7.1 Introduction
The coronavirus disease of 2019 (COVID-19) that became pandemic in 2020 reminds us poignantly about the possible consequences of spillover events of diseases from wildlife. Over recent decades, we have experienced the emergence of new or newly identified infectious disease such as severe acute respiratory syndrome (SARS), Ebola, Nipah, human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS), human ‘mad cow disease’ (variant Creutzfeldt–Jakob disease, CJD) and West Nile fever to name but a view. These diseases are directly or indirectly connected to wild and domestic meat and to wildlife in general. There is a huge variety of pathogens of animal origin including viruses, bacteria and parasites, all having different impacts ranging from mild to lethal. Because of the dramatic impact on the wider human population, we will focus in this chapter on those emerging zoonotic diseases which are directly linked to wild meat and which have the most serious impact on humans (mainly viral diseases). We will not focus on diseases which have had animal origins but are currently not directly linked with wild meat hunting. For example, malaria, caused by the parasite Plasmodium falciparum, had its likely origin in gorillas (Liu et al. Reference Liu, Li and Learn2010) and wild meat hunters will be particularly exposed to mosquitos that carry the malaria parasite, but there is no increased zoonotic risk by wild meat hunting to the resident human population. Similarly, we will not focus on parasites, such as helminths or bacteria, because their spillover risk is local, possibly affecting hunters and consumers (Kurpiers et al. Reference Kurpiers, Schulte-Herbrüggen, Ejotre, Reeder and Angelici2016), but without a direct health risk for the broader society.
A total of 1,415 species of human infectious organism has been described, of which 61% are zoonotic (Taylor et al. Reference Taylor, Latham and Woolhouse2001). Amongst all these pathogens, 175 are emerging, of which 75% are zoonotic. Whilst helminths are unlikely to cause emerging diseases, viruses and protozoa are overrepresented (Fig. 7.1). Almost all recent pandemics have a viral origin (Geoghegan et al. Reference Geoghegan, Senior, Di Giallonardo and Holmes2016; Jones et al. Reference Jones, Patel and Levy2008). The next pandemics will likely be caused by viruses again. About 263 viruses are known to affect humans (King et al. Reference King, Adams, Carstens and Lefkowitz2012). In mammals and birds about 1.67 million yet-to-be-discovered viral species from key zoonotic viral families are likely to exist of which between 631,000 and 827,000 have zoonotic potential (Carroll et al. Reference Carroll, Daszak and Wolfe2018). Currently, about one new disease is being detected per year (Cleaveland et al. Reference Cleaveland, Haydon, Taylor, Childs, 335Mackenzie and Richt2007; Woolhouse Reference Woolhouse2002). Thus, the potential for the emergence of new zoonotic diseases is enormous. In fact, the total number and diversity of zoonotic outbreaks and richness of causal diseases has increased significantly since 1980 even after controlling for disease surveillance, communications, geography and host availability (Smith et al. Reference Effiom, Birkhofer, Smith and Olsson2014).
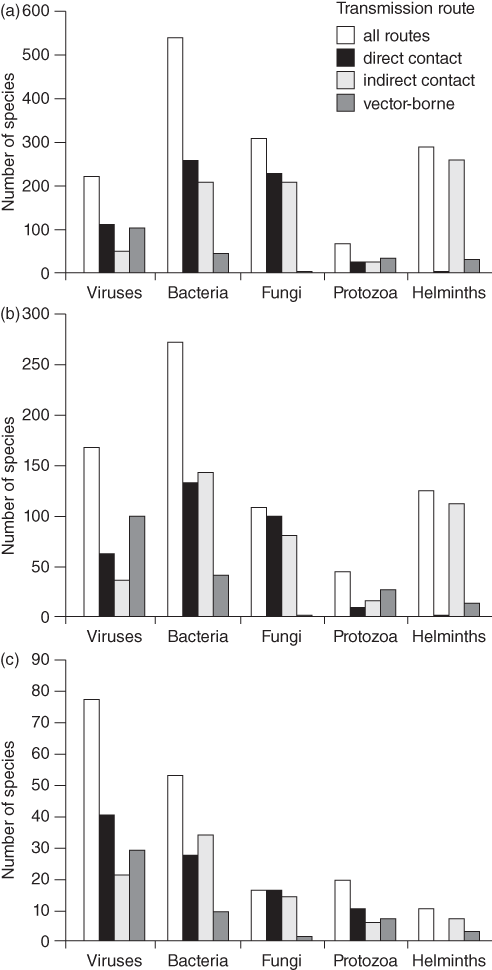
Figure 7.1 Numbers of species of infectious agent causing human disease, by taxonomic division and transmission route (noting that some species have more than one transmission route and for some the transmission route is unknown): (a) all infectious organisms (n = 1415); (b) zoonotic organisms (n = 868); (c) emerging organisms (n = 175).
Major anthropologic transitions with changes in human socio-economic and spatial organization, especially increases in human population density and concentration, increase of human–animal contacts, increase in human mobility and increase in anthropogenic movements of live domestic and wild animals have caused three historic and the current phases of emergence of new zoonotic diseases (McMichael Reference McMichael, McLean, May, Pattison and Weiss2005). Some diseases which spilled over into humans during the historic transitions are re-emerging again, including measles, plague and yellow fever.
7.2 Re-emergent Zoonotic Diseases
A re-emerging pathogen is one ‘whose incidence is increasing in an existing host population as a result of long-term changes in its underlying epidemiology’ (Woolhouse & Dye Reference Woolhouse and Dye2001). These pathogens emerged during the first three major historical phases of emerging infectious zoonotic disease (McMichael Reference McMichael, McLean, May, Pattison and Weiss2005). Before the domestication of livestock about 10,000–15,000 BP, hunter-gatherer-fisher communities were too small to maintain pathogens that spilled over from wildlife, let alone sustain epidemic or pandemic spread (Dobson & Carper Reference Dobson and Carper1996). The first opportunity for zoonotic pathogens to spillover into humans and then to adapt to and remain in human populations arose during the transition to agriculture and livestock herding and the period of early human settlements with emerging diseases staying on a local scale some 5,000–10,000 BP. The second phase was generated by increased military and commercial contact around 3,000–1,500 BP, triggering continent-wide spread of diseases. The third phase is marked by European expansionism over the past five centuries resulting in intercontinental disease spread. For example, measles seem to have emerged in humans around 8,000 BP spilling over from sheep or goats when they were domesticated, but the infection chain stayed within humans ever since (Weiss Reference Weiss2001). Thus, the formerly zoonotic disease adapted to person-to-person transfer and became anthroponotic. Together with smallpox and other diseases, their effect on Amerindian people after colonization by Europeans was highly devastating (McNeill Reference McNeill1976). These diseases likely allowed Cortéz to defeat the Aztec empire. Smallpox, whose exact animal origin remains unknown (Weiss Reference Weiss2001), has afflicted humans at least for 3,500 years but it has now been eradicated thanks to efforts that began with Edward Jenner’s pioneering vaccine prepared from cowpox in 1798 and were completed with the WHO-led programme to eliminate the disease (Fenner et al. Reference Fenner, Henderson, Arita, Jezek and Ladnyi1988; Mühlemann et al. Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). In contrast, measles is now re-emerging around the world (Misin et al. Reference Misin, Antonello and Di Bella2020).
7.2.1 Plague
The plague-causing bacillus Yersinia pestis is endemic among some species of rodents and is transmitted through human-to-human contact (pneumonic plague) or via fleas and lice between rodents, rodents-to-human and between humans as a common vector (bubonic or septicaemic plague). It emerged in humans at least 5,000 to 6,000 BP during the Neolithic decline in Asia and Europe followed by three major pandemics starting during the second historic disease period (Feldman et al. Reference Feldman, Harbeck and Keller2016; Rascovan et al. Reference Rascovan, Sjögren and Kristiansen2019; Rasmussen et al. Reference Rasmussen, Allentoft and Nielsen2015). The Justinian plague from 541 to around 750 BCE is the first detailed pandemic described in human history although mortality rates and socio-economic impact remain controversially discussed (Mordechai et al. Reference Mordechai, Eisenberg, Newfield, Izdebski, Kay and Poinar2019). Socio-economic devastation and a mortality of up to 50% during the Black Death has remained in public consciousness as the most widespread fatal pandemic in human history since it swept through Asia, the Middle East, North Africa and Europe in the 1340s (Benedictow Reference Benedictow2004). This pandemic lasted until the eighteenth century with several recorded waves such as London’s Great Plague (1665–1666 AD). The third epidemic started in the nineteenth century in China, spread around the world – over eight million people died in India between 1895 and 1914 – and is since a re-emerging infectious disease worldwide (Campbell & Hughes Reference Campbell and Hughes1995; WHO 2004a). Reservoir species are not only black rats, the principal species during the Black Death, but also diverse burrowing rodents such as chipmunks and woodchucks in the New World and marmots in Asia. Only in 2020, a teenage boy died of the disease in Mongolia after eating marmot hunted as wild meat (Associated Press 2020). Africa remains endemic for the pathogen with sporadic outbreaks (Davis et al. Reference Davis, Makundi, Machang’u and Leirs2006; Forrester et al. Reference Forrester, Apangu and Griffith2017).
7.2.2 Yellow Fever
Mosquito-borne yellow fever, caused by the yellow fever virus, arose in Africa during the last 1,500 years and became to prominence after it invaded the Americas from Africa via the slave trade in the seventeenth century (Bryant et al. Reference Bryant, Holmes and Barrett2007). Its natural reservoir is monkeys in Africa, but yellow fever established itself successfully in New World monkeys (Weiss Reference Weiss2001). Although largely under-researched and categorized as a neglected tropical disease, recent outbreaks in Angola in 2015–2016 and in Brazil in 2016–2017 have highlighted the threat posed by this zoonotic disease (Butler Reference Butler2016; Grobbelaar et al. Reference Grobbelaar, Weyer, Moolla, Jansen van Vuren, Moises and Paweska2016; Kleinert et al. Reference Kleinert, Montoya-Diaz and Khera2019). The zoonotic threat to hunters is not via consuming wild meat but being exposed to mosquitos whilst hunting.
7.3 Pandemic Zoonotic Emerging Infectious Diseases
An emergent disease is an ‘infectious disease whose incidence is increasing following its first introduction into a new host population’ (Woolhouse & Dye Reference Woolhouse and Dye2001). During the last quarter century, we have witnessed not only the resurgence of infectious disease but the emergence of novel or newly identified diseases. Rapidly increasing human population densities, social-economic and behavioural changes, the globalized economy, increased mobility, the ever increasing encroaching in and modification of the natural environment and ecological changes have triggered a fourth great transition phase which fosters the emergence of infectious disease (McMichael Reference McMichael, McLean, May, Pattison and Weiss2005). Whilst the first three periods were local, continental and intercontinental, this time the impact is global as the rapid pandemic spread of COVID-19 or the 2009 H1N1 swine-flu clearly demonstrate. Importantly, we encroach more and more on the last remaining pristine wilderness areas thereby destabilizing ecosystems, changing the population dynamics of animal reservoirs for pathogens and increasing human-pathogen contacts. These changes are particularly well demonstrated by COVID-19, HIV/AIDS, Ebola and SARS, which all have direct connections to wild meat exploitation and animal trade (see Loh et al. Reference Loh, Zambrana-Torrelio and Olival2015). After the original zoonotic transmission, all four diseases became anthroponotic and pandemic. A pandemic is ‘an epidemic occurring over a very wide geographic area, crossing international boundaries, and usually affecting a large number of people. The agent must be able to infect humans, to cause disease in humans, and to spread easily from human to human’ (Porta et al. Reference Porta, Greenland and Hernán2014).
