Recent global analyses have highlighted the substantial burden of major depressive disorder (MDD), with a 59% increase in incident cases from 1990 to 2019 despite a decline in age-standardised rates.Reference Mo, Qin, Ye, Hu, Wang and Zhao1 Women and older adults, particularly those in low-sociodemographic index regions, bear a disproportionate burden of MDD. The intricate interplay of risk factors, including intimate partner violence and childhood trauma, underscores the urgent need for targeted and personalised interventions.Reference Mo, Qin, Ye, Hu, Wang and Zhao1 Moreover, the strong comorbidity between MDD and physical diseases such as cardiovascular disorders and diabetes reveals the involvement of shared biological mechanisms, including systemic inflammation and dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which further compound the disease burden.Reference Berk, Köhler-Forsberg, Turner, Penninx, Wrobel and Firth2 Gaining a deeper understanding of the neurobiological underpinnings of MDD is essential for advancing treatment strategies and improving patient outcomes.
Neuroimaging insights into MDD
Resting-state functional magnetic resonance imaging (rs-fMRI) has emerged as a valuable neuroimaging modality for assessment of spontaneous or intrinsic brain activity in a non-invasive manner, without the need for task-based paradigms. One key rs-fMRI metric, the amplitude of low-frequency fluctuations (ALFF), quantifies the localised intensity of spontaneous fluctuations in blood oxygenation level-dependent (BOLD) signalsReference Zang, He, Zhu, Cao, Sui and Liang3 and serves as an indicator of intrinsic neural activity within specific brain regions.Reference Mohamed, Yousem, Tekes, Browner and Calhoun4 Owing to its temporal stabilityReference Kublbock, Woletz, Hoflich, Sladky, Kranz and Hoffmann5 and test–retest reliability,Reference Zuo and Xing6 ALFF has been proposed as a robust biomarker for identifying abnormalities in cerebral intrinsic activity associated with in psychiatric disorders.
Confounding effects of medication and illness duration
Prior neuroimaging research has demonstrated that MDD is associated with widespread alterations in ALFF across various brain regions, including the anterior cingulated cortex (ACC),Reference Guo, Liu, Xue, Xu, Wu and Ma7,Reference Fan, Wu, Yao and Dong8 parahippocampal gyrus,Reference Fan, Wu, Yao and Dong8 ventral median frontal gyrusReference Fan, Wu, Yao and Dong8,Reference Jing, Liu, Ma, Yan, Zhuo and Zhang9 and putamen.Reference Fan, Wu, Yao and Dong8 The inconsistencies observed among these studies may be attributed to the heterogeneity of the disorder, variations in medication status and differences in illness duration.Reference Zhou, Hu, Lu, Zhang, Chen and Gong10 Some studies involving MDD individuals receiving antidepressant treatment reported increased ALFF in the parahippocampal gyrus and putamen, along with decreased ALFF in the middle occipital gyrus.Reference Fan, Wu, Yao and Dong8,Reference Jing, Liu, Ma, Yan, Zhuo and Zhang9 Conversely, another study examining drug-naive individuals with MDD found reduced ALFF in the bilateral orbitofrontal cortex and increased ALFF in the bilateral temporal cortices.Reference Zhang, Zhu, Wang, Zhu, Zhong and Yi11 These cross-sectional findings suggest that medication status may contribute to the variability observed across studies. Moreover, an increasing body of longitudinal research has shown that antidepressants can normalise the abnormal activation of brain regions in MDD.Reference Delaveau, Jabourian, Lemogne, Guionnet, Bergouignan and Fossati12 For instance, antidepressant treatment has been shown to normalise hypoactivity in the dorsolateral prefrontal cortex (DLPFC) during cognitive tasks in MDD individuals.Reference Fales, Barch, Rundle, Mintun, Mathews and Snyder13 Additionally, treatment with escitalopram increased fractional ALFF (fALFF) in the DLPFC, where MDD individuals initially exhibited lower baseline signals compared with healthy controls.Reference Cheng, Xu, Arnone, Nie, Yu and Jiang14 Taken together, these findings suggest that prolonged exposure to antidepressants may serve as a confounding factor influencing neuroimaging results in MDD.
