For people with mental illness, high rates of smoking result in significantly increased risk of many smoking-related diseases, and it is likely that this is responsible for most of the excess natural mortality of this group (Reference Brown, Barraclough and InskipBrown et al, 2000). However, although they are about twice as likely to smoke, people with mental illness are also able to quit (Reference Lasser, Boyd and WoolhandlerLasser et al, 2000). Around half of smokers with mental health problems in the UK have expressed a desire to stop smoking (Reference Jochelson and MajrowskiJochelson & Majrowski, 2006). Those whose mental illness has remitted are at no greater risk for subsequent smoking than those with active disorders (Reference Breslau, Novak and KesslerBreslau et al, 2004). In the general population, smoking increases the risk of developing a mental illness even after correcting for major risk indicators of mental disorder (Reference Cuijpers, Smit and ten HaveCuijpers et al, 2007) and therefore smoking cessation may prevent mental as well as physical illness.
Although there has been less research specifically focusing on smokers with mental health problems, treatments are known to be effective in helping them to stop (Reference McNeillMcNeill, 2004). However, such treatments are not being offered routinely. In this article we summarise our findings on smoking cessation treatments in general, and then comment more specifically on particular mental health problems.
To review the literature on smoking cessation in people with mental health problems, Medline and the Cochrane reviews were searched using the keywords smoking, schizophrenia, depression and cessation. The findings are divided into those relevant to the general population and those for populations with mental health problems.
Smoking cessation interventions for the general population
The gold standard for help with smoking cessation (Box 1) is pharmacotherapy plus individual or group specialist behavioural support (Reference West, McNeill and RawWest et al, 2004), which approximately quadruples the chance of successfully stopping. Nicotine replacement therapy (NRT), bupropion and varenicline are proven cost-effective treatments, although the majority of smokers relapse after treatment (Reference Cahill, Stead and LancasterCahill et al, 2007; Reference Hughes, Stead and LancasterHughes et al, 2007; Reference Stead, Perera and BullenStead et al, 2008).
Box 1 Summary of effective smoking cessation medication
-
• NRT is effective for smoking cessation
-
• Combining different forms of NRT enhances cessation rates
-
• Pre-loading with NRT may increase cessation rates
-
• Use of NRT alongside a reduction in cigarette consumption may also be useful as a first step towards cessation for heavier dependent smokers
-
• Bupropion and nortriptyline are effective but SSRIs and anxiolytics do not improve cessation rates
-
• Bupropion reduces withdrawal symptoms as well as weight gain
-
• Varenicline is also effective and may be more effective than bupropion
Nicotine replacement therapy
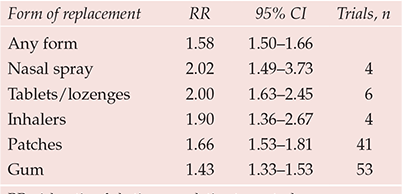
Six forms of NRT are currently available in the UK: gum, patch, inhalator, nasal spray, tablet and lozenge. These products come in a variety of doses and flavours. The products also vary in their speed of nicotine delivery. A recent review found that the risk ratio of abstinence for any form of NRT relative to control was 1.58 (Reference Stead, Perera and BullenStead et al, 2008). Although risk ratios of abstinence varied depending on the type of NRT, there was overlap of confidence intervals (Table 1). However, further analyses found that risk ratios of abstinence were significantly higher for tablets/lozenges than for gum (P = 0.014) and that nasal spray was marginally significant over gum (P = 0.055) (Reference Stead, Perera and BullenStead et al, 2008).
Table 1 Effectiveness of different forms of nicotine replacement therapy
| Form of replacement | RR | 95% CI | Trials, n |
|---|---|---|---|
| Any form | 1.58 | 1.50–1.66 | |
| Nasal spray | 2.02 | 1.49–3.73 | 4 |
| Tablets/lozenges | 2.00 | 1.63–2.45 | 6 |
| Inhalers | 1.90 | 1.36–2.67 | 4 |
| Patches | 1.66 | 1.53–1.81 | 41 |
| Gum | 1.43 | 1.33–1.53 | 53 |
Combined forms of NRT
Combining a nicotine patch with a rapid-delivery form of NRT is more effective than a single type of nicotine replacement (Reference Stead, Perera and BullenStead et al, 2008). Therefore, it would seem that rapid-delivery NRT such as nasal sprays deal with breakthrough urges, whereas nicotine patches provide background nicotine replacement. The effectiveness of NRT appears to be largely independent of the intensity of additional support provided to the individual (Reference Stead, Perera and BullenStead et al, 2008).
