How Evolution Works
Like every one of the many millions of other organisms with which we share our planet, the species Homo sapiens is the product of a long evolutionary history. The first very simple cellular organisms spontaneously arose on Earth close to four billion years ago, and their descendants have since diversified to give us forms as different as streptococci, roses, sponges, anteaters, and ourselves. That evolution was responsible for that variety was first formally articulated in 1858 by the British natural historians Charles Darwin and Alfred Russel Wallace, and the nature of the process(es) involved has been vigorously debated ever since (see the companion volume in this series, Understanding Evolution, by Kostas Kampourakis). As exhaustively documented by Darwin in his 1859 masterpiece On the Origin of Species by Natural Selection, the nested patterns of physical similarity that we see among organisms are best explained by a long history of branching descent from a succession of common ancestors: The essence of evolution is, as Darwin neatly put it, “descent with modification.” Living species, in other words, give rise to descendant species that are not exactly like them; and, with the passage of enough time, any initial differences between parental and offspring species will become hugely amplified. Predictably enough, this radical view of a dynamically changing biota initially raised a huge furor in the static mid-nineteenth-century Creationist world into which it exploded; but once the immediate fuss had died down, the notion that life had evolved was quite rapidly accepted by most Victorian scientists. Even the conservative British public and the ecclesiastical authorities were, by and large, not too far behind. What continued to be controversial was not the notion of evolution itself, but the process that Darwin put forward to explain it.
That process was “natural selection.” Darwin spent a lot of time studying the activities of animal breeders (he raised pigeons himself) and he saw how, by choosing which individuals were allowed to reproduce, those breeders could induce changes from one generation to the next in the physical characteristics of the animal stocks they wished to improve. He thus came to believe that a similar process of selection could happen naturally, and that it could help him to overcome the greatest obstacle he faced in convincing his fellow scientists and others that evolutionary relatedness was the explanation for the order seen in Nature: the received wisdom, enshrined in the Scriptures, that the living species on the planet now are just the same as when the Creator had placed them there. In short, Darwin saw that in each species individuals varied in their physical characteristics – an unarguable quality without which domestic animal breeding could never have worked. It thus logically followed that some individuals would be better suited – better adapted – to prevailing environmental circumstances than others were, enhancing not only their survival but also their ability to reproduce. And if those favorable adaptations were inherited – a reality that breeders had depended on from time immemorial, even if they didn’t have a clue how that inheritance worked – those fortunate individuals would pass those features along to their offspring. Provided that the environmental pressures remained constant, such blind and entirely situational reproduction bias would produce changes in the population over generations, as the favorable variations inexorably became more common; and, given enough time, a new species could emerge.
Humans are storytelling creatures, and natural selection proved to be an amazingly compelling story. Legend has it that Darwin’s colleague Thomas Henry Huxley slapped his forehead and exclaimed, “how stupid of me not to have thought of that!” when he heard about natural selection for the first time. Still, without knowing just how inheritance worked, the natural selection story was obviously incomplete; and it was not until the elucidation in 1900 of the principles of heredity that most of us associate with the Czech cleric Gregor Mendel that this gap began to be filled. Historian Robert Olby was the first to point out that Mendel’s 1866 conclusions were not identical to those that are generally attributed to him; but whenever biologists speak of “Mendelian” genetics they are referring to the fundamental notions that individual traits are controlled independently (of each other) by discrete hereditary elements that are passed on intact through the generations, and that remain discrete even though “dominant” alternative forms might mask the effects of “recessive” ones in the phenotype. And Mendel certainly deserves credit for rebuffing the vague assumption, prevalent in his day, that parental characteristics become somehow “blended” in the offspring.