Rationale for studying drug-naive first-episode patients
Furthermore, research suggests that illness duration and the number of depressive episodes influence the neurobiological alterations associated with MDD.Reference Guo, Liu, Yu, Zhang, Zhang and Liu15 One recent meta-analysis reported a correlation between ALFF-measured intrinsic brain activity and illness duration,Reference Zhou, Hu, Lu, Zhang, Chen and Gong10 suggesting that the latter may be a potential confounding factor in neuroimaging studies of MDD. Therefore, to comprehensively characterise alterations in cerebral intrinsic activity in MDD, these limitations must be addressed. Given that the majority of previous studies have focused on chronic MDD patients who had received antidepressant treatment prior to neuroimaging, it is essential to investigate cerebral intrinsic activity in first-episode, drug-naive individuals. Such an approach provides a more reliable means of elucidating the core neurobiological mechanisms underlying MDD, minimising the confounding effects of illness chronicity and medication exposure.Reference Wang, Dai, Su, Wang, Tan and Jin16,Reference Gao, Zou, He, Sun and Chen17
Study objectives and hypotheses
The objective of this study is to utilise voxel-based analysis of the amplitude of the ALFF technique to investigate cerebral intrinsic activity in first-episode, drug-naive individuals with MDD who have a relatively short illness duration. We hypothesise that abnormalities in ALFF activity will be observed in MDD patients compared with healthy controls. We also aim to examine the association between ALFF and clinical parameters such as symptom severity and illness duration. Specifically, we posit the following hypotheses. (a) First-episode, drug-naive MDD individuals will exhibit significant alterations in ALFF compared with healthy controls. (b) Specific brain regions, such as the dorsal anterior cingulate cortex (dACC) and cerebellum, will demonstrate increased ALFF in MDD individuals. This hypothesis is based on prior research indicating that these regions play key roles in cognitive and emotional processing. The dACC is a central component of the cognitive control network and has been shown to exhibit hyperactivation in drug-naive MDD individuals,Reference Liu, Ren, Womer, Wang, Fan and Jiang18 while the cerebellum has been implicated in attention and emotional regulation deficits associated with MDD.Reference Guo, Liu, Xue, Xu, Wu and Ma7 (c) The ALFF in certain brain regions, particularly the right DLPFC, will show a negative correlation with depression severity (Hamilton Rating Scale for Depression [HAMD] scores). This aligns with previous findings suggesting that hypoactivity in the DLPFC is associated with greater depressive symptom severity and impaired cognitive function.Reference Wang, Dai, Su, Wang, Tan and Jin16,Reference Fales, Barch, Rundle, Mintun, Mathews and Snyder13 (d) The observed ALFF changes will provide insights into the early neurobiological alterations associated with MDD, and highlight potential regions involved in cognitive dysfunction. By addressing these hypotheses, this study aims to enhance the understanding of the early neurobiological mechanisms underlying MDD and identify potential biomarkers for early diagnosis and intervention. The novelty of this study lies in its focus on first-onset, untreated MDD individuals, thereby eliminating the confounding effects of medication and providing direct evidence of brain activity alterations in the early stages of depression.
Method
Participants
In this research, 30 individuals with first-episode, drug-naive MDD and 52 healthy controls participated. These were a part of a large cohort research that looked at severe depressive disorder in Han Chinese people.Reference Williams20 The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) was used by two proficient psychiatrists to verify the diagnosis established for every person. Determining the level of depression included using the 17-item HAMD.Reference Chao-Gan and Yu-Feng21 A HAMD total score of >18 on the day of MRI scanning was among inclusion criteria. The following criteria were applied: history of significant medical illness, prior psychiatric treatment, age <18 or >60 years, use of vasoactive medicines, history of alcohol or drug misuse and the presence of psychiatric disorders other than MDD, as defined in DSM-IV. None of those enrolled had received antidepressant treatment before participation in the study. Healthy controls were recruited through public advertisements and matched to the patient group in terms of sociodemographic background. The Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-NP) was used to confirm the absence of psychiatric disorders in the control group. Additional eligibility criteria for healthy controls included the absence of a personal or family history of psychiatric or neurological disorders. All participants were of Han Chinese ethnicity and right-handed. Written informed consent was obtained from all participants prior to study enrollment. The authors affirm that all procedures conducted in this study adhered to the ethical standards of the relevant national and institutional committees on human research, and complied with the Helsinki Declaration 1975 revised in 2013. Ethical approval for this study was granted by the Ethics Committee of West China Hospital, Sichuan University Hospital.
