangi, Oonincx and Stouten6). Generally, non-haem iron and zinc in edible insects exist in protein-bound forms to ferritin, transferrin, and other transport and storage proteins(Reference Mwangi, Oonincx and Stouten6). Non-haem iron (mainly ferritin- and holoferritin-bound) is reportedly the most abundant form in edible insects but its bioavailability is relatively unknown(Reference Mwangi, Oonincx and Stouten6,Reference Mwangi, Oonincx and Hummel7) . However, the reviewed articles reported only total iron without indicating the amount of haem v. non-haem iron. The bioaccessibility and bioavailability of vitamin B12 could be influenced by processing and the form of the vitamin in the edible insects(Reference Fedosov, Nexo and Heegaard105). This review article only reported about total vitamin B12 (cobalamin) recorded for various edible insects without indicating the amount of the inactive form (cobalamin analogues) and the active form of cobalamin. In addition, there is scant data about the bioaccessibility and bioavailability of vitamin B12 in edible insects.
Contribution of edible insects to the improvement of the human nutrition status of iron, zinc and vitamin B 12
With regard to iron, zinc and vitamin B12, there is a dearth of human nutrition intervention studies that have determined the efficacy of consuming edible insects to improve nutritional outcomes. Only a few human nutrition intervention studies(Reference Kipkoech106–Reference Bauserman, Lokangaka and Gado108) have determined the efficacy of dietary edible insect inclusion to alleviate iron deficiency anaemia. Notably, all three studies were done in children. Following a cluster-randomised controlled trial among 6 month old infants fed on a caterpillar-cereal diet for 18 months, Bauserman et al.(Reference Bauserman, Lokangaka and Gado108) reported higher haemoglobin levels and fewer anaemia cases among the test group compared with those that were fed on a control (usual) diet without caterpillars, while there were no differences in body iron stores for both groups. However, Bauserman et al.(Reference Bauserman, Lokangaka and Gado108) did not specify the particular species and quantities of caterpillars used. A study conducted by Kipkoech et al.(Reference Kipkoech106) among 3–4·5-year-old children demonstrated that a 5% substitution of maize flour with cricket powder in porridge flour resulted in an improved nutritional status similar to milk-based porridge after 6 months of daily supplementation. This indicates that edible insects could improve the iron content of complementary foods similarly to milk. Conversely, Konyole et al.(Reference Konyole, Omollo and Kinyuru107) reported that after 9 months of feeding termite (Macrotermes subhyalinus)-containing complementary foods impaired iron status and led to higher prevalence of anaemia among 6-month-old infants as compared with maize fortified with micronutrients. Notably, the edible insects considered in the three studies were mixed with other food matrices, which could have impacted bioavailability, hence the specific impact of edible insects could not be isolated, as noted by Konyole et al.(Reference Konyole, Omollo and Kinyuru107).
Limitations of this study
The existing literature on the nutritional content of edible insects is limited with respect to iron and zinc contents, while data on vitamin B12 are also scarce. To the best of our knowledge, no data are currently available on the bioavailability of vitamin B12. Research on the bioavailability of iron and zinc from edible insects in humans also appears to be limited. In addition, there are few human intervention studies investigating the efficacy of edible insects as a source of iron, zinc, and vitamin B12 to alleviate deficiencies. Finally, substantial variations in the micronutrient levels studied have been observed, which may be due to the diversity of analytical techniques by different authors that are not validated for edible insect matrices, significant errors in laboratory analysis or data processing. This ultimately highlights the lack of official analytical methods for edible insects.
Conclusions
Edible insect species generally have high contents of iron, zinc and vitamin B12 and can potentially fulfil the nutrient gaps of a vast majority of the human population. However, there is limited research into the bioavailability and efficacy of iron, zinc and vitamin B12 to prevent deficiencies in the human population. In addition, data gaps are evident for a number of edible insects considering the different possibilities for processing edible insects. Furthermore, data inaccuracies are likely to have contributed towards the existing large variations in the available data. Therefore, more research is required to determine the micronutrient content of differently processed edible insects, and critical analytical considerations, such as data quality assessment, are required for better data quality. In addition, there is a need to develop and apply standard methods of analyses for edible insect matrices.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954422425000071
Financial support
This manuscript was funded by VLIR-OUS through the Global Minds – KU Leuven doctoral scholarship programme, Belgium, in a sandwich arrangement with Makerere University, Uganda.
Competing interests
The authors declare no conflicts of interest.
Authorship
Tom Bbosa: funding acquisition, conceptualisation, data collection, data curation, and original article writing. Dorothy Nakimbugwe: funding acquisition, supervision (supporting), project administration, and manuscript review and editing. Christophe Matthys: supervision (supporting), data curation, and manuscript review and editing. Mik Van Der Borght: funding acquisition, supervision (lead), data curation, project administration, manuscript review and editing, and the corresponding author.
Ethical standards
Ethical approval was not required.









