Introduction
The Patient Innovation project is an initiative that aims to create a knowledge commons for patients and nonprofessional caregivers to share and further develop their innovative solutions to medical care–related problems through an online platform, https://patient-innovation.com. Patients and nonprofessional caregivers are the largest, and most important, group of stakeholders in the health care value chain. After all, the system exists for their benefit. Traditionally, however, they have been perceived as passive recipients of medical care, merely buying and consuming the solutions and products that “medical producers” create and provide. This perspective has influenced the development of an entire health care ecosystem that reinforces the passive position of patients and caregivers.
The assumption of passivity is highly flawed, as demonstrated by research aimed at studying innovation activity by “users” in health care and understanding the role of patients of chronic diseases (or their nonprofessional caregivers) in developing innovative solutions to help them cope with their health conditions (e.g., Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015; Oliveira and Canhão Reference Oliveira, Canhão, Lakhani and Harhoff2016). That collaborative and interdisciplinary research effort demonstrated that patients and their nonprofessional caregivers are major sources of health care product and service “user innovations” (e.g., Oliveira and von Hippel Reference Oliveira and von Hippel2011; Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015; von Hippel Reference von Hippel1988, Reference von Hippel2005).
Most of the studies of innovation activity by patients build on several decades of “user innovation” research. This research demonstrated that ordinary users, not only commercial entities and research laboratories, are an important source of innovation. In his seminal work in this area, Eric von Hippel, defined user innovators as firms and individuals who innovate to benefit from using their innovation, rather than from selling it commercially (von Hippel Reference von Hippel1988). Translating this definition to the health care domain, we define patient innovators as those who come up with novel treatments, strategies, and equipment that help them cope with their health disorders or the disorders of those to whom they give care. For years, failure to appreciate the potential importance of user innovation led to a lack of attention and support for the development and diffusion of these innovations, a situation that is only beginning to be addressed. The assumption of patient passivity may be even more firmly rooted in the health care context (Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015). Moreover, we anticipate that diffusion of patient and caregiver innovations to others who might benefit from them may pose particular problems. While patients and caregivers, especially those coping with chronic or rare diseases, are highly motivated to create solutions for their problems, for a variety of reasons many do not invest the time and resources that would be required to share their innovations with other patients. As a result, many valuable patient-generated ideas may never reach their potential to become solutions and many valuable patient innovations may disappear without a record.
Our findings of the prevalence of patient innovation (Habicht et al. Reference Habicht, Oliveira and Shcherbatiuk2012; Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015) motivated us to seek ways to help patients innovate and share their innovations more effectively. Eventually, this led us to build an online platform where patients and caregivers can share their innovations and learn about and suggest improvements to innovations shared by others. That online platform is the central piece of the Patient Innovation project. While our rationale for the Patient Innovation platform is fairly simple, setting up such a platform and governing it are far from simple. It is an ongoing endeavor, from which we are continually learning. By sharing our experience and some of what we have learned so far, we hope to encourage others to join our efforts and to develop their ways of addressing the issues related to patient innovation.
In this chapter, we begin by explaining what patient innovation is and what we know about it, based on the user innovation and medical literature. We then discuss some of the challenges that individuals are dealing with as they innovate, and why it is important to help them if we want to obtain their full social benefits. To inform that discussion, we employ a simple process model of patient innovation. Next, we discuss how platforms such as the Patient Innovation project can help innovating patients, and describe the benefits they can provide and the major obstacles to running them successfully. We end with a policy-level discussion that we hope will stimulate further action to help taking patients and caregivers to the front lines of research and development in medical care.
13.1 The Importance and Potential of Patient Innovation
13.1.1 Do Patients and Caregivers Innovate?
Studies have documented that, for some diseases, innovations by patients and caregivers have made a strong impact on medical practice; some even represent state-of-the-art technology for the relevant disease (Scherbatiuk Reference Shcherbatiuk2012). To illustrate the patient innovation phenomenon, we begin by describing a few well-known examples of patients and caregivers who have created important medical innovations by building upon their deep intimate knowledge of their health disorders and the problems associated with them.
Tal Golesworthy, a process engineer, diagnosed with Marfan syndrome in 1992, is commonly referred to as a man who fixed his heart, for developing an external aortic root support to prevent its further dilatation (Treasure and Pepper Reference Treasure and Pepper2015). Marfan syndrome is a rare inherited disorder that affects the aorta. The term labels the phenotype of several connective tissue disorders, or “fibrillopathies.” Marfan syndrome results in decreasing functionality and resilience of the aorta, and progressive aortic root enlargement. Golesworthy’s disease progressed to the point where valve replacement surgery was his only treatment option. Under the standard of care at that time, after the surgery he would have required a lifetime of anticoagulation therapy. Golesworthy considered both the surgery and forgoing treatment to be unattractive. Since he was an engineer, he decided to develop a more suitable solution for himself. He thought of the issue as a plumbing problem, and, given his engineering expertise, he believed that he could devise something to keep his aorta functioning without resorting to valve replacement surgery. He invented the external aortic root support (ExoVasc), a device designed to fit a patient’s aorta exactly and reinforce it, thus avoiding both valve replacement and the lifetime anticoagulant drug regimen. In 2004, Tal became the first patient to have an ExoVasc implanted. In 2011, the External Aortic Root Support Project was the winner in the Medical & Healthcare category of the Engineer’s Technology and Innovation Awards; in 2015, Golesworthy won one of the Patient Innovation Awards from our project. By the end of 2015, 56 patients had received the aortic support ExoVasc (Treasure and Pepper Reference Treasure and Pepper2015).
Louis Plante, an electronics engineer with cystic fibrosis, developed a low-frequency waves-radiating device that helps clean mucus from the lungs (Shcherbatiuk Reference Shcherbatiuk2012). Removing thick mucus from the lungs is a frequent necessity for cystic fibrosis sufferers. The standard approach to mucous removal involves a caregiver thumping a patient’s chest for 20 to 40 minutes multiple times per day. Plante was attending a concert, seated near a large speaker when he was forced to leave the event because of a fit of coughing. His expertise in electronics helped him understand that low-frequency vibrations from the speaker had provoked his coughing (which had the effect of removing mucous from his lungs). He developed a device that generates similar vibrations without the accompanying loud sound. After developing the device for his use, he founded a firm (Dymedso) to commercialize his solution, thus becoming a user entrepreneur (Oliveira and Canhão Reference Oliveira, Canhão, Lakhani and Harhoff2016). Plante was one of the winners of the 2015 Patient Innovation Award.