MRI data acquisition
A 3.0T magnetic resonance imaging equipment with an eight-channel, phased array head coil (EXCITE, GE Signa, Milwaukee, USA) was used to scan each participant. The following settings were used to create a gradient-echo echo-planar imaging (EPI) sequence that produced rs-fMRI sensitive to variations in BOLD signal levels: field of view 240 × 240 mm2; matrix size 64 × 64; flip angle 90°; section thickness 5 mm; intersection gap 0; and voxel size 3.75 × 3.75 × 5.00 mm3. Each brain volume comprised 30 axial slices, and each functional imaging session included 200 image volumes preceded by 5 dummy volumes. Each participant utilised foam cushions and soft earplugs to reduce head motion and noise distraction during the rs-fMRI scan. Participants were instructed to relax with their eyes closed, while refraining from falling asleep or engaging in structured or deliberate thought processes. Compliance with these instructions was confirmed immediately after the scanning session. Data were excluded from the study if head movement exceeded 1.5 mm in translation or 1.5° in rotation.
Data preprocessing
Data Processing Assistant for Resting-State Functional MR Imaging (DPARSF, version 2.3; State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China; https://www.restfmri.net) was used to preprocess rs-fMRI data.Reference Lui, Li, Deng, Jiang, Wu and Tang22 The following steps were performed: idue to field homogeneity adjustments in the EPI scanning sequence, the initial images may exhibit signal fluctuations. During data processing, the first five whole-brain images from each scan were discarded. The remaining whole-brain images proceeded to the following step of data preprocessing. Slice timing and motion correction: first, slice timing correction was performed using interpolation to compensate for differences caused by varying acquisition times between two-dimensional images of different slices. Next, realignment was conducted using translation and rotation to minimise the effects of head movement between three-dimensional images acquired at different times. Throughout the scanning process, six-parameter rigid-body transformation was applied for head motion correction. Head movement quality control was conducted to ensure the integrity of rs-fMRI data. Participants with head motion exceeding 1.5 mm in translation (x, y or z directions) or 1.5° in rotation (pitch, yaw or roll) were excluded from further analysis. Additionally, framewise displacement was calculated for each time point to assess motion-related variability, and mean framewise displacement across the scan was reported for all participants. Scrubbing was applied to remove high-motion volumes with framewise displacement >0.5 mm, ensuring that only high-quality data were retained for subsequent analysis. These thresholds and procedures were chosen based on established standards for rs-fMRI motion correction, to minimise noise and artefacts. Normalisation: functional images were spatially normalised to the Montreal Neurological Institute (MNI) space using the EPI template. Voxel size was resampled to 3 × 3 × 3 mm³.
ALFF
Using DPARSF software and a methodology similar to our previous work, ALFF was computed.Reference Biswal, Yetkin, Haughton and Hyde23 The process included the following steps. Time-series transformation: the preprocessed time-series data of each voxel were transformed into the frequency domain using the fast Fourier transform (FFT) algorithm with zero-padding (taper percentage 0) to improve spectral resolution.Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman24 The power spectrum was computed as the square of absolute FFT coefficients. Frequency band selection: to isolate low-frequency fluctuations, the power spectrum was averaged across the frequency range of 0.01–0.08 Hz, corresponding to neuronal activity-related signals. High-frequency signals and scanner noise were excluded.