7.3.1 Covid-19
The coronaviruses SARS-CoV-2, SARS-CoV and MERS-CoV cause severe infections: COVID-19, the Severe Acute Respiratory Syndrome (SARS) and the Middle East respiratory syndrome (MERS), respectively. SARS-CoV-2, first termed 2019-nCoV, is the causative agent of COVID-19 and the seventh known coronavirus affecting humans. Except the above three, the other known coronaviruses affecting humans cause mild infections (Van der Hoek Reference Van der Hoek2007). All have animal origins with SARS-CoV-2, SARS-CoV, MERS-CoV, HCoV-NL63 and HCoV-229E likely originating from bats and HCoV-OC43 and HCoV-HKU1 from rodents (Cui et al. Reference Cui, Li and Shi2019). MERS-CoV and HCoV-229E have camelids, HCoV-OC43 cattle and SARS-CoV civets as intermediate hosts whilst intermediate hosts for HCoV-NL63, HCoV-HKU1 and SARS-CoV-2 are unconfirmed (Fig. 7.2). Since the spillover into humans, SARS-CoV-2 has been transmitted human-to-human. Genetic and epidemiological analysis have shown that the virus is not a laboratory construct or a purposefully manipulated virus, thus debunking the conspiratory hypothesis expressed by many that the virus is of artificial origin (Andersen et al. Reference Andersen, Rambaut, Lipkin, Holmes and Garry2020; Pekar et al. Reference Pekar, Magee and Parker2022; Worobey et al. Reference Worobey, Levy and Serrano2022). SARS-CoV-2 and coronaviruses discovered in bats are genetically very similar, making it likely that SARS-CoV-2 or its progenitor evolved in horseshoe bats with other mammals as a plausible conduit for transmission to humans (Boni et al. 2020; Zhou et al. Reference Chen, Zhou and Dong2020). After the emergence of SARS-CoV and MERS-CoV in the early twenty-first century, Afelt et al. (Reference Afelt, Frutos and Devaux2018a) predicted a new coronavirus to spillover from bats in Southeast Asia. The region is the world’s most affected region of deforestation. The human demographic growth – the human population in the region increased by 130 million between 2001 and 2011 – causes strong pressures on the land, increases demand on domestic and wild meat and is an ideal environment to sustain an epidemic once a zoonotic pathogen spilled over into humans. Afelt et al. (Reference Afelt, Frutos and Devaux2018a) also observed that the land-use changes triggered bat populations to move closer to human dwellings, in turn increasing the number and diversity of bat-borne viruses next to human dwellings and thus zoonotic risk (Afelt et al. Reference Afelt, Lacroix, Zawadzka-Pawlewska, Pokojski, Buchy and Frutos2018b; Plowright et al. Reference Plowright, Eby and Hudson2015). Whilst intermediate animal hosts for SARS-CoV-2 remain unknown, the virus can infect some other wildlife such as monkeys, rabbits and racoon dogs, and some domestic animals, such as cats, dogs, farmed American mink, ferrets and hamsters, but not pigs, chickens or ducks (El Masry et al. Reference El Masry, Dobschuetz and Plee2020; Shi et al. Reference Diamond and Wolfe2020). While experimentally infected cats, ferrets and hamsters infected other animals of the same species, dogs did not transmit the virus to other dogs in experimental settings (El Masry et al. Reference El Masry, Dobschuetz and Plee2020).
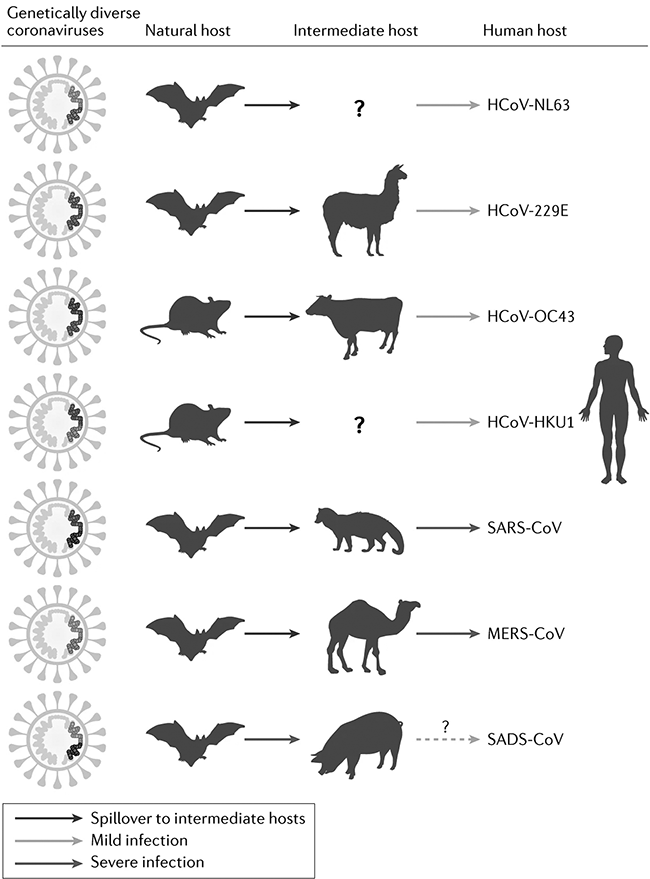
Figure 7.2 Animal origins of human coronaviruses prior the emergence of SARS-CoV-2
Since early December 2019, patients presenting with viral pneumonia due to an unidentified microbial agent were reported in Wuhan, China. Most patients worked at or lived around the local Huanan seafood wholesale market, where live animals were also on sale. The agent was subsequently identified as SARS-CoV-2 (Chen et al. Reference Chen, Zhou and Dong2020). Although COVID-19 was first detected officially at this market, epidemiological data indicate that early cases were not related to the market and thus that it may not necessarily be the site of emergence (Frutos et al. Reference Frutos, Lopez Roig, Serra-Cobo and Devaux2020). In November 2000, the WHO announced a Global Study of the origins of SARS-CoV-2 with field work to commence in China in early 2021. This study emphasizes that the origin of the virus and the spillover event remains unknown: ‘some countries have retrospectively identified cases of COVID-19 weeks before the first case was officially notified through surveillance, and unpublished reports of positive sewage samples could suggest that the virus may have circulated undetected for some time’¨ (WHO Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). The market might have acted as an amplification chamber for the human-to-human spread. The COVID-19 pandemic had caused 101,562,751 cases with 2,193,403 deaths worldwide as of 29 January 2021 and 456,956,790 cases with 6,042,210 deaths as of 13 March 2022 (https://coronavirus.jhu.edu).
7.3.2 Hiv/Aids
The first documented human HIV-1 infection dates from 1959 in Kinshasa (Worobey et al. Reference Worobey, Gemmel and Teuwen2008) and the AIDS was first recognized as a disease in 1981 (Barré-Sinoussi et al. Reference Barré-Sinoussi, Chermann and Rey1983). All the genetic evidence indicates that the human immunodeficiency virus type 1 (HIV-1) and the related type 2 (HIV-2) evolved after zoonotic transmission from non-human primates, specifically chimpanzee for HIV-1 and sooty mangabey for HIV-2, in West-Central Africa (Gao et al. Reference Gao, Bailes and Robertson1999; Van Heuverswyn & Peeters Reference Van Heuverswyn and Peeters2007). To account for the HIV’s genetic diversity (Fig. 7.3), at least 12 zoonotic transmission events must have occurred, four to account for the diversity of HIV-1 (Plantier et al. Reference Plantier, Leoz and Dickerson2009) and eight to account for the diversity of HIV-2 (Van Heuverswyn & Peeters Reference Van Heuverswyn and Peeters2007). HIV’s genetic diversity indicates that the zoonotic transmission of simian immunodeficiency viruses, (SIV), which then evolved into the respective HIV strains, is an ongoing, dynamic process and that new zoonotic transfers are real possibilities. A serological study of monkeys that were hunted in the rainforests of Cameroon for wild meat or kept as pets showed that a substantial proportion are SIV infected, thus exposing people to a plethora of genetically highly divergent SIV viruses (Peeters et al. Reference Peeters, Courgnaud and Abela2002). Although the exact circumstances of the zoonotic transmissions of SIV remain unknown, hunting and butchering of primate wild meat is the most parsimonious explanation (Hahn Reference Hahn2000; Van Heuverswyn & Peeters Reference Van Heuverswyn and Peeters2007). Wild meat hunters in Central Africa continue to be exposed to and possibly infected with SIV (Kalish et al. Reference Kalish, Wolfe and Ndongmo2005). Molecular clocks indicate that HIV-1 originated sometime near the beginning of the twentieth century (Worobey et al. Reference Worobey, Gemmel and Teuwen2008). This time frame corresponds with a period of the founding and rapid growth of colonial administrative and trading centres in West-Central Africa which might have facilitated the spread of the viruses in the human population, which eventually led to the global AIDS pandemic. The most dramatic effect is among the world’s poorest and most underprivileged communities, in which life expectancy has dropped by 20 years on average (Weiss Reference Weiss2003). By the year 2020, it is estimated that between 55.9 and 100 million people have become infected with HIV and that between 24.8 and 42.2 million people have died from AIDS-related illnesses since the start of the pandemic (UNAIDS Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020).
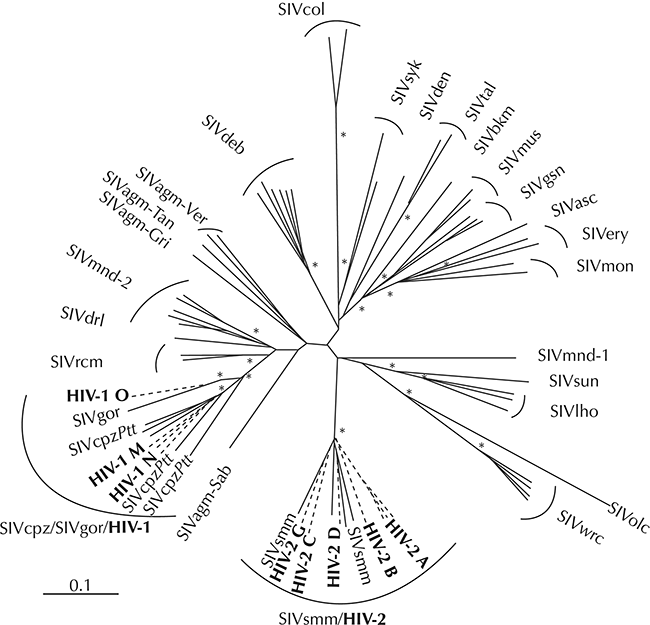
Figure 7.3 Evolutionary relationship among the different SIV and HIV lineages based on neighbour joining phylogenetic analysis of partial pol sequences. This phylogeny represents 26 of the 32 infected nonhuman primate species, for whom (partial) sequences are available. Asterisks indicate bootstrap replicates supporting the cluster to the right with values >85%. Within the branches with HIV sequences are sequences from gorilla (SIVgor), chimpanzee (SIVcpzPtt) and Sooty mangabey (SIVsmm).
7.3.3 Ebola
Six species of ebolavirus have been identified in West and Central Africa: Bombali virus, Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus and Zaire ebolavirus (Ebola virus) of which Bombali virus is the latest to be discovered (Goldstein et al. Reference Goldstein, Anthony and Gbakima2018). Note that the term ‘Ebolavirus’ can refer to the genus, when written in italics, and to the common name of the Zaire ebolavirus, if not written in italics. Only Bundibugyo ebolavirus, Sudan ebolavirus, and Ebola virus have caused disease outbreaks of severe haemorrhagic fever in humans with overall case fatality of 25%, 50% and 80%, respectively (Malvy et al. Reference Malvy, McElroy, de Clerck, Günther and van Griensven2019). Outbreaks of Ebola virus disease (EVD) have been recorded since 1976 when two consecutive outbreaks of fatal haemorrhagic fever occurred, first in the former Zaire in what is now the Democratic Republic of Congo, caused by the Ebola virus, and second in what is now South Sudan, caused by the Sudan ebolavirus (Fig. 7.4, Centers for Disease Control and Prevention 2020). Since then, an additional 26 outbreaks have been registered mostly caused by the Ebola virus. However, at least half of EVD spillover events were likely not being reported (Glennon et al. Reference Glennon, Jephcott, Restif and Wood2019). Fatalities ranged from zero in Ivory Coast in 1994, caused by the Taï Forest ebolavirus, up to 11,325 for the most severe outbreak across multiple countries in West Africa from 2014 to 2016, caused by the Ebola virus. The number of deaths recorded in the 2016 outbreak was 11,310 in the three most affected countries, Guinea, Liberia and Sierra Leone (WHO 2016a). This EVD outbreak was the largest amongst all outbreaks with almost ten times more fatalities than all previous outbreaks combined. In addition to West Africa, imported cases were reported from the seven countries (Italy, Mali, Nigeria, Senegal, Spain, UK and USA (WHO 2016b)). Ebola virus disease is also a rapidly fatal disease for non-human primates, for example killing 90–95% of the gorilla population at the Lossi Sanctuary in northwest Republic of Congo during a 2002–2003 outbreak (Bermejo et al. Reference Bermejo, Rodriguez-Teijeiro, Illera, Barroso, Vila and Walsh2006; Walsh et al. Reference Walsh, Abernethy and Bermejo2003).