In 1883 the German biologist August Weismann determined that in multicellular organisms the hereditary material is transmitted solely in the female ovum and the male sperm, a finding that opened the way for the independent (re)discovery of Mendelian genetic principles in three separate European laboratories at the turn of the century. In turn, this quickly led to the introduction of the term “mutation” for the spontaneous changes in the hereditary material that provide the potential for evolutionary change, and to the application of the term “gene” to the particulate unit of heredity that might mutate. There then followed a period of hyperactivity, by the end of which it had become generally recognized that most physical traits of individuals were determined not by single genes, as those studied by Mendel had conveniently been, but by many of them. It was also rapidly realized that the environment also played an important role in the determination of phenotypes. As early as 1915, the American geneticist Thomas Hunt Morgan and his colleagues had shown that at least 25 genes were responsible for eye color in the fruit fly Drosophila, and that an alteration in only one of them could bring about phenotypic change. By 1918 such considerations had led the statistician and population geneticist Ronald Fisher to his “infinitesimal model” of heredity, which saw most phenotypes (physical features of the individual) as the outcome of an interplay between the environment and large numbers of different genes, each of which was individually of minor effect. By admitting environmental agency, this model opened the door for natural selection to re-enter the scene as a potential agent of evolutionary change, a role that was solidified with the emergence during the 1920s and 1930s of what became known as the “Modern Evolutionary Synthesis.” This emerging consensus united geneticists, systematists, and paleontologists in seeing evolutionary change as principally driven by the gradual action of natural selection over vast periods of time: Species evolved as their members slowly adjusted under its influence to changing environments, or as they became better adapted to the ones they occupied. Applying this stately process to any evolutionary history would naturally transform it into a linear story of constant improvement, which rapidly became received wisdom about the process itself.
The reductionist appeal of the Synthesis was extremely seductive, but it was eventually forced to yield to empirical observations of the fossil record that suggested an absence of the linear evolutionary patterns it predicted. In the early 1970s, paleontologists began pointing out that instead of gradually transforming themselves out of existence as predicted by the Synthesis, well-known fossil species tended typically to linger in the record for extended periods, before being abruptly replaced, often by close relatives. This observation was hardly surprising in the light of a flood of paleoenvironmental data that increasingly revealed that climatic and biotic changes in the past had tended to occur rather suddenly, and with alarming frequency. Ancient environments had typically oscillated on timescales so short that it was difficult to imagine natural selection keeping up as a force for adaptive change. Certainly, the kind of steady, long-term environmental change that one might see as driving natural selection over the necessary timescales seems to have been rare, at best.
Realizations such as these opened the door to a new view of evolution as a complex and multifaceted process into which many influences intruded, not least among them random chance. Geneticists had long recognized the potential effect on evolutionary histories of what they called “drift,” namely, the incorporation of particular alleles (alternative versions of given genes) into small populations for reasons of nothing more than random chance (just as if you put equal numbers of black and white marbles in a bottle, you will nonetheless have a decent chance of shaking out of it two black ones in a row). What is more, new alleles arise spontaneously, by mutations that regularly occur without regard to their effects on the individuals in which they take place. We will return later to the nature of those mutations; for now, it is enough to note that they may be of three kinds: significantly disadvantageous, in which case there is a good chance that they will disappear from the population; significantly advantageous, which gives them a good chance of being conserved; and basically neutral, in which case they may disappear but may equally stay around if they don’t actively get in the way. If you add random elements of the last kind to the inherent instabilities of the environments in which populations live (which have the effect of periodically breaking up larger ones into the smaller fragments most susceptible to drift), then it becomes clear that evolution cannot be the burnishing process, always tending toward improvement, that the Synthesis promoted. Evolution is clearly not about optimization, as is often assumed. Rather, any population’s fate on the evolutionary stage is a result of its practical ability to get by (or not) with whatever Nature has dealt it, both in terms of its own inherent qualities and those of the environment (which includes any competition there may be around, as well as the resources available).