NRT while smoking
There is some evidence that using the nicotine patch for a short period before the quit attempt results in higher cessation rates (Reference Stead, Perera and BullenStead et al, 2008). Using NRT alongside a reduction in cigarettes is sometimes helpful for heavy smokers who find cessation in one step too difficult. Systematic reviews found that NRT while smoking significantly increases the likelihood of long-term abstinence (increase in risk ratio RR = 2.06, 95% CI 1.34–3.15; Reference Wang, Connock and BartonWang et al, 2008) and the odds of cessation (increase in RR =1.90, 95% CI 1.46–2.47; Reference Stead and LancasterStead & Lancaster, 2007). However, insufficient evidence exists about long-term benefits of interventions intended to help smokers reduce but not stop smoking.
The Medicines and Healthcare products Regulatory Agency & Committee on Safety of Medicines (2005) have enabled some NRT products (some forms of gum and inhalators) to be used for smoking reduction as a first step towards cessation and, more recently, some forms of gum and lozenge have been given indications for use during periods of temporary abstinence from smoking.
Bupropion and nortriptyline
A systematic review (Reference Hughes, Stead and LancasterHughes et al, 2007) showed that the antidepressant bupropion doubles smoking cessation rates (odds ratio OR = 1.94, 95% CI 1.72–2.19) as does nortriptyline (OR = 2.34, 95% CI 1.61–3.41). However, selective serotonin reuptake inhibitors (SSRIs) and anxiolytics do not improve cessation rates (Reference Hughes, Stead and LancasterHughes et al, 2007), suggesting that the mode of action of bupropion and nortriptyline is independent of their antidepressant effect. There is insufficient evidence on whether the combination of NRT with bupropion or nortriptyline increases cessation rates (Reference Hughes, Stead and LancasterHughes et al, 2007). Bupropion reduces withdrawal symptoms as well as weight gain (Reference Richmond and ZwarRichmond & Zwar, 2003).
Varenicline
Varenicline is a nicotine receptor partial agonist and is thought to act both as an agonist maintaining moderate levels of dopamine to counteract withdrawal symptoms and as an antagonist by reducing smoking satisfaction. It became available in the UK in December 2006. A systematic review (Reference Cahill, Stead and LancasterCahill et al, 2007) reported that varenicline increases the odds of cessation by 3.22 (95% CI 2.43–4.27) compared with placebo and by 1.66 (95% CI 1.28–2.16) compared with bupropion. Varenicline had fewer serious side-effects than bupropion and showed high patient satisfaction.
Rimonabant
Rimonabant is a selective type 1 cannabinoid receptor antagonist. A review concluded that rimonabant at 20 mg daily increased the odds of smoking cessation by 1.61 (95% CI 1.12–2.30), although at 5 mg it had no effect (Reference Cahill and UssherCahill & Ussher, 2007). It has potential to protect those who successfully stop from gaining weight, although the evidence for maintaining abstinence is inconclusive. However, concern has been raised over increased rates of depression and suicidal thoughts in those taking rimonabant.
Naltrexone
There are insufficient data regarding whether naltrexone is helpful for smoking cessation (Reference David, Lancaster and SteadDavid et al, 2006).
Adverse effects of pharmaceutical interventions
Nicotine replacement therapy
The effects of nicotine acquired through NRT are thought to be no different from those of smoking-derived nicotine. Nicotine replacement products can cause gastrointestinal disturbance, headache, dizziness, influenza-like symptoms, dry mouth, rash and palpitations. In addition, nasal spray can cause irritation of nasal mucosa, sneezing, epistaxis and watering eyes; patches can cause sleep disturbance, nightmares and local skin irritation, and their use is cautioned for people with widespread skin disorders such as psoriasis or dermatitis; nicotine gum can cause stomach irritation; inhalators can cause coughing; lozenges can cause stomach irritation, thirst, sleep disturbance and nightmares; gum, lozenges, sublingual tablets and inhalators can produce hiccups or throat irritation (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2008).