In 2006, The New England Journal of Medicine published an article (Elkins et al. Reference Elkins, Robinson and Rose2006) reporting on the benefits to cystic fibrosis sufferers of hypertonic saline therapy – inhaling sterilized salt water to clear the lungs of mucus – which has an interesting story behind it. Inhaling hypertonic saline was discovered by Australian surfer and cystic fibrosis patient Emily Haager (Peck Reference Peck2006). Haager observed that she felt much better during and after surfing. She traced the effect to her inhalation of the salty seawater. She disclosed this epiphany to her doctor, who formalized the salt therapy and reported the results in The New England Journal of Medicine.
To skeptics, these examples may seem exceptional. They also may suggest that, if they are important, patient innovations will somehow find their way into medical practice within the existing system. Our research suggests, however, that well-known examples such as these are only the tip of the iceberg. There is also substantial reason to believe that important patient and caregiver innovations will fail to make their way to wider adoption without systematic efforts to assist their diffusion.
Survey studies in the United States, Japan, Finland, and the UK suggest that approximately 0.5 percent of citizens have modified or created health-related products or services for personal use (de Jong et al. Reference de Jong, von Hippel, Gault, Kuusisto and Raasch2015; von Hippel et al. Reference von Hippel, de Jong and Flowers2012). In a study focusing on rare disease patients and their nonprofessional caregivers, whose needs tend to be underserved by the traditional medical innovation paradigm, we found a much higher occurrence of patient innovations: 8 percent of 500 survey respondents had developed innovations that were validated by two medical experts to be new to medical practice (Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015). These studies suggest that there may be large numbers of patient and caregiver innovators who are responding to pressing health-related needs by experimenting with new approaches and modifying existing solutions.
Of course, not all of these patient innovations are radical or sophisticated. (Indeed, radical innovation is rare even for well-supported professional researchers.) Even if it turns out that patient innovations are less likely to be radical than those made by professional medical researchers, that does not mean that they are insignificant. Good health is not merely the absence of disease or infirmity, but rather an overall well-being of an individual – physical, mental, and social well-being (WHO 1978). For individuals afflicted with a health disorder, radical treatments or cures thus are not the only important health care innovations.
The cumulative effect of various solutions that incrementally contribute to well-being can be extremely important. For those in need, a solution that alleviates the pressure of a disease or solves a practical problem is well worth having/using, be it simple or sophisticated.
13.1.2 Why Do Patients and Caregivers Innovate?
User innovation research has identified several factors driving users to innovate. One of the most important is “sticky information.” Users and manufacturers have different types of information that influence the types of innovations they produce. Users have accurate and detailed information about their day-to-day needs and experiences. As a consequence, they – the users – often tend to develop innovations that are functionally novel, which tend to require a great deal of user-generated need information and context of use information for their development (Riggs and von Hippel Reference Riggs and von Hippel1994; Ogawa Reference Ogawa1998). Commercial entities have detailed information about the needs of typical or average users, certain types of technical expertise, and expertise in marketing and diffusing profitable innovations. Synergies between innovative users and commercial entities are not uncommon, and producers sometimes even pick up user-developed solutions, improve them, and take them to the market (von Hippel Reference von Hippel2010). These synergies tend to be sporadic, however, and the academic literature in management offers plenty of evidence of under-diffusion of impactful solutions developed by users (Baldwin et al. Reference Baldwin, Hienerth and von Hippel2006; von Hippel et al. Reference von Hippel, DeMonaco and de Jong2017).
Patient innovation and innovation by health care professionals may also be expected to proceed along different, but complementary paths because of the different information and expertise available to each, which is determined in part by the way in which the health care system operates. A typical encounter of a health professional – a medical doctor, for example – with a patient afflicted with a health disorder is of a short duration. The doctor usually considers an individual’s medical history, information that the patient communicates about the reasons for the visit, and various other standard health indicators and bases treatment on that information. The doctor’s goal is to identify the problem that brought the patient into the office and to recommend a solution to remove the problem (cure) or to manage it in a way that will minimize its impact. The appointment ends, the doctor moves on to the next case, and the patient is left to cope with any remaining problem(s). Doctors necessarily must address most of their attention to the most urgent issues confronting their patients. Nonetheless, problems related to independent functioning on a daily basis, social integration, and maintaining long-term functionality may have a significant impact on quality of life and individual well-being. Moreover, though a health disorder may manifest itself differently from case to case, and some patients may have idiosyncratic needs related to the disorder, doctors are unavoidably limited, in the short time available, in their ability to ferret out and address all but the most pressing of these idiosyncrasies. Patients also do not seem to believe that only their doctors appropriately address certain kinds of problems. Survey results from the Pew Internet & American Life project suggest that patients rely upon different sources to address different needs: health professionals are more important for accurate diagnosis, drugs prescriptions, and treatments, while peers, family, and friends are the dominant sources for finding solutions to cope with their diseases on a daily basis, and for emotional support – the peer network is especially important for people with chronic conditions (Fox Reference Fox2011).
The paths of user innovation and seller innovation also may diverge because users and sellers have different motivations for innovating. In particular, commercial firms cannot be expected to invest in innovations that are not expected to produce significant profits. The mismatch of incentives can be acute in the medical arena. Consider the situation for those afflicted with one of more than 7000 rare diseases, whose sufferers constitute “orphan” markets. This designation means that patients can expect little help from producers in the form of specialized products to help them cope with their diseases, as the market incentives are perceived as too low to motivate vibrant research and development activity from commercial entities. Because many of these rare diseases are chronic and progressive, and particularly in light of the lack of commercial incentives, these patients and their caregivers have particularly strong incentives to develop their solutions – or to discover and adopt solutions developed by peers – to help them cope with their diseases and improve their daily lives. Consistent with this observation, our initial studies show an approximately 10 times higher rate of health related innovation among patients afflicted with rare diseases and their caregivers than among the public at large (Zejnilovic et al. Reference Zejnilovic, Canhão and Oliveira2016).
Our study of 500 patients with rare disease and caregivers provides further evidence of a correlation between innovative activity and patient/caregiver perception of need even among rare disease sufferers. The study also suggests, as have other user innovation studies, that innovative activity increases with level of education (Oliveira et al. Reference Oliveira, Zejnilovic, Canhao and von Hippel2015). Moreover, our data suggests that there may be an important subjective component to the perception of need and that it is an individual’s subjective perception of need that drives innovative activity. Additional insight into this possible subjective factor comes from an epidemiological study of a representative sample of adult Portuguese (Branco, Rodrigues, Gouveia et al. Reference Branco, Rodrigues and Gouveia2016). In that survey study, individuals were asked to assess their general state of health. Some individuals were known to have multiple, clinically confirmed health disorders; nonetheless, they indicated that their health was very good, while others, who did not report any significant physical limitations or illnesses, reported mediocre general health status. While more research is needed to explore the factors driving patient innovation, it may be that individual perceptions of the severity of the need, the likelihood that it will be addressed by others, and their capability to address it all jointly influence the likelihood that a patient or caregiver will innovate.