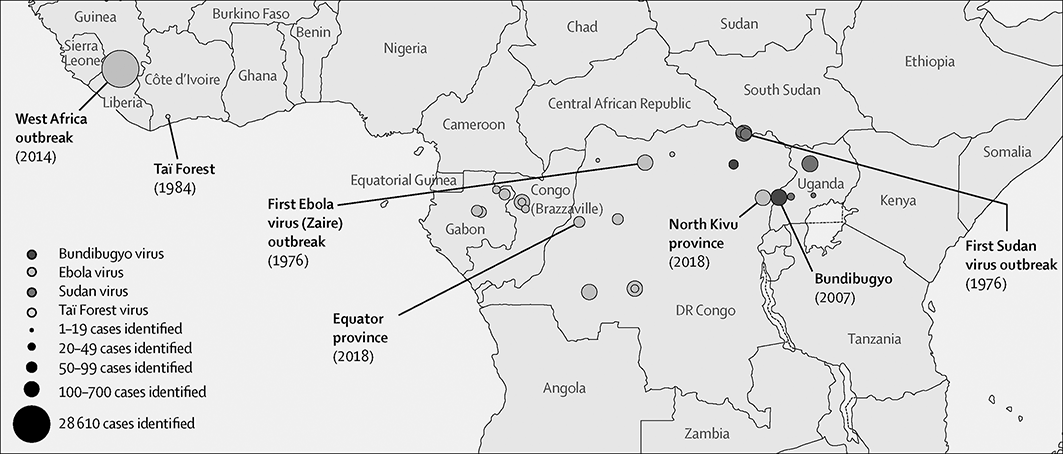
Figure 7.4 Outbreaks of Ebola disease in sub-Saharan Africa.
Wild meat has been implicated as a source of zoonotic spillover (Fig. 7.5, Rojas et al. Reference Rojas, Monsalve and Pacheco2020). All five human EVD outbreaks during 2001–2003 in the forest zone between Gabon and Republic of Congo began after humans handled the carcasses of gorillas, chimpanzees, and duikers (Rouquet et al. Reference Rouquet, Froment and Bermejo2005). In each case, mortality events in these species, which are also susceptible to Ebolavirus, began before each of the human outbreaks. These animal populations declined markedly during human EVD outbreaks. The first human victim of an EVD outbreak in the Democratic Republic of Congo in 2007 died after purchasing freshly killed fruit bats in a market (Leroy et al. Reference Leroy, Epelboin and Mondonge2009; Mann et al. Reference Mann, Streng, Bergeron and Kircher2015). Circumstantial evidence points to the source of the West African 2014–2016 outbreak to contact with secretions from wild fruit bats (Mann et al. Reference Mann, Streng, Bergeron and Kircher2015). Whilst all these species can harbour Ebola viruses the natural reservoirs of this virus remain unknown but is likely to be found amongst bats (Malvy et al. Reference Malvy, McElroy, de Clerck, Günther and van Griensven2019; Spengler et al. Reference Spengler, Ervin, Towner, Rollin and Nichol2016).
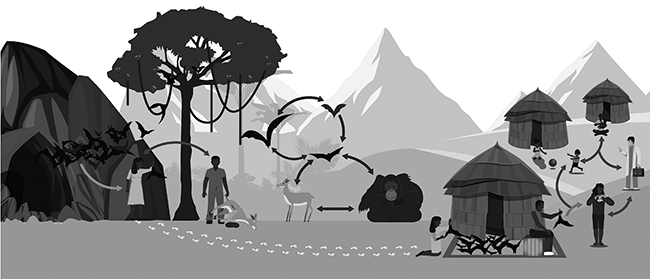
Figure 7.5 Ebola virus transmission. Fruit bats are considered natural reservoirs of Ebolaviruses EBOVs and these seem to infect non-human primates and duikers, which mostly constitute the spillover event. The virus disseminates from person to person, potentially affecting a large number of people. The virus spreads through direct contact with broken skin or mucous membranes in the eyes, nose, or mouth and semen. However, Ebolaviruses may spread through the handling and consumption of wild meat.
7.3.4 Sars
SARS was first recognized at the end of February 2003 in Hanoi, Vietnam involving a patient who had extensively travelled in Southeast Asia (WHO Reference Allan, Keesing and Ostfeld2003). In the same year, SARS spread to more than 30 countries across five continents (Guan et al. Reference Guan, Zheng and He2003). The coronavirus SARS-CoV was identified as the causative agent (Drosten et al. Reference Drosten, Günther and Preiser2003). This virus was much more pathogenic than the human coronaviruses (HCoV) known until then, which mainly cause mild respiratory disease (Section 7.3.1). The virus was traced back to a live-animal market in Guangdong, Southwest China, where it appears to have jumped from traded Himalayan palm civets that tested positive for a virus highly similar (99.8%) to SARS-CoV. Evidence of virus infection was also detected in other animals including a raccoon dog and Chinese ferret badger and in humans working at the same market (Guan et al. Reference Guan, Zheng and He2003). Furthermore, 40% of animal traders and 20% of animal slaughterers had detectable serum antibodies, compared to only 5% of vegetable traders. Subsequently, genetically diversified CoVs related to SARS-CoV were then found in diverse Chinese bat families albeit the reservoir population of bats for SARS has not been definitively identified (Drexler et al. Reference Drexler, Corman and Drosten2014; Lau et al. Reference Brook and Whitehead2005; Li Reference Altrichter2005). The likely infection scenario is that bats infected civets as intermediate and amplifying hosts, which then triggered the zoonotic spillover (Guan et al. Reference Guan, Zheng and He2003; Song et al. Reference Song, Tu and Zhang2005). The 2003–2004 pandemic infected 8,096 people worldwide and killed 774 (9.5%) of them (Drexler et al. Reference Drexler, Corman and Drosten2014).
7.4 Other Zoonotic Infectious Diseases
A number of zoonotic diseases are emerging but have not become pandemic or are endemic (see Jones et al. Reference Jones, Patel and Levy2008; Loh et al. Reference Loh, Zambrana-Torrelio and Olival2015). These include viruses, bacteria, helminths, protozoans, fungi and prions (Kurpiers et al. Reference Kurpiers, Schulte-Herbrüggen, Ejotre, Reeder and Angelici2016). The list of pathogens is so large that we restrict us here to some important and representative examples.
7.4.1 Anthrax
Anthrax is one of the oldest known zoonotic diseases, caused by the spore-forming bacterium Bacillus anthracis, which infects ruminants worldwide (De Vos & Bryden Reference De Vos and Bryden1996; Dragon et al. Reference Dragon, Renniie and Gates1996; Lindeque & Turnbull Reference Lindeque and Turnbull1994).Through direct contact, inhaling spores or by consuming meat from infected animals other species can be infected, including humans and primates (Leendertz et al. Reference Leendertz, Ellerbrok and Boesch2004; Sirisanthana & Brown Reference Sirisanthana and Brown2002). Use of contaminated carcasses and hides, which is a widespread practice amongst wild meat hunters, is the principle zoonotic risk (Beatty et al. Reference Beatty, Ashford, Griffin, Tauxe and Sobel2003; Hang’ombe et al. Reference Berrang-Ford, Dingle and Ford2012).
7.4.2 Hepatitis Viruses
Hepatitis E virus (HEV), transmission from wild boar meat to a human was reported in Japan confirming its zoonotic potential (Li et al. Reference Altrichter2005). Hepatitis E virus prevalence in Japanese wild boar and deer was 9% and 2%, respectively (Sonoda et al. Reference Sonoda, Abe and Sugimoto2004). Non-human primates harbour a range of hepatitis viruses, some of them closely related to human hepatitis B and C, HBV and HCV, respectively, but the zoonotic origin of human hepatitis viruses remains unclear (Simmonds Reference Simmonds2000). Hepatitis B-related viruses are also found in a range of other species, including rodents and birds (Marion et al. Reference Marion, Oshiro, Regnery, Scullard and Robinson1980; Mason et al. Reference Mason, Seal and Summers1980). Whilst HBV can be transmitted to non-human primates, there is no evidence of zoonotic transmission of the diverse primate hepatitis viruses even for zookeepers who are in close contact with primates (Noppornpanth et al. Reference Noppornpanth, Haagmans and Bhattarakosol2003). However, given the zoonotic transmission of HEV and the intensive contact of wild meat hunters with animal body fluid there is a clear existent zoonotic risk.
7.4.3 Lassa Virus
Lassa fever is endemic to West Africa and causes in approx. 30% of cases illness ranging from mild, flu-like symptoms to haemorrhagic fever with a mortality rate of 1–2%, but occasionally of 50% (McCormick et al. Reference McCormick, Webb, Krebs, Johnson and Smith1987; ter Meulen et al. Reference ter Meulen, Lukashevich and Sidibe1996). It has been known since the 1950s (Richmond & Baglole Reference Richmond and Baglole2003). The only known natural host is the multimammate mouse, a hunted rodent that associates closely with humans and is commonly found in and around African villages (Lecompte et al. Reference Lecompte, Fichet-Calvet and Daffis2006). Three risk factors affect Lassa virus transmission: rodent infestation, uncovered storage of food and hunting the mouse for wild meat (ter Meulen et al. Reference ter Meulen, Lukashevich and Sidibe1996).
7.4.4 Marburg Virus
The virus constitutes with Ebolavirus the family Filoviridae with insectivorous bat species as natural reservoirs (Allocati et al. Reference Allocati, Petrucci, Di Giovanni, Masulli, Di Ilio and De Laurenzi2016; Leendertz et al. Reference Leendertz, Gogarten, Düx, Calvignac-Spencer and Leendertz2016). It causes severe, often fatal, haemorrhagic fever in humans and primates. Marburg virus (MARV), is transmitted to humans through contact with body fluids and dead bodies of infected animals. Marburg virus was first identified in laboratory workers who had dissected an imported African green monkey (Martini et al. Reference Martini, Knauff, Schmidt, Mayer and Baltzer1968). The reservoir host is the Egyptian fruit bat with antibodies and viral DNA also found in other insectivorous and fruit bats (Amman et al. Reference Amman, Carroll and Reed2012; Swanepoel et al. Reference Swanepoel, Smit and Rollin2007). Egyptian fruit bats are hunted in West Africa for wild meat (Mickleburgh et al. Reference Mickleburgh, Waylen and Racey2009). Marburg virus is a prime example demonstrating that attempts to control the disease by persecuting the host species can fail (Amman et al. Reference Amman, Nyakarahuka and McElroy2014): after MARV infected gold miners in southwest Uganda at the Kitaka mine, the miners exterminated the bat colony. However, the bat colony re-established itself albeit at lower total size. The re-established colony had a significantly higher level of active infection than before the eradication and other studies in Uganda and Gabon have yielded similar results. Such failures are not without precedent. For example, badger culling in the UK to control bovine tuberculosis (TB) not only failed to control but also seems to increase TB incidence in cattle (Donnelly et al. Reference Donnelly, Woodroffe and Cox2003).
7.4.5 Mayaro Virus
Mayaro fever is a non-fatal dengue-like acute viral disease of tropical rainforest in Central and South America and the Caribbean, first detected in the 1950s (Anderson et al. Reference Anderson, Downs, Wattley, Ahin and Reese1957). The mosquito-borne virus is suspected to have monkeys as the principal reservoir (Pinheiro & Travassos da Rosa Reference Pinheiro, Travassos da Rosa and Beran1994). However, this illness being largely neglected, there is inadequate surveillance in endemic areas and limited epidemiological data available (Mota et al. Reference Mota, Ribeiro, Vedovello and Nogueira2015). People who are frequently within forest environments, such as wild meat hunters, are at a higher risk of being bitten by numerous mosquito species that can carry the virus. A study in Ecuador showed that mainly Amazonians are infected by the virus, indicating that deep forest hunting may selectively expose local men to zoonotic spillover (Izurieta et al. Reference Izurieta, Macaluso and Watts2011).