All of this puts natural selection in a new light. For, while selection is a mathematical certainty in any population in which more individuals are born than survive to reproduce (which is to say, all of them), its major role may in fact lie in stabilizing gene frequencies in populations, and thereby keeping those populations viable over time. That is something it achieves not by promoting directional change, but by trimming off the unfavorable extremes (say, individual vertebrates with two heads, or none). Indeed, it is hard to see how the gradual modification of many traits simultaneously might be achieved. For a start, individuals rarely succeed or fail in the reproductive stakes as the result of one or two favorable characteristics. That is because every individual is an incredibly complex integrated whole, and needs to function as such. It’s not ordinarily of much advantage, for example, to be the strongest or most fecund member of your species or society if you are also the slowest or the most short-sighted. From a survival point of view, especially in a highly social species, it is usually much better to be in the middle of the pack. And whether you survive or not is often a matter of random chance: An unfortunate encounter with a predator, a falling tree branch, or even a slip of the foot can easily carry away even the most magnificently endowed individuals. What’s more, most features of any individual are controlled developmentally by many genes, each of which may be involved in turn with the development of many characteristics, so that changing one allele will probably have ramifying effects, and every change will involve a trade-off. It is thus very hard to see how natural selection can home in on one feature to favor over the ages, except, perhaps, in the rare event that the feature involved is absolutely key to survival or reproduction.
What this means is that we need to get away from the idea that populations within species are composed of individuals all engaged in constant competition to be the most optimized in some respect or another. Rather, both those individuals and the populations they belong to are reasonably characterized as grab-bags of features and alleles that have historically proven good enough to get by, and many of which are present in them purely as random occurrences. As we’ll see, this certainly appears to be the case in Homo sapiens.
Species and Subspecies
Nature is wonderfully diverse. And the most fundamental unit within that mind-boggling diversity is the species. Our species is Homo sapiens, the first component of this two-part “binomen” being the name of our genus, Homo, and the second designating our species in particular. Genera are the larger groups into which related species are assembled; and in obeisance to the nested pattern of resemblances produced by the branching evolutionary process, genera are in turn grouped into subfamilies, which are grouped into families, which belong to orders, and so on up until all living things are subsumed. Just for the record, a unit at any level of this hierarchy can be referred to as a “taxon” (plural “taxa”), hence the term “taxonomy” for the study of natural diversity.
The intuitive recognition of species as the basic unit into which Nature is organized goes back a long way, perhaps as far back as words themselves. And in some respects, species recognition has remained intuitive. In 1859 Charles Darwin wrote that “No one definition [of species] has satisfied all naturalists; yet every naturalist knows vaguely what he means when he speaks of a species.” That sage declaration still rings true: There are currently on offer almost three dozen different formal definitions of what sexually reproducing species are. What most of those modern definitions have in common, though, is that they mostly recognize those species less by what their members look like than by their ability to interbreed with each other and produce fully viable and fertile offspring. In other words, most biologists would nowadays concur that, among sexually reproducing organisms, species are the largest fully interbreeding populations – an idea whose origins go back as far as the seventeenth-century speculations of the English natural historian John Ray.
Unfortunately, that doesn’t get us too far in practice, hence the plethora of definitions, most of which apply to specific circumstances. That’s largely because the inability to reproduce successfully (i.e., to produce fully viable and fertile offspring), which is what confirms that two populations have gone off on their own independent paths, can express itself in many ways. A handful of acts of interbreeding between individuals from two populations, for example, does not guarantee the production of viable and fully fertile offspring, and does not necessarily mean that the parental populations of both individuals would reintegrate if they came into contact. In fact, it may well be that what is or is not a species is only determinable in long retrospect, because any reproductively isolated new species inevitably starts with the splitting of a single species, the members of which may continue sending out reproductive signals and interbreeding should the opportunity arise, even after having embarked on an individuated evolutionary trajectory. This is what appears to have happened in the case of Homo sapiens and H. neanderthalensis, two very physically distinctive species of the genus Homo that initially went their separate evolutionary ways (in Africa and Eurasia, respectively) well over half a million years ago. The two apparently interbred on numerous occasions once H. sapiens had eventually reached the Neanderthals’ European heartland after leaving Africa at around 70,000 years ago. The resulting encounters resulted in the exchange of various genes between the two species (most of them later weeded out of the gene pools of both), but they did nothing to affect the evolutionary fate of either party in any really significant way. The Neanderthals became extinct pretty much as they were, while H. sapiens went on to conquer the rest of the world as the entity we know today (with high-altitude populations on the Tibetan Plateau apparently receiving a genetic assist from a mysterious Neanderthal relative known currently only as the Denisovans). At around the same time, all other remaining archaic hominin populations went extinct as well, presumably because they, too, were unable to cope with competition from our species.
