1. Introduction
The prevalence of obesity and overweight is increasing across almost all countries of the world (NCD Risk Factor Collaboration 2016; Wang et al. Reference Wang, McPherson, Marsh, Gortmaker and Brown2011). This is considered to constitute a major global public health challenge. Despite the societal importance of the topic, there is a dearth of well-developed explanatory theories for why some people become obese. Weight gain occurs when individuals habitually consume more energy than they use. Thus, decision making – in particular, decision making about how much and which foods to eat – is a central and necessary node on the causal pathway to weight gain. Decision making must, in turn, be underlain by decision-making mechanisms. It is the operating principles of these mechanisms that we need to understand: Under what circumstances will individuals recurrently make decisions that lead to their habitual consumption of more calories than they immediately require?
In this article, we advance and review one particular hypothesis concerning obesity. We will call this the insurance hypothesis (IH). We will lay out the hypothesis and its predictions over the course of the article, but it is worth stating its main constituent claims up-front:
-
• Storage of body fat is an adaptive strategy used by many vertebrates, including humans, to buffer themselves against periods during which food is unavailable.
-
• Fat storage also has costs.
-
• The optimal level of body fat to store, therefore, depends on security of access to food: If food is guaranteed to be always available, relatively little fat storage is necessary, but as the risk of temporary unavailability of food increases, the amount of fat the individual should optimally store also increases.
-
• Humans and other vertebrates possess decision-making mechanisms that adaptively regulate their fat storage. These mechanisms cause them to increase their energy intake above their level of energy expenditure when they receive cues from their environment that access to food is insecure, and reduce their energy intake to close to their expenditure when they receive cues that access to food is secure.
-
• A major driver of obesity and overweight among contemporary humans is exposure to cues that, over evolutionary time, would have reliably indicated that access to food was insecure. Exposure to these cues engages evolved decision-making mechanisms and leads to increased food consumption relative to expenditure, thus resulting in greater fat storage and higher body weights.
It is the final claim that constitutes the IH for the distribution of obesity in contemporary humans. However, the plausibility of the final claim depends logically on establishing each of the earlier points. Thus, in this article, we will consider each of the earlier points before reviewing the evidence supporting the final one.
We must stress that the IH does not originate with us. The adaptive ideas underlying it were developed within behavioural ecology more than two decades ago (see sect. 3) and have been most thoroughly tested empirically in birds (see sect. 4). There is already an extensive human social science literature on the relationship between obesity and food insecurity (see sect. 5); here, the idea tends to be known by such names as the food-insecurity hypothesis or hunger-obesity paradigm. However, this human literature makes no reference to the adaptive ideas from behavioural ecology and little reference to the empirical evidence from non-human animals. Thus, our goal in this article is to bring together the models from behavioural ecology, the non-human findings, and the empirical evidence from humans to provide an integrative statement and assessment of the IH, including its strengths, its limitations, and its possible extensions and applications.
2. Existing approaches to the psychology of human obesity
The IH is fundamentally a psychological hypothesis, given that it concerns mechanisms, presumably in the brain, that sense cues in the individual's experience and use those cues to regulate energy intake and/or expenditure. Before turning to the IH, then, we will examine some of the other psychological approaches to obesity that have been proposed. A first influential idea is the evolutionary mismatch hypothesis (e.g., Nesse & Williams Reference Nesse and Williams1995, p. 48): Roughly speaking, the idea that human decision-making mechanisms are optimized for ancestral environments where calories were usually scarce. In contemporary environments, these mechanisms produce overconsumption, especially of energy-dense foods. Obesity in contemporary populations is thus the by-product of a mind evolved to deal with frequent scarcity living now in constant abundance (for a recent version of this argument, see McNamara et al. Reference McNamara, Houston and Higginson2015). A variant of the evolutionary mismatch hypothesis states that it is energy expenditure, rather than food supply, in modern environments that falls outside of the ancestral range (Prentice & Jebb Reference Prentice and Jebb1995). Because ancestral energy expenditure was always high, we do not down-regulate food intake sufficiently when this is not the case.
Consistent with the evolutionary mismatch hypothesis is the overwhelming evidence that mean body weight increases as the population's lifestyle comes to resemble that of the urban developed world (NCD Risk Factor Collaboration 2016). However, the evolutionary mismatch hypothesis alone is incomplete, because it fails to account for the patterned variability in the incidence of obesity. If, as a species-typical fact, humans lack mechanisms to appropriately limit their intake of energy-dense foods when these foods are constantly abundant, then more or less all humans living under conditions of affluence should be overweight or obese. This is not the case. In countries such as France, Italy, Spain, Austria, Canada, and Korea, the majority of people have body mass indexes (BMIs) of less than 25, the conventional cutoff for classification as overweight (Wang et al. Reference Wang, McPherson, Marsh, Gortmaker and Brown2011). Even in the United States, which has very high rates of obesity, around one third of adults are neither overweight nor obese (Wang et al. Reference Wang, Beydoun, Liang, Caballero and Kumanyika2008). Moreover, the evolutionary mismatch hypothesis provides no account of why there should be such dramatic differences between affluent countries in obesity prevalence. Widespread obesity is concentrated in countries with relatively high levels of economic inequality (Pickett et al. Reference Pickett, Kelly, Brunner, Lobstein and Wilkinson2005), or (relatedly) where large numbers of individuals face economic insecurity (Offer et al. Reference Offer, Pechey and Ulijaszek2010). For example, whereas the 2014 rate of adult female obesity (BMI ≥ 30) is 34.9% in the unequal United States, it is only 3.0% for Japan and 17.3% for Switzerland (World Health Organization 2015). Yet it would be hard to argue that most people in Japan or Switzerland lack access to abundant energy-dense food if they want it.
Just as the evolutionary mismatch hypothesis alone fails to predict the between-country variation in obesity prevalence, it also fails to predict the within-country variation, too. Within high-income countries, obesity has been consistently linked to a low socioeconomic position, especially in women, whether this is defined by income, education, or occupation (McLaren Reference McLaren2007; Sobal & Stunkard Reference Sobal and Stunkard1989). Living in a disadvantaged community increases the risk of obesity above and beyond the effects of individual-level socioeconomic status (Black & Macinko Reference Black and Macinko2008). The simplest rendering of the evolutionary mismatch hypothesis would predict that the more financial resources people have, the more they would be able to satisfy their evolved food motivations, and the fatter they would be. In fact, the opposite is true: It is those social groups with the greatest constraints on available resources to spend on food that carry the most body fat. Thus, while the evolutionary mismatch hypothesis correctly draws attention to the obesogenic potential of the food landscape in developed countries, it needs augmenting to account for the fact that obesity is concentrated under particular types of social conditions.
A separate literature links obesity to a group of related psychological traits such as impulsivity, inhibitory control, or sensitivity to reward (e.g., Guerrieri et al. Reference Guerrieri, Stanczyk, Nederkoorn and Jansen2012; Nederkoorn et al. Reference Nederkoorn, Braet, Van Eijs, Tanghe and Jansen2006; Weller et al. Reference Weller, Cook III, Avsar and Cox2008). The central finding of this literature is that obese individuals are relatively impulsive (present-oriented, unable to delay gratification, sensitive to reward, etc.). Because this approach is rooted in the psychology of individual differences, it has greater potential to explain why some people become obese and others do not. However, when researchers have measured, in the same study, impulsivity for food and impulsivity in non-food domains, it is only the food-related impulsivity measure that is associated with obesity or food consumption patterns, not the more general measure (Dassen et al. Reference Dassen, Houben and Jansen2015; Houben et al. Reference Houben, Nederkoorn and Jansen2014). Thus, the finding essentially comes down to the fact that people who are obese or eat unhealthily place a high motivational value on getting food soon. Although this is plausible, it fails to provide a very deep explanation: What is the cause of some people placing a higher motivational value on immediate food than others do?
In view of the foregoing discussion, it seems clear that our existing understanding of the drivers of obesity is incomplete. Any satisfactory approach needs to account for the strong ecological patterning of obesity and overweight (socioecological factors such as income inequality or individual poverty increase the risk), but also explain why people respond to these particular contexts by increasing their energy intake relative to their expenditure. In the next section, we return to evolutionary first principles of what fat storage is for, in order to develop the foundations of the IH.
3. A functional approach to fat storage: The insurance hypothesis
3.1. Background
Specialized lipid stores are found in the bodies of all well-nourished animals (McCue Reference McCue2010). Lipid storage is an evolved adaptation that allows individuals to continue to survive and reproduce in the face of temporary shortfalls in energy intake from food (Higginson et al. Reference Higginson, McNamara and Houston2012; Reference Higginson, McNamara and Houston2014; Norgan Reference Norgan1997; Pond Reference Pond1998). When glycogen reserves from immediate food intake become depleted, animals generate energy mostly through the oxidation of their lipid stores until food becomes available again, though they switch to the catabolism of protein when the level of adiposity drops low enough (McCue Reference McCue2010). Lipid stores are thus beneficial to the organism and, other things being equal, the greater the extent of stored lipids, the longer the period of energy shortfall an individual is able to buffer.
However, storing lipids also has disadvantages. First, as body weight increases, so too do energy requirements. The positive scaling of energy requirement with body weight is well established across species (White & Seymour Reference White and Seymour2003), but energy requirements and body weight also co-vary within a species, including within humans (Garby et al. Reference Garby, Garrow, Jorgenssen, Lammert, Madsen, Sorensen and Webster1988; Johnstone et al. Reference Johnstone, Murison, Duncan, Rance and Speakman2005; Leibel et al. Reference Leibel, Rosenbaum and Hirsch1995; Prentice et al. Reference Prentice, Black, Coward, Davies, Goldberg, Murgatroyd, Ashford, Sawyer and Whitehead1986). Not all of this evidence is correlational: Leibel et al. (Reference Leibel, Rosenbaum and Hirsch1995) measured energy expenditure in human participants at baseline and then after a 10% weight gain or a 10% weight loss, and found that energy expenditure responded to changes in body weight. Thus, an individual storing more body fat will increase his or her ability to buffer periodic shortfalls, but do so at the cost of requiring greater energy intake to maintain his or her body weight.