ALFF calculation: the square root of the averaged power spectrum was taken to derive the ALFF value for each voxel:
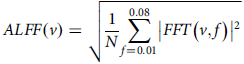 $$ ALFF \left(v\right)=\sqrt{{1 \over N}\sum _{f=0.01}^{0.08}\left| FFT\left(v, f\right)\right| ^{2}} $$
$$ ALFF \left(v\right)=\sqrt{{1 \over N}\sum _{f=0.01}^{0.08}\left| FFT\left(v, f\right)\right| ^{2}} $$
where v represents the voxel and N is the number of frequency points within the selected range. Normalisation: ALFF values were normalised by dividing each voxel’s ALFF by global mean ALFF across the entire brain, resulting in a dimensionless ratio to control for inter-individual variations:Reference Woo, Krishnan and Wager25
Spatial smoothing: ALFF maps were spatially smoothed using an 8 mm, full-width-at-half-maximum (FWHM) Gaussian kernel to enhance signal-to-noise ratio and reduce spatial variability. Cluster thresholding: a rigorous statistical threshold was applied (P < 0.001, family-wise error [FWE] corrected) to identify significant ALFF changes.
Statistical analysis
Clinical and demographic differences between MDD individuals and healthy controls were analysed using IBM SPSS Statistics version 27 (Armonk, NY, USA). Continuous variables were compared using independent-samples t-tests, while categorical variables were assessed using chi-square tests with a significance threshold of P < 0.05. To control for potential confounding factors, age, gender and head movement (quantified using mean framewise displacement) were included as covariates in the voxel-based analysis of ALFF differences between groups. This analysis was conducted in Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, University College London, UK; https://www.fil.ion.ucl.ac.uk/spm/). Group differences in ALFF were evaluated using two-sample t-tests, with statistical thresholds set to minimise type II errors and improve spatial specificity.Reference Monkul, Hatch, Sassi, Axelson, Brambilla and Nicoletti26 Specifically, a voxel-level threshold of P < 0.001 was applied, followed by FWE correction at the cluster level and with a significance threshold of P < 