Bupropion
The most common side-effect of bupropion is insomnia (30–40% of individuals), dry mouth (10%) and nausea. Other documented side-effects include pruritis, irritability, tremor, impaired concentration, headache, dizziness, depression, agitation, anxiety, and less commonly, confusion and visual disturbances (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2008). Rarely, hallucinations, depersonalisation, dystonia, ataxia, abnormal dreams, memory impairment, and very rarely Stevens–Johnson syndrome, delusions, and aggression have been reported. Seizure is the most significant and important potential side-effect and it occurs at a rate of 1 in 1000, although no randomised controlled trial of bupropion has reported seizures (Reference Hughes, Stead and LancasterHughes et al, 2007). Adverse effects of withdrawal from bupropion include rash, insomnia, tremor, headache, dry mouth, anxiety, psychological problems, drowsiness, weight loss, thinking difficulties, dizziness and palpitations (Reference Woolacott, Jones and ForbesWoolacott et al, 2002). Bupropion is contraindicated in people with epilepsy (Reference Richmond and ZwarRichmond & Zwar, 2003) and a history of bipolar disorder (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2008).
Varenicline
Side-effects of varenicline include gastrointestinal disturbance such as nausea, dry mouth, taste disturbance, headache, drowsiness, dizziness, sleep disorders and abnormal dreams (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2008). Less commonly documented side-effects include weight gain, panic attack, abnormal thinking, mood swings, dysarthria, tremor, incoordination, hypertonia and restlessness. It is recommended that abrupt withdrawal of varenicline be avoided because of risk of relapse, irritability, depression and insomnia, and exacerbation of underlying mental illness, including depression.
Case reports describe varenicline's induction of a manic episode in a patient with bipolar affective disorder (Reference Kohen and KremenKohen & Kremen, 2007) and its exacerbation of the symptoms of schizophrenia (Reference FreedmanFreedman, 2007). Depression and suicidal ideation have been reported (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2008). These are being reviewed by the European Medicines Agency, which has advised that patients should stop varenicline if they develop suicidal ideation and should contact their doctor immediately.
Rimonabant
Side-effects of rimonabant include nausea and upper respiratory tract infections (Reference Cahill and UssherCahill & Ussher, 2007).
Potential interactions with other medication
The Drugs and Therapeutics Bulletin (Anonymous, 2000) advises ‘extreme caution’ when considering prescribing bupropion to people taking epileptogenic drugs such as tricyclic antidepressants and typical antipsychotics other than butyrophenones. It also advises avoidance in alcohol misuse and amphetamine use because of seizure risk. Carbamazepine reduces levels of bupropion and increases levels of its main metabolite hydroxybupropion (Reference Martinez-Raga, Keaney and SutherlandMartinez-Raga et al, 2003); bupropion can also affect blood plasma levels of medications, including some antipsychotics (e.g. risperidone, haloperidol, phenthiazines) and antidepressants (e.g. tricyclic antidepressants, trazodone and SSRIs) (see ‘Effects of smoking on psychiatric medication’ below).
Non-pharmacological interventions for the general population
Counselling
Meta-analyses show that simple advice from a physician has a small but significant effect on smoking cessation (OR = 1.74, 95% CI 1.48–2.05) (Reference Lancaster and SteadLancaster & Stead, 2004). Smoking cessation advice and/or counselling given by nurses significantly increase the likelihood of quitting (RR = 1.28, 95% CI 1.18–1.38) (Reference Rice and SteadRice & Stead, 2008), although the evidence is weaker with brief interventions provided by nurses and when counselling is provided by nurses whose main role is not health promotion or smoking cessation. Smoking cessation counselling given face to face by a counsellor trained in smoking cessation is effective, although the evidence is insufficient to demonstrate that intensive counselling is more effective than brief counselling (Reference Lancaster and SteadLancaster & Stead, 2005a ). Further meta-analysis found that group therapy was more effective than self-help and other less intensive interventions (Reference Stead and LancasterStead & Lancaster, 2005). However, there was not enough evidence to evaluate whether groups are more effective than individual counselling. Meta-analysis shows that telephone support to assist cessation is also effective (Reference Stead, Perera and LancasterStead et al, 2006) and multiple call-back counselling improves long-term cessation for smokers who contact cessation services (Reference Stead, Perera and LancasterStead et al, 2007).