Another consequence of increased body weight is reduced locomotor performance. In birds, for example, it is well established that extra mass impairs flight performance (Kullberg et al. Reference Kullberg, Fransson and Jakobsson1996; O'Hagan et al. Reference O'Hagan, Andrews, Bedford, Bateson and Nettle2015; Witter et al. Reference Witter, Cuthill and Bonser1994). In terrestrial animals, too, the cost of locomotion increases with body mass, albeit following a decelerating function (Rubenson et al. Reference Rubenson, Heliams, Maloney, Withers, Lloyd and Fournier2007). The BMI distribution of successful human runners is sharply curtailed at the heavier side, and the more elite the selection of athletes, the lower the variance in BMI (Sedeaud et al. Reference Sedeaud, Marc, Marck, Dor, Schipman, Dorsey, Haida, Berthelot and Toussaint2014). For running events of 3,000 meters and more, the BMI associated with maximal elite performance is around 20, which is towards the bottom end of the normal weight range. (Elite competitors in events shorter than 400 meters have higher BMI values, sometimes in the overweight range, but this is due to muscularity rather than adiposity.) Reduced locomotor performance is likely to affect fitness: For a prey species, locomotor abilities are central to escaping predators, whereas for predators, particularly cursorial predators like humans, locomotor abilities are central to getting enough to eat. Increased body weight also increases the risk of injury or death due to the forces and loads involved in maintaining a larger body (e.g., osteoarthritis; see Bray Reference Bray2004; Felson Reference Felson1988).
In view of the consequences of increased body weight, behavioural ecologists have long accepted that increased fat storage has benefits, in terms of enhanced ability to buffer shortfalls, as well as costs, in terms of increased energy requirements, health risks, and impairments to locomotion (e.g., Witter & Cuthill Reference Witter and Cuthill1993). The optimal level of fat reserves to carry thus depends on how the beneficial aspects of increased adiposity trade off against the detrimental ones, and the shape of this trade-off will depend on the environment experienced by the individual. Beginning with Lima (Reference Lima1986), a series of theoretical papers has shown, using slightly different assumptions and approaches, that the optimal level of fat an animal should carry depends on the risk of shortfall in the food supply (Bednekoff & Houston Reference Bednekoff and Houston1994; Higginson et al. Reference Higginson, McNamara and Houston2012; Reference Higginson, McNamara and Houston2014; Reference Higginson, McNamara and Houston2016; Lima Reference Lima1986; McNamara & Houston Reference McNamara and Houston1990). If there is no risk of shortfall, the individual can maintain a minimal level of fat and need not incur the drawbacks of carrying any more than that. If the risk of shortfall is substantial, then the individual has to carry fat as insurance – insurance that is to be paid for in terms of the drawbacks of increased body weight. This is the adaptive principle central to the IH and to this article.
3.2. An illustrative model
As the insurance principle is so fundamental to our claims, we wish to illustrate how it arises in quite a general way from principles of fitness maximization. We will therefore present a simple theoretical model here. The text presents the model in verbal form, and Online Appendix A provides the details. Our model uses an approach similar to several of the prior published ones, but sacrifices some realism in favour of generality and ease of exposition. Readers are referred to the papers on which we have built (Bednekoff & Houston Reference Bednekoff and Houston1994; Higginson et al. Reference Higginson, McNamara and Houston2012; Reference Higginson, McNamara and Houston2014; Reference Higginson, McNamara and Houston2016; Lima Reference Lima1986; McNamara & Houston Reference McNamara and Houston1990) for a sense of the elaborations that have been explored and, more importantly, for how similar results appear again and again in models set up in slightly different ways.
In our model, individuals must decide in each time period how much they will eat if they find food (from 0 to a maximum capacity of N energy units; N is always 10 for the results presented in this section). They have a metabolic requirement per time period, and anything they eat above this will be converted into fat and stored, increasing reserves but adding weight. (Weight and level of fat reserves are synonymous in our model.) The metabolic requirement is fixed at 1 unit per time period regardless of current body weight for the results presented here. (For the consequences of varying this, see Online Appendix A. Results are qualitatively unchanged by varying the metabolic requirement as long as that requirement remains substantially less than the amount an individual is able to eat in one time period, and unless it increases extremely steeply with increasing body weight. It is reasonable to assume that it should not do so, given that the main determinant of metabolic rate is lean mass; metabolic rate increases only slowly with additional fat mass.)
The individual may fail to survive the time period for two reasons. It may starve to death. As a default, we implement the probability of starvation as increasing very steeply as reserves approach zero: The probability is 0 at reserves of 2 units, 0.5 at reserves of 1 unit, and 1 (i.e., certainty) at reserves of 0 units. If the individual does not starve to death, then there is a probability of death from other causes such as predation or injuries. This probability increases by 1% for every extra unit of body weight. Thus, we are assuming an asymmetric survival function in relation to weight (Fig. 1A): There is a cliff-edge at the critically low threshold and a gentler slope with increasing weight, producing maximal survival just above the critical threshold. This asymmetric, inverted-V shape is biologically plausible and central to all of the theoretical models in this literature. Our model allows us to independently vary the location of the cliff-edge, its steepness, and the size of the fitness cost of each extra unit of reserves (see Online Appendix A and sect. 6.2 for the consequences of doing this).
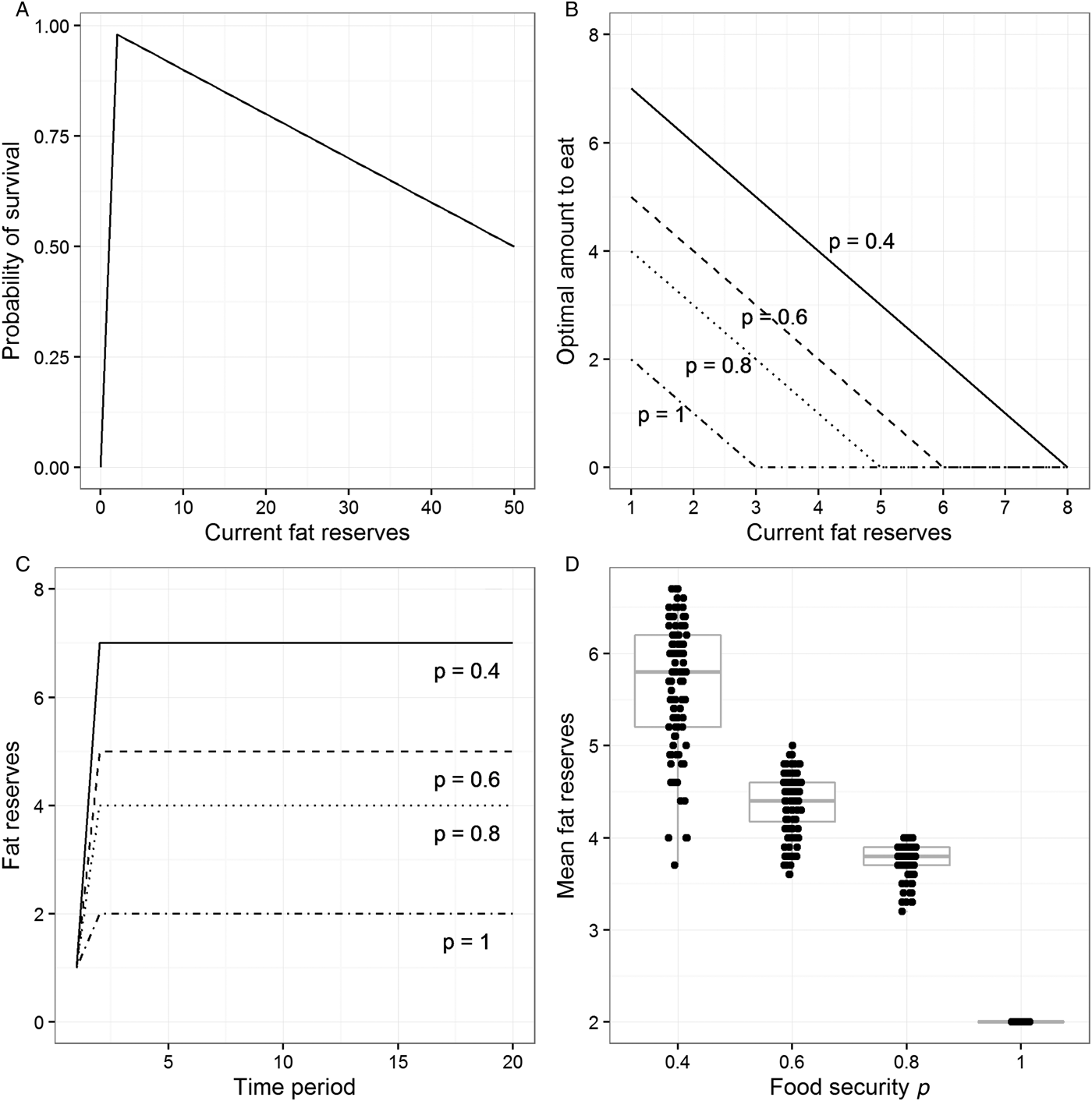
Figure 1. Output from the model described in section 3 (for details, see Online Appendix A). (A) The assumed probability of survival against current level of fat reserves. (B) The optimal amount to eat for different levels of fat reserves and four values of the food security parameter p. (C) Fat reserves over 20 time periods for individuals who begin with reserves of 1 unit, follow the optimal eating policy for their level of food security, and find food in every period. (D) Mean fat reserves over 40 time periods for simulated individuals who find food each period with probability p and follow the optimal eating policy for their level of food security. Points have been jittered in the horizontal dimension to make them more visible.