7.4.6 Monkeypox Virus
Monkeypox is an emerging zoonotic disease with clinical symptoms of fever and a severe rash similar to smallpox (Parker et al. Reference Parker, Nuara, Buller and Schultz2007; Sklenovská & Van Ranst Reference Sklenovská and Van Ranst2018). Mortality rates can be as high as 17%, but a vaccine exists (Di Giulio & Eckburg Reference Di Giulio and Eckburg2004). It is endemic in the Democratic Republic of Congo, but human and animal cases have also been reported from elsewhere in Central and West Africa (Rimoin et al. Reference Rimoin, Mulembakani and Johnston2010). The disease was imported once into the USA (Hutson et al. Reference Hutson, Lee and Abel2007). Frequency and geographical spread of human monkeypox have increased in recent years (Rimoin et al. Reference Rimoin, Mulembakani and Johnston2010), but the epidemiology and ecology remain understudied (Sklenovská & Van Ranst Reference Sklenovská and Van Ranst2018). Transmission likely occurs by direct contact with infected animals or their bodily fluids (Jezek et al. Reference Jezek, Arita, Mutombo, Dunn, Nakano and Szczeniowski1986). The virus was first isolated in primates (Arita & Henderson Reference Arita and Henderson1968), but the main host appears to be wild squirrels (Hutin et al. Reference Hutin, Williams and Malfait2001; Parker et al. Reference Parker, Nuara, Buller and Schultz2007). It has been isolated from diverse rodents, including imported and domestic rodents during a US monkeypox outbreak (Hutson et al. Reference Hutson, Lee and Abel2007). The virus’s broad host range may permit additional species to become reservoirs or incidental hosts, increasing the zoonotic risk (Parker et al. Reference Parker, Nuara, Buller and Schultz2007). Human-to-human transmission occurs but the disease requires continuous reintroduction from the wild reservoir to be maintained in a human population (Hutin et al. Reference Hutin, Williams and Malfait2001; Jezek et al. Reference Jezek, Arita, Mutombo, Dunn, Nakano and Szczeniowski1986).
7.4.7 Nipah Virus
The paramyxovirus causes encephalitis and respiratory disease (Chua et al. 2000). It spilled over in 1998 from fruit bats first to pig livestock and then from pigs to farm workers in Malaysia causing 265 cases of encephalitis, including 105 deaths (Chua et al. 2000). Since, it has spread in Southeast Asia, especially to Bangladesh where spillover events now occur regularly (Gurley et al. Reference Gurley, Hegde and Hossain2017). Nipah is a prime example of how habitat change can cause spillover events. Deforestation and climate change are likely drivers for these events (Chua et al. Reference Chua, Chua and Wang2002). Following decades of deforestation combined with a severe drought following an El Niño Southern Oscillation event, Pteropid fruit bats, which are the natural reservoir of the virus, compensated for the loss of flowering and fruiting forest trees by an unprecedented encroachment into cultivated fruit orchards. These orchards also house ever increasing piggeries, allowing the transmission from fruit bats to pig livestock (Chua et al. Reference Chua, Chua and Wang2002; Field Reference Field2009). In Bangladesh, areas with reported Nipah outbreaks are characterized by higher human density and forest fragmentation than areas without outbreaks (Epstein et al. Reference Epstein, Gurley and Patz2014). Although the outbreak did not involve wild meat hunters in this case, these are likewise at risk as fruit bats are regularly hunted across Africa and Asia (Mickleburgh et al. Reference Mickleburgh, Waylen and Racey2009). Moreover, antibodies and henipavirus-related RNA, that is RNA from the same virus genus as Nipah, has been identified in straw-coloured fruit bat, the largest and most abundant African fruit bat species, in Ghana and in a wild meat market in the Republic of Congo (Drexler et al. Reference Drexler, Corman and Gloza-Rausch2009; Hayman et al. Reference Hayman, Wang and Barr2011; Weiss et al. Reference Weiss, Nowak and Fahr2012). In Africa, no human infection associated with bat henipavirus has been reported but continuing monitoring is advised to diminish the threat of a novel zoonotic disease especially as Nipavirus is associated with high mortality rates.
7.4.8 Simian Foamy Virus
Although there is no disease reported in humans (Switzer et al. Reference Switzer, Bhullar and Shanmugam2004), Simian foamy virus,(SFV), infections are an increasing public health concern (Calattini et al. Reference Calattini, Betsem and Froment2007). Simian foamy virus is endemic in most African primates (Peeters & Delaporte Reference Peeters and Delaporte2012; Switzer et al. Reference Switzer, Salemi and Shanmugam2005; Wolfe et al. Reference Wolfe, Switzer and Carr2004). It is transmitted by intensive contact between non-human primates and hunters (Calattini et al. Reference Calattini, Betsem and Froment2007; Wolfe et al. Reference Wolfe, Escalante, Karesh, Kilbourn, Spielman and Lal1998, Reference Alvard, Gillespie and Alvard2004), zookeepers, veterinarians and scientists (Switzer et al. Reference Switzer, Bhullar and Shanmugam2004) and people living near macaques in Asia (Jones-Engel et al. Reference Jones-Engel, Engel and Schillaci2005, Reference Albertí, Panea and Sañudo2008). In southern Cameroon, less than 0.4% of the general population was seropositive to SFV, but 24% of those people who had contact with great apes (gorillas or chimpanzees) and 3.6% of those who had contact with monkeys, highlighting the zoonotic potential of SFV (Calattini et al. Reference Calattini, Betsem and Froment2007). A serological survey of 1,099 rural Cameroonian villagers that had contact with primates identified that 1% had antibodies to SFV (Wolfe et al. Reference Wolfe, Switzer and Carr2004), suggesting a constant exposure to animal reservoirs (Pike et al. Reference Pike, Saylors and Fair2010). Simian foamy virus is one of the pathogens that were diagnosed in confiscated primates at US airports, highlighting the global zoonotic risk posed by the illegal wild animal trade (Smith et al. Reference Smith, Anthony and Switzer2012).
7.4.9 T-lymphotropic Viruses
Two lineages of human T-lymphotropic viruses, HTLV-1 and HTLV-2, are anthroponotic transmitted via body fluids and can cause adult T-cell lymphoma or one of several inflammatory disorders (Proietti et al. Reference Proietti, Carneiro-Proietti, Catalan-Soares and Murphy2005). Wild meat hunters and primate pet owners in Central Africa are infected not only with HTLVs including the newly discovered HTLV-3 and HTLV-4 lineages, but also with a wide variety of simian T-lymphotropic viruses (STLVs) of non-human primates (Wolfe et al. Reference Wolfe, Heneine and Carr2005b). The lineage HTLV-3 falls into the phylogenetic clade of STLV-3, supporting the suspected multiple zoonotic origin of the different HTLV lineages (LeBreton et al. Reference LeBreton, Pike, Saylors, Aguirre, Ostfeld and Daszak2012; Wolfe et al. Reference Wolfe, Heneine and Carr2005b). Prevalence of HTLV-1 in Pygmy hunter-gatherers was higher than amongst non-hunting villagers in Cameroon (Ndumbe et al. Reference Ndumbe, Okie, Nyambi and Delaporte1992), confirming the observation that HTLVs are more prevalent in populations which are exposed to wild primates (Delaporte et al. Reference Delaporte, Peeters, Simoni and Piot1989).
7.4.10 Tularaemia
Described in the 1910s, the tularaemia-causing bacterium Francisella tularensis has been reported in a range of vertebrates including mammals – in particular rodents and especially rabbits and hares – birds, amphibians and fish, and in invertebrates across the northern hemisphere (Ellis et al. Reference Ellis, Oyston, Green and Titball2002; Yeatter & Thompson Reference Yeatter and Thompson1952). A wide range of arthropod vectors have been implicated in the transmission between mammalian hosts. Infection can occur by handling animal skins or carcasses and less frequently from tick or deer fly bites; it is also possible to acquire the disease from drinking water contaminated with animal faeces and urine, or by eating undercooked contaminated meat (Higgins et al. Reference Higgins, Hubalek and Halouzka2000). Rural people, especially hunters but also farmers, walkers and forest workers, are most at risk of contracting tularaemia. Therefore, it is also variously known as rabbit fever, hare fever and deerfly fever. A study in a suspected endemic region of Germany showed a seroprevalence among hunters (1.7%) that was higher than in the general population (0.2%) (Jenzora et al. Reference Jenzora, Jansen, Ranisch, Lierz, Wichmann and Grunow2008). Outbreaks of disease in humans often parallel disease occurrences in wildlife as seen in Sweden where an association between peaks in vole and hare populations and outbreaks of tularaemia in humans have been reported (Tärnvik et al. Reference Tärnvik, Sandström and Sjöstedt1996).
7.4.11 Others
Besides the above-listed diseases, many more pathogens with zoonotic risk are found in species used as wild meat. For example an unknown proportion of the about 25,000 yearly fatalities from rabies in Africa, caused by a lyssavirus, might be via wild meat species although the majority of cases stems from domestic dogs (Dodet et al. Reference Dodet, Tejiokem, Aguemon and Bourhy2015; Kurpiers et al. Reference Kurpiers, Schulte-Herbrüggen, Ejotre, Reeder and Angelici2016). Rabies also occurs in a variety of species other than canids, including primates that are hunted as wild meat (Gautret et al. Reference Gautret, Blanton and Dacheux2014) and bats (Kuzmin et al. Reference Kuzmin, Bozick and Guagliardo2011). Many other lyssaviruses exist including Duvenhage virus, which causes fatal encephalitis and is transmitted by bats (Allocati et al. Reference Allocati, Petrucci, Di Giovanni, Masulli, Di Ilio and De Laurenzi2016; van Thiel et al. Reference Van Thiel, de Bie and Eftimov2009).
In addition to the already mentioned, B. anthracis and F. tularensis, a large variety of bacteria can affect wild meat species and can be transmitted to humans. Bacteria constitute 54% of emerging infectious diseases (Jones et al. Reference Jones, Patel and Levy2008). Bachand et al. (Reference Bachand, Ravel, Onanga, Arsenault and Gonzalez2012) confirmed the intestinal-infection causing Campylobacter, Salmonella and Shigella at low frequencies from wild meat carcasses in two markets in Gabon, emphasizing the potential transmission risk although the overall risk is low. Transmission of bacteria can occur through direct exposure to faeces or bodily fluids, to which hunters are exposed, or indirectly via fleas and ticks as in the case of F. tularensis or for ticks collected from duikers and a pangolin that harboured the bacterium Rickettsia africae, which causes African tick-bite fever, and, thus, pose a zoonotic risk (Mediannikov et al. Reference Mediannikov, Diatta and Zolia2012). Another example of bacterial infection is Mycobacterium ulcerans that is transmitted from plants to grasscutters (greater cane rats) and then to people who hunt and use them as wild meat, causing Buruli ulcer in the skin and subcutaneous tissues (Hammoudi et al. Reference Hammoudi, Dizoe and Saad2020). The disease is endemic especially in West Africa, but the impact is much more small-scale compared to the above introduced viral emerging zoonoses as it is noncontagious.
Spillover of many helminth species is likely (Kurpiers et al. Reference Kurpiers, Schulte-Herbrüggen, Ejotre, Reeder and Angelici2016). For example, very high prevalence rates of helminth ova were found in greater cane rats and bush duikers from wild meat markets in Nigeria (Adejinmi & Emikpe Reference Adejinmi and Emikpe2011). Because humans and non-human primates share susceptibility to many parasitic helminth species (Pedersen et al. Reference Pedersen, Altizer, Poss, Cunningham and Nunn2005), it is highly relevant that high loads of gastrointestinal parasites were present in the monkey species traded in a wild meat market in Cameroon (Pourrut et al. Reference Pourrut, Diffo and Somo2011). A similar risk as helminths is posed by protozoans, for example the diarrheal disease-causing Amoebozoa which have been confirmed in wild meat species (Pourrut et al. Reference Pourrut, Diffo and Somo2011). No transmissions of fungi and prions have been documented, but these constitute potential zoonotic risk nevertheless (Kurpiers et al. Reference Kurpiers, Schulte-Herbrüggen, Ejotre, Reeder and Angelici2016). It has not only been difficult to find undisputable evidence to demonstrate the zoonotic transmission of specific pathogens from specific host species, but the exact risk and the frequency of transmission to wild meat hunters remains unknown for many pathogens.