0.05. Within each significant cluster, peak voxel coordinates (in MNI space) and associated t-values were reported to highlight the most robust group differences in ALFF. Correlation analyses between ALFF and clinical measures, including HAMD scores and illness duration, were performed using the simple regression function in SPM12.
Results
Table 1 presents an overview of the clinical and demographic characteristics of all participants. There were no statistically significant differences in age (P = 0.754) or gender (P = 0.114) between those with MDD and healthy control groups. The results of the voxel-based ALFF analysis are summarised in Table 2 and Fig. 1(a). Compared with healthy controls, individuals with MDD exhibited significantly increased ALFF in the dACC and vermal subregion V3 of the cerebellum.Reference Pizzagalli27 All significant clusters survived FWE correction at P < 0.05, following a voxel-wise threshold of P < 0.001. No significant decreases in ALFF were observed in the MDD group.
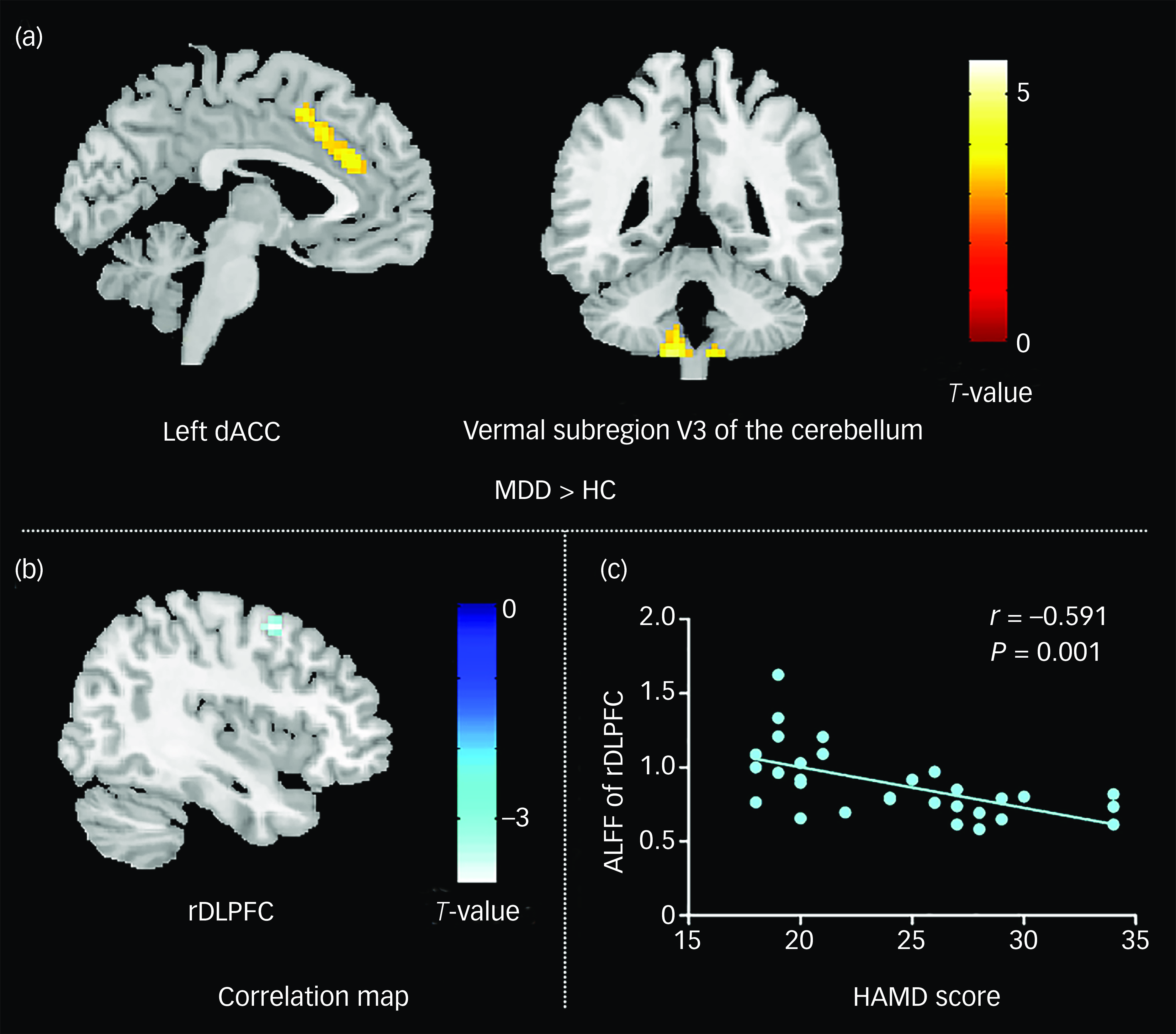
Fig. 1 ALFF differences and correlation analyses in first-episode, drug-naive MDD patients. (a) Compared with healthy controls, individuals with MDD had higher ALFF in the bilateral cerebellum and left dACC. (b) Negative correlation between ALFF in the DLPFC and HAMD scores. (c) Scatter plot illustrating the significant negative relationship between ALFF in the right DLPFC and HAMD scores, with the regression line indicating the trend of association. At the individual voxel level, the statistical threshold was set at P < 0.001 and, at the cluster level, it was adjusted for multiple comparisons using family-wise error correction (P < 0.05). HAMD, Hamilton Rating Scale for Depression score; rDLPFC, right dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate cortex; HC, healthy controls; ALFF, amplitude of low-frequency fluctuation; MDD, major depressive disorder.
Table 1 Demographic and clinical characteristics of all participants
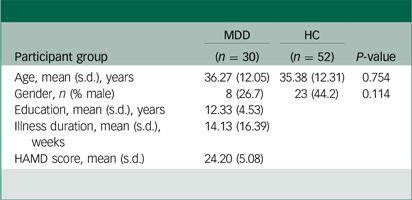
MDD, major depressive disorder; HC, healthy controls; HAMD, Hamilton Rating Scale for Depression.