Each time period, the individual finds food with probability p. We can think of p as the individual's level of food security. If p is 1, then access to food is totally secure, whereas if p is, say, 0.6, then access to food is very insecure: there is a 40% chance there will be no food. Given that we are concerned with computing optimal behaviour, we treat individuals as knowing the value of p for their environment perfectly. The question we set our model is: What is the best amount to eat if the individual does find food, given its level of food security p, and its current level of reserves? To find this optimal eating policy, we use a dynamic programming approach (Clark & Mangel Reference Clark and Mangel2000; Houston & McNamara Reference Houston and McNamara1999; Mangel & Clark Reference Mangel and Clark1988). This involves starting at the final time period in a long sequence and computing, for each value of p and possible level of reserves, what the probability of surviving beyond that period would be if the individual ate 0 units, 1 unit, 2 units, and so on (if food can be found). This produces a look-up table specifying for every level of reserves the amount to eat that maximizes the probability of survival. We then move to the previous period and ask, for every level of possible reserves the individual might have, and given that in the next period it will follow the already-calculated optimal policy for the reserves it will have at that point, what is the probability of survival associated with every possible eating decision? This in turn gives a look-up table linking reserves to the amount to eat for the penultimate period. The backwards iteration is repeated for 100 periods, and the output is the look-up table from the earliest time point. What we report as the optimal policy for each possible value of p thus represents the mapping between current reserves and amount to eat (if food can be found) that maximizes the probability of survival into the distant future.
Note that, although we have described the catastrophic fitness event that occurs when reserves fall below a critical threshold as death by starvation, and the maximand of the model as survival into the distant future, the catastrophic event could equally be thought of as loss of reproductive capacity; the maximand, the probability of successful reproduction. The computations and predictions would be the same under this interpretation. This is important because temporary energetic shortfall may lead to loss of reproductive capacity long before death by starvation is reached; this may be an equally important way in which energetic shortfall is detrimental to fitness. We return to this issue in section 6.2.
3.3. Model results
For all levels of p, the optimal policy produced by our model has the same basic form: If current reserves are very high, don't eat anything, and instead burn down some reserves. As reserves get lower, there comes a point where it is optimal to eat something and thus maintain or increase reserves (Fig. 1B). Both the level of reserves at which eating should begin and the optimal amount to eat when reserves are low depend on the level of food security p. When p = 0.4, for example, the individual should start to eat when reserves drop to 7 units, and when reserves drop to 1, the individual should take in 7 units per period. When p = 0.8, eating only kicks in when reserves drop to 4 units, and the most that the individual should ever eat is 4 units in a period. When p = 1, complete food security, the individual only eats when reserves drop to 2 units.
The optimal policies illustrated in Figure 1B amount to “trying” to maintain a constant fat buffer whose size is related to the level of food security p: The lower p is, the larger the buffer should be. We can illustrate this by simulating individuals who follow the optimal policies for different values of p and find food every time period (Fig. 1C). As Figure 1C shows, individuals initially eat more than their energetic requirements, then stabilize at a certain level of reserves. For p = 1, this is simply the level of reserves that maximizes survival in the current period (2 units), but for lower values of p, individuals carry more than this, and the lower p is, the more they carry.
Under food insecurity, by definition, individuals may not find food in every time period. Thus, a more realistic investigation is to simulate individuals who have a probability p of finding food each period and follow the optimal eating policy for that value of p. Given that this simulation has a stochastic component, no two individuals have exactly the same sequence of experiences or weights (as long as p < 1). We therefore simulate 100 individuals at each level of food security for 40 periods each. All individuals begin with 5 units of reserves, and individuals not surviving for 40 periods are excluded. Figure 1D plots individuals’ mean body weights/fat reserves, removing the first 10 periods to eliminate initialization artefacts. As the figure shows, mean weights/reserves become higher as p becomes lower.
Thus, our very simple model recovers the insurance principle often described in the theoretical behavioural ecology literature. High levels of stored reserves ought to be found not among those whose access to food is assured, but exactly among those whose access to food is insecure. The more insecure this access is, the heavier their target weight should be, essentially because it is in their interest to bear the costs of some extra weight to insure themselves against the more catastrophic cost of possible starvation. The consequence of following this optimal policy is that individuals should in practice become heavier as their access to food becomes more insecure. This result is very robust to numerical variation in the parameters chosen (see Online Appendix A).
4. Non-human evidence that food insecurity causes weight gain
The insurance principle described in section 3 was well known in behavioural ecology at least as early as the publication of Lima (Reference Lima1986). Evidence consistent with it was available from observational comparisons both within and between species. For example, Rogers (Reference Rogers1987) showed that bird species whose winter food supplies were unpredictable (insecure, in the language used in this article) carried more fat than those whose winter food supply was predicable (secure). More recent work confirms the basic effect of food security and demonstrates an additional effect of predation risk (Rogers Reference Rogers2015). Species facing higher predation risk, other things being equal, carry relatively less fat than those whose risk is lower. Because one of the major costs of additional fat in birds is the reduction in predator escape performance, this makes sense in the light of the theoretical literature: Birds trade off the risk of predation if they are fat against the risk of starvation if they are thin. Other early work showed that within bird species, fat storage increases at those times of year when insecurity of food supply is likely (for a review, see Witter & Cuthill Reference Witter and Cuthill1993).
The real breakthrough arose when the experimental method began to be applied to fat storage. This allowed the unequivocal demonstration that fat storage was plastic within individuals and could be deployed strategically as a response to environmental experience. Ekman and Hake (Reference Ekman and Hake1990) experimentally manipulated the food-access regime of captive greenfinches Carduelis chloris, by either giving them food ad libitum or an equal total quantity of food appearing intermittently at unpredictable times of the day. They found that 10 of 11 birds significantly increased their weight in response to the unpredictable regime; the lightest and leanest individuals showed the strongest response. Witter et al. (Reference Witter, Swaddle and Cuthill1995) subjected an experimental group of adult European starlings Sturnus vulgaris to unpredictable daily periods of food deprivation: Birds in this group increased their weight, while those in an ad libitum control group did not. This result was confirmed in a later experiment in juveniles (Witter & Swaddle Reference Witter and Swaddle1997); here again, the largest response was seen in those individuals whose weight was lowest prior to the manipulation.
A related set of findings concerns the effect of dominance on weight regulation. Ekman and Lilliendahl (Reference Ekman and Lilliendahl1993) showed in willow tits Parus montanus that it was subordinate individuals who carried the greatest fat reserves (for a related theoretical model, see Clark & Ekman Reference Clark and Ekman1995). Moreover, experiments in which dominant individuals were removed from flocks showed that this relationship was causal: Subordinates lost weight when the dominants were removed. Witter and Swaddle (Reference Witter and Swaddle1995) showed that in European starlings, too, subordinates carried more weight than dominants and lost weight when dominants were removed from their group. They also replicated the effect of imposing food insecurity on weight, but showed that the weight gain in response to insecurity was greatest among subordinates. Subordinate birds are, by definition, prone to being displaced or excluded from resources that are available. Thus, any insecurity in access to food is likely to fall particularly strongly on them, and so it is consistent with the IH that their levels of fat storage would be raised. This is a very interesting finding in light of the human epidemiological evidence that within affluent societies, it is the most disadvantaged social groups in which obesity is most common (Black & Macinko Reference Black and Macinko2008; McLaren Reference McLaren2007; Sobal & Stunkard Reference Sobal and Stunkard1989).
Thus, the evidence from small birds shows that when individuals receive cues suggesting that their access to food is likely to be insecure – and hence that there might be periods of shortfall – they increase their stored fat reserves to provide insurance. Moreover, the use of experimental approaches demonstrates that the association between insecurity and fat storage is causal, and that individuals can dynamically increase or decrease stored fat in response to variation in their experience of the world. The implication is that birds have evolved psychological mechanisms that integrate information received concerning metabolic demands and likely security of access to food, and these mechanisms up-regulate levels of food consumption – or down-regulate energy expenditure – as perceived security of access to food decreases.
The evidence reviewed thus far is all from birds. The costs of excess mass might be particularly high in a small flying animal; terrestrial animals might thus tune their reserves less finely to their current expectations of shortfall. A recent experimental study showed that weight increased in mice whose food access was restricted, compared to a control group (Li et al. Reference Li, Cope, Johnson, Smith and Nagy2010). Thus, the insurance principle works in at least one species of mammal as well as birds. This does not, of course, guarantee that humans possess similar mechanisms. However, there is a large empirical literature on food insecurity and fatness in humans, and it is to this literature we now turn.
5. Empirical evidence for the IH
5.1. Background
In 1995, William H. Dietz published a paper in the journal Pediatrics with the title “Does hunger cause obesity?” (Dietz Reference Dietz1995). Dietz presented a case study of an obese young girl whose impoverished parents (also obese) received welfare assistance. They frequently lacked money to buy food in the period just before their welfare cheque arrived. They apparently compensated by consuming many calories whenever they could, leading to their high body weights. Dietz speculated that what was at work in this family might be “an adaptive response to episodic food insufficiency” (p. 766).