In any event, though many questions about the extent and characteristics of patient innovation remain, the available evidence leads us to believe that a substantial amount of patient and caregiver innovation occurs. Unfortunately, there is also reason to believe that only a small fraction of patent innovations are ever shared with other patients and caregivers, adopted by physicians, or picked up by commercial providers. Given the fact that, globally, many individuals are innovating to cope with their health disorders, we believe it is important to consider how best to support patient innovation so that it will be as effective, safe, and socially beneficial as possible.
In the next two sections, we discuss the challenges and opportunities of patient innovation, from individual-level and platform-level perspectives and how the Patient Innovation project seeks to support it.
13.1.3 An Individual-Level Model of Patient Innovation
To gain a better understanding of the innovation activity of patients and caregivers and identify ways to improve it, we model it with the Patient Innovation value chain shown in Figure 13.1. The underlying goal of this value chain is that the drivers of patient innovation will lead to the development of new treatments, therapies, and medical devices, which should be validated for safety and efficacy, and then diffused more broadly. Ideally, the economic and social impact of the output of the value chain should then be measured and evaluated. The integration of patient innovation into health care innovation represents a paradigm shift. While this shift is already happening, no studies yet assess its economic and social impact.

Figure 13.1 The Patient Innovation value chain.
The Patient Innovation value chain starts with an identified need – a driver of patient innovation – that prompts the innovation itself. The process of patient innovation is easy to imagine, but it does not proceed according to the kind of structured process often implemented by commercial firms. The process of user innovation is likely to be an unstructured, iterative decision-making sequence, similar to the model proposed by Mintzberg et al. (Reference Mintzberg, Raisinghani and Theoret1976), involving experiential learning, serendipitous jumps over the stages, and events that originate from the environment in which the users are embedded and which influence the process. Nonetheless, when considering how best to intervene in the Patient Innovation value chain, it will be helpful to devise an easy to interpret process model to help guide our interventions.
To develop our model, we conducted a qualitative study in Portugal, by interviewing 15 patients afflicted with chronic diseases or lesions (Oliveira Reference Oliveira2014). From these 15 interviews, we identified 30 cases of patient innovation, adoption, or passive behavior by patients. Our analysis, grounded in the existing literature on user innovation and individual decision making, yielded a simple stylized develop-or-adopt process model of patient innovation (Figure 13.2). In Figure 13.2 we also show, in parallel, how this model compares to two other representations of the innovation process, the stages of development of a user innovation (Lüthje et al. Reference Lüthje, Herstatt and von Hippel2005), and a decision-making model – the trichotomy identification-development-selection model (Mintzberg et al. Reference Mintzberg, Raisinghani and Theoret1976).
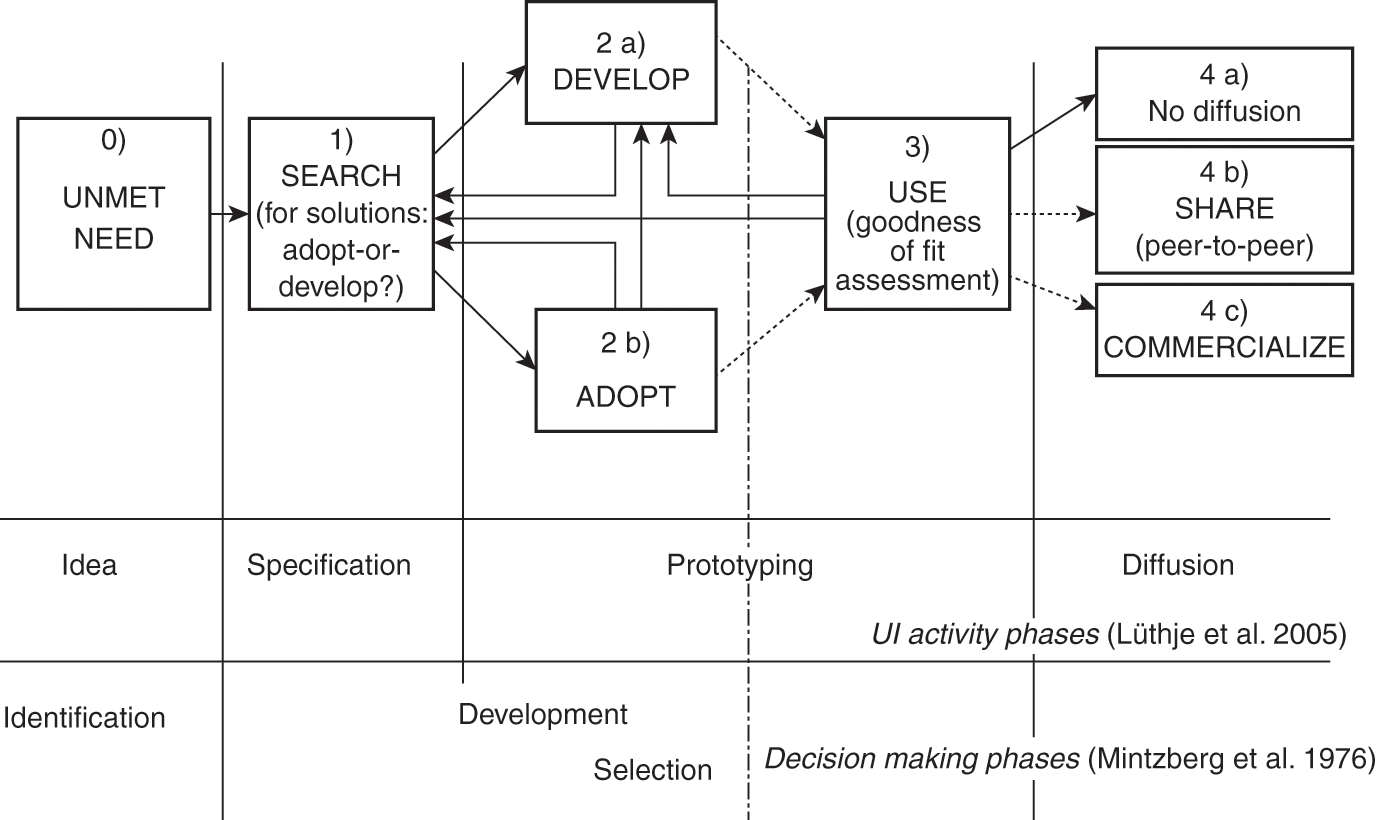
Figure 13.2 A stylized model of a patient’s adopt-or-develop process with corresponding phases of user innovation activity and decision-making processes. The arrows indicate potential paths of problem solving – the transitions from one stage to another (UI - User Innovation).