Other interventions
Standard self-help materials are likely to have only a small effect in the general population (Reference Lancaster and SteadLancaster & Stead, 2005b ). They have no additional benefit when used alongside other interventions such as NRT or advice from a healthcare professional. Incentives and competitions do not improve long-term cessation rates (Reference Hey and PereraHey & Perera, 2005). Meta-analysis of trials also reveals no benefit for acupuncture (Reference White, Rampes and CampbellWhite et al, 2006) and hypnotherapy (Reference Abbot, Stead and WhiteAbbot et al, 1998). However, there is some evidence for the effectiveness of internet support to aid cessation in those already using NRT (Reference Zwartz, Noell and SchroederZwartz et al, 2006).
Relapse prevention
Smoking cessation has high relapse rates: many people in the general population return to smoking within a year of giving up (Reference Lancaster, Hajek and SteadLancaster et al, 2006). Evidence does not yet support the adoption of skills training or other specific interventions to help those who have given up smoking to avoid relapse.
Exercise
A systematic review of 12 studies that compared exercise with a passive condition found positive effects on cigarette cravings, withdrawal symptoms and smoking behaviour. This suggests that exercise can be a useful aid to managing cigarette cravings and withdrawal symptoms (Reference Taylor, Ussher and FaulknerTaylor, A.H. et al, 2007).
Adolescents and young people
Most tobacco-control programmes for adolescents are based on prevention of uptake, since those who do not smoke before the age of 20 are significantly less likely to start as adults. Further high-quality trials are needed to identify effective tobacco cessation interventions for young people (Reference Grimshaw and StantonGrimshaw & Stanton, 2006). In the UK, the only smoking-cessation medication licensed for young people is NRT, and then only from 12 years of age.
Smoking cessation for people with mental health problems
There is less research on smoking cessation in those with mental health problems despite this group's higher rates of smoking. The following section highlights research evidence specifically among people with different mental health problems and it is worth noting that most of the medication trials also included behavioural support.
Depressive disorders
A number of medical and psychological interventions for smoking cessation used in the general population can be implemented in psychiatric settings for those in treatment for depression. These are summarised in Box 2 and discussed in a little more detail below.
There is evidence that a minority of people experience an increase in depressive symptoms after they stop smoking (Reference HughesHughes, 2007), so smokers with depression would benefit from closer monitoring following smoking cessation. Increases in depressive symptoms during an initial period of abstinence have been associated with a return to smoking (Reference Hayford, Patten and RummansHayford et al, 1999). However, meta-analysis shows that a lifetime history of major depression is not an independent risk factor for failure in smoking cessation treatments (Reference Hitsman, Borrelli and McChargueHitsman et al, 2003).
Box 2 Summary of medication and psychological treatment for those with depression
-
• NRT can double cessation rates and lower self-reported depression in depressed smokers
-
• One study showed that extended use of bupropion for relapse prevention was effective for smokers with and without a history of major depression
-
• Psychological and lifestyle strategies assisted in mood regulation over and above the standard smoking cessation treatments for smokers with a depression history
-
• Structured psychological approaches to smoking cessation added to the benefits of antidepressants and NRT particularly for smokers with high nicotine dependence and repeated episodes of major depression
-
• CBT doubled abstinence rates in a trial of smokers with recurrent depression
-
• Increased cessation rates for those being treated for depression occurred through addition of counselling and computerised motivational feedback
Nicotine replacement therapy
Nicotine patches were associated with significantly greater positive mood over time compared with nicotine gum in a study of 335 smokers from the general population (Reference Strasser, Kaufmann and JepsonStrasser et al, 2005). A systematic review concluded that nicotine gum reduces total withdrawal discomfort, irritability and anxiety, with some evidence for a positive effect on depressed mood and craving (Reference West and ShiffmanWest & Shiffman, 2001).