Dietz's empirical insight was followed up, but his adaptive logic was not. Hundreds of papers have subsequently been published on the association between food insecurity and high body weight in humans, as we shall see later in this section. Ironically, they often describe the association as paradoxical (e.g., Crawford & Webb Reference Crawford and Webb2011; Scheier Reference Scheier2005; Tanumihardjo et al. Reference Tanumihardjo, Anderson, Kaufer-Horwitz, Bode, Emenaker, Haqq, Satia, Silver and Stadler2007). For example, Basiotis and Lino (Reference Basiotis and Lino2003, p. 57) asked, “How can a person report that in her household sometimes or often they do not have food to eat, yet be overweight? … A definitive solution to this paradox must await additional research.” In fact, the association follows from the adaptive theoretical models developed years earlier in behavioural ecology. Unfortunately, not a single paper from the human social science literature that we have been able to find cites any of the theoretical models from behavioural ecology discussed in section 3.
The empirical studies that began to appear after Dietz's paper used either large, representative population surveys, or smaller opportunity samples of particular social groups, to investigate whether participants’ reports of their food insecurity were associated with their body mass. Within this literature, food insecurity is defined as “limited or uncertain ability to acquire nutritionally adequate and safe food in socially acceptable ways” (Castillo et al. Reference Castillo, Ramsey, Yu, Ricks, Courville and Sumner2012; Dinour et al. Reference Dinour, Bergen and Yeh2007). It is typically measured using self-report questionnaires, of which the most widely used examples are the Radimer/Cornell Hunger and Food Insecurity Instrument (Kendall et al. Reference Kendall, Olson and Frongillo1995; Radimer et al. Reference Radimer, Olson, Greene, Campbell and Habicht1992) and its derivative, the U.S. Department of Agriculture's Core Household Food Security Module (Nord et al. Reference Nord, Andrews and Carlson2009). These questionnaires address both the experience of sometimes having insufficient food (e.g., “The food that we bought just didn't last, and we didn't have money to buy more.”) and also the cognitive evaluation that an episode of insufficient supply is likely (“We worried whether our food would run out before we got money to buy more.”). Thus, what these instruments measure is some kind of running cognitive estimate of the variable p in our model: that is, the likelihood of a temporary shortfall in the food supply. Both questionnaires yield a continuous food insecurity score, although in practice this is often reduced to a food-secure versus food-insecure dichotomy, or a three-way – occasionally a four-way – classification.
The human literature on food insecurity and body weight has become so extensive that several reviews have appeared (Dinour et al. Reference Dinour, Bergen and Yeh2007; Eisenmann et al. Reference Eisenmann, Gundersen, Lohman, Garasky and Stewart2011; Franklin et al. Reference Franklin, Jones, Love, Puckett, Macklin and White-Means2012; Laraia Reference Laraia2012; Larson & Story Reference Larson and Story2011; Morais et al. Reference Morais, Dutra, Franceschini and Priore2014). The general consensus of these reviews is that there is a positive association between food insecurity and high body weight in women, but the association is less clear or absent in men. This may well relate to the wider finding that low socioeconomic position is a more consistent predictor of overweight or obesity in women than in men (Sobal & Stunkard Reference Sobal and Stunkard1989). The previous reviews have also concluded that the relationship between food insecurity and high body weight may not be detectable in children, and that developing countries may not show the same pattern as the developed countries (especially the United States) from which most of the evidence comes.
5.2. Meta-analysis methods
Although the level of consensus within the existing review articles is fairly high, none has used meta-analytic techniques to estimate the overall strength of the association or examine potential moderators of association strength. Instead, they based their conclusions on tallying up which studies reported statistically significant associations and which ones did not. Because individual studies may have fairly low statistical power, this approach does not definitively answer the question of whether, for example, the association is significantly less strong in men and children than in adult women. We thus undertook a meta-analytic review of the human food insecurity–body weight literature to 2015. The full methods and results of the meta-analysis are presented as Online Appendix B. This and the next section provide a short summary.
We used PubMed and Scopus searches, enriched with all papers citing and cited by key previous reviews of the literature, to identify papers reporting quantitative data on an association between a measure of food insecurity and a measure of body weight. The initial candidate set identified by our searches was 173 papers. Review of the full text of these led to a final set of 125 papers included in the meta-analysis. The 48 excluded papers either did not present original data on a relevant association or did not present them in a form statistically comparable to the other studies. The standard measure of association used in this literature is the odds ratio (OR) or its logarithm (LOR) for high versus normal body weight for participants reporting food insecurity as compared to security. The exact definition of high body weight varies from association to association (e.g., for some associations it is obesity [BMI ≥ 30] versus normal weight, for others overweight [BMI ≥ 25] versus normal weight), as does the exact specification of the food-insecurity variable. In the majority of cases, ORs or LORs were provided directly by the study's authors. In the remaining cases, we converted correlations, frequencies, or means and standard deviations into LORs using standard transformations. Papers often presented multiple associations (e.g., separate comparisons for men and women, for obesity vs. normal weight and overweight vs. normal weight, or for severe food insecurity vs. security and moderate food insecurity vs. security). Thus, there were a total of 301 reported associations from the 125 papers. We dealt with the statistical non-independence of multiple associations from the same study using multilevel meta-regression.
As well as asking whether the evidence supports an association between food insecurity and high body weight overall, we explored the effects on association strength of a wide variety of moderating factors. These included aspects of the study design (longitudinal vs. cross-sectional, whether the authors controlled for co-variates such as socioeconomic position); the analysis (whether the high body-weight outcome was obesity or overweight, whether the predictor was continuous, dichotomous, or multinomial), and the participants (whether the sample was male, female, or mixed sex; adults or children; World Bank–defined high-income country or not). Full statistical results are presented in Online Appendix B. Here, we summarize the main findings qualitatively and illustrate them graphically in Figure 2 by showing central LOR estimates and their 95% confidence intervals, for a series of different subsets of the data.
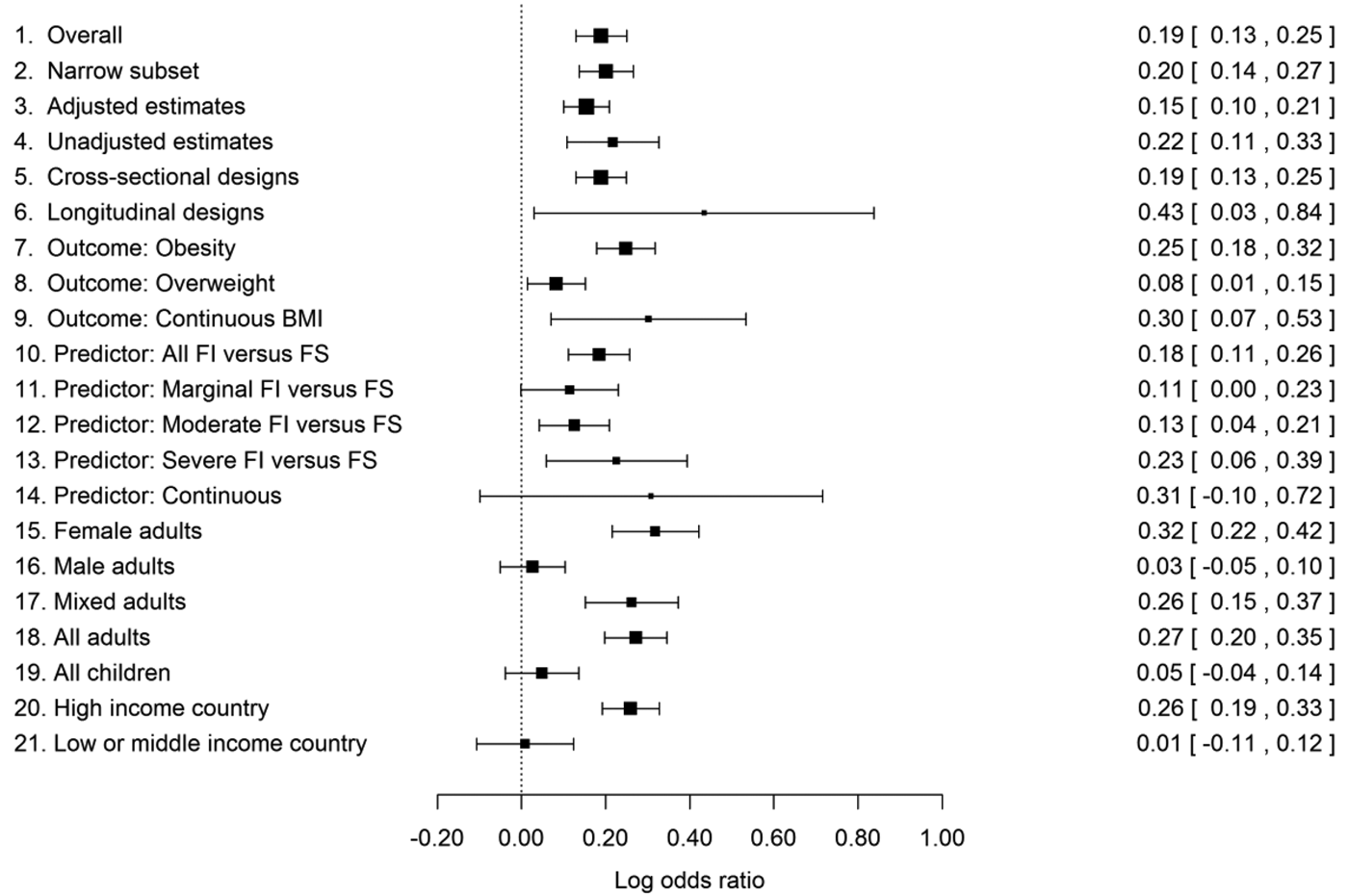
Figure 2. Estimated log odds ratios (LORs) for high versus normal body weight in food-insecure versus food-secure individuals, plus their 95% confidence intervals, from the data set overall (line 1) and from various subsets of the data. Zero represents no association. The high body-weight outcome varies from association to association (e.g., obesity, overweight), as does the exact specification of the food-insecurity variable. For details, see section 5 and Online Appendix B.