In this representation, the patient’s problem-solving activity is a dynamic, iterative process that ends in either adoption or innovation. The linear representation of the stages is not meant to imply that every problem-solving activity by patients passes through all the steps in the order shown, as problem-solving is inherently an unstructured process embedded in a dynamic stochastic environment (Cooper Reference Cooper2008; Langley et al. Reference Langley, Mintzberg, Pitcher, Posada and Saint-Macary1995). Sometimes, for example, a patient may simply develop a solution, without any search. This simple model nonetheless provides a useful structure within which potential interventions – aimed at helping patients make better decisions and obtain better outcomes from their innovation activity – may be considered. We will use this model to also describe our intervention, the Patient Innovation platform. However, before we move to the platform-related discussion, let us briefly describe the phases of the model, supporting them with selected interview excerpts.
13.1.3.1 Stage 0: Unmet need
As any problem-solving process starts with the identification of unmet need, this is the initial stage of the patient innovation develop-or-adopt process. There are two ways for individuals to become aware of a need: (1) through experiential learning – a form of learning-by-doing/using where a patient observes an unmet need and then attempts to formulate the problem informally (von Hippel Reference von Hippel2005) or (2) by observing a solution simultaneously with identifying a need (von Hippel and von Krogh 2013). In 8 out of the 30 cases we analyzed, upon observing a problem, the patients immediately came up with a solution and pursued its development. A need may also be recognized by observing a solution (von Hippel and von Krogh Reference von Hippel and von Krogh2015), and this was the case in 3 out of the 30 cases we analyzed; patients saw a solution and then realized that the solution was a match for a latent need or for a problem they had attempted to solve in the past:
As I was watching TV, I saw a university professor talking about a software he developed to control computers with eye movements. He developed the software to help his wife in her work with people afflicted with cerebral paralysis. The software was free to use, and I thought about my difficulties to use computers. So, I gave it a try.
In this example, the patient was aware of difficulties when using a solution, but the need was latent as he was not actively pursuing or searching for a solution. Hearing about the software, the patient’s latent need got activated and by adopting the solution, his quality of life has been improved. One takeaway from this example is that increasing the availability and salience of solutions may help people in unanticipated ways.
13.1.3.2 Stage 1: Search for information and solutions
If a solution does not immediately present itself upon recognition of unmet need, the next step is often to undertake a search for information and solutions. Real-life decisions are complex, and in response to a problem, people often do not search for the best possible solution, but for one that satisfices – fixes the problem to an extent that is satisfactory for their needs (Simon Reference Simon1977). Descriptive models of decision making include a search for solution alternatives as part of a development phase. In decision-making procedures, the relevant subroutine or procedure (Mintzberg et al. Reference Mintzberg, Raisinghani and Theoret1976) employs an algorithm to develop alternatives that are subjected to a cost-benefit analysis against the previous best solution (Newell and Simon Reference Newell and Simon1972). Having identified an unmet need, patient innovators may engage in a similar, though usually less formal, search process. The dynamics of the search for health-related information change as patients and caregivers gain experience about the health condition of interest:
When she was diagnosed, I sought for support and information about epidermolysis bullosa. However, it is a rare disease, and with so few patients like her, it was difficult to find help. I searched everywhere, online fora, international associations, journals, pharmaceutical companies. I do it nowadays as well, but more to learn about a specific thing or to find new solutions. [Apart from innovating] I also adopted a solution developed by another patient, and I found it on an international online forum – to add table salt to bathing water, as skin contact with pH neutral or isotonic solution lessens the sting. I also experimented with sea salt instead of table salt, and it works even better for my daughter.
In the beginning, I would search a lot for information about my condition, whereas nowadays I only search for specific information or solutions, when I feel it is needed. Usually, I search online (e.g., YouTube or scientific papers), but also, I ask other patients with spinal cord injuries and doctors.
In the early stages, when an ailment is contracted, patients and caregivers search mostly for information about the nature of their ailment and the prospects of living with it. As they accumulate knowledge about their health condition and learn how to function with it on a daily basis, patients tend to engage more intensively in searching for specific solutions to their unmet needs.
It was obvious from our interviews that there is a stark contrast between active and passive patients. Active patients detailed about search behavior, solution development, tinkering, and adoption. Passive patients demonstrated strongly reliance on doctors only:
I do not search for health-related information. All that I need, I get from health professionals in the rehabilitation center.
We also asked the interviewees for their opinions about solutions that patients develop for themselves. We wondered whether patients’ lack of confidence in their expertise would lead them to eschew solutions developed by other patients. The non-active patients we interviewed (such as the one from interview L) were not opposed – in principle – to the idea of adopting a solution developed by a patient. Rather, they expressed no need for such solutions, finding their existing medical care sufficient.
Not all patients who identify an unmet need begin their searches for solutions. In what is referred to as the “community push,” patients may instead share the problem with others, who may then suggest solutions they know about or are using. We identified two such cases, which we elaborate upon in our discussion of the diffusion phase, since responding to inquiries from other patients is one mechanism for diffusing innovations.
13.1.3.3 Stages 2 and 3: Develop-or-adopt and then use a solution, an iterative trial-and-error process
The stage 1 search for a potentially satisfactory solution ends with a decision to adopt or develop a possible solution. This initiates a solution development (stage 2a), or acquisition of an existing solution (stage 2b). After obtaining the firsthand experience using the solution (stage 3), or even during the solution acquisition, the goodness-of-fit of the solution to the need is assessed (stage 3) based on the patient’s personal cost-benefit assessment (e.g., Damanpour Reference Damanpour1991; Rogers Reference Rogers2003). There is no guarantee that the outcome will be a satisfactory need-solution pair. In cases of mismatch, the patient may return to stage 2a for further development or to stage 1 for further search. Searching for a match is often a dynamic, iterative, trial-and-error process, both for solutions developed independently by the patient innovator or adopted from preexisting technology:
Every time we encounter a new problem, we start by searching online to see if there is a solution, and we consider if we can come up with something by ourselves.