Table 2 Region-specific differences in amplitude of low-frequency fluctuations between first-episode, drug-naive patients with major depressive disorder and healthy controls
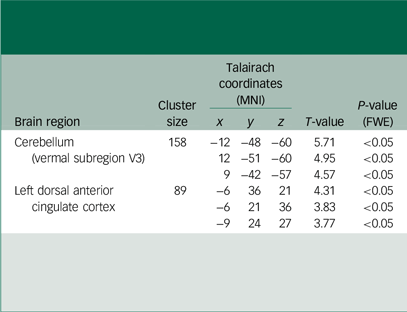
MNI, Montreal Neurological Institute; T-value, statistical value of peak voxel showing differences in amplitude of low-frequency fluctuations (ALFF) between patients with major depressive disorder and healthy controls who were not receiving therapy at the time of their first episode. The statistical threshold was established at P < 0.001 for individual voxels, and modified to P < 0.05 for clusters based on family-wise error (FWE) correction for multiple comparisons.
Following analysis of the correlation between MDD clinical measures and ALFF parameters, patients showed a negative association (r = −0.591, P < 0.001) between HAMD score and ALFF in the right DLPFC (Fig. 1(b) and (c)). There were no associations found between ALFF and length of illness.
Discussion
This study investigated resting-state cerebral intrinsic activity in first-episode, drug-naive individuals with MDD who had a relatively short illness duration. The results revealed increased ALFF in the dACC and vermal subregion V3 of the cerebellum, suggesting dysregulated cerebral intrinsic activity in the early stages of MDD, independent of medication effects. Furthermore, a negative correlation was observed between ALFF in the right DLPFC and HAMD scores, indicating that altered intrinsic activity in the right DLPFC could serve as a potential marker of depression severity in MDD patients. This study provides novel insights into the neurobiological alterations underlying the early stages of MDD, contributing to a better understanding of the disorder’s pathophysiology and potential biomarkers for early diagnosis and intervention.
To the best of our knowledge, the ACC can be functionally and anatomically subdivided into an ‘affective subdivision’, which includes the rostral ACC and subgenual ACC, and a ‘cognitive subdivision’, which encompasses the dACC.Reference Pizzagalli27 The dACC serves as a critical hub within the cognitive control network, which is responsible for higher-order cognitive processes such as decision-making, working memory and conflict resolution.Reference Dutta, McKie and Deakin28 A recent meta-analysis of neuroimaging studies has shown that cognitive control tasks consistently engage the dACC.Reference Niendam, Laird, Ray, Dean, Glahn and Carter29 Additionally, meta-analytic findings on cognitive deficits in MDD have identified impaired executive function as a hallmark of first-episode MDD.Reference Zhou, Hu, Lu, Zhang, Chen and Gong10
In individuals with executive dysfunction, the dACC may require compensatory hyperactivation to maintain normal cognitive functions, such as decision-making and conflict resolution. In the present study, we observed increased ALFF in the dACC of first-episode, drug-naive MDD patients, suggesting aberrant intrinsic activity in this region during the early course of the disorder.
In comparison with healthy controls, first-episode, drug-naive MDD individuals showed greater ALFF activity in the dACC. Furthermore, Liu et al discovered that drug-naive MDD patients have higher ALFF activity in the dACC.Reference Liu, Ren, Womer, Wang, Fan and Jiang30 However, a previous meta-analysis of eight ALFF studies demonstrated that ACC activity increase was detected in treated patients but not in drug-naive MDD patients.Reference Zhou, Hu, Lu, Zhang, Chen and Gong10 That meta-analysis included five original studies on drug-naive patients, and four of these studies failed to find ALFF activation differences in the ACC between MDD patients and healthy controls; the tiny sample size might have been the cause of this disparity,Reference Wang, Dai, Su, Wang, Tan and Jin16 and lower field strength of the magnetic resonance scannerReference Guo, Liu, Xue, Xu, Wu and Ma7 in those studies than ours. Besides, the discrepancies between the findings of the present study and those reported in meta-analyses may be attributed to the lack of detailed subregional analyses in the latter, as well as to differences in patient sample characteristics, particularly regarding illness duration. In comparison with the study by Liu et al,Reference Liu, Ren, Womer, Wang, Fan and Jiang30 our study specifically focuses on first-episode, drug-naive MDD patients with a mean illness duration of only 14 weeks, allowing for a more precise investigation of early-stage neural alterations before disease progression occurs. Additionally, the use of a more stringent statistical threshold (P < 0.001 with FWE correction) enhances the robustness and reliability of our findings, minimising the likelihood of false-positive results. Moreover, the observed significant negative correlation between ALFF in the right DLPFC and HAMD scores suggests a potential biomarker for depression severity, which may contribute to early diagnosis and clinical monitoring of MDD.