Nicotine replacement doubled cessation rates and significantly lowered self-reported depression in 269 smokers with current depression (Reference Kinnunen, Doherty and MilitelloKinnunen et al, 1996). Negative mood peaked in the week following cessation and was linked to higher relapse rates, which highlighted the importance of NRT in those with depression.
Bupropion is effective for smoking cessation for people with and without a history of depression or alcoholism (Reference Richmond and ZwarRichmond & Zwar, 2003). One study has shown that extended use of bupropion for relapse prevention was effective for smokers with and without a history of major depression (Reference Cox, Patten and NiauraCox et al, 2004). Varenicline has not been assessed in trials specifically for smokers with depression. A recent case report advises that varenicline induced a manic episode in a patient with bipolar affective disorder (Reference Kohen and KremenKohen & Kremen, 2007).
For smokers with a history of depression, psychological and lifestyle strategies such as motivational interviewing, relaxation exercises and mood charts can help to regulate mood over and above the standard smoking cessation treatments (Reference Wilhelm, Arnold and NivenWilhelm et al, 2004). This group requires more attention to relapse of both depression and smoking after cessation.
Structured psychological approaches to smoking cessation add to the benefits of effective smoking cessation pharmacotherapies particularly for smokers with high nicotine dependence and repeated episodes of major depression (Reference Ferguson, Goodwin and HorwoodFerguson et al, 2003). In a trial of over 400 smokers with recurrent depression, 35% of those receiving cognitive–behavioural therapy (CBT) remained abstinent, compared with 18% receiving health education (Reference Haas, Munoz and HumfleetHaas et al, 2004).
Staged (stepped) care can also improve cessation rates for people receiving treatment for depression. In one such trial showing positive results, the 322 participants with current depression were offered computerised motivational feedback about their smoking at baseline, 3, 6 and 12 months. They also had provision for 6 sessions of psychological counselling and pharmacotherapy at the appropriate stage of readiness (Reference Hall, Tsoh and ProchaskaHall et al, 2006). Reference Wilhelm, Wedgwood and NivenWilhelm et al (2006) had similar success using a series of tiered interventions.
Schizophrenia
Rates of cessation for smokers with schizophrenia are half those for the general population, partly because of their lower motivation to quit, fewer cessation attempts, increased level of nicotine dependence and reduced access to treatment (Reference Williams and FouldsWilliams & Foulds, 2007). Neuropsychological deficits in schizophrenia are also implicated (Reference Dolan, Sacco and TermineDolan et al, 2004).
Nevertheless, the literature shows that increased rates of cessation occur with combinations of medication, psychosocial interventions and integration of treatment into overall care plans to reduce the risk of relapse. Box 3 summarises our search findings, which we discuss in a little more detail below.
Box 3 Smoking cessation in schizophrenia
General
-
• Cessation rates are lower for people with schizophrenia than for the general population
-
• Specialist cessation services for those with mental illness can achieve higher rates of abstinence
Medication
-
• Nicotine replacement is effective, although less than in the general population
-
• Bupropion contributed to reduced smoking and did not worsen clinical symptomsalthough relapse rate was high after treatment discontinuation
-
• Combination NRT and bupropion treatment is effective in abstinence and reduction
Combined medication and psychological therapy
-
• Specialised group therapy plus NRT is effective, and atypical antipsychotics improve abstinence with such treatment
Harm reduction
-
• Harm reduction approaches include NRT and NRT plus bupropion to reduce the level of smoking, which may improve the chance of future cessation
Nicotine replacement therapy may be more successful at improving abstinence in combination with atypical than with typical antipsychotics (Reference George, Ziedonis and FeingoldGeorge et al, 2000).
Pharmacotherapy
Nicotine replacement is effective for people with schizophrenia, although not as effective as it is for the general population (Reference Williams and HughesWilliams & Hughes, 2003). There is some evidence that the rapid nicotine delivery of a nasal spray is most successful (Reference Williams and FouldsWilliams & Foulds, 2007) and cessation rates are likely to be enhanced when it is combined with nicotine patches.