In this book we fortunately do not have to deal with the larger questions of human identity raised by complications such as these coexistences. Because, as a result of the late Ice Age purges of the competition, all modern humans belong to the single freely interbreeding species Homo sapiens. There is no question about that. What is more, as we will see later, most of the variations we see today in the extraordinarily dense and widespread populations of our species are no more than epiphenomena of the last 70,000 years or so, and none of them can predate its origin in Africa at about 200,000 years ago. And because our forebears eliminated all human competition on our planet a pretty long time ago, we modern Homo sapiens are not faced with having to philosophize about who is human, and who is not. We all are. The relevant question is: Is our young but widespread species currently divisible in any meaningful way?
Old-time zoologists used to recognize species, the basic “kinds” of organisms, on the basis of physical similarity. Doing this raised all kinds of issues since, as Charles Darwin was one of the first to make explicit, all species consist of individuals that vary among themselves. You might be able to demonstrate that a measured physical characteristic varies around a mean value (a value that might not be observed in even one individual in the sample available); but there is certainly no single individual out there that, in every particular, represents the “ideal” of the species. That such an ideal exists is a tenacious notion that has hung around since Aristotle, but it is obviously problematic since any member of a species can be accused of falling short in some respect – but remains a member, nonetheless.
Still, the “population thinking” that Darwin advocated raised issues as well, because as more was learned about the behaviors of nonhuman animals in their natural environments, the plainer it became that populations of closely related organisms that appeared different to the human eye sometimes – even often – could, and would, interbreed if given the chance. From this there eventually emerged the idea that among most animals, including mammals, distinctive features and reproductive incompatibilities evolved in small populations in isolation, the implication being that if a species was divided into discrete populations by the formation of some impassable geographic boundary such as a major river, over time they would eventually accumulate enough genetic differences to become reproductively incompatible with their former conspecifics. But even as those reproductive incompatibilities accumulated, behavioral attraction might remain among those close relatives, so that if the populations somehow reestablished physical contact, some interbreeding behaviors might take place. If effective speciation – the establishment of effective reproductive incompatibility at any level – had occurred, that behavioral integration would continue only until the greater reproductive success of those who declined to interbreed edged out any inclinations to do so in the population at large. In this perspective, for all their physical differences, Neanderthals and modern humans first encountered each other before behavioral isolating mechanisms had completely evolved. But it is the bottom line that is critical, and the Neanderthals are nonetheless long gone.
The hominin case is a fairly extreme one in terms of the extent of the substantial physical differences between the quasi-reproductively compatible taxa involved. Still, it is nevertheless pretty typical among living species to exist as a number of readily recognizable varieties that inhabit particular geographic areas, but that appear to hybridize readily if and when they come into physical contact. In diurnal vertebrate species, especially, such varieties are often strikingly distinguished by the markings and coloration of their pelage, and they are often allocated to their own “subspecies.” Subspecies are designated by a “trinomen,” with a third italicized name added after the species name. In the human case this was done, for example, by those who at one time believed that the extinct Neanderthals should be included in the modern human species, as Homo sapiens neanderthalensis. That opened a huge can of worms, because if the Neanderthal variety of humankind should formally be recognized as a subspecies, shouldn’t the other “kinds” of humans be similarly distinguished? Fortunately, it has turned out that the Neanderthals amply justify recognition as their own species, so the issue is moot. Subspecies are taxa that are readily recognized from their physical and often genomic characteristics, but that are nonetheless genetically wholly compatible reproductively with members of other populations of the same species. When you are deciding whether it is worth making a subspecies distinction in a particular case, it is important to remember that the differences that cause taxonomists to recognize different subspecies are wholly maintained by their possessors’ physical separation. They will rapidly disappear, through interbreeding, if and when geographical circumstances allow the populations to merge. If they don’t merge under those circumstances, you are not looking at subspecies, but instead at differentiated species.