5.3. Meta-analysis results
Overall, there was a positive association between food insecurity and high body weight (line 1 of Fig. 2). The central LOR estimate of 0.19 corresponds to an OR of 1.21 (95% CI [confidence interval] 1.14–1.29); the odds of high body weight are around 21% higher for food-insecure than food-secure participants. This estimate was almost unchanged when we restricted the analysis to just those associations where the OR or LOR had been stated in the original paper, rather than converted by us from other kinds of statistics (line 2 of Fig. 2). An important possibility is that this association is just a consequence of both food insecurity and obesity both being related to a common third variable, most obviously income or socioeconomic position (Gundersen et al. Reference Gundersen, Kreider and Pepper2011a). If this was the mechanism producing the association, we would expect estimated associations from analyses that control for socioeconomic and demographic factors to be substantially weaker than those from unadjusted analyses. This was not the case: The adjusted LORs in the data set were only slightly less strong than the unadjusted ones and still significantly greater than 0 (lines 3 vs. 4).
A previous narrative review suggested that longitudinal evidence for the association (which gives a stronger suggestion of causality) has not been as convincing as cross-sectional evidence to date (Larson & Story Reference Larson and Story2011). We found no evidence that longitudinal associations are any weaker than cross-sectional ones (lines 5 and 6). There are just many fewer longitudinal studies (seven that we were able to include, and several of these concerned the specific situation of longitudinal studies of pregnancy). Correspondingly, there is less precision in their estimate of the association. We note that most of the few longitudinal studies are only longitudinal in a partial sense: They examine change in body weight over time by food-insecurity status. We are aware of only one study employing the stronger “doubly longitudinal” approach, in which change in body weight is examined by change in food insecurity (Whitaker & Sarin Reference Whitaker and Sarin2007). Given that change in food-insecurity status may be relatively rare, such studies are difficult and require large samples. However, it is these designs that come as close to the experimental approaches used in birds as is possible with human participants. More longitudinal evidence, particular doubly longitudinal studies, is thus a priority.
There is considerable variation across studies in how the data are analysed. We found that associations are significantly stronger when the outcome variable is obesity (BMI ≥ 30) than when it is the less extreme outcome overweight (BMI ≥ 25; lines 7, 8, and 9 of Fig. 2). We found no significant differences in association strength according to exactly which predictor was used (lines 10–14 in Fig. 2). This is of note because in one of the most influential studies (Townsend et al. Reference Townsend, Peerson, Love, Achterberg and Murphy2001), it was the milder but not the most severe levels of food insecurity where increased odds of obesity were found. Our analysis suggests that this is not a general pattern. However, the division points between marginal, moderate, and severe food insecurity are made in different ways by different authors, even those using the same measurement questionnaire. Thus, the lack of clear patterning of association strength by level of food insecurity may simply result from variation between studies in the definition of each level.
All-male adult samples showed significantly weaker associations than all-female or mixed-sex ones (which are often female-biased; lines 15–17 of Fig. 2). Moreover, the LOR in just the all-male adult samples did not differ significantly from zero. There has been a particular focus on women and girls in this literature, with 117 all-female associations reported compared to 41 male and 143 mixed-sex associations. However, this is likely to be a consequence of the sex difference in association – an influential early paper showed that food insecurity was particularly relevant to women's obesity (Townsend et al. Reference Townsend, Peerson, Love, Achterberg and Murphy2001), and this inspired further research – rather than its cause. Forty-one papers still constitute a good sample size for detecting an association in men.
Child samples showed significantly weaker associations than adult ones. The LOR did not differ from zero in all children considered separately from the adults (lines 18 and 19). We also examined whether the age of children made any difference; overall, it did not, though there was some evidence that the sex difference in association characteristic of adults begins to be detectable in older children (see sect. B3.2 in Online Appendix B).
We also examined the moderating effect of the level of economic development of the study country. This made a significant difference to the association strength, with a positive LOR in high-income countries and an overall LOR close to zero in low- and middle-income countries (lines 20 and 21). The overall null effect in the low- and middle-income countries masks variability: Some individual studies have found significant positive associations in line with the high-income country evidence (e.g., Chaput et al. Reference Chaput, Gilbert and Tremblay2007 in urban Kampala), although there are several associations in the opposite direction (i.e., food insecurity reduces odds of overweight: Dubois et al. Reference Dubois, Francis, Burnier, Tatone-Tokuda, Girard, Gordon-Strachan, Fox and Wilks2011; Isanaka et al. Reference Isanaka, Mora-Plazas, Lopez-Arana, Baylin and Villamor2007) in children. The geographical coverage of the data set is very uneven: 209 of the 301 associations came from high-income countries, and 178 of these from the United States. More evidence is thus needed from different kinds of samples in the developing world and also from non-U.S. high-income countries.
A serious problem for the interpretability of meta-analytic results is publication bias. If significant positive associations are more likely to be published than null ones, then any data set assembled through a search of the literature will overestimate the true association. We examined whether publication bias was likely to be operative in two ways. First, we compared estimates from appropriate parts of our data set to those from two individual studies that used authoritative methods (Gundersen et al. Reference Gundersen, Garasky and Lohman2009; Townsend et al. Reference Townsend, Peerson, Love, Achterberg and Murphy2001). These both featured large, nationally representative samples (from the U.S. National Health and Nutrition Examination Survey) and high-quality measurement of both food insecurity and body weight. The results of Townsend et al. (Reference Townsend, Peerson, Love, Achterberg and Murphy2001) produced a combined LOR of 0.27 (95% CI 0.10–0.44) for U.S. women and an LOR not significantly different from 0 (exact value and CI unstated) for U.S. men. The aggregated studies from high-income countries in our data set give LORs of 0.42 (95% CI 0.29–0.55) for women and 0.03 (95% CI−0.05 to 0.10) for men. The individual LOR from Gundersen et al. (Reference Gundersen, Garasky and Lohman2009) for U.S. children (0.13, 95% CI−0.17 to 0.43, using the BMI-based measures) is extremely similar to the meta-analytic LOR for all children in high-income countries (0.11, 95% CI 0.01–0.21). Our aggregated estimates for high-income countries are thus broadly in line with the evidence from high-quality individual studies.
Second, we performed a standard statistical test for publication bias based on the asymmetry of the distribution of associations (Egger et al. Reference Egger, Davey Smith, Schnieder and Minder1997; see sect. B3.3 in Online Appendix B for details). The test was significant, suggesting publication bias might be operative. We then used the “trim and fill” method to impute the associations required to make the distribution symmetrical (Duval & Tweedie Reference Duval and Tweedie2000). This procedure reduced the central estimate of the LOR by around one third, but it remained significantly different from zero (0.12, 95% CI 0.07–0.17). Moreover, the differences between women, men, and children, and between high-income and other countries, survive imputation of extra associations via the trim-and-fill procedure (see sect. B3.3 in Online Appendix B).
In summary, our meta-analysis of the literature leads to several conclusions. The large body of available evidence supports the view that food insecurity is a predictor of high body weight in humans. This is unlikely to be an artefact of food insecurity and high body weight both being associated with some third variable, such as socioeconomic position. However, the association is far from uniform. Specifically, the overall association is driven by adult women in high-income countries; it is weaker or absent in men, in children, and in low- and middle-income countries. These conclusions are largely consistent with those of previous reviews (Dinour et al. Reference Dinour, Bergen and Yeh2007; Eisenmann et al. Reference Eisenmann, Gundersen, Lohman, Garasky and Stewart2011; Franklin et al. Reference Franklin, Jones, Love, Puckett, Macklin and White-Means2012; Laraia Reference Laraia2012; Larson & Story Reference Larson and Story2011; Morais et al. Reference Morais, Dutra, Franceschini and Priore2014). This is reassuring, given that we assembled a larger and more comprehensive data set than any previous reviews and used quantitative meta-analytic techniques for the first time. With the meta-analytic evidence in hand, we are now in a position to make an evaluation of the IH as an explanation for the distribution of obesity in the contemporary human population. That evaluation is presented in the next section.
6. Evaluating the IH as an explanation for human obesity
To begin evaluating the IH, it is worth restating exactly what its claims are. The hypothesis proposes that humans possess evolved mechanisms that respond to cues or experiences indicating that access to sufficient food is uncertain by increasing energy intake relative to expenditure, and hence storing more fat. Exactly how these mechanisms work at the proximate level (e.g., what the cues are, the relative contributions of increased intake and reduced energy expenditure, whether it is motivation for food overall or for energy-dense foods in particular that is affected) requires further specification. Note that the hypothesis does not need to claim that being obese is a currently adaptive strategy for people in food-insecure social groups. That is, it need not predict that in food-insecure social groups, fatter people have better survival than leaner people. Such a pattern would be very interesting in the light of the hypothesis, but the absence of such a pattern would not refute it. This is because the hypothesis claims psychological mechanisms that increase fat storage in response to cues of food security have, on average, been fitness-promoting over evolutionary time. It is agnostic on whether they still promote fitness in, say, the contemporary United States. For example, the mapping between cues of food insecurity and evolutionary fitness might be quite different in contemporary environments than in historical ones.
Although the evidence reviewed in section 5, taken overall, finds the association predicted by the IH, there are still important grounds for scepticism or at least qualification. Below we discuss some of these, before concluding with an overall evaluation.