… we were desperately trying to find a solution … something that would help KK to hold the dish when he places a spoon or a fork in it, or … in whatever he is eating. We searched everywhere and tried several things. For example, we tried available silicone adherent mats, but they were too big and did not hold well. However, once, when we bought a big bowl in a shop, I realized that it had a small silicone adherent mat inside, to hold the bowl, and it came to my mind that I should give it a try with KK … we used it and it worked so well for him. It holds whatever he is eating, he can eat by himself, and it is small, so he is OK to use it at school too (it is discrete). He now uses it for every meal.
The guys in Brazil shared everything with me for free, about their surfboard, the chair attached to it, and how to make it. However, making it was a lengthy and costly process. It was even more difficult as their solution was custom made, and I decided that it was better to make a general purpose solution, something that anyone can use.
If patients are not satisfied with the cost-benefit performance of potential solutions, they can conduct a new stage 1 search or return to stage 2 to refine or modify the solutions they have developed or adopted. In 8 of the 30 cases we analyzed, patients returned to stage 1 search after being dissatisfied with a potential solution, while in 11 out of 30 cases patients engaged in iterative refining or modifying the adopted or developed solutions. Patients, who initially attempted to solve their problems by adopting an available solution (stage 2b) uncovered during their search, may recognize the need to develop their innovative solution (shifting to stage 2a). This usually happens after a trial use of a recommended available solution, finding it to be inadequate:
They [the doctors] gave us the orthopedic cast for my son’s functional arm, but it was heavy, not practical, and did not quite do what we expected … I realized that I could just sew the sleeves on the functional arm, and that worked much better.
Rosenberg (Reference Rosenberg and Rosenberg1982) describes the trial-and-error process that we represent as iteration among stages 1, 2, and 3 as “learning-by-using.” The accumulation of use experiences creates tacit knowledge, or what von Hippel (Reference von Hippel1994) refers to as sticky information. The cases we encountered show that users, based on their sticky information, may come up with more effective solutions than their commercial counterparts (Morrison et al. Reference Morrison, Roberts and von Hippel2000; von Hippel Reference von Hippel2010).
13.1.3.4 Stage 4: Diffusion of patient-developed solutions
Most patient innovations are potentially valuable to other, similarly situated patients and caregivers. Thus, the full social value of innovation is obtained only if it is shared with and used by other patients. Making solutions publicly available allows others to find solutions to problems they are attempting to solve (contributing to stage 1) and for need-solution pairs to arise (contributing to stage 0). In 13 out of the 22 cases that involved a developed or adopted solution, the patients made efforts to diffuse further the solutions. In diffusion efforts, solution developers may share information in a relatively passive way, such as by making an online post or preparing other types of written information. They can also actively suggest the use of a solution, a “market-push” approach, in which they bring the solution to the attention of people with whom they interact. We identified two such market-push cases. In such cases, we found that those who suggested a potential solution to a particular individual also provided help to reduce the solution’s acquisition cost. The diffusion channel in these examples was a two-way interaction between peers, suggesting that social interactions with peers may increase the likelihood of adoption of patient-developed solutions.
At times, particularly when there is uncertainty about the safety and effectiveness of a potential solution, the patients may also involve medical doctors in the innovation process:
We (the parents) exchange information about drugs, and the experiences with different combinations of drugs, what worked and for what. We experiment with different combinations of drugs to see which one works better. I did it at least once for my kid. First, I informed myself about a combination of drugs that other parents suggested. Then I went to my doctor and explained what I found, the reasons why I need it, and the doctor was enthusiastic and supportive about it. Therefore, I tried it, and it worked.
This example demonstrates the importance of the doctor-patient relationship. Namely, hazardous solutions can be identified with higher confidence if they are openly discussed with experienced professionals, and not solely within the community of patients.
Although most of the innovators in our sample made some efforts to share their solutions – after all, this is how we found them – in 2 out of 13 cases of developed solutions, the developers did not share their solutions, even though they were well connected to other patients and medical professionals. The patient innovators reported either that they had no time to engage in diffusion or simply that they did not share their solutions because no one asked. These observations are in line with the general arguments about potential market failure in the diffusion of user innovations presented by de Jong et al. (Reference de Jong, von Hippel, Gault, Kuusisto and Raasch2015). It was very rational for some innovators not to share information about their solutions with others – after solving their problems they had no interest in investing time or money in diffusing the solutions. Where diffusion of a valuable solution does not occur, another patient or caregiver looking for a solution to a similar problem must replicate the whole development process. That replication is a waste of resources, and the patients may or may not succeed in finding or developing a solution. The cases of non-diffusion that we analyzed demonstrate that failure to diffuse may occur even when developers would be willing to share them for free – when patient innovators find sharing too burdensome or are unaware that others might be interested in knowing about them. The cases thus suggest that actively soliciting solutions from patients and educating them about the value of sharing the outcomes of their creative activity, as well as creating the infrastructure to reduce the costs of sharing, may be important to correct this market failure.
The model presented here provides an overview of the patient innovation process from the perspective of the individual innovator. In our design, implementation, and ongoing efforts to improve the Patient Innovation platform, we use this model to help us identify ways to move forward – in other words, to learn how an online platform can be used to address some of the challenges patients encounter in the process and to contribute to the development of patient innovation on a larger scale.
13.2 The Patient Innovation Online Platform: Infrastructure for a Knowledge Commons
Next, we discuss how the Patient Innovation platform can help overcome some of the barriers to successful patient innovation and diffusion of those innovations by providing the infrastructure for a knowledge commons.
13.2.1 The Landscape of Health Care–Related Online Platforms
Health care systems are under serious pressure to deliver good medical care despite capacity and budget constraints. Total spending on health accounted for 9.5 percent of GDP in the Organisation for Economic Co-operation and Development countries in 2012, and the cost is still rapidly rising, particularly because the population is aging and, relatedly, because more people are living longer with chronic diseases. Chronic diseases are the most common cause of death globally, accounting for 75 percent of direct health care costs in the United States, and taking a significant toll on the quality of life of the elderly (Thrall Reference Thrall2005; Vos et al. Reference Vos, Flaxman, Naghavi and Lozano2013). There is a common understanding that novel solutions and business models are necessary to help overcome constraints, contain costs, and unlock new opportunities. Technology, and especially digital platform-based business models, may be an important contributor to better and more cost-effective health care.
In the Merriam-Webster dictionary, a platform is defined as “a place or opportunity for communicating ideas and information.” A “digital platform” is a set of components including a core, upon which third parties can build (Parker and Van Alstyne Reference Parker and Van Alstyne2010). The value of a digital platform is characterized by network effects, meaning that it becomes more valuable when more third parties use and contribute to it. Popular examples of digital platforms include hardware and software environments such as Google Play Store, as well as websites where sellers and buyers are matched, such as Amazon, eBay, or Uber, which matches available amateur taxi drivers with people who need a ride. The Internet itself is a platform – an infrastructural platform. It enables the development of efficient, sustainable online resources that individuals can use to create and exchange knowledge, research pressing questions, communicate with one another, and even act upon their ideas.