So, while subspecies, having emerged in isolation, undoubtedly provide the fodder for new species (without them, it is difficult to know how existing species could split to form new evolutionary lineages to create the diversity of species we see), we have also to remember that they are by nature ephemeral, with an entirely transient existence until they are historically established as discrete units by speciation – the establishment of that key reproductive isolation that allows them to embark upon independent historical existences. Crucially, this means that we are unable to recognize subspecies in the species Homo sapiens. That’s because it could hardly be more evident that the many geographical variants within it are busily intermingling right now, worldwide. And, as we will see, they have a long history of doing so.
Human Evolution
At around the middle of the twentieth century, the Modern Evolutionary Synthesis found its way into paleoanthropology, the branch of science devoted to the evolution and diversification of human beings and their close relatives. The new view of this process brought with it an elegantly simple and linear story of human phylogeny that had the appeal of all the best stories from time immemorial, featuring as it did a single protagonist that, with the aid of natural selection, single-mindedly battled its way across the eons from the status of primitive bipedal ape to that of the perfected product that some believe Homo sapiens is today. The triumph of this interpretation brought with it a welcome clearing-out of the clutter of names that had accumulated for the denizens of what was then a fairly limited fossil record. But subsequent discoveries have changed the picture once more; and although the seductiveness of the linear story still shows significantly in the desire of many paleoanthropologists to minimize the diversity of species and genera they are prepared to recognize in the human fossil record, almost three-quarters of a century down the line it is hard for anyone to deny that the actual story of human evolution involved a lot of trial and error, rather than the burnishing of a single central lineage.
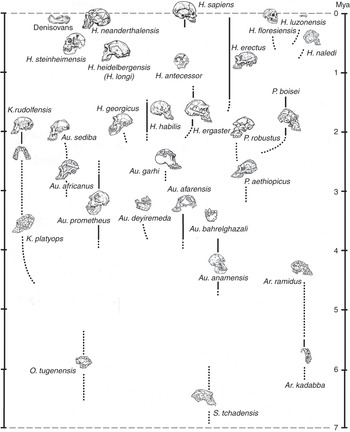
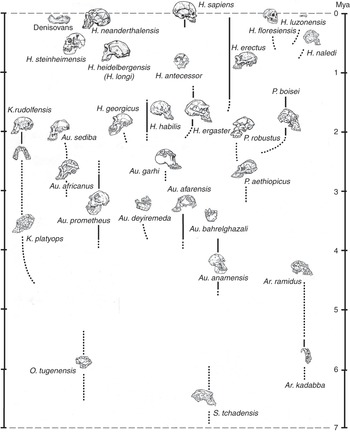
In a book about human beings and race, it is important to point out that, while subspecies may be quite readily distinguishable among living animals on the basis of external features, it is usually difficult or impossible to spot them if you only have the bones and teeth that typically fossilize. In many cases it is tricky even to identify fully differentiated fossil species. Looking in the human fossil record for taxa below the level of the species is, therefore, likely to be a pretty unproductive endeavor, although in the literature you may still spot the occasional trinomen (and, in one spectacular instance, a quadrinomen: Homo erectus ergaster georgicus, for what it’s worth). In the last decade or two the availability of genomic information has made it possible to talk about population movements of early Homo sapiens subsequent to about 30,000 years ago; but as far as extinct kinds of human are concerned (with the partial exception of the Neanderthals), all the traditional paleontologist can say is that, although we can often clearly observe a lot of variation in bony features among individuals that may or may not have belonged to the same species, we have no way of judging if, let alone how, they were differentiated below that level. What the human fossil record does give us, though, is evidence that in the past several different kinds of hominin typically occupied the planet – and sometimes the very same landscape – at the same time. The situation we naturally enough take for granted – that Homo sapiens is the only hominin around – is, in fact, highly unusual. So unusual, in fact, that our current lonely splendor is very probably entirely unprecedented in the seven million years since the first member of our subfamily – the first hominin – took to walking upright on terra firma. This means that, in our own case at least, we need to be cautious in taking the past as a guide to the present.