7.5 Risk Factors for Zoonotic Disease Emergence
7.5.1 Hosts
In terms of numbers of pathogens, rodents, followed by bats are the most abundant and most species-rich mammal order (Fig. 7.6, Han et al. Reference Dixon, Loh, Davidson, Beltrame, Freeman and Walpole2016). They also include a greater number of zoonotic hosts than any other order, carrying 85 known zoonotic diseases. However, zoonotic viruses are most abundant in domesticated species, primates and bats (Johnson et al. Reference Johnson, Hitchens and Pandit2020). The relative risk of disease emergence is highest for bats, followed by primates and then ungulates and rodents (Cleaveland et al. Reference Cleaveland, Haydon, Taylor, Childs, 335Mackenzie and Richt2007). More than 200 viruses are harboured in bats, many of them causing zoonotic disease (Allocati et al. Reference Allocati, Petrucci, Di Giovanni, Masulli, Di Ilio and De Laurenzi2016). For example, coronaviruses including SARS-CoV, SARS-CoV-2 and MERS-CoV likely originated in bats, but dromedary camels are intermediate hosts, a current natural reservoir and potential source for zoonotic transmission of MERS-CoV. Because bats host many coronaviruses, which represent 31% of their virome (Chen et al. Reference Chen, Liu, Yang and Jin2014), and because they are remarkably resistant to viruses (Storm et al. Reference Storm, Jansen Van Vuren, Markotter and Paweska2018), the risk of emergence of a novel bat-CoV disease is high (Afelt et al. Reference Afelt, Frutos and Devaux2018a). Bats are widely hunted in Africa and Asia (Kamins et al. Reference Kamins, Baker, Restif, Cunningham, Wood, Paulsen, Bauer, Vodnansky, Winkelmayer and Smulders2014; Mickleburgh et al. Reference Mickleburgh, Waylen and Racey2009; Mildenstein et al. Reference Mildenstein, Tanshi, Racey, Voight and Kingston2016).
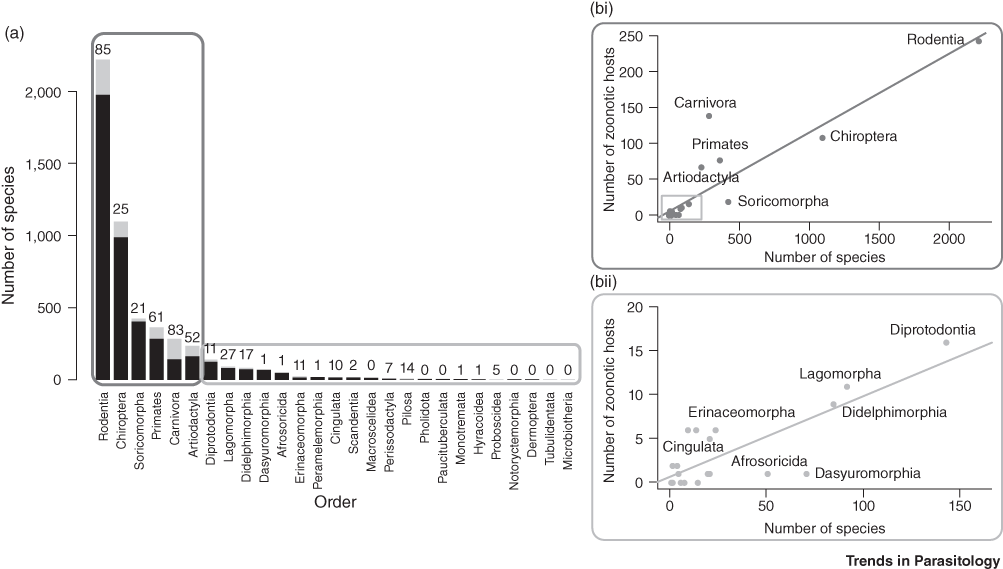
Figure 7.6 The number of zoonotic hosts increases with total species richness of the order. (a) This split bar plot shows the total number of host species (black plus grey) and the fraction of species that are confirmed zoonotic hosts for one or more zoonotic diseases (grey). The number above each bar represents a tally of the total unique zoonoses per order. Mammal orders are arranged in descending order of species richness. (b) The number of zoonotic host species in each order is represented by scatterplots. (i) The most-speciose orders being are shown in the upper chart (R2 = 0.81); (ii) all other orders in lower chart (regression R2 = 0.63).
7.5.2 Wild Meat Hunting and Trade
In the early stages of the COVID-19 pandemic, China banned wildlife trade and consumption of wild meat through the 16th meeting of the Standing Committee of the 13th National People’s Congress, on ‘Comprehensively Prohibiting the Illegal Trade of Wild Animals, Eliminating the Bad Habits of Wild Animal Consumption, and Protecting the Health and Safety of the People’ of 24 February 2020. The Wildlife Conservation Society hailed the decision ‘for not only solving the COVID-19 outbreak but in preventing future risks through legislative reform and improved enforcement and management’ (WCS Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). On 25 February 2020, one of the first international actions to address the danger of zoonotic disease in the wake of the COVID-19 pandemic was the demand to close wildlife markets as outlined in an open letter to the World Health Organization, the UN Environment Programme and the Office International Epizoologie (Born Free Foundation 2020). The letter, undersigned by 236 international organizations and individuals, emphasizes the increasing risks to global human and animal health and the animal welfare problems. Whilst the open letter’s primary demand is to close wildlife markets and to ban trade of live wild animals in order to protect human health, it implicitly extends to ‘products derived from them’, thus wild meat in general since wildlife markets primarily rely on animals taken from the wild but with slaughtering taking place at the market or the buyer’s place rather than in the wild. Wild meat hunting and wildlife trade are two sides of the same coin. Indeed, subsequent bans on trade of wildlife included life wild animals and any products derived from them, for example, in Vietnam (Ratcliffe Reference Ratcliffe2020).
Although the exact pathways of the zoonotic emergence remain unsolved, the 2003 SARS and, possibly, the 2019/20 COVID-19 coronavirus outbreaks demonstrate the wildlife trade’s zoonotic disease risk. Especially when markets sell live animals, the so-called ‘wet’ markets, the combination of high wildlife volumes, taxonomic diversity, crammed and stressful conditions for the captive wildlife, taxa with high risk for zoonoses, poor biosafety and close contact between wildlife, domestic animals and humans contribute to a high potential for pathogen transmission. Often, live wild animals and domestic animals are housed alongside each other, with domestic animals also implicated in the transmission of zoonotic disease such as the avian influenza A H7N9 virus (Li et al. Reference Li, Zhou and Zhou2014; Yu et al. Reference Aliaga-Rossel and Fragoso2014). Turnover of live and dead animals is enormous. For example, after the outbreak of SARS in November 2002 more than 800,000 endangered animals were confiscated from the markets in China‘s southern province of Guangdong, where SARS originated, up to April 2003 (BBC Reference Allan, Keesing and Ostfeld2003). During 25 weekends of the Bangkok Weekend Market approx. 70,000 birds of 276 species and approx. 3,500 native animals of at least 24 species were sold (Round Reference Round1990). Numbers of wild meat outlets, that is markets, restaurants, butchers and street vendors, in the Kinshasa–Brazzaville metropolitan area are estimated at 366 wild meat outlets per 100,000 inhabitants in Brazzaville and just over 700 per 100,000 inhabitants in Kinshasa (Fa et al. Reference Auzel2019). Only the trade in narcotics exceeds illegal wildlife trade in volume in the worldwide black market (Toledo et al. Reference Toledo, Asmüssen and Rodríguez2012).
These conditions in wet markets create perfect storms for pathogen cross-species and zoonotic transmission. Taxa sold as wild meat in restaurants, roadside stalls and markets in Malaysia potentially contain 51 zoonotic pathogens (16 viruses, 19 bacteria and 16 parasites), highlighting the extent of the problem (Fig. 7.7, Cantlay et al. Reference Cantlay, Ingram and Meredith2017). All samples from illegally imported African wild meat confiscated at Paris Charles de Gaulle airport had viable counts of bacteria above levels considered safe for human consumption including the pathogens Staphylococcus aureus and Listeria monocytogenes which are associated with food-borne illnesses (Chaber & Cunningham Reference Chaber and Cunningham2016). Trade of West African rodents to the USA triggered a local outbreak of monkeypox in prairie dogs and eventually zoonotic transmission to humans (Reed et al. Reference Reed, Melski and Graham2004). The potential effect of trading activities along the market chain is demonstrated by a study on the prevalence of SARS-CoV in civets, the likely intermediate host responsible for the initial zoonotic SARS-CoV spillover. Whilst civets on farms were largely free from SARS-CoV infection, the prevalence in one animal market in China‘s Guangzhou was approx. 80% (Tu et al. Reference Cowlishaw, Mendelson and Rowcliffe2004). Another study demonstrated that the transmission risk increases along wildlife supply chains for human consumption in Vietnam (Huong et al. Reference Huong, Nga and Long2020): for field rats, the odds of coronavirus RNA detection significantly increased along the supply chain from animals sold by traders by a factor of 2.2 for animals sold in large markets and by a factor of 10.0 for animals sold and served in restaurants.
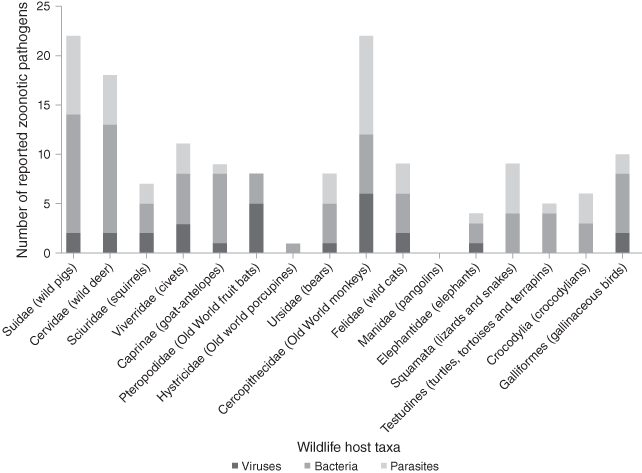
Figure 7.7 Total numbers of viral, bacterial and parasitic pathogens reported in traded wildlife taxa in Malaysia.
The opportunities for zoonotic spillover have increased in parallel with the increase in the intensity and extent of wild meat trade over the last decades (Karesh & Noble Reference Karesh and Noble2009). Encroaching of remaining intact forests by road building, forestry and mining have made vast new areas accessible for wild meat hunting, thus increasing the zoonotic risk by not only bringing humans in contact with hitherto undisturbed host and pathogen populations, but also by increased wild meat hunting. For example, Poulsen et al. (Reference Clark, Poulsen, Malonga and Elkan2009) monitored the supply and household consumption of wild meat in a logging concession in the Congo Basin and observed a 69% increase in the population of logging towns and a 64% increase in wild meat supply. It is not only the increasing human population density in the logging areas, but also the increase of disposable incomes and few other dietary options which drives demand for wild meat in logging camps (Auzel & Wilkie Reference Auzel, Wilkie, Robinson and Bennett2000). Commercial logging has encouraged subsistence hunters to engage in or contribute to hunting as a commercial enterprise (Walsh et al. Reference Walsh, Abernethy and Bermejo2003). Armed conflicts also contributed to the scaling up of wild meat extraction. For example, the sales of protected species in urban markets in the Congo Basin increased five-fold in wartime (De Merode & Cowlishaw Reference De Merode and Cowlishaw2006).
Wild meat hunting certainly carries a high zoonotic risk, whether it is the hunting activity in the forest such as in the case of Mayaro virus and tularaemia, the butchering of infected animals, such as in the case of zoonotic emergence of HIV via spillover of SIV to humans, or whether by capture of wild animals who then enter the live animal markets, such as likely in the case of SARS and COVID-19. A pre-COVID-19 review of transmission pathways for emerging zoonoses from 1940 onwards identified only four cases where wild meat was likely the causative agent for the spillover: Monkeypox virus, SARS, Sudan Ebola virus and Zaire Ebola virus (Loh et al. Reference Loh, Zambrana-Torrelio and Olival2015). This places wild meat only in ninth place, which is shared with the breakdown of public health services, of 11 primary drivers of zoonotic disease events (Loh et al. Reference Loh, Zambrana-Torrelio and Olival2015). Figure 7.8 shows the geographic distribution of zoonotic diseases and the underpinning drivers (Keesing et al. Reference Keesing, Belden and Daszak2010).