In the health care arena, patients have been empowered by the resources available on the Internet to assume more responsibility for their medical care. Greater involvement of patients in managing their health may also decrease the burden on the health care system (Hibbard et al. Reference Hibbard, Stockard, Mahoney and Tusler2004). In recent years, patient organizations, providers, commercial entities, and nonprofit organizations have developed a variety of online communities and virtual fora where patients can not only discuss their health concerns but also capture, record, and exchange valuable health-related information. In a similar vein, the US Food and Drug Administration made an additional effort to bring closer to patients their existing platform for reporting adverse effects of medical devices and drugs, MedWatch (FDA 2013). It introduced an easy-to-understand report form that can be downloaded from MedWatch website, and a way to submit the report online, aiming at consumers with little medical knowledge. Other interesting examples of online fora include the Braintalk community for neurological diseases and Building User Involvement in Motor Neuron Disease (BUILD) for amyotrophic lateral sclerosis (ALS). Going beyond communication and knowledge exchange, pharmaceutical companies are also keen to organize virtual clinical trials, in which the data collected is self-reported by patients (Roehr Reference Roehr2011).
Major technology companies, such as Apple, Google, IBM, Microsoft, and Samsung, also have stepped in to offer health care–related initiatives. Their strategies include open source frameworks for creating health-related software applications, providing medical data storage and analysis, and processing large amounts of health-related scientific data. Connecting users to report and share health data is also a business model for platform-based companies, which are harnessing the increasing availability of Internet connectivity to assist patients in finding and interacting with other people suffering from similar conditions. For example, PatientsLikeMe is a for-profit health data-sharing platform that aggregates patient-contributed health data to perform observational studies. PatientsLikeMe has more than 150,000 active users sharing data on more than 1000 health disorders. A study performed using this platform disproved a previous scientific paper regarding the effects of lithium carbonate in amyotrophic lateral sclerosis (ALS), by systematically collecting and analyzing self-experimentation information provided by patients (Wicks et al. Reference Wicks, Vaughan, Massagli and Heywood2011). Genomera, another online project, is “crowd-sourcing health discovery by helping anyone create group health studies” (Genomera 2013). These new platforms seek to enable patients, caregivers, and others whose input to medical research previously has been limited to a passive role. They create and enable communities that can help in coming up with suitable designs for testing hypotheses or answering questions by connecting knowledge but also locating peers willing to serve as testers, implementing the test design and sharing their symptoms and outcomes. These existing efforts demonstrate the potential for the Internet and related technology to enable the engagement of patients and patient associations, nonprofessional caregivers, health professionals, and even medical doctors who have, so far, been considerably excluded from the health innovation process.
13.2.2 The Patient Innovation Platform
The Patient Innovation initiative differs from prior online health platforms in that it aims to recognize and enhance the capacity of users – patients, caregivers, and collaborators with complementary skills who are intrinsically motivated to help people in need – to contribute to innovation in medical care. To that end, we developed and deployed the Patient Innovation platform, https://patient-innovation.com, a non-profit digital platform designed to provide infrastructure to support a knowledge commons for identifying, sharing, commenting on, improving, and diffusing the work of these medical user innovators. The Patient Innovation platform is international, multilingual, open, and free for use by patients and caregivers dealing with any disease. The platform was launched in February 2014 and relies on partnerships with numerous patient associations and medical research centers in the five continents to reach international audiences.
To increase public awareness of the potential benefits of patient innovation, we established the Patient Innovation Award. Each year we invite patient innovators to submit their best innovations to the competition. A jury composed of six highly reputable scientists reviews the proposed solutions and selects the innovator of the year in three categories – patient, caregiver, and collaborator. The awards are presented at a ceremony, which provides an opportunity to raise awareness of the Patient Innovation project. At the same time, this is an excellent occasion to gather individual patients, patient associations, health professionals, innovation experts, entrepreneurs, media, companies, and health authorities to discuss patient and caregiver innovation.
The Patient Innovation platform design invites patients and caregivers to submit their self-developed solutions as video files, sets of pictures, textual narratives, or a combination of these media. Other members of the network are free to evaluate each proposed solution, make their judgments about its applicability, safety, and to which problems it could usefully be applied. Also, members may engage in a co-creation process and upload modified versions of others’ solutions, so that the path of development can be traced by users of the site. Tagging mechanisms and advanced search capability allow efficient search by patients and interested parties and may enable cross-pollinating of solutions across health disorders. The platform has the potential to become a unique repository of open knowledge, created and co-developed in a patient-to-patient trusted environment, about innovations to enhance the health and quality of life of chronic disease patients.
The Patient Innovation platform is intended to enable patients to assume more responsibility for the collaborative development and diffusion of their innovations, and to allow patients to contribute more knowledgeably to medical care. To further that goal, we designed the Patient Innovation platform to mesh strategically with the stages of patient innovation mapped out in the process model presented in Figure 13.2. The relationships between the design of the Patient Innovation platform and the stage of innovation activity are summarized in Table 13.1.
Table 13.1 Stages of innovation activity by patients and the associated challenges and opportunities for the Patient Innovation platform
| Stage | The function provided by patient innovation | The expected improvements in the patient innovation process |
|---|---|---|
| UNMET NEED | Solution tagging and search by tags: disease, symptom, location, and activity tags | Faster identification of potential problems/solution |
| Need identification | ||
| SEARCH | Faster and more successful search for a solution | |
| Search for solutions | Spillover of knowledge from different diseases/solution categories; e.g., solutions for digestive problems as a primary health disorder can serve to those who have it as a side effect of a cancer treatment | |
| ADOPT | Safeguard mechanism for obviously dangerous solutions | More solutions/versions to choose from |
| Adoption | Descriptions of the solutions: textual, audio, and visual | Low investment in the acquisition of a solution |
| Social networking feature: commenting | ||
| DEVELOP | Comparison with the existing solutions, and incremental buildups | |
| Development | ||
| USE | Reviewing and updating use-related information /experiences from the users | Collection of evidence about solutions |
| Goodness-of-fit assessment | ||
| DIFFUSE | Free-of-charge and easy to use/post solutions platform | Higher rate of diffusion of patient-developed solutions |
| Sharing and/or commercializing | Promotion |
We see Patient Innovation and similar initiatives as useful efforts to bring health care closer to the patients, to enhance the participation of patients in the management of their health, to incorporate the patients’ and caregivers’ valuable use-based knowledge into the improvement of medical care, and to provide conduits for practical learning about how to improve struggling health care systems on a global scale. Because such initiatives involve humans and their well-being, they are embedded in complex legal, regulatory, and social environments. In such a landscape, new approaches to medical innovation may be in some tension with existing legal and social norms. We believe that such tensions, where they arise, should not discourage us from seeking means to incorporate patients and caregivers into the medical innovation ecosystem. Rather, they highlight the need to adapt health care systems and public policies to provide a meaningful framework for integrating patients and caregivers (and their important use-based local knowledge) more fully in the development of safe and effective health care solutions.