The hominin subfamily (hominid family, if you prefer – for our purposes, the difference is notional) was born in Africa at a time when an increasing aridity and seasonality of rainfall was transforming the continent’s environment. Especially to the east of the elevated Great Rift Valley that runs in a ragged line from Djibouti to Mozambique, the formerly ubiquitous humid African forests were drying out and giving way to more open woodlands and bushy formations. All this put pressure on the ancestral apes that inhabited those forests, while at the same time presenting new ecological opportunities on the ground; and in the period between about seven and four million years ago we have African fossil evidence of several different early hominin genera, all of which appear to have walked erect on the ground. As far as we can tell, they all also shared another peculiarity with us: the reduction of the canine teeth that has important consequences for what we can chew, and thus eat. Whether all – or even any – of these forms were directly ancestral to later hominins can be debated. What is clear, though, is that the open terrestrial environment offered valuable resources to any apes willing to spend significant amounts of time away from the trees; and that some of those apes, at least, were already accustomed to holding their trunks upright because they were highly suspensory climbers in the trees. Their preexisting upright proclivity would then have translated to the ground – as it does, for example, among today’s gibbons. Aside from the immediate challenges of earthbound existence, such as figuring out how to dig out the underground tubers that were to be a major component of their diets, these terrestrial pioneers also faced some pretty difficult realities: Moving on two legs was a slow way of getting around, and the new environment teemed with fast and ferocious predators.
At around 4.4 million years ago we find the first evidence of a much better-documented group of early hominins known colloquially as the “australopiths” (for the best-known genus of the group, Australopithecus, which translates as “southern ape”). Some australopiths persisted until well under two million years ago (see Figure 1.1, which shows the diversity of hominins over time). As Figure 1.1 shows, a dozen australopith species are known from fossils found in eastern, central, and southern Africa, and all have in common short stature, brains not a lot bigger than those of apes, projecting faces with large chewing teeth, and lower bodies showing a commitment to bipedality when on the ground. They retained climbing adaptations in their upper bodies, however, and appear to have divided their lives between the trees and the ground. Nonetheless, it was almost certainly an australopith that made the first stone tool at some time over about 2.5 million years ago – probably the most consequential invention ever in hominid history. Hominids were therefore already stone-tool makers well before the time when the first species of our genus Homo emerged in Africa, at some point just under two million years ago. The ancestry of this species, commonly known as Homo ergaster, probably lay somewhere within the larger australopith group, though specific claims about this ancestry are probably better treated with caution.
Homo ergaster – taller and slenderer than any australopith, and significantly larger-brained – initially made stone tools like those made by the australopiths: simple sharp flakes obtained by bashing one fist-sized cobble with another. But soon they were making “handaxes,” larger stone tools that were made to a specific (usually teardrop) shape that must have existed in the toolmaker’s mind before the work began. These hominids were also the first that were committed by their anatomy (much like our own) to life away from the trees: They were “obligate” rather than “facultative” bipeds, meaning that they were dedicated to walking upright rather than just being able to do so as the occasion demanded. They are known to have used their stone tools to butcher the animal carcasses that would have been necessary to feed their energy-hungry large brains (though it is an inference that they actively hunted them), and it has been convincingly argued that they would have needed to have cooked animal and plant parts – obviously using domesticated fire – to extract that energy. In the two-to-one-million-year period we have plenty of evidence for a diversity of “early Homo” hominids around, suggesting that active experimentation was going on with the potential inherent in belonging to Homo; but we have nothing that would help us to understand how their populations were organized biologically. As an aside, it is worth noting that some paleoanthropologists still classify African H. ergaster as H. erectus, even though it is becoming ever more apparent that the latter, despite its historical importance as the second species of extinct hominin to be discovered, was a much later and exclusively eastern Asian descendant of H. ergaster.