6.1. Is the association strong enough?
To convincingly claim the IH was supported by the epidemiological data would require a strong association between food insecurity and high body weight. Our observed association, although statistically highly significant, is moderate: For adult women in high-income countries, the odds of high body weight are about 50% higher for food-insecure individuals compared to food-secure ones. To put this in context, it is larger than the increase in odds of high body weight due to carrying a risk allele of the FTO gene (Frayling Reference Frayling2013; see sect. 6.5). Moreover, it is generally accepted that the existence of measurement error leads to the underestimation of associations. In classical psychometric theory, the best estimate of the true association is the observed association divided by the square root of the product of the reliabilities of the two measures, where reliability is the proportion of variation in the measure that reflects variation in the underlying quantity (Spearman Reference Spearman1910). Thus, if the reliabilities of the measures are 0.5, the true association is twice as strong as the observed association.
In the food-insecurity–obesity literature, there is likely to be considerable measurement error in both outcome and predictor. The limitations of BMI and its derivatives as measures of fatness are well known: They do not measure adiposity directly, and people of quite different body compositions can have the same BMI (Prentice & Jebb Reference Prentice and Jebb2001). On the predictor side, the questionnaires used to assess food insecurity are unlikely to capture the required causal variable very accurately. The causal variable is presumably some implicit integration of multiple cues and experiences over an extended period of time. Questionnaires simply may not be able to capture this well; indeed, it may not be the kind of psychological variable that is available to explicit self-report with any precision. Thus, the relatively modest association strength does not, in our view, necessarily undermine the IH; rather, we are struck that any clear evidence emerges from such noisy measures.
6.2. Why is there a sex difference?
Our meta-analysis finds no association between food insecurity and high body weight in men. On the face of it, this is problematic for the IH, which should be generally applicable. In this section, we consider how differences between women's and men's life histories could explain why the predictions of the model described in section 3 are met in the one case but not the other. There is a clear sex difference in human adiposity, with fat representing around 27% of body weight in women to about 15% in men (Norgan Reference Norgan1997). The sex difference is generally attributed to the energetic requirements of reproduction for women (Norgan Reference Norgan1997; Zafon Reference Zafon2007). However, what we are concerned with here is not women's greater average adiposity, which appears readily explained by reproductive demands, but the greater responsiveness of their adiposity to food insecurity.
The best way to try to explain the sex difference within the model presented in section 3 is to make the shape of the function mapping reserves to fitness (Fig. 1A) different for men and women. The model allows three ways of doing this (see sects. A3.2–A3.4 in Online Appendix A). First, we can move the location of the fitness cliff-edge further to the right for women (Fig. 3A). This would make sense if the level of adiposity below which it is costly to drop is higher for them than for men, due to the need to be able to fund pregnancy and lactation. Moving the cliff-edge to the right increases steady-state adiposity at every level of p, and hence can account for women's greater adiposity overall (Fig. 3B). However, it does not increase responsiveness to food insecurity: The gradient of the relationship between p and steady-state fat reserves is unchanged by moving the cliff-edge to the right, as Figure 3B shows.
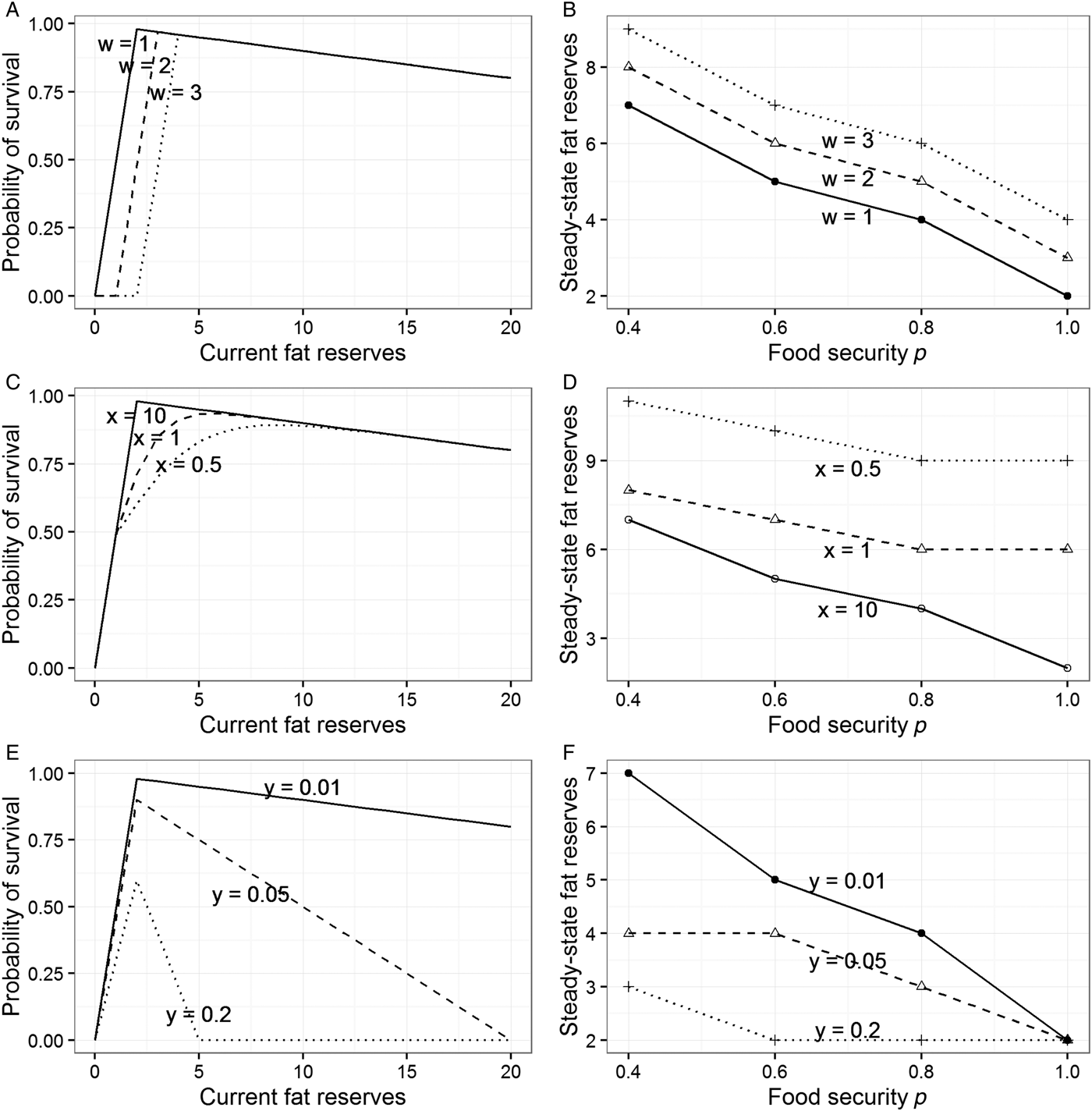
Figure 3. Modifications to the model from section 3 to explore potential explanations for sex differences. See sections A3.2–A3.4 in Online Appendix A for full details. (A) Three different locations for the cliff-edge below which starvation becomes likely (controlled by parameter w). (B) Steady-state target levels of fat reserves at different values of p for the different cliff-edge locations shown in panel A. (C) Three different shapes of the left part of the survival function (controlled by parameter x). (D) Steady-state target levels of fat reserves at different values of p for the different shapes shown in panel C. (E) Three different slopes of the right part of the survival function, the cost of carrying each additional unit of weight (controlled by parameter y). (F) Steady-state target levels of fat reserves at different values of p for the slopes shown in panel E.
Second, we can make the probability of fitness loss increase in a more graded way as reserves become low, rather than the step-function used thus far (Fig. 3C). This is another way of capturing the intuition that for women there are costs of low reserves that manifest short of the point of death by starvation. A more graded diminution leads to individuals maintaining higher levels of fat reserves (this is because the effect of introducing the more graded function is to move the point of maximal survival in each period somewhat to the right; see Fig. 3C). However, it does not lead to greater responsiveness to changes in the level of food security p. On the contrary, a more graded survival function leads to fatter individuals who are somewhat less sensitive to the prevailing value of p (Fig. 3D). Thus, in our model, allowing women to have a greater minimal required level of adiposity, or a more graded relationship between low fat levels and reproductive success, correctly predicts that they will be fatter on average, but fails to shed any light on why they should be more sensitive to the experience of food insecurity.
The third way of altering the model is to make the slope at the right of the survival function steeper for men than for women. To recap, this slope represents the degree to which survival declines with each extra unit of weight. Steeper slopes (as shown in Fig. 3E) produce individuals who maintain lower average reserves and are also less responsive to the current level of food security p (Fig. 3F). This lack of responsiveness arises because with a heavy penalty for each extra unit of weight, it becomes too costly to carry a substantial buffer, regardless of the risks. Sexually differentiated foraging and mobility patterns are widely documented in hunter-gatherer societies and assumed to be typical of past human societies: Men range more widely, partly through pursuing more mobile prey (Marlowe Reference Marlowe2007) and partly for other reasons (MacDonald et al. Reference MacDonald and Hewlett1999). Men are also much more likely to be involved in intraspecific violent conflict, thought to be an important selection pressure in ancestral human societies (McDonald et al. Reference McDonald, Navarrete and Van Vugt2012). Thus, one tentative possibility is that men's activities meant that the costs of extra body weight were more severe for them than for women over evolutionary time. If this were correct, our model would predict both lower average adiposity in men and reduced responsiveness to current food insecurity.