Additionally, we discovered that individuals with MDD had considerably higher ALFF than controls in vermal subregion V3 of the cerebellum. The functional abnormalities of the cerebellum are a common manifestation of MDD.Reference Phillips, Hewedi, Eissa and Moustafa31 Several fMRI studies have reported significantly increased ALFF and regional homogeneity (ReHo) in the cerebellum, including vermal subregion V3, in MDD patients compared with healthy controls,Reference Guo, Liu, Xue, Xu, Wu and Ma7,Reference Guo, Sun, Liu, Xu, Wu and Liu32 findings that align with the results of our study. Additionally, a study on medication-naive MDD patients demonstrated enhanced cerebellar connectivity with the ACC during the resting state, further supporting the involvement of the cerebellum in the neuropathophysiology of MDD.Reference Ma, Zeng, Shen, Liu and Hu33 The cerebellum receives projections from DLPFC, the medial frontal cortex, ACC, the parietal and superior temporal areas and the hypothalamus, largely via the thalamus relay, all of which are relevant to cognitive processing.Reference Rapoport, van Reekum and Mayberg34 Vermal area V3 of the cerebellum involves various domains of cognition such as language processing, memory and attention.Reference Park, Pipitone, Baer, Winterburn, Shah and Chavez35 Studies of cognitive deficits demonstrated that attention was a trait marker and reduced in first-episode MDD.Reference Lui, Wu, Qiu, Yang, Kuang and Chan19 Hence, the increased ALFF activity in vermal subregion V3 of the cerebellum could be interpreted as a result of counteracting or delaying the decline of attention. For those with reduced attention, the cerebral intrinsic activity of vermal subregion V3 of the cerebellum may need to be enhanced to maintain near-normal function. Our finding of higher ALFF activity in vermal subregion V3 of the cerebellum may be of significant clinical interest. Future studies combining structural and functional MRI are warranted to clarify the findings.
In this investigation, we discovered a negative correlation between HAMD scores and ALFF in the right DLPFC, suggesting that cerebral-intrinsic activity of the right DLPFC may be applied as an indicator of depression severity in first-episode, drug-naive MDD patients. Specifically, ALFF activity of the right DLPFC would decrease as depression symptoms worsen in individual with MDD. Because an important central component in cognitive control networks is involved in MDD and participation in maintaining and manipulating working memory, goal-directed action and attentional control,Reference Miller and Cohen36 the DLPFC has also attracted attention in research into the pathophysiology of MDD. Several studies reported hypoactivation of the DLPFC in patients while performing cognitive tasks such as planning, word generation and working memory.Reference Fincham, Carter, van Veen, Stenger and Anderson37–Reference Rajah and D’Esposito39 Wang et al found that ALFF activity in the DLPFC was decreased in first-episode, drug-naive patients with MDD relative to controls,Reference Wang, Dai, Su, Wang, Tan and Jin16 although this result was not found in our study. The observed discrepancies may be attributed to differences in depressive symptom severity among study populations. In the study of Wang et al, patients exhibited more severe depressive symptoms than those in our cohort, which may have contributed to the disparity in findings. Our research highlights the critical role of the DLPFC in the manifestation of depressive symptoms in MDD. Additionally, it is important to acknowledge that years of education have been identified as a significant factor influencing the onset and progression of MDD. While our study collected data on education years for MDD individuals, similar information was not available for healthy controls, precluding a direct statistical comparison. This represents a limitation of the current study, and future research should aim to systematically collect education-related data across all participant groups to fully assess its potential impact on MDD.