Figure 7.8 Drivers and locations of emergence events for zoonotic infectious diseases in humans from 1940 to 2005. (a) Worldwide percentage of emergence events caused by each driver. (b) Countries in which the emergence events took place, and the drivers of emergence.
The report by UNEP and the International Livestock Research Institute (2020) on preventing the next pandemic lists seven human-mediated factors as the most likely driving the emergence of zoonotic diseases:
increasing human demand for animal protein;
unsustainable agricultural intensification;
increased use and exploitation of wildlife;
unsustainable utilization of natural resources accelerated by urbanization, land use change and extractive industries;
increased travel and transportation;
changes in food supply; and
climate change.
Wild meat features in the factor ‘increasing human demand for animal protein’ as intensified forestry and mining causes increased demand for wild meat. It also features in ‘increased use and exploitation of wildlife alongside recreational hunting and consumption of wildlife as a status symbol, trade in live animals for recreational use (pets, zoos) and for research and medical testing, and use of animal parts for decorative, medicinal and other commercial products. Nevertheless, the majority of these factors are not related to wild meat, whether dead or alive.
7.5.3 Environmental Change
An analysis of correlates with zoonotic diseases demonstrated that zoonotic risk is elevated in forested tropical regions with high mammal species biodiversity which experience land-use changes (Allen et al. Reference Allen, Murray and Zambrana-Torrelio2017). Risk of disease emergence is elevated in tropical regions in North and Central America, Asia, Central Africa, and regions of South America (Fig. 7.9). The mechanisms underlying this process are complex. Greater host biodiversity and their associated larger diversity of pathogens increase the potential for novel zoonotic disease emergence (Murray & Daszak Reference Carroll, Daszak and Wolfe2013). On the other hand, increased biodiversity has been hypothesized to decrease zoonotic risk and vice versa because of a dilution effect. This has been demonstrated for Lyme disease (Allan et al. Reference Allan, Keesing and Ostfeld2003), hantavirus (Suzán et al. Reference Fuentes-Montemayor, Cuarón, Vázquez-Domínguez, Benítez-Malvido, Valenzuela-Galván and Andresen2009) and West Nile virus (Ezenwa et al. Reference Ezenwa, Godsey, King and Guptill2006). However, the general applicability of this has been widely refuted (Clay et al. Reference Clay, Lehmer, Jeor and Dearing2009; Salkeld et al. Reference Salkeld, Padgett and Jones2013). Empirical and modelling data have demonstrated high complexity with declining habitat, and thus declining biodiversity, leading to either increasing or decreasing infectious disease risk, depending on the pathogen transmission mode and how host competence scales with body size (Faust et al. Reference Faust, Dobson and Gottdenker2017). Lyme disease is the best-known example that has been assumed to follow the dilution effect (Allan et al. Reference Allan, Keesing and Ostfeld2003). The pathogen is a spirochete bacterium Borrelia burgdorferi, which is transmitted by ixodid ticks vectors. These ticks feed on white-footed mice when young and on white-tailed deer as the primary host when adult. Detailed analyses have now shown a much more complex and scale-dependent disease dynamics for Lyme disease (Wood & Lafferty Reference Wood and Lafferty2013). The recent hypothesis of the ‘coevolution effect’ suggests that anthropogenically created forest fragments serve as islands harbouring wildlife hosts of pathogens that undergo rapid genetic diversification, leading to greater probability that one of these pathogens will spillover into human populations (Keesing et al. Reference Keesing, Belden and Daszak2010; Zohdy et al. Reference Zohdy, Schwartz and Oaks2019).
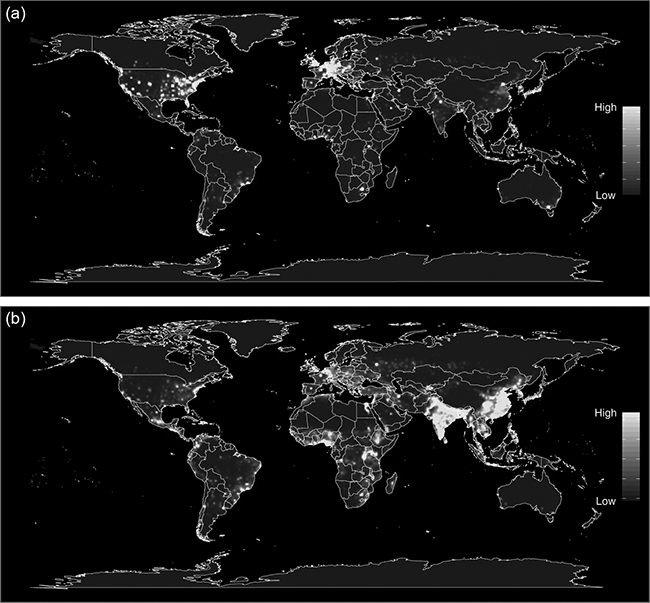
Figure 7.9 Heat maps of predicted relative risk distribution of zoonotic emerging infectious disease events: (a) the predicted distribution of new events being observed; (b) the estimated risk of event locations after factoring out reporting bias.
A meta-analysis of publications on the effect of anthropogenic land use change on infectious disease dynamics revealed that 57% of studies documented increased pathogen transmission, 10% decreased pathogen transmission, 30% demonstrated complex pathogen responses and 2% showed no detectable changes (Gottdenker et al. Reference Gottdenker, Streicker, Faust and Carroll2014). Examples for increased pathogen transmission include Ebola and Nipah as outlined above. Others are yellow fever and rabies with expansion into the forest by human settlements being a frequent cause of outbreaks (Wilcox & Ellis Reference Wilcox and Ellis2006), or the tapeworm Echinococcus multilocularis which is correlated with overgrazing of pastures resulting in increases of small mammal and disease densities (Craig Reference Craig2006) to name but a few. The mosquito genera Aedes, Anopheles and Culex, which include the most important vectors for mosquito-borne diseases such as malaria, dengue and yellow fever, were more commonly encountered in disturbed habitats and had higher virus prevalence than forest mosquitoes did (Junglen et al. Reference Junglen, Kurth and Kuehl2009). An analysis of 6,801 ecological assemblages and 376 host species worldwide showed that sites under substantial human use had wildlife hosts of human-shared pathogens and parasites with a greater proportion of local species richness (18–72% higher) and total abundance (21–144% higher) compared with nearby undisturbed habitats (Gibb et al. Reference Gibb, Redding and Qing Chin2020). The effect was strongest for rodent, bat and passerine bird zoonotic host species. Mammal species harbouring more pathogens overall are more likely to occur in human-managed ecosystems.
Ecotones, the boundary between ecological systems, play key roles in the ten diseases for which information exists (Despommier et al. Reference Despommier, Ellis and Wilcox2007). These ten diseases are caused by viruses (sin nombre, yellow fever, Nipah, influenza, rabies), bacteria (Lyme disease, cholera, leptospirosis) and protozoa (malaria, sleeping sickness), and are in most cases zoonotic. These diseases are ecologically similar to about half of the known zoonotic emerging infectious diseases, indicating a general importance of ecotones, particularly their anthropogenic origination or modification (Despommier et al. Reference Despommier, Ellis and Wilcox2007). Olivero et al. (Reference Olivero, Fa and Real2017) analysed 27 EVD outbreak sites and 280 comparable control sites and showed that outbreaks along the edges of the rainforest biome were significantly associated with forest losses within the previous three years (Olivero et al. Reference Olivero, Fa and Real2017).
Gottdenker et al.’s (Reference Gottdenker, Streicker, Faust and Carroll2014) meta-analysis identified the most common types of land use change related to zoonotic disease transmission as deforestation, habitat fragmentation, agricultural development, irrigation and urbanization. Human encroachment has caused some bat species to become peridomestic, thus making them more vulnerable to hunting and increasing the zoonotic risk such as in the case of Nipah and Hendra (Kamins et al. Reference Kamins, Restif, Rowcliffe, Cunningham and Wood2011b; Plowright et al. Reference Plowright, Foley and Field2011). Bats are also highly susceptible to deforestation, which isolates or divides populations, changes contact rates with other bat species, alters behaviour, compromises ecosystem functions and increases emergence of pathogens (Willig et al. Reference Willig, Presley and Plante2019). For example, in Brazil bats near human settlements in deforested areas have a viral prevalence of coronaviruses of 9.3% compared to 3.7% in forested areas (EcoHealth Alliance & University of Sao Paulo 2015). Changes of animal guild compositions such as for bats due to deforestation (Willig et al. Reference Willig, Presley and Plante2019) also happen due to selective hunting. For example, the removal of large carnivores from a savanna ecosystem in East Africa caused rodent and, consequently, flea abundance to double and, thus, elevating the risk for zoonotic transmission of Bartonella bacteria, which cause bartonellosis (Young et al. Reference Dirzo, Young, Galetti, Ceballos, Isaac and Collen2014).
Climate change will not only alter climatic conditions but also habitat structure and distribution. Alongside, it is likely that the geographic distribution of zoonotic diseases will change, especially for vector-borne diseases, such as Rift Valley fever, yellow fever, malaria and dengue, which are all highly sensitive to climatic conditions (Martin et al. Reference Martin, Chevalier and Ceccato2008). For example, change in rainfall patterns triggered malaria re-emergence in Anhui Province, China (Gao et al. Reference Gao, Wang and Liang2012). The geographic area of many infectious diseases will expand into previously disease-free areas. Between 1998 and 2005, changes in European climate have caused bluetongue virus, which causes an insect-borne disease of ruminants, to spread 800 km northward in Europe as a consequence of the northward expansion of the African midge Culicoides imicola, the main bluetongue virus vector, and the recruitment of indigenous European Culicoides species as vectors (Purse et al. Reference Purse, Mellor, Rogers, Samuel, Mertens and Baylis2005). Ecological niche modelling showed that the habitat range and distribution of the bat reservoir species for Nipah will likely change under climate change scenarios, increasing risk for zoonotic transmission (Daszak et al. Reference Carroll, Daszak and Wolfe2013). Changes in avian migratory routes as a consequence of temperature changes of aerial streams can explain the outbreak of West Nile virus in Southeast Europe (Mills et al. Reference Mills, Gage and Khan2010). Climate change will impose very complex changes on zoonotic disease distribution and evolution of novel susceptible immunocompromised populations including the very complex dynamics of evolution of virulence/resistance and genomic variability of zoonotic agents (Cascio et al. Reference Cascio, Bosilkovski, Rodriguez-Morales and Pappas2011).
7.5.4 Poverty
A number of zoonotic diseases disproportionately affect poor and marginalized populations but are largely ignored by public health and veterinary services. The WHO has designated them as ‘neglected diseases’ (Molyneux et al. Reference Molyneux, Hallaj and Keusch2011). Although treatments exist, action is often lacking (Wielinga & Schlundt Reference Wielinga, Schlundt, Mackenzie, Jeggo, Daszak and Richt2013). For example, rabies remains a neglected disease in Africa and Asia and, despite that there being vaccinations for humans and wildlife, the mortality rate is about 55,000 per year (Knobel et al. Reference Knobel, Cleaveland and Coleman2005). Parasitic diseases including schistosomiasis, cysticercosis, trematodiasis, taeniasis and echinococcosis are predominant amongst the neglected tropical diseases. Wild meat hunters are amongst the poorest people and any zoonotic infection remains often treated only with traditional and not modern medicine. For example, the factors that best predict lemur hunting are poverty, poor health and child malnutrition, whereas knowledge of laws, level of education, involvement in ecotourism, traditional cultural values, taste preferences, opportunity and human–wildlife conflict had no impact (Borgerson et al. Reference Borgerson, McKean, Sutherland and Godfrey2016). In Tanzania, questionnaires confirmed a strong linkage between poverty and poaching (Knapp et al. Reference Knapp, Peace and Bechtel2017). In Uganda, those arrested for unauthorized activities in a national park were significantly poorer than others (Twinamatsiko et al. Reference Twinamatsiko, Baker and Harrison2014). Similarly, one of the most effective ways to reduce illegal wildlife hunting in Uganda is poverty alleviation (Harrison et al. Reference Harrison, Roe and Baker2015).