13.2.3 Challenges and opportunities in developing the Patient Innovation platform
We encountered (and continue to encounter) many challenges in designing and maintaining the Patient Innovation platform. Here, we discuss a few of the most significant challenges, and our response to overcome them. The most basic challenge for any digital-platform business is the creation of a community of users. The community supplies the content, without which the platform has no value. In the case of the Patient Innovation platform, the content consists of information about health-related patient innovations and the record of improvements to these solutions made by other patients. The community also plays the role as the demand side of the Patient Innovation platform. Those with health disorders may use the platform to learn about potential solutions to their needs by browsing descriptions of existing solutions developed by their peers. As they browse, community members can provide new content, feedback, and validation of usefulness (or not) of the posted solutions. As more community members participate as content providers or users of the content provided by a platform, the community becomes stronger, and the platform that supports the community becomes more valuable.
We engaged in developing the Patient Innovation platform in the face of substantial evidence that users – and in this case, the patients, caregivers, and collaborators – often do not have strong enough incentives to share their content, given the time, effort, and resources that sharing requires. By creating a specialized venue for sharing peer-developed solutions, Patient Innovation lowers the barriers to diffusing patient innovations. An equally important role of the platform is to increase awareness about the potential importance of the patient innovation phenomenon, thereby bolstering patient innovators’ intrinsic motivations to help others by sharing their innovations. During Patient Innovation’s three years of operation so far, more than 700 solutions have been posted on the platform.
When we began developing the Patient Innovation platform, we had to make decisions about the governance of the platform. We desired to maintain as much openness as possible in light of our overall goals. We also hoped to create a sustainable knowledge commons based on the platform. Sustainability is dependent on maintaining a vibrant community and also on the mechanisms for funding and supporting the platform itself. We believed that sustainability would be enhanced by aligning the intended identity of the community with the mechanisms used to fund its development and maintenance The goal of sustainability thus had implications for our decisions about the platform provider, its legal structure, and the platform sponsors. We believed that a commons based on our fundamental commitment to openness and free peer-to-peer sharing would only be viable in an environment of trust. To encourage a trusting environment, we decided to structure Patient Innovation as a not-for-profit organization and to fund platform development and maintenance only through research grants and donations. Maintaining trust also requires that we keep the commons governance free of conflicts of interest, by working only with partners who share the same values and are dedicated to and invest efforts in better empowering patients within the health care system.
We also confronted decisions about the type and degree of openness to employ in governing the Patient Innovation platform. The openness of a platform (and the knowledge commons it supports) is multidimensional. For Patient Innovation, decisions had to be made about who could contribute content, what types of content could be contributed, and which contributed content would be displayed. (It went without saying that the content of the platform would be available for anyone to explore and use.) In line with our primary goal of promoting better medical care, we imposed some limitations along various dimensions of openness.
Patient Innovation is completely open about who can contribute, but we had to decide whether to allow anonymous participation or to employ some means of authenticating the identities of those who post on the site. There are benefits and weaknesses to authenticating participants in a virtual community versus allowing anonymous participation, including questions about the quality and value of anonymous peer-review feedback mechanisms. Trust among those who create and use the content of the knowledge commons supported by the Patient Innovation platform is also important for sustainability. Inappropriate or otherwise deleterious posts could easily erode this trust. User authentication is one means of discouraging disruptive (and, in this case, potentially dangerous) contributor behavior and incentivizing higher-quality participation. However, we did not want to discourage patients from participating, even if they preferred to remain anonymous. We thus decided to permit anonymous contributions, while incorporating other mechanisms of quality assurance to address our concerns about sustainability, trust, and safety.
We address the issue of quality assurance primarily by imposing some limits on what types of content can be posted and which particular contributed content is displayed. Like many other online communities, we employ community ratings and feedback to identify disruptive or otherwise inappropriate contributions. The medical context raises additional quality control concerns, and specifically related to safety, that we feared might not be adequately addressed by relying only on community feedback. We employ two additional mechanisms to alleviate these concerns. First, because of the particular difficulty in assessing the safety and efficacy of drugs and other ingestible treatments, Patient Innovation’s terms of service simply forbid the posting of solutions involving drugs or other ingestible products. Second, each post is reviewed by a medical professional before it becomes visible on the site. This review ensures that contributions comply with the terms of service and satisfy the basic requirements of safety. The involvement of medical professionals in reviewing posts serves the additional purpose of beginning to bridge the gap between patient innovation and the medical professions. Bridging this gap is a worthwhile goal, given the important role that medical professionals play in validating and diffusing medical innovations.
Another dimension of openness relates to the potential uses of the content available on the site. One way to enhance the value of the content pooled in a knowledge commons is to permit third parties to design tools that interface with that content. A pertinent example of such a tool might be an application that allows users to report their health indicators before and after using a specific solution posted on the platform. Patient Innovation is open so that third parties can create tools that interface with the platform to enable collection and sharing of evidence about the effectiveness of the solutions. Our decision to make the platform open along this dimension was guided by our commitment to advancing paradigm change in health care in the direction of distributed innovation and patient-centric medical care.
Validating the safety and efficacy of patient innovations remains a major challenge, and we continue to consider ways in which a platform such as Patient Innovation might contribute to that task. The usual path for bringing medical innovations to the market requires clinical trials, defined as sets of tests that generate data on the safety and efficacy of health interventions. The merits of the clinical trial procedure are undoubted, but traditional clinical trials have serious drawbacks as well (e.g., Appel Reference Appel2006). One major issue is the delay in patient access to newly developed treatments caused by regulatory constraints and clinical trial practices. Another related concern stems from the fact that safety and efficacy questions are most easily answered when a disease is common, and the outcome of interest has a high chance of occurring. In the case of rare diseases, the challenges are great, as trials of sufficient size are often impossible because of the difficulty of recruiting a sufficiently large sample of patients with a rare disease of interest (Hayes Reference Hayes2012).