Bupropion
Bupropion can help to reduce smoking in people with schizophrenia (Reference Williams and HughesWilliams & Hughes, 2003), and it appears not to exacerbate clinical symptoms of the disorder (Reference Evins, Cather and DeckersbachEvins et al, 2005). Its modest success in smoking cessation in the latter study, involving 53 individuals, was not maintained after treatment discontinuation, when the relapse rate was high. In a subsequent study (Reference Evins, Culhane and BirnbaumEvins et al, 2007), a combination of NRT and bupropion improved abstinence and reduced cigarette consumption during treatment.
Varenicline
There are no trials of varenicline for smokers with schizophrenia, but a case report (Reference FreedmanFreedman, 2007) describes exacerbation of symptoms of the disorder during its use.
Group interventions
Specialised group therapy programmes and NRT can be effective in smokers with schizophrenia, although less so than in smokers in the general population (Reference Williams and HughesWilliams & Hughes, 2003).
Combination interventions
As previously noted, the majority of trials used a combination of pharmacotherapy and other interventions. A trial of 298 smokers with psychosis found that an eight-session, individually administered smoking cessation intervention using NRT, motivational interviewing and CBT significantly increased the proportion of participants who stopped smoking (Reference Baker, Richmond and HaileBaker et al, 2006).
Specialist services for smokers with schizophrenia and other mental illnesses have used a combination of medication and counselling, and achieved abstinence rates as high as those for people without a history of mental illness (Reference Foulds, Steinberg and RichardsonFoulds et al, 2006). These services did not limit the number of contacts, did not require a cessation date and employed an addictions psychiatrist and a mental health social worker who was also a certified tobacco treatment specialist.
Harm reduction approaches
Nicotine replacement is also used to reduce levels of smoking. A 2-year follow-up study (Reference Evins, Cather and RigottiEvins et al, 2004) showed that people with schizophrenia who reduce the amount they smoke are more likely to quit. As already mentioned, NRT combined with bupropion reduces cigarette consumption (Reference Evins, Culhane and BirnbaumEvins et al, 2007). Since smokers with schizophrenia spend a considerable amount on tobacco (in the USA, about a third of their monthly income; Reference Steinberg, Williams and ZiedonisSteinberg et al, 2004), there are significant financial benefits particularly relevant to the heavier, more dependent smokers found in mental health settings.
Effects of smoking on psychiatric medication
Smoking affects the metabolism of various psychiatric medications by inducing enzymes in the cytochrome P450 (CYP) system, potentially lowering serum levels of medication by as much as 50% (Reference Wilhelm, Arnold and NivenWilhelm et al, 2004). Medication metabolised by CYP1A2 includes diazepam, haloperidol (partial), olanzapine (partial), clozapine, mirtazapine (partial) and tricyclic antidepressants (Reference BazireBazire, 2003). Liver enzyme induction has also been documented with opiates, barbiturates and benzodiazepines (Reference Schall, Pries and KattaSchall et al, 1996). Smoking can significantly lower serum levels of such medication. Reference Haslemo, Eikeseth and TanumHaslemo et al (2006) estimate that a daily consumption of 7–12 cigarettes is probably sufficient for maximum induction of clozapine and olanzapine metabolism, and recommend a 50% lower starting dose in non-smokers to avoid side-effects.
Effect of smoking cessation on psychiatric medication
Significantly, if smokers who are stable on a drug metabolised by CYP1A2 enzymes stop smoking, enzyme activity reduces over several days, less medication is metabolised and plasma levels increase. Toxic levels of medication can accumulate in a matter of days. Similarly, plasma levels reduce over days if smoking is resumed.
Clozapine plasma levels have been found to be 40% lower in smokers than in non-smokers (Reference Seppala, Leinonen and LehtonenSeppala et al, 1999). Clozapine toxicity has also been observed during early tobacco abstinence in patients being treated with clozapine (Reference Zullino, Delssert and EapZullino et al, 2002). It is therefore recommended that plasma levels are measured before smoking cessation and, for clozapine, olanzapine and fluphenazine, the dose of medication is gradually reduced to 75% over the subsequent week and a further plasma level is taken 1 week after cessation (Reference Taylor, Ussher and FaulknerTaylor, D. et al, 2007). For benzodiazepines, doses may be reduced by up to 25% in the first week, and for tricyclic antidepressants dose reductions of 10–25% over the first week may be required. For all these medications, further dose reductions may be needed.