Poverty is linked with human health and access to health care systems. A study in Madagascar showed that consuming more wildlife was associated with significantly higher haemoglobin concentrations and that removing wild meat would triple anaemia cases among children in the poorest households (Fig. 7.10; Golden et al. Reference Brashares, Golden, Weinbaum, Barrett and Okello2011). Yet, wild meat hunters such as the Baka Pygmies face health challenges due to their limited access to and discrimination in public health centres and being more likely than their non-Pygmy neighbours to mention not using modern health care due to cost (Carson et al. Reference Carson, Kentatchime and Sinai2019). Baka Pygmies in Cameroon are also particularly disadvantaged and in general exhibit poor health. They are the Indigenous group with the largest difference in life expectancy, 22 years, compared with their non-Indigenous neighbours amongst all studied populations (Anderson et al. Reference Anderson, Robson and Connolly2016). For Indigenous Peoples, such as the Baka, consuming and also selling wild meat remains the backbone of their ways of life and food security (Fa et al. Reference Fa, Olivero and Real2015b), despite the fact that numerous groups are no longer fully nomadic but have been dragged into our economic system. This reliance on wild meat combined with lack of access to modern health care means that Pygmy people are not only especially exposed to zoonotic diseases because of their hunting activities, but zoonotic spillovers will remain undetected until any resulting infectious disease has reached the non-Pygmy neighbours and people who can afford modern health care.
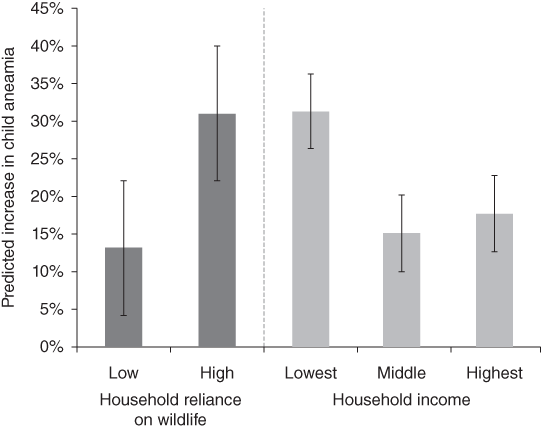
Figure 7.10 Wildlife loss induces major increases in childhood anaemia that is modified by household-level characteristics. Predictive models of the association between wildlife consumption and children’s haemoglobin concentrations (n = 77) demonstrate that removing wildlife from the diet engenders a disproportionate risk of developing anaemia in households with a high reliance on wildlife and in low-income households.
7.6 Solutions
The establishment of diseases throughout history has been described as ‘a side effect of the growth of civilisation’ (Dobson & Carper Reference Dobson and Carper1996). Yet, the enormous human and socio-economic costs cry for solutions. The pandemics of COVID-19, Ebola, HIV and SARS have sharpened humanities’ perception of the worldwide misery caused by these diseases. It is not only the mortality rate, which can be very high (e.g. up to 88% for Ebola), but the disruption of society and commerce to control the disease as poignantly laid bare by COVID-19. Knock-on effects, such as loss of investment, reduced international tourism and unemployment, to name but a few make it notoriously difficult to estimate the total economic cost (Smith et al. Reference Marlowe and Kent2019). For example, the 2014–2016 Ebola crisis in West Africa caused at least 28,616 suspected cases and 11,310 confirmed deaths in Guinea, Liberia and Sierra Leone, the mainly affected countries (WHO 2016a). The overall economic cost has been estimated at US$2.8 billion for these three countries including decreases of Gross Domestic Product (GDP) growth, declining government revenues and loss in private and foreign investors’ confidence (World Bank 2016). The loss of investor confidence alone cost US$600 million. The international cost for fighting the epidemic by the end of 2015 was more than $3.6 billion (Centers for Disease Control and Prevention 2016). All these numbers, however, do not include indirect effects. For example in West Africa, the entire healthcare workforce declined and led to an estimated 10,600 additional deaths due to untreated conditions, childhood vaccination coverage decreased by 30%, 17,300 children lost one or both parents and more than 33 weeks of education were lost due to school closures (Centers for Disease Control and Prevention 2016). Moreover, local quarantine and travel restriction measures and enforcement led to illegal poaching, logging and mining and negatively impacted previous advances in environmental protection (Smith et al. Reference Marlowe and Kent2019). All of these costs for Ebola are, however, overshadowed by COVID-19 whose economic damages have been estimated at US$8.1–15.8 trillion with at least US$5 trillion for 2020 (Dobson et al. Reference Dobson, Pimm and Kaufman2020). The large uncertainty in the cost estimate is because the estimate was conducted only seven months into the pandemic and without knowledge whether and when a vaccine against COVID-19 would be available (Dobson et al. Reference Dobson, Pimm and Kaufman2020).
Finding a solution to the zoonotic crisis is difficult because so many stakeholders and competing interests are involved. For example, China‘s ban on trade and consumption of terrestrial wild animals has met with support from various quarters, especially the international conservation and animal welfare lobby (Born Free Foundation 2020; Diamond & Wolfe Reference Diamond and Wolfe2020; WCS Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). Others have called for much more cautionary approaches (FAO 2020a,b; SWM Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). A successful regulation or ban of live and butchered wild meat will indeed avoid zoonotic risk especially for those involved in the wild meat chain and provide a cost-effective approach to decrease the risks for disease for humans, domestic animals, wildlife and ecosystems (Karesh et al. Reference Karesh, Cook, Bennett and Newcomb2005). However, there are three major problems with the approach.
First, such bans have been implemented in many countries, but limited law enforcement have either rendered these laws as paper tigers or enforcement actually drove the trade into illegality. For example, following the 2014–2016 outbreak of Ebola virus disease in West Africa, governments imposed such bans on the hunting and consumption of meat from wild animals jointly with information campaigns on the infectious potential of wild meat (Bonwitt et al. Reference Bonwitt, Dawson and Kandeh2018). The three mainly affected countries Guinea, Liberia and Sierra Leone banned the sale of wild meat (Samb & Toweh Reference Samb and Toweh2014). However, the criminalization of wild meat consumption entrenched distrust towards outbreak responders and governments whilst messaging contradicted people’s own experience because they had always eaten wild meat without any incident (Bonwitt et al. Reference Bonwitt, Dawson and Kandeh2018). Subsequently, informal and thus illegal networks of wild animal trade proliferated and undercut any meaningful ‘development of acceptable, evidence-based surveillance and [made] mitigation strategies for zoonotic spillovers almost impossible’ (Bonwitt et al. Reference Bonwitt, Dawson and Kandeh2018). Indeed, informality and illegality are major obstacles to implementing policies on health and sustainable wildlife management.
Second, a generalized ban ignores both the dependency on wild meat of many people and the rights of Indigenous Peoples, who have hunted for millennia. Consumption of wild meat is the basis for food security in many rural communities (Friant et al. Reference Friant, Ayambem and Alobi2020). Overhunting and unsustainability are driven by modern market economies by people who buy wild meat as luxury items (Wolfe et al. Reference Wolfe, Daszak, Kilpatrick and Burke2005a) whilst Indigenous Peoples reacting to rather than causing the excessive demand. Under the pressures of poverty ‘it is no wonder that hunters are lured into commercial’ wild meat (Volpato et al. Reference Volpato, Fontefrancesco, Gruppuso, Zocchi and Pieroni2020). Therefore, we have to distinguish hunters and subsistence hunting on one hand and buyers and commercial hunters on the other hand. We need to find solutions for each group.
Buyers from urban, national and international markets are typically driving unsustainable exploitation where income generated from this livelihood activity will likely be short-lived, following a boom–bust cycle but where the depletion of wildlife is long-lasting (Fa et al. Reference Fa, Currie and Meeuwig2003). This ultimately risks increasing malnutrition and poverty for rural populations who rely on this resource for their subsistence and cultural identity. Here we need adequate legislation that limits trade to sustainable levels. Legislation must enable management and monitoring of harvesting, use and trade of wildlife. To avoid the pitfalls of illegality, which are difficult to counteract as amply demonstrated by the narcotics trade, ‘well-regulated and well monitored wildlife use and trade will encourage the long-term conservation of biodiversity, ensure good animal and human health, as well as combat illegal, unhealthy or unsustainable practices’ (FAO 2020a,b). Moreover, total bans will often drive the market into illegality as demonstrated by the unintended consequences of the wild meat ban in West Africa following the 2014–2016 Ebola epidemics (Bonwitt et al. Reference Bonwitt, Dawson and Kandeh2018).
From the hunters’ perspective we first of all need to acknowledge that Indigenous Peoples, who have hunted for millennia and critically depend on wild meat for their protein intake, have an inalienable right to harvest wild meat akin to Indigenous whaling rights (Fitzmaurice Reference Fitzmaurice2010). According to the UN Secretary-General: ‘It is critical for countries to marshal the resources to respond to their needs, honour their contributions and respect their inalienable rights’ (Guterres Reference Guterres2020). Consequently, it is essential that Indigenous Peoples are not only included in the COVID-19 response but that they are consulted and empowered to contribute and participate in policy planning and the drafting and execution of new laws that aim to avoid or better manage future spillovers. However, the use must be sustainable. Sustainable use of biodiversity is a key component of the UN Convention on Biological Diversity. The sustainable use should also include a trading component that is geographically restricted to the rural areas of origin. However, unsustainable use of wild meat may also decrease human welfare where people are dependent on wild meat (Duffy et al. Reference Duffy, St John, Büscher and Brockington2016; Golden et al. Reference Brashares, Golden, Weinbaum, Barrett and Okello2011). The ultimate aim is to find a balance between people’s rights and conservation whilst minimizing zoonotic risk. Concrete actions should include the following and see also (FAO 2020a,b), SWM (Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020) and UNEP & International Livestock Research Institute (2020):
(i) Wildlife legislation needs to adequately protect and regulate the sustainable use of wildlife whilst taking into account the environmental and social needs and practices of local people and zoonotic risk. In Africa, such laws typically exist but wildlife is hunted as an unregulated open access resource (Bennett et al. Reference Bennett, Blencowe and Brandon2007). Importantly, this legislation needs enforcement and monitoring but also needs to support the protection of livelihoods of those communities dependent on wild animals for food and income.
(ii) Animal health legislation for cases where trade in live animals remains permitted needs to be based on international standards and regulations as advocated by the World Animal Health Organization (OIE), founded in 1924. It is the intergovernmental organization responsible for improving animal health worldwide and has a total of 182 Member Countries as of 2018 (www.oie.int).
(iii) Legislation for food safety and surveillance along the wild meat chain are key factors in controlling zoonotic risks associated with wildlife meat consumption and trade. Again, it cannot be overstated that it is important to work with communities and stakeholders as one-sided imposition of laws and regulations can achieve the opposite of intended results (Grace et al. Reference Grace, Dipeolu and Alonso2019).