It was a million years or more after the invention of the handaxe that the next major stone tool type was invented, this time the “prepared-core” tool that required a stone “nucleus” to be laboriously shaped until a final blow would detach a more-or-less finished tool, with a continuous cutting edge around its periphery. This development took place within the tenure of Homo heidelbergensis, the first cosmopolitan hominin species we know of, with fossil representatives in Africa, Europe, and eastern Asia. Homo heidelbergensis had a brain not too far short of modern size, and it showed a whole panoply of complex behaviors, such as hunting with spears, the construction of artificial shelters, and the fabrication of compound tools with stone points mounted in wood or bone handles. But it exhibited few, if any, inklings in its recorded behaviors of the unusual way in which modern humans handle information in the mind. We are “symbolic,” which is to say that we deconstruct the world around and within us into a vocabulary of discrete mental symbols that we can shuffle around, according to rules, to imagine alternatives. We do not simply live in the world that Nature presents directly to us: We live for much of the time in the worlds we create in our minds, and much of the discord we observe in society results from the fact that we each do this in our own idiosyncratic way.
Interestingly, we only arguably find evidence for symbolism in the complex and extensive record left behind by Homo neanderthalensis, a close human cousin that had a lineage in Europe extending back to over 400,000 years, and a brain as large as that of contemporaneous Homo sapiens. This latter fact suggests that our mental peculiarities result from a unique internal organization of the brain, rather than from raw brain size; and indeed, the earliest Homo sapiens fossils, known from Ethiopia at about 200,000 years ago, left no evidence of substantive cognitive difference from the Neanderthals (and, suggestively, modern human brains got smaller once the symbolic way of processing information had been adopted). Only at around 100,000 years ago do we begin to find indications (again, in Africa) that humans were beginning to think symbolically. Those indications come mainly in the form of objects of bodily decoration and geometric engravings that evidently held meaning for their makers; and since such objects appear significantly after the event of biological reorganization that gave rise to the very anatomically distinctive species Homo sapiens, the conclusion must be that a new biological potential acquired in that reorganization had been finally released by some behavioral factor, most plausibly the spontaneous emergence of spoken language. Such an event would certainly not have been evolutionarily unprecedented, or even unusual: The dinosaur ancestors of birds, for example, possessed feathers for many tens of millions of years before co-opting them for flight. And once those rudimentary symbolic expressions had begun to appear, the blossoming Homo sapiens rapidly left Africa and took over the entire world, displacing all other hominins in the process – and, in short order, producing some of the most powerful artistic expressions ever made, on the cave walls of France and Spain (and now of Sulawesi and Borneo).
The adoption of symbolic thinking changed the entire relationship of human beings to the world around them, and it quite probably changed the rules of the evolutionary game as we play it. But it probably didn’t immediately affect the way in which human populations were organized. Like the members of any other species, local human population nuclei were buffeted hither and yon by the climatic vicissitudes of the late Ice Ages, fragmented at one moment by drought, and reunited at another when the rains returned. All along, hominins had been hunters and gatherers, necessarily living off whatever bounty the landscape offered, even after their new cognitive powers had allowed them to exploit with unprecedented intensity the environments that they happened to find themselves in. At certain points they were limited to tiny refuges that could sustain them even in the hardest times, and it was probably in one such isolated place that the anatomically recognizable species Homo sapiens originated in one local population, as explained in greater detail in the companion volume in this series, Understanding Human Evolution. That population would have been small enough to allow the fixation of whatever genomic change it was that had resulted in the highly unusual new anatomy (modern humans are much slenderer and more lightly built than even their closest known fossil relatives, and have radically reorganized skull proportions). The fledgling species was then poised to acquire symbolism (again, in one small subpopulation), and subsequently to spread throughout Africa and beyond. It is hard to know exactly what to make of the various fossils from around the Old World that document this diaspora; and fortunately, as we will see, that story is much better told by the molecular record.