In summary, in addition to the other health benefits of stopping smoking, lower doses of medication may be required. Attempts to stop smoking should be closely monitored as a clinical issue.
Health economics of smoking cessation
We could find no published economic evaluations of smoking cessation interventions for people with mental illnesses. However, evaluations for the general population consistently indicate that smoking cessation interventions are relatively cost-effective in terms of cost per life-year saved. One UK analysis (Reference Woolacott, Jones and ForbesWoolacott et al, 2002) estimated that the incremental cost per life-year saved is £1000–2400 for NRT, £640–1500 for bupropion and £900–2000 for NRT plus bupropion. This makes pharmacotherapy for smoking cessation very good value since the average cost per life-year for other interventions is more than £17 000 (Reference RafteryRaftery, 2001).
Guidance on smoking cessation in mental illness
National guidance has been published for health professionals helping people with mental health problems to stop smoking (Reference McNeillMcNeill, 2004). This stresses the importance of increasing the accessibility of cessation treatments and monitoring attempts to quit, particularly as stopping smoking may affect the metabolism of antipsychotic medication.
Since 2000, a national network of National Health Service (NHS) smoking cessation services has provided support and training to healthcare professionals who interact with smokers in primary and secondary care, as well as offering intensive specialist support for smokers themselves. These services, resources permitting, can provide support and treatment for patients and staff on an ongoing basis, particularly when the NHS's smoke-free policy is being implemented.Footnote † Health professionals working within mental health settings should receive training on smoking cessation interventions. Although patients can be referred to specialist advisors within the NHS Stop Smoking Services, it would make most sense to integrate and coordinate smoking cessation services within mental health settings where those with more severe mental illness are receiving treatment. This is important since to date, smoke-free policies in mental health settings appear to have had little effect on smoking cessation in the longer term, possibly in part because of poor coordination between in-patient, out-patient and smoking cessation services. The Disability Discrimination Act 2005 should assist those with mental health problems to access the same level of smoking cessation service as the general population.
Conclusions
People with mental health problems tend to smoke more heavily and therefore have higher levels of smoking-related morbidity and mortality. The diagnosis of nicotine dependence and its management with a range of effective treatments will help such individuals to stop smoking. Smoke-free legislation encourages the use of these interventions, which have not been offered routinely to this group. Increased training opportunities for psychiatrists in smoking cessation will assist this process. The result will reduce the negative effects that smoking has on patients' physical and mental health, and will therefore encourage a more health-promoting culture within mental health settings.
Declaration of interest
None.
MCQs
-
1 Evidence does not exist for the following smoking-cessation interventions:
-
a simple advice by a physician
-
b smoking cessation advice and/or counselling given by nurses
-
c acupuncture
-
d group therapy
-
e telephone support.
-
-
2 Which of the following is false?
-
a half of smokers with mental illness have expressed a desire to stop smoking
-
b smoking increases the risk of developing future mental illness
-
c increased smoking in those with mental illness is responsible for a large proportion of this group's excess mortality
-
d smoking cessation treatments are ineffective for those hospitalised with mental illness
-
e those who recover from mental illness are not at increased risk of subsequent smoking.
-
-
3 The following drugs are not affected by smoking-related liver enzyme induction:
-
a olanzapine
-
b risperidone
-
c mirtazapine
-
d clozapine
-
e diazepam.
-
-
4 The following interventions are not helpful in smoking cessation:
-
a NRT
-
b nortriptyline
-
c fluoxetine
-
d bupropion
-
e CBT.
-
-
5 The following interventions are not helpful for assisting those with schizophrenia to stop smoking:
-
a NRT
-
b bupropion
-
c combination NRT and bupropion
-
d harm reduction with NRT
-
e SSRIs.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | F | a | F | a | F |
| b | F | b | F | b | T | b | F | b | F |
| c | T | c | F | c | F | c | T | c | F |
| d | F | d | T | d | F | d | F | d | F |
| e | F | e | F | e | F | e | F | e | T |
Acknowledgements
We are grateful to Professor Ann McNeill (University of Nottingham) for her advice and suggestions.




eLetters
No eLetters have been published for this article.