(iv) Education and awareness building are cornerstones for behavioural change (Kuisma et al. Reference Kuisma, Olson and Cameron2019; Monroe & Willcox Reference Monroe and Willcox2006). Often hunter behaviours – for example, eating animals found dead or sick (Smiley Evans et al. Reference Smiley Evans, Myat and Aung2020) – are highly risky. Risk-reduction education programmes can help hunters and consumers minimize their risk, for example, by encouraging hunters not to butcher when there are injuries on their hands or limbs, to avoid all contact with animals found dead in the forest or to avoid riskier species, such as bats and primates (LeBreton et al. Reference LeBreton, Pike, Saylors, Aguirre, Ostfeld and Daszak2012; Pike et al. Reference Pike, Saylors and Fair2010). In general, awareness of zoonotic risk amongst hunters, butchers, vendors and consumers is, however, low (Kamins et al. Reference Kamins, Rowcliffe, Ntiamoa-Baidu, Cunningham, Wood and Restif2015; Ozioko et al. Reference Ozioko, Okoye, Obiezue and Agbu2018; Pruvot et al. Reference Pruvot, Khammavong and Milavong2019; Smiley Evans et al. Reference Smiley Evans, Myat and Aung2020; Subramanian Reference Subramanian2012). Where knowledge exists, people might be less likely to engage in wild meat hunting and butchering (LeBreton et al. Reference LeBreton, Prosser and Tamoufe2006; Subramanian Reference Subramanian2012), but knowledge is often not translated into behaviours which needs to be addressed by a culturally sensitive intervention programme, designed and implemented through collaboration between the education, public health, veterinary and wildlife authorities with wild meat stakeholders (Alhaji et al. Reference Alhaji, Yatswako and Oddoh2018; LeBreton et al. Reference LeBreton, Prosser and Tamoufe2006; Muehlenbein Reference Muehlenbein2017; Wilkie Reference Wilkie2006). For example, although the knowledge about anthrax was very high among butchers, owners, herdsmen and consumers in Ghana, 64% of respondents thought that meat from cattle suspected of having died from anthrax was suitable for consumption (Opare et al. Reference Opare, Nsiire, Awumbilla and Akanmori2000). The pitfalls are also highlighted by Ebola awareness campaigns which contradicted people’s perceptions of low life-time risk of wild meat (Samb & Toweh Reference Samb and Toweh2014), thus squandering trust in governments and driving the wild meat market into illegality (Bonwitt et al. Reference Bonwitt, Dawson and Kandeh2018). Behavioural change can be short-term. For example in Nigeria, wild meat consumption crashed after the 2014–2016 West African Ebola outbreak but immediately returned to pre-Ebola levels in some areas after the country was declared Ebola-free (Ogoanah & Oboh Reference Ogoanah and Oboh2017; Onyekuru et al. Reference Onyekuru, Ezea and Ihemezie2018). On the other hand, trade of wild meat in other Nigerian markets did recover only slightly up to 2020 but never reaching pre-Ebola levels (Funk et al. Reference Fa, Nasi and Funk2021). Especially young and urban people stopped consuming wild meat, indicating that mild wild meat consumers can be highly sensitized and that further education campaigns might achieve long-term behavioural change (Funk et al. Reference Fa, Nasi and Funk2021). Education also needs to address the mistaken idea that persecution of animals suspected to transmit disease will solve the problem. We not only need to acknowledge that wildlife and humans are inter-dependent – for example, gorillas and chimpanzees suffered also from the same Ebola outbreak as humans or were infected by humans with respiratory pathogens (Spelman et al. Reference Spelman, Gilardi and Lukasik-Braum2013) – and that eradication measures might actually make the problem worse (as in the case of the Marburg virus, outlined above) but also that humans critically depend on the same species that carry pathogens, such as the pollination ecosystem service by bats. Often, education projects are implemented on the ground but fail to measure whether these efforts resulted in actually changed behaviour (e.g. Kuisma et al. Reference Kuisma, Olson and Cameron2019). Therefore, monitoring the effectiveness of education programmes and changing approaches, if applicable, are vital.
(v) Pathogen surveillance and research are needed to establish a sufficient knowledge basis on the diversity of pathogens in different guilds of wild and domestic animals in their concrete site-specific settings. Only this knowledge will allow the development of sufficiently accurate risk assessment models that predict pathogen transmission from wild animals to domestic animals and humans.
(vi) Because zoonotic diseases emerge not only from wildlife hunting but also from our modern livestock production systems, such as pig farming for Nipah virus or the 2009 H1N1 influenza pandemic, which originated in North American pig farms (Mena et al. Reference Mena, Nelson and Quezada-Monroy2016), a general reconsideration and restructuring of our food systems is required (FAO 2020a,b; IPES-Food Reference Friant, Ayambem and Alobi2020). The International Panel of Experts on Sustainable Food Systems highlights that intensive livestock production amplifies the risks of diseases emergence and spread and that commercial agriculture exacerbates zoonotic risk by commercial agriculture driving habitat loss and creating the conditions for viruses to emerge and spread (IPES-Food Reference Friant, Ayambem and Alobi2020). Increased substitution of wild meat with domestic animals, whether from global commercial systems or local subsistence husbandry, appears logical, but might accelerate the zoonotic problems because of the emergence of new pathogens and hosts. Commercial systems are intrinsically connected with the conversion of land for agricultural use, which constitutes the highest risk factor for the emergence of zoonotic disease. Even when domestic animals are raised locally in the tropics and subtropics in a sustainable manner, zoonotic risk may be increased, because these animals are raised in areas also frequented by wildlife.
Third, regarding the avoidance of future disease emergence and pandemics, the regulation or ban of live and butchered wild meat might demonstrate political actions and reassurance to the general public that something is being done by governments and politicians during an actual epidemic such as COVID-19. However, if the aim is to prevent pandemic zoonotic diseases, it will certainly not be sufficient – and might constitute nothing but a political smokescreen – considering that the vast majority of cases are based on anthropogenic environmental change and agricultural intensification. ‘Although enforcement of hunting laws and promotion of alternative sources of protein may help curb the pressure on wildlife, the best strategy for biodiversity conservation may be to keep sawmills and the towns that develop around them out of forests’ (Poulsen et al. Reference Clark, Poulsen, Malonga and Elkan2009). The UN report prepared in the wake of COVID-19 highlights that we currently treat the symptoms of the COVID-19 pandemic but not the underlying issues (UNEP & International Livestock Research Institute 2020). Indeed the significantly increased number of incidences of emerging infectious diseases since the 1940s (Fig. 7.11; Jones et al. Reference Jones, Patel and Levy2008; Smith et al. Reference Effiom, Birkhofer, Smith and Olsson2014) coincides with the increased acceleration of socio-economic human activities (Steffen et al. Reference Steffen, Broadgate, Deutsch, Gaffney and Ludwig2015a). Habitat change and destruction is not only increasing the species richness and abundance of species sharing pathogens and parasites with humans (Gibb et al. Reference Gibb, Redding and Qing Chin2020), but it also is driving species out of their natural habitats and into manmade environments, where they can interact and breed new strains of diseases such as in the case of Nipah. Therefore, the key to prevent or minimize future spillovers of zoonotic disease is that countries actively participate in the development and implementation of the CBD targets.
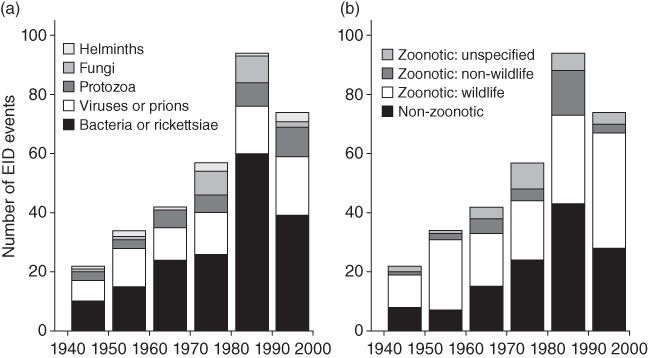
Figure 7.11 Number of emerging infectious disease events per decade according to (a) pathogen type and (b) transmission type.
The accurate prediction of when, where and how a spillover will emerge is impossible because of the ecological complexity. However, it is clear that business-as-usual will inevitably lead to new zoonotic disease emergence. In 2018, the emergence of a new coronavirus was predicted to happen from bats in Southeast Asian areas most affected by deforestations (Afelt et al. Reference Afelt, Frutos and Devaux2018a) and this is, indeed, what happened. However, we have the knowledge – albeit the toolbox needs constant refinement by research and monitoring – to make better risk assessments and to reduce and mitigate the risk. According to UNEP Executive Director Inger Andersen: ‘The science is clear that if we keep exploiting wildlife and destroying our ecosystems, then we can expect to see a steady stream of these diseases jumping from animals to humans in the years ahead’ (Carrington Reference Carrington2020).
Many activities involving zoonotic disease control are at risk because of a failed investigative infrastructure or financial base (Murphy Reference Murphy1998). Yet in the face of the enormous cost, prevention is significantly more cost-effective than response (UNEP & International Livestock Research Institute 2020). Dobson et al. (Reference Dobson, Pimm and Kaufman2020) estimate that the gross annual costs of programmes to reduce deforestation and the wildlife trade and build pandemic surveillance in disease hotspots would be $17.7–26.9bn. The programmes would include monitoring wildlife trade, reducing spillovers from wildlife, early detection and control, reducing spillover via livestock, reducing deforestation by half, and ending wild meat trade (see also Box 7.1) in China. This is more than three orders of magnitude smaller than the current estimated cost of Covid-19 economic damages, of $8.1–15.8 trillion (Dobson et al. Reference Dobson, Pimm and Kaufman2020).
Box 7.1 Ending or managing wild meat trade?
Whether it is possible or advisable to fully end the wild meat trade is debatable. From a public health point of view, trade in ‘high-risk’ species, particularly bats, which harbour a wide array of coronaviruses (Afelt et al. Reference Afelt, Frutos and Devaux2018a), and primates (see Section 7.5.1; Cleaveland et al. Reference Cleaveland, Haydon, Taylor, Childs, 335Mackenzie and Richt2007), should no longer be permitted anywhere in the world. Moreover, wet markets are prone to promulgation of animal viruses and zoonotic disease spillovers (see Section 7.5.2) and need to be either severely restricted and controlled, or closed down altogether. Wildlife trade including trade in wild meat is a major cause of population decline. In a recent meta-analysis (Morton et al. Reference Morton, Scheffers, Haugaasen and Edwards2021), species abundance declined by 62% on average with the reductions greatest when national or international trade was involved (76% and 66%, respectively). From a conservation point of view, improved management and control is trade is urgently required to stem the negative impacts of trade-related population declines.
Indeed, the worldwide ban of wild meat hunting and trade was suggested early in the COVID-19 pandemic because of the link between COVID-19 and wet markets (Born Free Foundation 2020). However, it is important to consider that a strict global ban on wild meat hunting and any type of market trade including local trade will affect the food security and livelihoods of millions of the poorest people (Fa et al. Reference Coffaci de Lima2021; SWM Reference Andermann, Faurby, Turvey, Antonelli and Silvestro2020). For Indigenous Peoples and myriad rural communities, consumed and also sold wild meat remains the backbone of their ways of life (Fa et al. Reference Fa, Olivero and Real2015b) despite the fact that numerous groups are no longer fully nomadic but have been dragged into our economic system. Hence, stopping short food supply chains can be a blunt tool which will imperil vulnerable peoples even more. This is not to say that urban wild meat consumption and any illegal and unregulated wildlife trade that endangers human health, animal welfare and biodiversity should not be banned, but extra care is required so that we can protect the already precarious food security of vulnerable Indigenous Peoples such as the Pygmies who rely on hunting and consumption of wild meat. For example, in the case of the Twa Pygmies in Uganda, exclusion from their traditional land in the 1990s caused severe poverty and hardship and high mortality rates amongst under-five year olds. It was only after Twa families were given land and hunting rights that mortality rates dropped from 59% to 18%, demonstrating the crucial importance of land for survival (Jackson Reference Jackson2006).
Allowing communities subsistence hunting and local trade requires effective laws to regulate subsistence and commercial hunting practices, which is lacking or remains unenforced in many countries. Wildlife legislation is often unclear in defining subsistence hunting for one’s own food and local small-scale trade versus commercial hunting and trade. Moreover, legal guidance of disease risk assessment or public health protection is mostly lacking for informal or illegal hunting and trade. Thus, the development, promotion and enforcement of strong animal health guidelines and legislation are urgently required in many tropic and subtropic countries. The development of such animal health legislation can utilize the standards and recommendations of the OIE, including its Terrestrial and Aquatic Animal Health Codes, as a general framework. Training and education and investment in appropriate facilities are essential to translate such legislation into meaningful actions on the ground to prevent spillovers of zoonotic disease along the bush-to-table chain (hunting, slaughter, processing and handling, storage and distribution in food markets).
After Ebola and SARS, scientists hoped that these diseases would be eye-openers and warned that the next pandemic of zoonotic origin stood around the corner (Afelt et al. Reference Afelt, Frutos and Devaux2018a; Singh et al. Reference Singh, Dhama and Malik2017). Hopefully, COVID-19 will be the final trigger for implementing holistic solutions, whether under the umbrella of the ‘One Health’, ‘EcoHealth’ or ‘Planetary Health’ concepts (Lerner & Berg Reference Lerner and Berg2017). Future costs in dealing with zoonotic emerging infectious diseases, especially because of the pandemic risk, can be substantially reduced if global actions to lessen zoonotic risk are taken globally now to safeguard human health and conserve biodiversity.