The findings of this study indicate that first-episode, drug-naive individuals with MDD exhibit significantly increased ALFF in vermal subregion V3 of the cerebellum and dACC. These results suggest that abnormal changes in cerebral intrinsic activity occur in the early stages of MDD. The practical implications of these findings are significant, because they highlight the potential for brain imaging assessments in the early stages of depression to identify potential cognitive dysfunctions, thereby providing a basis for early intervention and treatment. Additionally, this study provides new insights into the early neurobiology of depression, which may contribute to the development of novel diagnostic and therapeutic strategies. However, several limitations should be acknowledged. First, the small sample size may limit the generalisability and statistical power of the findings. Future research should include larger cohorts to enhance the reliability of the results. Second, the cross-sectional design precludes the ability to track dynamic changes in brain activity over time. Future studies should employ longitudinal designs to assess neurobiological alterations before and after treatment, or across different stages of the disorder. Third, physiological noise may affect the accuracy of rs-fMRI data. To mitigate potential confounding effects, future studies should incorporate simultaneous monitoring of heart rate and respiratory rate to improve data reliability. Moreover, this study primarily focused on intrinsic brain activity alterations in first-episode, drug-naive MDD individuals, using ALFF as the primary measurement index. Due to the study design and resource constraints, specific assessments of attentional changes were not included. This decision was based on the study’s primary objective: to explore the relationship between ALFF and clinical parameters such as depression severity and illness duration, rather than specific cognitive function changes. Additionally, measuremnt of attention would require additional behavioural assessments or cognitive tasks, which could increase study complexity and duration. However, future research should incorporate attentional assessments, particularly investigating the relationship between attention and cerebellar ALFF changes. Such investigations could provide a more comprehensive understanding of cognitive impairments in MDD individuals and potentially identify biomarkers for early diagnosis and intervention.
By addressing these limitations, future studies can further elucidate the neurobiological mechanisms of MDD and provide stronger empirical evidence to support clinical practice.
Our study identified the dACC and vermal subregion V3 of the cerebellum as being closely associated with drug-naive MDD individuals when they first experienced depression, implying a disturbance of cerebral intrinsic activity in the early course of MDD without the interference of medication. Given the critical role of these regions in cognitive function,Reference Niendam, Laird, Ray, Dean, Glahn and Carter29,Reference Miller and Cohen36 it is speculated that early increase in cerebral intrinsic activity within these regions may help delay or mitigate the progression of cognitive impairment in MDD individuals during the early stages of the disorder. This finding has important clinical implications, particularly for the early screening and timely intervention of MDD. Additionally, our study suggests that altered ALFF in the right DLPFC may serve as a potential biomarker for evaluation of depression severity in first-episode, drug-naive MDD individuals. This finding provides new insights into the neurobiological mechanisms underlying the early course of depression, which may contribute to the development of more effective diagnostic and therapeutic strategies.
Data availability
The data-sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Xiaoxiao Hu: methodology, formal analysis, data curation, conceptualisation, supervision, resources and writing of original draft. X. Huang: methodology, formal analysis, data curation, supervision, resources and writing of original draft. Xinyu Hu: methodology, formal analysis, data curation, conceptualisation and writing of original draft. H.L.: formal analysis, data curation, conceptualisation and writing of original draft. L.Z.: formal analysis, data curation, conceptualisation, supervision and writing of original draft. L.L.: formal analysis, data curation, resources and writing (review and editing). X.B.: methodology, formal analysis, data curation and writing (review and editing). S.T.: formal analysis, data curation and writing (review and editing). Q.G.: formal analysis, data curation and writing (review and editing).
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Declaration of interest
None.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation, and with the Helsinki Declaration of 1975 as revised in 2013. All procedures involving human subjects/patients were approved by the Ethics Committee of West China Hospital, Sichuan University Hospital.
Consent statement
Written informed consent from all subjects was obtained for any experimental work with humans.







eLetters
No eLetters have been published for this article.