This explanation is not definitive, because one can imagine a differently implemented model leading to different conclusions. Furthermore, the sex differences in the mappings between body weight and fitness need to be established empirically. Nonetheless, it illustrates how principled refinement to the model presented here can generate hypotheses for further investigation. Our tentative suggestion on sex differences is at the very least incomplete, because the model parameter values required to make males insensitive to food insecurity also lead to them being extremely lean under all circumstances. Though men are leaner than women, globally, male body weights have increased just as steeply in recent years as female ones (NCD Risk Factor Collaboration 2016). This means that something in the environment can drive substantial increases in male body weight, even though that something is apparently not food insecurity. Recourse to candidate explanations other than the IH is required. Once we admit that other candidate explanations are important for men, the door is open to their invocation in women, too. Hence, the failure of the IH for men implies that our explanations for the contemporary distribution of obesity must be multifactorial, with food insecurity playing only a part.
6.3. Why is the association found only in high-income countries?
Our meta-analysis showed that food insecurity predicts high body weight only in high-income countries. In low- and middle-income countries, the average association is zero. In high-income countries, the available diet generally has higher energy density than the food available in lower-income countries (Drewnowski & Popkin Reference Drewnowski and Popkin1997). This means that food-insecure individuals will be able to consume high levels of calories in periods when they do have access to food, even if these periods are intermittent. In a low-income country, not only might food access be insecure, but also when food is available, it may not be energy-dense enough to allow the buildup of fat reserves before the next period of scarcity strikes.
In section A3.5 in Online Appendix A, we explore the consequences of low energy density of food in our model. We do this by placing a sharp constraint on N, the number of units of energy that can be consumed in one time period when food is available. Constraining N has interesting consequences; when p is low, the steady-state target level of reserves is higher when N is small than when it is large. On the other hand, in simulations, the actual body weights that individuals maintain are much more variable when N is low and are often well below the steady-state target (Fig. 4). This is because, under food insecurity, stochastic periods without food deplete individuals’ reserves, and it takes them much longer to build those reserves back up again when food is available, because the amount by which their intake can exceed their expenditure in any one period is constrained. Essentially, in a low-p, low-N world, individuals should aspire to carry high reserves, but are often unable to get as fat as they should want because their food supply is not energy-dense enough. This means that, when N is small, p becomes a relatively poor predictor of body weight. In the data underlying Figure 4, p predicts 77% of the variance in reserves in the left panel and only 20% of the variance in the right panel.

Figure 4. Mean level of reserves over 40 days for simulated individuals experiencing different levels of food security p, for two different values of the maximum energy available from food per period, N. When N is small, p becomes a poor predictor of body weight as the variability between individuals at the same level of p becomes greater. Points have been jittered in the horizontal dimension to make individual data points more visible.
If the interaction between food insecurity and energy density of food is indeed an explanation for the lack of observed association in low-income countries, then interesting predictions follow. We should predict that food-insecure individuals in these countries would often wish to be fatter than they can actually manage to be. The existence of heavier body ideals in subsistence populations is well documented and stands in contrast to the ideals of thinness typical of high-income societies (Anderson et al. Reference Anderson, Crawford, Nadeau and Lindberg1992; Tovee et al. Reference Tovée, Swami, Furnham and Mangalparsad2006; Wetsman & Marlowe Reference Wetsman and Marlowe1999). More specifically, Gulliford et al. (Reference Gulliford, Nunes and Rocke2006) found that food-insecure individuals in Trinidad and Tobago did not have higher BMIs than food-secure individuals. However, food-insecure study participants were more likely to report that they were trying to gain weight. Reporting trying to gain weight was quite common in Trinidad and Tobago, whereas it would presumably be very rare in a high-income country.
The idea that, under low-income conditions, the available food is insufficiently energy-dense for food-insecure individuals to maintain high body masses offers a reasonable explanation for why the association is restricted to high-income countries. In fact, we need to go further: The very high body weights seen in high-income countries probably represent the operation of decision-making mechanisms optimized to deal with food insecurity in energy-sparse ancestral food environments in contemporary environments where widely available foods are energy-dense. That is, rather than levels of food insecurity per se explaining the contemporary distribution of obesity, it is the combination of high levels of perceived food insecurity with historically unprecedented energy density of widely available foods; the IH needs to be synthesized with some form of evolutionary mismatch argument to explain the extent of contemporary obesity. Such a synthesis makes sense of why widespread obesity should be an epidemic of affluence, but particularly of affluent countries characterized by high levels of inequality and/or economic insecurity. It also predicts rapid increases in obesity as unequal developing countries make the transition to urban living and an industrialized food supply, as has indeed been observed (Drewnowski & Popkin Reference Drewnowski and Popkin1997).
6.4. Why is there no association in children?
Our meta-analysis showed that the association between food insecurity and high body weight was not generally detectable in children, even older children. (In the child samples from high-income countries considered separately, the OR was just significantly different from 1 [1.11, 95% CI 1.01–1.24], though even here, it was significantly smaller than that for adults [1.41, 95% CI 1.30–1.53].) It is not clear how the IH as currently formulated could account for this. There are some methodological issues that may contribute to the absence of a detectable association. First, studies on children generally measure food insecurity through parental reports. Thus, the measure of food insecurity is even further removed from the causal psychological state than is true in studies in adults, weakening the ability to discover a relationship. Second, measurement of fatness in children is itself complicated by growth. Growth trajectories will be related to the food supply, with individuals with better food access tending to grow faster. Simple BMI-type measures may be particularly problematic for assessing adiposity in growing children (Freedman et al. Reference Freedman, Wang, Maynard, Thornton, Mei, Pierson, Dietz and Horlick2005). However, explaining the weaker association in children stands as a challenge to the IH.
6.5. Why are there genetic influences on obesity?
There is abundant evidence of heritable genetic effects on body-weight adiposity (Maes et al. Reference Maes, Neale and Eaves1997), with a number of specific genetic loci having been implicated through association studies (Frayling Reference Frayling2013; Locke et al. Reference Locke, Kahali, Berndt, Justice, Pers, Day, Powell, Vedantam, Buchkovich, Yang, Croteau-Chonka, Esko, Fall, Ferreira, Gustafson, Kutalik, Luan, Mägi, Randall and Winkler2015). At first blush, this seems at variance with the IH, which gives causal primacy to environmental inputs in explaining who gets fat and who does not. However, we do not see the existence of heritable variation as a fundamental challenge to the hypothesis. Genetic variation is ubiquitous in all kinds of morphological and physiological traits. In terms of the IH, we should expect mutation to produce variation in the mechanisms governing weight regulation, such as those perceiving cues of food insecurity and governing the rate of fat storage in response to them (McNamara et al. Reference McNamara, Houston and Higginson2015). In addition, there might be genetically based variation in such parameters as metabolic rate and the mobility costs of carrying extra fat. Thus, the framework outlined in this article not only allows, but also leads us to expect, that genetic variation in such traits would lead to variation in adiposity. When genetic variation is explicitly incorporated into adaptive dynamic models, the predicted outcome is often genotype by environment interactions (Thorpe et al. Reference Thorpe, Mangel, Metcalfe and Huntingford1998). Thus, it would be of interest to investigate whether known obesity-proneness genetic variants increase obesity risk under all circumstances or especially where food insecurity is also present.
The genetic variability maintained in the mechanisms underlying weight regulation will be greater if the strength of selection against deviations from optimal weight regulation is relaxed. Such a relaxation of selection in recent human evolution, specifically an elimination of the fitness costs of carrying too much weight over the last two million years, is proposed by the “drifty genotype” hypothesis for human obesity (Speakman Reference Speakman2008). Contrary to Speakman (Reference Speakman2008, p. 306), we find it implausible that the fitness costs of high weight have been completely abolished in humans. The claim that ancestral humans completely eliminated predation as a source of mortality cannot be sustained; moreover, the costs of high body weight arise not just from predation but from foraging, agonistic interactions, and many other sources (see Higginson et al. Reference Higginson, McNamara and Houston2016, p. 6). However, it is possible that selection has been relatively relaxed in recent human evolution, and that, consequently, there is greater non-adaptive genetic variation in weight-regulation mechanisms in humans than other species. (We note also that weaker selection against carrying slightly too much fat than against carrying slightly too little fat is already integral to the model from sect. 2 and models like it, due to the asymmetry of the survival function shown in Fig. 1A. Thus, within the IH, there is already greater scope for genetic drift of variants that lead to reserves being a little too high than variants with the opposite effect; see Higginson et al. Reference Higginson, McNamara and Houston2016.) In any case, relaxation of selection in recent human evolution would not completely abolish phylogenetically older weight-regulation mechanisms; the basic functioning of those mechanisms should still remain detectable on average, even if there is individual genetic variability in the response (a point on which Speakman Reference Speakman2004 concurs). The IH and the drifty genotype hypothesis could thus coexist in a multifactorial explanation of the contemporary distribution of obesity.
6.6. Overall evaluation
The IH is attractive because of the way it incorporates both the biological and social roots of obesity. It incorporates the biological roots by deriving from well-developed adaptive principles, positing species-typical evolved adaptations, and drawing on comparative evidence from other species. It incorporates the social roots by locating a key proximate cause of obesity in the social-structural factors that lead to some individuals being food insecure within societies that are very affluent overall. The empirical evidence that food insecurity predicts high body weight in adult women in high-income countries is clear, and a reasonable rationale can be given for why it is only in high-income countries that the association can be observed. On the other hand, the lack of an association in men, although potentially explicable, undermines any claim that the IH by itself is sufficient as an explanation for the current distribution of human obesity. The constellation of food insecurity and an energy-dense food landscape is an obesogenic one, but not all contemporary obesity can be explained by the presence of this constellation.