This discussion suggests a need for developing new methods and more pragmatic practices for validating the safety and efficacy of at least some types of medical innovations, including (1) treatments, therapies, or medical devices developed by patients, who lack incentives to diffuse their innovations and do not have access to the resources needed to perform standard clinical trials and whose innovations may be tailored to circumstances encountered by relatively small numbers of patients and (2) innovations aimed at rare diseases, for which it is very hard to apply common methods of clinical trials for uncommon medical conditions.
Experiences with the complementary and alternative medicine (Ernst Reference Ernst2000) market may be informative. Even for complementary medicine, where out-of-pocket payments generated are measured in billions of dollars annually (Dodds et al. Reference Dodds, Bulmer and Murphy2014; Harris et al. Reference Harris, Cooper, Relton and Thomas2012; Xue et al. Reference Xue, Zhang, Lin, Da Costa and Story2007), no satisfactory mechanism ensures that adequate efficiency and safety evidence is made available to consumers (Harvey Reference Harvey2012). Instead, patients often rely on anecdotes provided by laypeople, peers, or relatives or acquired over the Internet in deciding to try a health-related solution, despite the fact that these stories do not represent reliable evidence and the solution has not been endorsed by any expert authority (Harvey Reference Harvey2012; Williamson et al. Reference Williamson, Tudball, Toms, Garden and Grunseit2008). The safety problem is perhaps even more complex regarding solutions developed for personal use by patients which, if they diffuse at all, usually spread for free through informal peer-to-peer networks. The existing literature and practices do not offer reliable answers to the question of how to validate the safety and efficacy of patient-developed solutions.
In light of this situation, we hope to extend the role of the Patient Innovation community to include both the roles of innovator and tester of the self-developed innovations. We are exploring innovative ways of collecting safety and efficacy data that might create new opportunities for validating new treatments, therapies, or medical devices. We are currently considering two possible approaches: the idea of a “toolkit” for patient self-management of chronic medical conditions and the potential to implement observational trials of innovative treatments using our platform.
User innovation toolkits are integrated sets of specialized tools that enable end users of a product or service to develop or modify products for themselves (von Hippel Reference von Hippel2005). Shifting a problem-solving task to users via a toolkit lowers costs when users possess the information required to solve the problem in raw form and when it is less expensive to transfer the tools needed to process that information to the user than it is to transfer the user’s information to an expert.
This same economic logic may apply to medical toolkits devised for specific purposes. Here we consider the possibility of toolkits for patient self-management of chronic medical conditions. Such toolkits would offer patients the tools needed to process their personal “raw” disease-related data into information that helps them manage their illnesses. The toolkit would give patients the information needed to (1) appropriately recognize when to apply a prescribed or over-the-counter medical treatment, (2) apply the treatment, (3) receive feedback on the results of the treatment, and (4) make appropriate treatment adjustments without the involvement of a health care specialist (DeMonaco et al. Reference DeMonaco, Ali and Von Hippel2006). We are currently working on developing toolkits for allowing patients of some selected chronic diseases to test their self-developed treatments, therapies, or medical devices. Such toolkits, interfacing with the Patient Innovation platform, might help overcome the difficulties faced by clinical trials of rare disease treatments: the need to “recruit” patients spread across different areas and follow them for a longer period than typically needed for clinical trials aimed at more common diseases. To enable better collection of data, we aim to attract developers of cross-platform (Android, iOS, Windows) mobile applications, which would be integrated with the Patient Innovation solution-sharing platform, for patients to record vital health indicators and associate the recordings with the solutions shared in the platform. Shifting the validation of user-developed treatments, therapies, or medical devices to patients spread across different areas, using a toolkit for data collection, would significantly lower the costs of generating data on the safety and efficacy of the innovation.
Simply by providing an infrastructure for reporting patient innovations and centralizing information about patient innovations in an easily accessible location, Patient Innovation lowers barriers to the diffusion of innovation. The Patient Innovation platform also lowers the barriers to diffusion because it facilitates the patient-initiated search for solutions. Health needs often are heterogeneous, even among different patients suffering from the same disease. Patients and caregivers know their circumstances and needs better than anyone else and are well aware of when their needs are addressed by conventional health care and health systems and when they must adopt or discover solutions to fill in the gaps. By using an open platform, Patient Innovation helps diffuse solutions proposed by patients and caregivers, after careful screening for compliance with our terms of service. We are currently exploring ways to develop an ecosystem, which would enable even wider diffusion of solutions that have been proven to be of clinical efficacy.
Conclusions
The global sharing of innovative solutions and treatments developed by patients and their caregivers can potentially be a “game changer” in the health industry. The innovation efforts by patients and their valuable solutions can potentially save significant resources, reduce costs, promote the exchange of knowledge among target stakeholders, and ultimately have an important social and economic impact. Recognition of patient innovation also has the potential to enable patients to assume more responsibility for the collaborative development and diffusion of medical innovations. We have approached patient innovation along two complementary paths: an interdisciplinary scholarly research effort aimed at understanding the patient innovation phenomenon and the design and launch of the Patient Innovation online platform to encourage and facilitate the development and diffusion of patient innovations. Our academic research helped frame the development of the Patient Innovation platform. Implementing the platform has raised new research questions in turn.
Feedback between scholarly research and practical application in the patient innovation arena has the potential to produce great societal benefit. Consider how a similar virtuous cycle has operated in the recently developed field of “entrepreneurship.” Individuals have always created new firms. However, only recently have researchers started studying entrepreneurship and firm creation in a systematic way. The topic has become so popular that schools now offer courses on the topic. The “buzz” has motivated people to become entrepreneurs, while the improved understanding of entrepreneurship can be employed to empower them to do so. Something similar can perhaps happen with patient innovation. With technology and guidance, we can empower patients to innovate, and this is Patient Innovation’s mission.
We have shown that patients are innovators, and we have developed the basic infrastructure for a patient innovation knowledge commons, but much remains to be done. Many patients do not even understand that they have the potential to create valuable innovations – even if they are already doing so. Greater awareness of the potential for patient innovation might motivate patients who have various potentially relevant skills to become deeply involved in using those skills to improve their health care – just as Tal Golesworthy employed his engineering skills to design his aortic root external support. Numerous interesting questions for researchers in many disciplines remain. Our experience implementing the Patient Innovation platform uncovered questions lacking clear-cut solutions concerning intellectual property, legal responsibility, medical ethics, and the methodology for assessing safety and efficacy, just to name a few. We are only at the beginning of understanding patient innovation and realizing its potential to benefit society. We invite those with the skills and will to join our efforts, helping us understand and enable patient innovators and to level the field for meaningful patient participation at all levels of integrated health care.