7. Further applications of the IH
In this section, we briefly discuss some possible extensions of the IH to explain other phenomena related to fatness and the management of body weight.
7.1. Understanding developmental influences on obesity
In recent years, it has become increasingly clear that experiences in early life can predispose individuals to maintaining high levels of body fat, not just as children but subsequently as adults. These experiences can include poor in utero nutrition (Law et al. Reference Law, Barker, Osmond, Fall and Simmonds1992; although see Rogers Reference Rogers2003), childhood exposure to food scarcity (Olson et al. Reference Olson, Bove and Miller2007), or psychosocial stress more generally (D'Argenio et al. Reference D'Argenio, Mazzi, Pecchioli, Di Lorenzo, Siracusano and Troisi2009; Greenfield & Marks Reference Greenfield and Marks2009; Gundersen et al. Reference Gundersen, Mahatmya, Garasky and Lohman2011b; Gunstad et al. Reference Gunstad, Paul, Spitznagel, Cohen, Williams, Kohn and Gordon2006b). Such phenomena are not restricted to humans. We have recently found that European starlings Sturnus vulgaris made to compete hard for food as nestlings develop into adults with a “hungry phenotype”: They are hyperphagic, indiscriminate about what they eat, and heavy for their skeletal size (Andrews et al. Reference Andrews, Viviani, Egan, Bedford, Brilot, Nettle and Bateson2015; Bloxham et al. Reference Bloxham, Bateson, Bedford, Brilot and Nettle2014). There are similar experimental findings from rats and monkeys (Kaufman et al. Reference Kaufman, Banerji, Shorman, Smith, Coplan, Rosenblum and Kral2007; Qasem et al. Reference Qasem, Yablonski, Li, Tang, Pontiggia and D'mello2012).
Rather than seeing these developmental phenomena as separate from the IH, we can see them as part of it. Under the IH, the individual's task is to build up an estimate of the likelihood of periodic shortfall in the food supply over its lifetime, so that it can maintain appropriate reserves. Early experience provides the first data contributing to such an estimate. How much importance it makes adaptive sense to give to early life relative to later experience in setting adult phenotype is a topic of active research (Fawcett & Frankenhuis Reference Fawcett and Frankenhuis2015; Frankenhuis & Panchanathan Reference Frankenhuis and Panchanathan2011; Nettle & Bateson Reference Nettle and Bateson2015; Nettle et al. Reference Nettle, Frankenhuis and Rickard2013; Stamps & Krishnan Reference Stamps and Krishnan2014). It depends, among other things, on the temporal consistency of environmental conditions. Nonetheless, it is plausible to suggest that the empirically observed associations between early life adversity and later obesity reflect some initial calibration or prior setting of the mechanisms that estimate the dangers of starvation from food shortfall in adulthood.
7.2. Explaining dieting-induced weight gain
A number of studies suggest that restrictive dieting, as a strategy for weight loss, is not only ineffective but also counterproductive in the majority of individuals (Mann et al. Reference Mann, Tomiyama, Westling, Lew, Samuels and Chatman2007; Pietiläinen et al. Reference Pietiläinen, Saarni, Kaprio and Rissanen2012; Siahpush et al. Reference Siahpush, Tibbits, Shaikh, Singh, Sikora Kessler and Huang2015). Most individuals who practice restrictive diet regimes regain more weight than they lose, increasing their risk of obesity in the long term. From the food-insecurity perspective, this makes sense. By following a restrictive diet, individuals are intentionally exposing themselves to restricted food availability. Thus, it is very likely that the effect of dieting episodes is to provide the mechanisms governing weight regulation with cues of food insecurity (Nesse Reference Nesse1984; Williams & Nesse Reference Williams and Nesse1991). Under the IH, weight gain as soon as food becomes available again is the predicted result.
7.3. Understanding anorexia nervosa
Although obesity is a major public health concern in affluent countries, about 1% of young people in these countries (mostly women) significantly impair their survival chances by maintaining low body weight in anorexia nervosa. Anorexia is defined by a low body mass index, as well as the sufferer imposing a low body mass target on themselves, above which they dread going and feel it would be inappropriate to do so (Bulik et al. Reference Bulik, Reba, Siega-Riz and Reichborn-Kjennerud2005). Although a full discussion is beyond the scope of this article, the IH is potentially relevant to anorexia in two ways. First, in terms of aetiology, the hypothesis predicts that anorexia will occur where the person's estimate of their food security is unusually high. That is, if an individual has developed the perception that shortfalls will never occur, he or she should favour an extremely lean body and be motivated to maintain it. We have not been able to find any epidemiological studies of food insecurity in relation to anorexia, but we would predict that anorexia sufferers will be at the high-security end of the spectrum, diametrically opposite the obese. Some support for this prediction comes from the evidence that anorexia risk, in contrast to obesity risk, is highest in families of relatively high socioeconomic position (Goodman et al. Reference Goodman, Heshmati and Koupil2014). Note that the IH is agnostic about why individuals might have unusually high perceptions of food security; thus, the hypothesis is not incompatible with a neuropsychological literature investigating general decision-making deficits in some anorexia sufferers (Danner et al. Reference Danner, Sanders, Smeets, van Meer, Adan, Hoek and van Elburg2012). Given that anorexia shows substantial genetic heritability (Bulik et al. Reference Bulik, Sullivan, Tozzi, Furberg, Lichtenstein and Pedersen2006), it could be that genetic factors affect the formation of food-insecurity estimates. The hypothesis merely predicts that low perceived food insecurity might be an important psychological mediator between anorexia risk factors and anorexia symptoms.
A second potential area of relevance is in anorexia treatment. If perceived food insecurity is causally important in promoting weight gain, as the IH asserts, then inducing some food insecurity, for example, by randomly varying feeding routines, might be useful in combating low body weight. This is a contentious proposal, because anorexia patients are at considerable risk of starving themselves to death, and the understandable caregiver response is to try to provide all kinds of foods at all times in the hope that the person will eat them. However, it might be that making at least some kinds of food unavailable at least some of the time is a better strategy for motivating long-term gains in body weight. Given that anorexia tends to have a chronic and disabling course, with a tendency of patients to defend and return to their weight-management practices (Abbate-Daga et al. Reference Abbate-Daga, Amianto, Delsedime, De-Bacco and Fassino2013), the food-insecurity perspective deserves further, if cautious, exploration.
8. Implications of the IH
We conclude by considering the implications of the IH. Despite abundant research on human obesity, there is rather little evidence for effective, scalable interventions that prevent obesity or lead to weight loss that is maintained in the long term (Glenny et al. Reference Glenny, Omeara, Melville, Sheldon and Wilson1997). The IH does not in itself change this situation, of course. However, it ought to change our framing of the problem. If (adult female) obesity results from the psychological mechanisms posited by the IH fulfilling their evolved function, then there is no reason to expect simple information giving, food labelling, or explicit exhortation to be able to override them. Certain interventions, such as restrictive dieting, in fact look potentially counterproductive. Indeed, the IH suggests that the interventions most likely to work are the very antithesis of restrictive dieting: In the words of Dietz's original paper, the IH suggests that “the prevention of obesity in impoverished populations may require increased food supplementation rather than food restriction to achieve a more uniform pattern of food consumption” (Dietz Reference Dietz1995, p. 767).
Perhaps the major virtue of the IH is summed up in the following oxymoron. The IH is a hypothesis about individual decision-making mechanisms, but it ends up pushing the focus in terms of explaining obesity away from individual decisions and onto society. Surely, the key question is why, in countries of historically unprecedented affluence, there are millions of people who feel they might not have enough to eat. These people need not less food, but more: Better food access and less uncertainty in their lives. If the IH has any merit, then tackling these societal problems should lead to a melioration of the obesity epidemic.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0140525X16000947.
ACKNOWLEDGMENTS
Our research has been supported by the Biotechnology and Biological Sciences Research Council under grant BB/J016446/1 and the European Research Council under grant AdG 666669 (COMSTAR).








Target article
Food insecurity as a driver of obesity in humans: The insurance hypothesis
Related commentaries (25)
A game theory appraisal of the insurance hypothesis: Specific polymorphisms in the energy homeostasis network as imprints of a successful minimax strategy
A life-history theory perspective on obesity
Anorexia: A perverse effect of attempting to control the starvation response
Anti-fat discrimination in marriage more clearly explains the poverty–obesity paradox
Appraising food insecurity
Children respond to food restriction by increasing food consumption
Committed to the insurance hypothesis of obesity
Eating and body image: Does food insecurity make us feel thinner?
Epidemiological foundations for the insurance hypothesis: Methodological considerations
Episodic memory as an explanation for the insurance hypothesis in obesity
Expanding the insurance hypothesis of obesity with physiological cues
Future research directions for the insurance hypothesis regarding food insecurity and obesity
Household-level financial uncertainty could be the primary driver of the global obesity epidemic
Implicit attitudes, eating behavior, and the development of obesity
Mapping multiple drivers of human obesity
Obesity as self-regulation failure: A “disease of affluence” that selectively hits the less affluent?
Obesity is not just elevated adiposity, it is also a state of metabolic perturbation
Potential psychological accounts for the relation between food insecurity and body overweight
Predicting human adiposity – sometimes – with food insecurity: Broaden the model for better accuracy
Social nature of eating could explain missing link between food insecurity and childhood obesity
The life history model of the insurance hypothesis
Toward a mechanistic understanding of the impact of food insecurity on obesity
Towards a behavioural ecology of obesity
Using food insecurity in health prevention to promote consumer's embodied self-regulation
“It's a bit more complicated than that”: A broader perspective on determinants of obesity
Author response
Adaptive principles of weight regulation: Insufficient, but perhaps necessary, for understanding obesity